Abstract
Bioenergetics is central to our understanding of living systems, yet has attracted relatively little attention in origins of life research. This article focuses on energy resources available to drive primitive metabolism and the synthesis of polymers that could be incorporated into molecular systems having properties associated with the living state. The compartmented systems are referred to as protocells, each different from all the rest and representing a kind of natural experiment. The origin of life was marked when a rare few protocells happened to have the ability to capture energy from the environment to initiate catalyzed heterotrophic growth directed by heritable genetic information in the polymers. This article examines potential sources of energy available to protocells, and mechanisms by which the energy could be used to drive polymer synthesis.
Capturing energy from the environment would have been critical for early life to synthesize genetic polymers and may have involved formation of formaldehyde in response to UV light or electrical discharges.
Previous research on life's origins has for the most part focused on the chemistry and energy sources required to produce the small molecules of life—amino acids, nucleobases, and amphiphiles—and to a lesser extent on condensation reactions by which the monomers can be linked into biologically relevant polymers. In modern living cells, polymers are synthesized from activated monomers such as the nucleoside triphosphates used by DNA and RNA polymerases, and the tRNA-amino acyl conjugates that supply ribosomes with activated amino acids. Activated monomers are essential because polymerization reactions occur in an aqueous medium and are therefore energetically uphill in the absence of activation.
A central problem therefore concerns mechanisms by which prebiotic monomers could have been activated to assemble into polymers. Most biopolymers of life are synthesized when the equivalent of a water molecule is removed to form the ester bonds of nucleic acids, glycoside bonds of polysaccharides, and peptide bonds in proteins. In life today, the removal of water is performed upstream of the actual bond formation. This process involves the energetically downhill transfer of electrons, which is coupled to either substrate-level oxidation or generation of a proton gradient that in turn is the energy source for the synthesis of anhydride pyrophosphate bonds in ATP. The energy stored in the pyrophosphate bond is then distributed throughout the cell to drive most other energy‐dependent reactions. This is a complex and highly evolved process, so here we consider simpler ways in which energy could have been captured from the environment and made available for primitive versions of metabolism and polymer synthesis. Because the atmosphere of the primitive Earth did not contain appreciable oxygen, this review of primitive bioenergetics is limited to anaerobic sources of energy.
BACKGROUND
Fundamental Considerations from Thermodynamics and Kinetics
In general, life is characterized by the fact that the catalytic and genetic polymers exist in a steady state far from equilibrium. It follows that the origin of life can be understood in terms of a process in which the flow of energy through relatively simple systems of molecules produced a more complex set of polymeric molecules that had specific physical and chemical properties. The origin of life occurred when a subset of these molecules was captured in a compartment and could interact with one another to produce the properties we associate with the living state.
Energy flow, and the changes it produces, are described by the fundamental laws of thermodynamics and kinetics. These concepts are familiar to most readers, but it is less obvious how they can be applied to our understanding of the prebiotic environment and the increase in chemical complexity driven by energy flux. We will briefly recapitulate them here in relation to the origin of life.
1. The amount of energy released as a reaction proceeds toward equilibrium and is referred to as free energy, which has components of enthalpy and entropy. Both must be taken into account to understand how systems of molecules can become more complex. On the prebiotic Earth, immense numbers and varieties of chemical reactions were taking place because the Earth itself was in a state of thermodynamic disequilibrium. To understand the origin of life, it is essential to sort out which of the many energy sources were primary factors in driving the increasing complexity from which life emerged.
2. All reactions in principle are reversible and can approach equilibrium from either direction. This means that a potentially destructive reaction such as hydrolysis of polymers can proceed toward polymer synthesis if there is a way to remove water molecules so that covalent bonds can form. Their enzyme-catalyzed biosynthesis requires an input of metabolic energy, primarily delivered as pyrophosphate bonds of ATP, but the linking bonds are also thermodynamically unstable, which means that enzymes can also catalyze spontaneous hydrolysis of the bonds. The result is that life incorporates a continuous and controlled synthesis and breakdown of its polymeric components. Similar reactions were presumably occurring in the prebiotic environment, so it is essential to establish plausible mechanisms by which polymers could be synthesized and accumulate despite hydrolytic back reactions.
3. Because the concentration of reactants strongly affects reaction rates, it seems likely that for a prebiotic reaction to proceed at a useful rate, there must have been concentrating processes such as evaporation or adsorption. A dilute solution of activated monomers is unlikely to form polymers because at low concentrations the rate of monomer condensation is less than the rate of their hydrolytic deactivation. However, adsorption of activated monomers on mineral surfaces could enhance their polymerization by increasing their concentration and possibly orienting them in a way that favors condensation.
4. The reactants in a given reaction must overcome an energy barrier called activation energy that limits the rate at which the reaction can occur. Elevated temperatures provide activation energy to a potentially reactive system of molecules. The global temperature when life began is estimated to be in the range of 55 and 85°C (Kauth and Lowe 2003) but there would be considerable variability around this mean, ranging from cold polar regions to extensive high temperature vulcanism. It is reasonable to consider that thermal activation energy was likely to be abundant, so that the major hurdles to be overcome in promoting polymerization reactions would be to concentrate, organize, and chemically activate monomers.
5. Catalysts reduce the activation energy barrier so that a reaction can proceed more rapidly toward equilibrium. There were no protein enzymes on the prebiotic Earth, so simpler catalysts like mineral surfaces, metal ions, and small polymers presumably acted as catalysts in primitive metabolic pathways.
6. Chemical kinetics defines the rates at which a given reaction occurs, and allows thermodynamically unstable molecular structures to exist far from equilibrium. A protein or nucleic acid in water, for instance, will ultimately hydrolyze to its component amino acids. However, in the absence of a catalyst, this is a slow reaction, so that faster catalyzed reactions of biosynthesis can keep up with the slower degradative rate of hydrolysis. The difference in reaction rates is referred to as a kinetic trap. On the early Earth, if there was a relatively fast process that could produce chemical bonds between monomers, kinetic traps would allow the resulting polymers to have a transient existence even if they were thermodynamically unstable.
Overview of Contemporary Bioenergetics
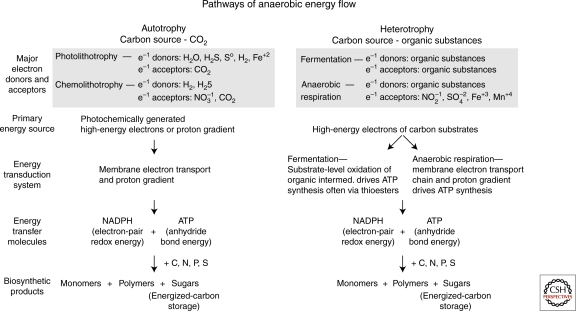
The bioenergetic pathways of contemporary anaerobic life are well understood. As depicted in Figure 1, the light energy captured by photolithotrophs is used to activate and release electrons from inorganic donors like H2S, So, or H2, thereby producing the electrochemical energy that reduces carbon dioxide and yields the organic molecules required by life. Similarly, chemolithotrophs transfer high energy electrons from H2 and H2S to lower energy states in electron acceptors such as CO2 and NO3−, and use the derived free energy to reduce carbon dioxide to organic molecules. The organic products of such reactions are subsequently used by heterotrophic life as electron donors in their energy metabolism based on either anaerobic respiration or fermentation.
Figure 1.
Energy sources and processing by anaerobic autotrophs and heterotrophs. See text for discussion.
In anaerobic photolithotrophy, chemolithotrophy, and respiration, the acquired electrochemical energy is used to pump protons across membranes in such a way that an electrochemical proton gradient is produced, equivalent to approximately 0.2 volts of electrical potential. This energy of the proton potential is coupled to ATP synthesis catalyzed by an ATP synthase embedded in the membrane. The pyrophosphate bond energy in the ATP is then transferred by diffusion to the rest of the cell where it drives a variety of essential metabolic reactions, motor molecule functions, ion transport processes, and polymerization reactions of biosynthesis.
In contrast, in fermentation, the electrochemical energy stored in organic substrates activates the transfer of high energy electrons from carbon groups (electron donors) to lower energy states provided by other carbon groups (electron acceptors). This substrate-level electron transfer oxidizes the electron donor to create an anhydride intermediate (usually a thioester) that in turn drives the synthesis of ATP (Gottschalk 1986)
The coupling of ATP synthesis to electron transfer and proton gradients is clearly a highly evolved system. The bioenergetic processes used by the first forms of cellular life must have been very different even though the same sources of energy were available, with the exception of electron transport to molecular oxygen. To understand the origin of life, we need to establish plausible sources of energy that would drive synthetic reactions.
Energy Sources on the Prebiotic Earth
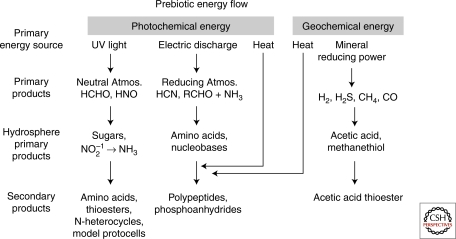
Three kinds of energy are considered in this article. As shown in Figure 2, the first is the relatively high energy required to synthesize small molecules that have the potential to serve as monomers for polymerization reactions and feedstock for a primitive metabolism. These include photochemical energy available in ultraviolet light, atmospheric electric discharge, and geological electrochemical energy. The second is a series of relatively low energy reactions that incorporate condensation processes by which monomers are assembled into random polymers. These include anhydrous heat, mineral-catalyzed synthesis, and sugar-driven reactions. The third is the energy flow in metabolic networks in which an energy source in the local environment is captured and then transferred to standardized energy carriers (like ATP and NAD[P]H). These are catalytically coupled to a series of intracellular reactions that ultimately activate monomers required for catalyzed polymerization.
Figure 2.
Possible pathways for synthetic prebiotic reactions. See text for discussion.
Table 1 shows the kinds and relative amounts of energy on today's Earth. It is a reasonable assumption that similar energy sources were available 4 billion years ago at the time of life's origin. Light energy is by far the most abundant, and in fact photochemistry drives virtually all life today. Could light have been a primary energy source for the first forms of life? This is an obvious possibility, yet there is a major conceptual problem: In modern life, capturing visible light requires a pigment system and a mechanism for transducing the energy content of photons into chemical energy to be used in metabolism, and there is as yet no plausible way to do this in a prebiotic scenario.
Table 1.
Most of the energy flux on the early Earth was in the form of light energy from the sun, just as it is today.
| Energy sources on the early Earth (kilojoules m−2yr−1) | |
| Solar radiation (UV<250 nM) | 24,000 |
| Shock waves from impacts | 200 |
| Radioactivity | 117 |
| Electrical discharges | 2.9 |
| Volcanoes | 5.4 |
| Chemical energy | Significant for the origin of life, not yet estimated. |
| The value shown in the table is the energy of ultraviolet radiation in a wavelength range that is absorbed by common organic compounds such as PAH, and therefore capable of activating photochemical reactions. Table adopted from estimates given by Chyba and Sagan (1992) and Miller and Urey (1959). There is considerable uncertainty in these estimates, which are presented only to illustrate order-of-magnitude approximations for energy flux in meter-scale localized environments. | |
However, ultraviolet light is clearly a potential source of high energy for small molecule synthesis, together with the energy released by shock waves generated by impacting comets and asteroid-sized objects (Chyba and Sagan 1992). The electrical discharge used in the original Miller-Urey experiments, and elevated temperature and pressures associated with geothermal environments (>200°C) can also do chemical work, but these sources represent a tiny fraction of the total energy flux. Electrical discharge is meant to simulate lightning in reducing gas mixtures, and Miller (1953) found that small amounts of highly reactive HCN and HCHO were produced in the discharge, which afterwards underwent Strecker reactions to produce several amino acids. This experiment revolutionized origins of life research, because for the first time, biologically relevant molecules were synthesized in a simulated prebiotic environment using a plausible source of energy.
Formaldehyde can also be synthesized by UVlight interacting with water vapor and carbon dioxide in the upper atmosphere, and a plausible photochemical reaction was proposed by Pinto et al. (1980). From reasonable assumptions about UV flux and composition of the early atmosphere, these workers calculated that formaldehyde was added to the oceans at a rate of 1011 moles/year. It has long been known that formaldehyde (HCHO) in alkaline solutions readily reacts to form a variety of carbohydrates, as is discussed later. Cyanide (HCN) is another common product of UV and electrical energy impinging on mixtures containing nitrogen and gases such as CO and CO2, and cyanide readily reacts with itself to produce other biologically relevant molecules. For instance, a few years after the Miller experiment was published, John Oro (1961) discovered that at high concentration, HCN could undergo pentamerization to form adenine.
Elevated pressures and temperatures associated with geothermal conditions can also promote significant chemical reactions. In particular, the Fischer-Tropsch (FT) reaction uses elevated temperatures to provide activation energy for a reaction in which carbon monoxide and hydrogen combine to produce long-chain hydrocarbons. Nooner et al. (1987) were among the first to test this reaction as a potential prebiotic source of hydrocarbon derivatives, using meteoritic iron as a catalyst, which lowers the activation energy of the reaction.
Although it is a reasonable assumption that the synthesis of organic compounds required for the origin of life was driven by energy available in the prebiotic environment, another source of potential chemical energy was available in organic compounds delivered by comets and meteoritic infall during late accretion. Chyba, Sagan, and co-workers (1990, 1992) estimated the total amount of organic carbon compounds that could be delivered in this way, and within an order of magnitude, it was in the range of the amounts estimated to be synthesized by Miller-Urey reactions under the most favorable conditions. The energy content would be present in the form of reduced carbon compounds that could undergo chemical modification if they were exposed to mineral surfaces in geological settings of appropriate fugacity (Shock 1990) (See also Pizzarello and Shock 2010). This possibility is largely unexplored and is likely to be a fruitful direction for future research.
In summary, early investigations, beginning with Stanley Miller's experiments, showed that the energy available in electrical discharge, UV light, and elevated temperatures, when impinging on simple gas mixtures, can produce reactive compounds like HCN and HCHO, which in turn react to form amino acids, purine and pyrimidine bases, lipid-like amphiphiles, and carbohydrates. Assuming that inorganic phosphate was also available, all of the monomers required for the synthesis of major polymers of life would be available for the next stage of increasing complexity in the prebiotic environment.
Thermal Energy
Moderate heating at temperatures below 200°C is not a direct source of useful chemical energy. Heat speeds up the rate at which a reaction approaches chemical equilibrium, but does not change the position of equilibrium to favor one side of a reaction. However, heat can move the equilibrium of a reaction if it changes the concentration of the reactants by removing solvent, or by evaporating (volatilizing) a product of one side of a reaction, such as water from a condensation reaction. Under these conditions, net polymer synthesis becomes favorable because the monomer concentration is increased, and once the solvent water has evaporated, continued heating favors polymerization by removing the water produced by condensation reactions.
Early researchers investigating the origin of life attempted, with some success, to drive condensation reactions by drying potential reactants such as amino acids and nucleotides at elevated temperatures. The advantage of using anhydrous heating to drive condensation is that it is by far the simplest way to produce the polymers required for life to begin. Although the amino acid polymerization studied by Fox and colleagues is a prominent example (Fox and Harada 1958), there were also early attempts to drive phosphodiester bond formation under these conditions. For instance, Verlander and Orgel (1974) found that oligonucleotides up to six nucleotides long could be synthesized from cyclic 2′,3′-AMP when it was dried and heated. Usher (1977) suggested that the presence of a template should promote such reactions. Earlier McHale and Usher (1976) had shown that 6-mers of adenylic acid could form a phosphodiester bond to give a 12-mer, if a polyuridylic acid template was present.
Although anhydrous heat would appear to be a plausible source of energy to drive prebiotic condensation reactions, there are two problems with this approach. The first is obvious: In a dry state, potential reactants are trapped in a solid and diffusion is minimized, so that bond formation is limited to interactions between neighboring molecules. The second problem is that multiple reactions become activated as temperature increases, with the result that nonspecific chemical bonds begin to form, ultimately producing polymerized tars. But at temperatures between 60° and 80°C, it may be possible to organize reactants in such a way that bond formation is more specific. Cycling between hydrated and anhydrous conditions could then drive the synthesis of specific kinds of polymers. Furthermore, if the dry phase of a condensation reaction is cycled repeatedly through a hydrated phase, the reaction can lead to the accumulation of more complex products, as is discussed in the next section.
It seems reasonable to expect that small amounts of organic compounds would occur in the prebiotic environment, but they would be present as complex mixtures with inorganic anions and cations. The prebiotic environment was likely to have thousands of different organic compounds dissolved in a global ocean and lacustrine bodies of fresh water that accumulated on volcanic land masses rising above the ocean. The mixture would contain biologically relevant compounds such as amino acids and sugars, largely as racemic mixtures of their d and l enantiomers. Because the organic compounds would be present as very dilute solutions, it is essential to discover processes by which such compounds could be concentrated and organized to participate in intermolecular reactions such as polymer synthesis and protocell assembly needed for the origin of life.
There are two obvious ways in which dilute solutions can be concentrated. The simplest is the input of heat energy to evaporate the solution onto mineral surfaces. For instance, evaporating a volume of water containing one micromole of an organic solute such as an amino acid evenly spread onto a mineral surface area of 100 cm2 will form a film approximately 10 molecules thick. During the last stages of evaporation, the concentration of solutes pass through molar concentrations, thereby promoting any chemical reactions that could not occur spontaneously in dilute solutions. Subsequent wetting cycles would release the products into the environment for further processing.
The second process, originally proposed by Bernal (1951), is that clay surfaces strongly adsorb organic compounds from dilute solutions and thereby concentrate them. Furthermore, the orderly arrangement of charged groups in the crystal structure of the clay can impose order on the adsorbed solutes and thereby promote polymerization reactions. A good example is the polymerization of activated nucleotides into short molecules of RNA, to be discussed later in this article (for review, see Ferris 1999, 2002).
It is interesting to note that simple freezing is also a process that concentrates potential reactants in an otherwise dilute solution. As a solution freezes, the microscopic crystals that initially form are nearly pure ice, so that solutes are concentrated into fluid eutectic phases between the crystals. Freezing has been used to promote nucleobase synthesis in frozen cyanide solutions (Miyakawa et al. 2002) and Kanavarioti et al. (2001) found that oligomers of RNA were synthesized from activated nucleotides frozen at −18°C. (See Orgel 2004 for review.) Although extensive ice on a hot early Earth seems unlikely, there is still uncertainty about actual temperatures available in specific sites, so ice eutectics cannot be dismissed as a means to concentrate dilute reactants in solution.
Thermal Energy Storage in Pyrophosphate Bonds
Most chemical energy in cells today circulates in the cytoplasm as ATP. Although cells use ATP to drive synthetic reactions, ATP is not a primary energy source, but rather is an energy transfer molecule that picks up energy from an energy source and then delivers it to energy-requiring reactions. This constant resynthesis (cycling) of ATP inside the cell is revealed by estimates showing that to synthesize 1 g of cell mass requires the energy of about 20–100 g of ATP (Stouthamer 1977). The chemical energy content of ATP is present in the pyrophosphate bonds that link the second and third phosphate groups of ATP. These are anhydride bonds, and their chemical energy is released by energetically downhill group transfer reactions of the phosphate group to other molecules, an activating process called phosphorylation. The second molecule gains chemical energy and can in turn undergo reactions that otherwise will not occur. Classic examples include the formation of aminoacyl-tRNA in protein synthesis, or acetyl-CoA in fatty-acid synthesis.
The question is whether pyrophosphate bond energy could have been a significant source of chemical energy in the reactions leading to the origin of life. In fact, phosphate is such an integral part of all contemporary life that phosphorylation reactions must have been incorporated in primitive metabolic pathways. Baltscheffsky (1996) has argued that this is plausible, in part because pyrophosphate and polyphosphates are readily produced simply by heating inorganic phosphate under anhydrous conditions, a process known to occur under natural conditions. Pyrophosphate-containing minerals, canaphite and wooldridgeite, have been discovered in quarries, albeit in minute quantities and in the form of microscopic crystals. Furthermore, Baltscheffsky found that the coupling membranes of a photosynthetic bacterial species—Rhodospirrilum rubrum—synthesize pyrophosphate instead of ATP. The R. rubrum use the pyrophosphate as an energy source, much as other organisms use ATP.
Despite the ease of capturing thermal dehydration energy as pyrophosphate bonds, a plausible pathway for incorporating it as an energy source in early life has not yet been established. This is well worth further study, as is discussed in the last section of this article.
Sunlight and Photochemical Energy
Light only becomes a useful energy source if there is a pigment to absorb the photons. When photons are absorbed by a pigment molecule, they interact with the electronic structure of the molecule and add energy to the bonds to produce an excited state. This seemingly simple photochemical reaction is the energetic foundation of most life on the Earth, because this is what happens when a chlorophyll molecule absorbs light. Starting with chlorophyll in its ground state, a photon of red light is absorbed and increases the energy content of chlorophyll. The added energy causes it to go to an excited state that then donates an electron through multiple reactions to carbon dioxide, which ends up after further reactions as a carbohydrate. In addition, the transfer of the electron to lower energy states is coupled to the generation of a proton gradient across the membrane that is used to drive ATP synthesis. In this way, the original light energy is conserved in the form of chemical energy. After it loses the electron, chlorophyll is positively charged and the electron is replaced from a water molecule in the “water splitting reaction,” which releases oxygen. This is the source of virtually all of the oxygen in the Earth's atmosphere.
If photochemistry is essential for life today, could it also have provided energy for the origin of life? Perhaps, but there is a problem: What pigment molecules were available? Certainly not chlorophyll, which is a very complex molecule requiring multiple enzymatic steps for its synthesis. Furthermore, even if primitive pigments were present, the capture of light energy would not be possible unless there were membranes available that could contain the pigment molecules. To process the absorbed light energy, a primitive cell would also require a system of electron transport molecules to transduce the light energy into chemical energy. Future research might someday discover a mechanism by which this seemingly complex series of reactions could arise, but until then it seems plausible that an energy source other than light was used by the first living cells. In other words, the first life was likely to be heterotrophic.
Geological Electrochemical Energy
The electrochemical reactions used by aerobic life today require oxygen produced by plants. In mitochondria and aerobic bacteria, an electron transfer from reduced substrates to molecular oxygen produces 0.2 volts and enough protonic current to synthesize ATP. It is improbable that a complete chemiosmotic system was available to the first forms of life, but simpler electrochemical reactions are still conceivable mechanisms in which donors and acceptors of electrons provide an energy source. Several potential donors were likely to be available in the prebiotic environment. Perhaps the most plausible is hydrogen gas itself, as well as hydrogen sulfide (H2S) and methane. A variety of microorganisms today use these gases as a source of electrons, a good example being the abundant bacteria present in hydrothermal vents. There is a consensus that little or no oxygen was present in the early atmosphere, so what electron acceptors might have served instead of oxygen? A useful list of potential anaerobic donors and acceptors was presented by Gaidos et al. (1999), who considered the interesting question of energy sources that might be available in a putative ocean beneath Europa's icy surface. Terrestrial anaerobes use sulfate, nitrate, iron (III), and manganese (IV) as electron acceptors, with CO, nitrite, and hydrogen sulfide as electron donors. Sulfate would be abundant on the early Earth, as it is today. Nitrate and nitrite could have been made on the prebiotic Earth from nitrogen gas by photochemical synthesis and aqueous processes (Summers and Khare 2007) and in high pressure geochemical processes (Brandes et al. 1998). In addition, Cody et al. (2000, 2004) have shown how sulfide minerals could have been involved in primitive metabolic pathways.
Energy and Sulfur Chemistry
Sulfur chemistry is of particular relevance to the origin of life, because the minimal amount of molecular oxygen in the prebiotic environment would allow sulfur to be abundant as an element, as a reduced gas (hydrogen sulfide), and as iron sulfide minerals. Taking into consideration this prebiotic context, Wächtershäuser (1988) proposed that life did not arise by assembly of pre-existing organic compounds, but instead as two-dimensional synthetic chemistry on an iron sulfide mineral surface called pyrite. According to this idea, when hydrogen sulfide reacts with iron in solution to produce insoluble iron sulfide mineral, the reaction generates electrons that can be donated to the bound compounds and thereby drive a series of energetically uphill chemical reactions that otherwise cannot take place in solution. Wächtershäuser sees these reactions as the beginning metabolism, which occurred on a mineral surface rather than in the volume of a cell. To test this idea, Huber and Wächtershäuser (1997) heated a dispersion of iron and nickel sulfides together with a source of carbon and analyzed the products. There was no evidence of a long chain of integrated reactions, but using CO and H2S, acetic acid was produced, and from CO and CH3SH the thioester CH3-CO-SCH3 was synthesized. In later work in which amino acids were added to the simulation (Huber and Wächtershäuser 1998; Huber et al. 2003), the amino acids were observed to be chemically activated and formed peptide bonds.
DeDuve (1991) has proposed another version of sulfur chemistry as a source of chemical energy related to the origin of life, which involves thioesters that could have served to activate energy-dependent steps in primitive metabolic pathways before the advent of ATP. Thioesters are synthesized when a water molecule is lost during the reaction of an organic acid and a thiol: R-COOH+R'-SH –> R-CO-SR'. Unlike the relatively low-energy content of an ester bond, the thioester bond has an energy content equivalent to the pyrophosphate bond of ATP. Earlier Weber (1984, 1998) had shown that α-hydroxy acid and α-amino acid thioesters could be synthesized by reacting sugars (or sugar precursors) with ammonia and a thiol under plausible prebiotic conditions.
Several investigators have incorporated sulfur chemistry in their research. Wieland (1953) originally showed that amino acid thioesters spontaneously form peptide bonds in aqueous solution, and Maurel and Orgel (2000) showed the elongation of a decapeptide of oligoglutamic acid up to 15 mers in the presence of thioglutamic acid. Zepik et al. (2006) extended this reaction to protocells by encapsulating decaglutamic acid in liposomes composed of dimyristoylphosphatidylcholine, and found that externally added thioglutamic acid was able to permeate the vesicle membrane at a rate sufficient to support elongation of the decapeptide within the vesicles. It is clear that sulfur chemistry has considerable potential for expanding our understanding of early bioenergetic processes.
Chemiosmotic Energy Conversion to Anhydride Energy
Life today uses either chemiosmosis or substrate-level phosphorylation to convert electrochemical energy to anhydride chemical energy of ATP (Mitchell 1961, 1966). Was chemiosmosis an ancient process used by the first cells, or a later discovery? Arthur Koch (1985) first addressed this question and speculated that chemiosmosis may have arisen in the first forms of primitive cellular life, and this conjecture is worth considering here. Three relevant postulates of chemiosmosis are listed below:
Coupling membranes maintain a proton gradient, and the gradient must be of sufficient magnitude to drive ATP synthesis.
Coupling membranes pump protons by electron transport using either light or electron-transfer as an energy source.
Coupling membranes contain an ATPase that is also a proton pump.
The lipid composition of biological membranes today contains specific lipids that are products of highly evolved metabolic pathways. A stable hydrophobic lipid bilayer barrier composed of hydrocarbon chains 16–18 carbons in length is required to maintain ionic concentration gradients, particularly protons in the case of coupling membranes. Lipid bilayers are notoriously leaky to protons (Nichols and Deamer 1980; Paula et al. 1996), so the first self-assembled membranes composed of relatively short-chain amphiphiles would be unable to maintain significant proton gradients, as recently shown by Chen and Szostak (2004). Mansy (2010) discusses the role of membrane permeability in primitive cellular systems. Even if a plausible primitive barrier membrane can be discovered, an electron transport system and ATP synthase would need to be incorporated in the bilayer for chemiosmosis to be a source of chemical energy. In our judgment, the complex requirements for a functioning chemiosmotic system weighs against the proposition that primitive life used chemiosmosis for converting electrochemical energy to the anhydride energy of ATP.
Chemical Energy of Organic Substrates: Carbohydrates
The environment of the prebiotic Earth was far from equilibrium, so that a variety of chemical reactions were occurring simultaneously. The problem is to gain some understanding of which of these was relevant to the origin of life, and how they were incorporated. Living systems today use chemical reactions to release energy in small steps called metabolism, which can be defined as a series of chemical reactions linked in a molecular system that provides energy and small molecules required for growth. Each step is catalyzed by a specific enzyme, and the reaction rates are controlled by feedback loops in which a product is an allosteric inhibitor of the enzyme to be regulated. If the first life was heterotrophic, what nutrients might have been available as a source of chemical energy?
Of all the organic substrates, sugars are by far the most attractive organic energy substrate of primitive anaerobic life, because they are able to provide all the energy and carbon needed for the growth and maintenance of a fermentative metabolism. In fact, the sugars that are the first substrates of the glycolytic pathway can be considered to be optimal biosynthetic substrates because they contain mainly alcohol groups that have maximum self-transformation energy, and a single carbonyl group (aldehyde or ketone) that makes them reactive and able to form covalent adducts to enzyme active sites (Weber 2004). Moreover, in fermentation, the energy content of sugars is converted to the anhydride energy of ATP by substrate-level oxidation phosphorylation, a process that does not require the organized membrane structures of phosphorylation coupled to electron transfer. As discussed later, the energy content and reactivity of sugars also allows them to act as substrates for chemically spontaneous synthetic processes that yield many of the molecular products required for the origin of life. Such sugar-driven syntheses require no external source of chemical energy (Weber 2000).
RECENT EXPERIMENTAL STUDIES
Sugar-driven Prebiotic Synthesis
In addition to being the sole energy and carbon source of fermentative organisms today, sugars have chemical properties that make them very attractive substrates for synthetic processes needed for the origin of life. First, sugars can be synthesized under plausible prebiotic conditions from formaldehyde and glycolaldehyde by the formose reaction (Schwartz and de Graaf 1993; See also Benner et al. 2010). Second, sugars are reactive and contain considerable self-transformation energy, properties that allow them to react with ammonia, yielding many types of molecules needed for the origin of life. These sugar-driven syntheses require no additional source of chemical energy (Weber 2000).
The synthetic versatility of sugars is shown by their spontaneous reactions in the presence of ammonia that yield catalytic amines, biomonomers (amino acids), metabolites (pyruvate, glycolate), energy molecules (hydroxy and amino acid thioesters), alternative nucleobases (2-pyrazinones that resemble uracil), heterocyclic molecules (furans, pyrroles, imidazoles, pyridines, and pyrazines), polymers (polypyrroles and polyfurans), and cell-like organic microspherules (Weber 2001-2008, refs. therein). Sugars have also been shown to drive the prebiotic synthesis of ammonia from nitrite. Remarkably, these prebiotic synthetic processes based on sugar chemistry can evolve directly into modern sugar-driven biosynthesis without violating the principle of evolutionary continuity.
Finally, sugar synthesis from formaldehyde and glycolaldehyde, and the subsequent conversion of sugar products to carbonyl-containing products can be catalyzed by small molecules (ammonia and amines including amino acids and peptides). In fact, small l-dipeptides (the isomer found in proteins) stereoselectively catalyzed the formation of d-ribose (Pizzarello and Weber 2010). These ammonia and amine-catalyzed reactions yielded aldotriose (glyceraldehyde), ketotriose (dihydroxyacetone), aldotetroses (erythrose and threose), ketotetrose (erythrulose), pyruvaldehyde, acetaldehyde, glyoxal, pyruvate, glyoxylate, and several unidentified carbonyl products. The uncatalyzed control reaction yielded no pyruvate or glyoxylate, and only trace amounts of pyruvaldehyde, acetaldehyde, and glyoxal. With l-alanine, the rates of triose and pyruvaldehyde synthesis were about 15-times and 1200-times faster, respectively, than the uncatalyzed reaction (Weber 2001). Because amines are also products of sugar–ammonia reactions, these studies suggested that the sugar–ammonia reaction could be autocatalytic. This possibility was tested in a later study, which showed that reaction of the triose sugar (glyceraldehyde) with ammonia yielded a crude product mixture capable of catalyzing a 10-fold acceleration of the same sugar–ammonia reaction that produced the catalytic products (Weber 2007).
Amphiphile Synthesis Using Geothermal Energy
Franz Fischer and Hans Tropsch discovered in the 1920s that a gaseous mixture of carbon monoxide and hydrogen, when passed over a hot iron catalyst, produced excellent yields of hydrocarbons. Oro and coworkers (Nooner et al. 1987) first showed that the Fischer-Tropsch type synthesis (FTT) also worked with meteoritic iron-nickel as a catalyst, and proposed that long-chain fatty acids may have been produced this way on the early Earth. McCollom et al. (1999) and Rushdi et al. (2001) found that the FTT reaction also worked simply by treating oxalic acid to elevated temperatures 150°–250°C and corresponding pressures in a stainless steel chamber, producing a mixture of fatty acids and alcohols as products. At elevated temperatures, oxalic breaks down into a mixture of CO, CO2, and H2, the reactive gases required for FTT synthesis. In a later paper, Simoneit et al. (2006) found that if stoichiometric glycerol was present in the mixture along with a variety of fatty acids, the same conditions produced good yields of monoglycerides.
These results are significant because the majority of membrane-forming lipids today are glycerol esters. Furthermore, monoglycerides by themselves can assemble into lipid bilayers, and when mixed with fatty acids are able to form stable membranes (Monnard et al. 2002; Mansy and Szostak 2008). Future research in this area should be directed toward characterizing such membranes in terms of long-term stability and capacity for maintaining ionic concentration gradients of protons and other cations.
Phosphodiester Bond Synthesis Driven by Anhydrous Cycles
Clay mineral is a common example of an organizing surface. Clays have a surprisingly large crystalline surface area, over 100 m2/g, and have a multilamellar structure that seems likely to adsorb, concentrate, and organize potential monomers on and between the lamellae. Ferris and coworkers (1996) have made extensive studies of montmorillonite clay as an organizing agent, and established that activated monomers of mononucleotides can in fact polymerize on clay surfaces into RNA-like polymers up to 50 nucleotides in length containing both 3′–5′ and 2′–5′ phosphodiester bonds. However, the resulting polymers are tightly bound to the clay surfaces, and if they are to participate in the origin of cellular life, the RNA products must somehow be associated with membranous vesicles to form a protocellular system. Significantly, Hanczyc et al. (2003) found that clay particles with bound RNA were readily encapsulated in fatty-acid vesicles.
It is not generally realized that lipids also form multilamellar structures that have the potential to organize and concentrate monomers. The most common image of lipids is in the form of microscopic vesicles bounded by a lipid bilayer membrane, usually referred to as liposomes. However, in the dry state, lipids are present as multilamellar matrices consisting of stacked bilayers, or less often as hexagonal phases in which the lipids form indefinitely long tubes that tend to have a hexagonal packing, rather than lamellar (Reiss-Husson and Luzzati 1967; Deamer et al. 1970). Furthermore, if lipid vesicles undergo drying, the bilayers fuse into the multilamellar phase, and any solutes present are trapped and concentrated between the lipid head groups of bilayers. Unlike the solid matrix of clay surfaces, the bilayers are liquid crystals, which means that trapped solutes have diffusional mobility.
Rajamani et al. (2008) investigated the possibility that the order imposed on mononucleotides by multilamellar lipid matrices could promote polymerization. The nucleotides used were ordinary 5′-ribonucleotides that had not been chemically activated. Instead, the chemical potential for ester bond formation was provided by cycling through anhydrous conditions at moderately elevated temperatures in the range of 60°–90°C. Thermal cycling of the mononucleotides in mass ratios from 2:1 to 1:2 with the phospholipids was shown to yield relatively long chains of linear RNA-like polymers, ranging from 20 to 100 nucleotides in length. The linearity was determined by nanopore analysis of the products, and the RNA-like character was established by 32P-end labeling with T7 RNA kinase. A variety of phospholipids could promote the polymerization, but in the absence of lipids, only short nucleotide oligomers were detected.
These results show that there is sufficient chemical potential available in anhydrous conditions to drive phosphate ester synthesis as long as the nucleotide monomers are concentrated and organized within a fluid liquid crystalline matrix that permits diffusional mobility. Furthermore, at the end of the reaction when the lipid matrix is rehydrated, the products of the reaction are encapsulated in lipid vesicles. This appears to be a plausible pathway to protocells, which could be generated at the edges of volcanic geothermal pools where wet–dry cycles would be common.
Carbonyl Sulfide as a Plausible Prebiotic Condensing Agent
At some point, polymerization reactions must have evolved from simple processes driven by an input of physical energy to a more complex mechanism involving primitive versions of activation occurring in an aqueous environment. For many years, research has focused on discovering a plausible condensing agent that can perform this feat, and recent discoveries suggest that carbonyl sulfide is a likely candidate. Carbonyl sulfide (COS) is a reactive compound that has been detected in volcanic gas and mineral ash (Rasmussen et al. 1982), along with its chemical relatives, carbon disulfide and carbon dioxide. Leman et al. (2004) found that if COS is present in an aqueous solution of amino acids, di- and tripeptides are synthesized with yields up to 80%. In a second paper, Leman et al. (2006) reported that amino-acyl phosphate anhydrides up to 30% yields were synthesized in mixtures of amino acids, phosphate, and COS.
These results offer a strong clue to the manner in which phosphate was initially incorporated into primitive metabolic pathways, particularly those leading to peptide bond formation and the synthesis of small oligopeptides that may have served as catalysts and structural components of early life.
Novel Prebiotic Synthesis of Nucleotides
The nucleotide monomers of nucleic acids consist of a purine or pyrimidine linked through a nitrogen–carbon bond to a pentose sugar, which in turn is phosphorylated through an ester bond on the 5′ hydroxyl group. Each of the component molecules of nucleotides was presumably present in organic mixtures on the prebiotic Earth, but must be linked into the more complex molecular structure of nucleotides before they can be incorporated into nucleic acid polymers. One might imagine that phosphorylation of a ribose sugar could occur, because ester bonds are not difficult to synthesize by simple condensation reactions, but synthesis of the C-N bond between a sugar and a nucleobase has been much more challenging.
A recent paper by Powner et al. (2009) (see also Sutherland 2010) reported a remarkably efficient series of reactions that leads to mononucleotide synthesis. Instead of attempting to produce C-N bonds between an existing pyrimidine such as cytosine and ribose, the reaction uses sequential additions of cyanamide, cyanoacetylene, glycolaldehyde, and glyceraldehyde, all in the presence of phosphate. Under these conditions, spontaneous reactions first form arabinose aminooxazoline and anhydronucleoside intermediates, which then add phosphate and condense into cytosine monophosphate. Although it is unlikely that such a complex mixture of reactants and phosphate might occur in the prebiotic environment, the fact that the reaction can occur at all in aqueous solution, using only the chemical energy of the reactants, opens a new direction for future investigations that may reveal a simpler process.
CHALLENGES AND FUTURE RESEARCH DIRECTIONS
Identifying a Source of Condensation Energy
In this article, we briefly touched on processes by which certain kinds of energy could have driven chemical reactions related to the origin of life. There is a consensus that electrical discharge and ultraviolet light could drive the synthesis of reactive molecules like cyanide and formaldehyde, which in turn would react to produce more complex molecules that could serve as potential monomers for primitive forms of life. However, a plausible energy source for polymerization remains an open question. Condensation reactions driven by cycles of anhydrous conditions and hydration would seem to be one obvious possibility, but seem limited by the lack of specificity of the chemical bonds that are formed. On the other hand, there may be conditions yet to be discovered that organize monomers in such a way that the formation of specific chemical bonds is promoted. The organizing effect of clay mineral surfaces is one well-known example, and incorporation of monomers into orderly lipid matrices also promotes specific polymerization reactions. Future studies of conditions that can add order to reactive monomers are likely to reveal new clues to plausible polymerization processes that could occur in the prebiotic environment.
An alternative to anhydrous heat is a condensing agent such as carbonyl sulfide, which can activate peptide bond and phosphanhydride bond synthesis in aqueous solutions. This discovery certainly deserves further attention. So far, only short oligomers have been produced using COS and dilute monomers, but conditions that can concentrate and organize the reactants are likely to produce much longer polymers. Related to carbonyl sulfide as a condensation agent is the energy available in thioester bonds (DeDuve 2005). Although molecular oxygen was virtually absent from the prebiotic atmosphere, its neighbor in the periodic table—sulfur—was abundant, both as an element and in common gases such as H2S and COS. Therefore, it seems probable that sulfur was incorporated into a variety of organic molecules, and further studies of the thioester bond as a plausible intermediate in primitive metabolic pathways should be fruitful.
A third source of energy for polymerization processes is the chemical potential of simple carbohydrates. Sugars, when heated near 70°C in the presence of ammonia or amines, are known to produce polymers containing furan and pyrrole residues, and cell-like microspherules (Weber 2005, refs. therein). Sugars also drive the synthesis of α-hydroxyacid thioesters and α-amino acid thioesters that are known to oligomerize forming polyesters and peptides, respectively (Weber 1998, refs. therein). In addition, in his description of the sugar-driven synthesis of uracil-like 2-pyrazinone, Weber (2008) proposed that sugars also have the potential to form pyrazinone monomers with α‐hydroxycarbonyl side-chain groups (CH2OH-CO-CH2‐pyrazinone) that could spontaneously polymerize to give oligomers joined by enol ether linkages or dehydrated aldol linkages. In contrast to other prebiotic syntheses, the previously cited sugar-driven processes are “one-pot” reactions that yield reactive monomers capable of spontaneous oligomerization without being coupled to an additional source of chemical energy, like ATP.
Phosphate Reactions
There are good reasons why phosphate is central to energy metabolism today. These were described by Westheimer (1987) in an excellent review that should be required reading for students interested in the origins of life. An important, still unanswered question concerns how phosphate might have first become involved in life processes. Phosphate today is mostly present in the form of a mineral called apatite, the same combination of calcium and phosphate that composes tooth enamel and bones. Apatite has a very low solubility, so what was the original source of a soluble form of phosphate? There are no convincing explanations yet, but it is possible that life began in a low pH environment similar to that of volcanic hydrothermal springs. Calcium phosphate readily dissolves at acidic pH ranges to release phosphate anions. The presence of free phosphate in solution may have permitted incorporation into organic compounds as phosphate esters, followed by a second set of chemical reactions that initiated primitive metabolic pathways involving phosphate. For instance, Prabahar and Ferris (1997) reported that adenine itself is able to activate phosphate under certain conditions. Identifying a plausible mechanism for prebiotic phosphorylation represents an important problem for future research.
Pigments and Photosynthesis
Sunlight drives virtually the entire biosphere today, but when and how did life first begin to capture light as an energy source? Although today's photosynthetic process seems much too complex to have been a source of energy for early life, there must have been some sort of pigment available that could begin capturing light energy in a useful way and initiate the evolutionary path toward modern photosynthesis. Pteridines are examples of relatively simple pigment molecules that can be generated from amino acids exposed to anhydrous heat. This reaction and potential roles of pteridines in the origin of life have been extensively explored by Kritsky and coworkers and deserve further study (for review, see Kritsky and Telegina 2004).
An alternative scenario is that pigments existed in the environment that partitioned into lipid bilayers of membranes in such a way that light energy could be transduced into chemical energy. Were organic molecules present in the prebiotic environment that might serve as membrane-bound pigments? Polycyclic aromatic hydrocarbons (PAH) are abundant forms of organic carbon, and most PAH species absorb light in the near UV and blue region of the spectrum. After accepting photons, the excited states can act as reducing agents and release protons, thereby generating chemiosmotic pH gradients (for review, see Deamer et al. 1994). Another interesting photochemical reaction of PAH derivatives is photocarboxylation. For instance, when exposed to near UV light, phenanthrene reacts directly with carbon dioxide to produce phenanthrene carboxylic acid, probably the simplest possible example of carbon dioxide fixation (Tazuke et al. 1986; See Deamer 1997 for review). These properties of PAH compounds have yet to be explored thoroughly and surely represent a fruitful area for future research.
Footnotes
Editors: David Deamer and Jack Szostak
Additional Perspectives on The Origins of Life available at www.cshperspectives.org
REFERENCES
- Baltscheffsky H 1996. Origin and evolution of biological energy conversion New York: Wiley VCH [Google Scholar]
- Benner SA, Kim H-J, Kim M-J, Ricardo A 2010. Planetary organic chemistry and the origins of Biomolecules. Cold Spring Harb Perspect Biol 2:a003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal JD 1951. The physical basis of life London: Routledge and Kegan Paul [Google Scholar]
- Brandes JA, Boctor NZ, Cody GD, Cooper BA, Hazen RM, Yoder HS 1998. Abiotic nitrogen reduction on the early Earth. Nature 395:365–336 [DOI] [PubMed] [Google Scholar]
- Chen IA, Szostak JW 2004. A kinetic study of the growth of fatty acid vesicles. Biophys J 87:988–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyba CF, Sagan C 1992. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origin of life. Nature 355:125–113 [DOI] [PubMed] [Google Scholar]
- Chyba CF, Thomas PJ, Brookshaw, Sagan C 1990. Cometary delivery of organic molecules to the early Earth. Science 249:366–373 [DOI] [PubMed] [Google Scholar]
- Cody GD 2004. Transition metal sulfides and the origin of metabolism. Ann Rev Earth Planetary Sci 32:569–599 [Google Scholar]
- Cody GD, Boctor NZ, Filley TR, Hazen RM, Scott JH, Sharma A, Yoder HS 2000. Primordial carbonylated iron-sulfur compounds and synthesis of pyruvate. Science 289:1337–1340 [DOI] [PubMed] [Google Scholar]
- Deamer DW 1991. Polycyclic aromatic hydrocarbons: Primitive pigment systems in the prebiotic environment. Adv Space Res 12:183–189 [DOI] [PubMed] [Google Scholar]
- Deamer DW 1997. The first living systems: A bioenergetic perspective. Microbiol Mol Biol Rev 61:239–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer DW, Harang-Mahon E, Bosco G 1994. Self-assembly and function of primitive membrane structures. In: Early life on Earth Nobel Symposium No. 84 Bengston S. ed. [Google Scholar]
- Deamer DW, Leonard R, Tardieu A, Branton D 1970. Lamellar and hexagonal lipid phases visualized by freeze-etching. Biochim Biophys Acta 219:47–60 [DOI] [PubMed] [Google Scholar]
- DeDuve C 1991. Blueprint for a cell: The nature and origin of life New York: Neil Patterson Publishers [Google Scholar]
- DeDuve C. Singularities: Landmarks on the pathway of life. Cambridge University Press; 2005. [Google Scholar]
- Ferris JP 1999. Prebiotic synthesis on minerals: Bridging the prebiotic and RNA worlds. Biol Bull 196:311–314 [DOI] [PubMed] [Google Scholar]
- Ferris J 2002. Montmorillonite catalysis of 30–50 mer oligonucleotides: Laboratory demonstration of potential steps in the origin of the RNA world. Orig Life Evol Biospheres 32:311–332 [DOI] [PubMed] [Google Scholar]
- Ferris JP, Hill AR, Liu R, Orgel LE 1996. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59. [DOI] [PubMed] [Google Scholar]
- Fox SW, Harada K 1958. Thermal copolymerization of amino acids to a product resembling protein. Science 128:1214. [DOI] [PubMed] [Google Scholar]
- Gottschalk G 1986. Bacterial metabolism New York: Springer-Verlag, New York, pp. 1–11, 210–317 [Google Scholar]
- Gaidos EJ, Nealson KH, Kirschvink JL 1999. Life in ice-covered oceans. Science 284:1631–1633 [DOI] [PubMed] [Google Scholar]
- Hanczyc MM, Fujikawa SM, Szostak JW 2003. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science 302:618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves WR, Deamer DW 1978. Liposomes from ionic, single-chain amphiphiles. Biochemistry 17:3759–3768 [DOI] [PubMed] [Google Scholar]
- Huber C, Wächtershäuser G 1997. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 276:245–247 [DOI] [PubMed] [Google Scholar]
- Huber C, Wächtershäuser G 1998. Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: Implications for the origin of life. Science 281:670–672 [DOI] [PubMed] [Google Scholar]
- Huber C, Eisenreich W, Hecht S, Wächtershäuser G 2003. A possible primordial peptide cycle. Science 301:938–940 [DOI] [PubMed] [Google Scholar]
- Kanavarioti A, Monnard P-A, Deamer DW 2001. Eutectic phases in ice facilitate nonenzymatic nucleic acid synthesis. Astrobiology 1:271–281 [DOI] [PubMed] [Google Scholar]
- Knauth LP, Lowe DR 2003. High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. GSA Bulletin 115:566–580 [Google Scholar]
- Koch A 1985. Primeval cells: Possible energy-generating and cell-division mechanisms. J Mol Evol 21:270–77 [DOI] [PubMed] [Google Scholar]
- Koch AL, Schmidt TM 1991. The first cellular bioenergetic process: Primitive generation of a proton motive force. J Mol Evo 33:297–304 [DOI] [PubMed] [Google Scholar]
- Kritsky MS, Telegina TA 2004. Role of nucleotide-like coenzymes in primitive evolution. In Cellular origin, life in extreme environments and astrobiology Seckbach J., Ed. Springer; Netherlands [Google Scholar]
- Leman L, Orgel L, Ghadiri MR 2004. Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306:283–286 [DOI] [PubMed] [Google Scholar]
- Leman LJ, Orgel LE, Ghadiri MR 2006. Amino acid dependent formation of phoshate anhydrides in water mediated by carbonyl sulfide. J Am Chem Soc 128:20–21 [DOI] [PubMed] [Google Scholar]
- Lohrmann R, Bridson PK, Orgel LE 1980. Efficient metal-ion catalyzed template-directed oligonucleotide synthesis. Science 208:1464–1465 [DOI] [PubMed] [Google Scholar]
- Mansy SS 2010. Membrane transport in primitive cells. Cold Spring Harb Perspect Biol 2:a002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy SS, Szostak JW 2008. Thermostability of model protocell membranes. Proc Natl Acad Sci 105:13351–13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M-C, Orgel LE 2000. Oligomerization of α-thioglutamic acid. Orig Life Evol Biospheres 30:423–430 [DOI] [PubMed] [Google Scholar]
- McCollom TM, Ritter G, Simoneit BRT 1999. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig Life Evol Biospheres 29:153–166 [DOI] [PubMed] [Google Scholar]
- Miller SL 1953. Production of amino acids under possible primitive Earth conditions. Science 117:528–529 [DOI] [PubMed] [Google Scholar]
- Miller AL, Urey HC 1959. Organic compound synthesis on the primitive Earth. Science 130:245–51 [DOI] [PubMed] [Google Scholar]
- Mitchell P 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148 [DOI] [PubMed] [Google Scholar]
- Mitchell P 1966. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev 41:445–501 [DOI] [PubMed] [Google Scholar]
- Miyakawa S, Cleaves HJ, Miller SL 2002. The cold origin of life: Implications based on pyrimidines and purines pruduced from frozen ammonium cyanide solutions. Orig Life Evol Biosph 32:209–218 [DOI] [PubMed] [Google Scholar]
- Monnard PA, Apel CL, Kanavarioti A, Deamer DW 2002. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2:139–152 [DOI] [PubMed] [Google Scholar]
- Nichols JW, Deamer DW 1980. Net proton-hydroxide permeability of large unilamellar liposomes measured by an acid-base titration technique. Proc Natl Acad Sci 77:2038–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner DW, Oro J 1987. Synthesis of fatty acids by a closed system Fischer-Tropsch process. Adv Chem 178:159–171 [Google Scholar]
- Orgel L 2004. Prebiotic adenine revisited: Eutectics and photochemistry. Orig Life Evol Biospheres 34:361–369 [DOI] [PubMed] [Google Scholar]
- Oro J 1961. Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive Earth conditions. Nature 191:1193–1194 [DOI] [PubMed] [Google Scholar]
- Paula S, Volkov AG, Van Hoek AN, Haines TH, Deamer DW 1996. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys J 70:339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JP, Gladstone GR, Yung YL 1980. Photochemical production of formaldehyde in Earth's primitive atmosphere. Science 210:183–185 [DOI] [PubMed] [Google Scholar]
- Pizzarello S, Shock E 2010. The organic composition of carbonaceous meteorites: The evolutionary story ahead of biochemistry. Cold Spring Harb Perspect Biol 2:a002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzarello S, Weber AL 2010. Stereoselective syntheses of pentose sugars under realistic prebiotic conditions. Orig Life Evol Biosph (in press). [DOI] [PubMed] [Google Scholar]
- Powner MW, Gerland B, Sutherland JD 2009. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459:239–242 [DOI] [PubMed] [Google Scholar]
- Prabahar KJ, Ferris JP 1997. Adenine derivatives as phosphate-activating groups for the regioselective formation of 3',5'-linked oligoadenylates on montmorillonite: possible phosphate-activating groups for the prebiotic synthesis of RNA. J Am Chem Soc 119:4330–4337 [DOI] [PubMed] [Google Scholar]
- Rajamani S, Vlassov A, Benner S, Coombs A, Olasagasti F, Deamer D 2008. Lipid-assisted synthesis of RNA-like polymers from mononucleotides. Orig Life Evol Biosphere 38:57–74 [DOI] [PubMed] [Google Scholar]
- Rasmussen RA, Khalil MAK, Dalluge RW, Penkett SA, Jones B 1982. Carbonyl sulfide and carbon disulfide from the eruptions of Mount St. Helens. Science 215:665–667 [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F, Luzzati V 1967. Phase transitions in lipids in relation to the structure of membranes. Adv Biol Med Phys 11:87–107 [DOI] [PubMed] [Google Scholar]
- Rushdi AI, Simoneit BRT 2001. Lipid formation by aqueous Fischer-Tropsch-type synthesis over a temperature range of 100 to 400°C. Orig Life Evol Biosphere 31:103–118 [DOI] [PubMed] [Google Scholar]
- Schwartz AW, de Graaf RM 1993. The prebiotic synthesis of carbohydrates: A reassessment. J Mol Evolution 35:101–106 [Google Scholar]
- Shock E 1990. Geochemical constraints on the origin of organic compounds in hydrothermal systems. Orig Life Evol Biospheres 20:331–367 [DOI] [PubMed] [Google Scholar]
- Simoneit BRT, Rushdi AI, Deamer DW 2006. Abiotic formation of acylglycerols under simulated hydrothermal conditions and self-assembly properties of such lipid products. Adv Space Res 11:1649–1656 [Google Scholar]
- Sleep NH, Zahnle K, Kasting JF, Morowitz HJ 1989. Annihilation of ecosystems by large asteroid impacts on the early Earth. Nature 342:139–142 [DOI] [PubMed] [Google Scholar]
- Stouthamer AH 1977. Energetic aspects of the growth of microorganisms. In Microbial Energetics Haddock BA, Hamilton WA, Eds. Cambridge University Press; London: pp. 285–315 [Google Scholar]
- Stribling R, Miller SL 1987. Energy yields for hydrogen cyanide and formaldehyde synthesis: The HCN and amino acid concentration in the primitive ocean. Orig Life Evol Biospheres 17:261–273 [DOI] [PubMed] [Google Scholar]
- Summers DR, Khare B 2007. Nitrogen fixation on early Mars and other terrestrial planets: Experimental demonstration of abiotic fixation reactions to nitrite and nitrate. Astrobiology 7:333–341 [DOI] [PubMed] [Google Scholar]
- Sutherland JD 2010. Ribonucleotides. Cold Spring Harb Perspect Biol 2:a005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak JW, Bartel DP, Luisi PL 2001. Synthesizing life. Nature 409:387–390 [DOI] [PubMed] [Google Scholar]
- Tazuke S, Kazama S, Kitamura N 1986. Reductive photocarboxylation of aromatic hydrocarbons. J Org Chem 51:4548–4553 [Google Scholar]
- Usher DA 1977. Early chemical evolution of nucleic acids: a theoretical model. Science 196:311–313 [DOI] [PubMed] [Google Scholar]
- Usher DA, McHale AH 1976. Nonenzymic joining of oligoadenylates on a polyuridylic acid template. Science 192:53–54 [DOI] [PubMed] [Google Scholar]
- Verlander MS, Orgel LE 1974. Analysis of high molecular weight material from the polymerization of adenosine cyclic 2′, 3′-phosphate. J Mol Evol 3:115–120 [DOI] [PubMed] [Google Scholar]
- Wachtershauser G 1988. Before enzyme and template: Theory of surface metabolism. Microbiol Rev 52:452–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walde P, Wick R, Fresta M, Mangone A, Luisi PL 1994. Autopoietic self-reproduction of fatty acid vesicles. J Am Chem Soc 116:11649–11654 [Google Scholar]
- Weber AL 1998. Prebiotic amino acid thioester synthesis: Thiol-dependent amino acid synthesis from formose substrates (formaldehyde and glycolaldehyde) and ammonia. Orig Life Evol Biospheres 28:259–270 [DOI] [PubMed] [Google Scholar]
- Weber AL 1984. Nonenzymatic formation of “energy-rich” lactoyl and glyceroyl thioesters from glyceraldehyde and a thiol. J Mol Evol 20:157–166 [DOI] [PubMed] [Google Scholar]
- Weber AL 2000. Sugars as the optimal biosynthetic carbon substrate of aqueous life throughout the universe. Orig Life Evol Biospheres 30:33–43 [DOI] [PubMed] [Google Scholar]
- Weber AL 2001. The Sugar Model: Catalysis by amines and amino acid products. Orig Life Evol Biospheres 31:71–86 [DOI] [PubMed] [Google Scholar]
- Weber AL 2004. Kinetics of organic transformations under mild aqueous conditions: Implications for the origin of life and its metabolism. Orig Life Evol Biosphere 34:473–495 [DOI] [PubMed] [Google Scholar]
- Weber AL 2005. Growth of organic microspherules in sugar-ammonia reactions. Orig Life Evol Biospheres 35:523–536 [DOI] [PubMed] [Google Scholar]
- Weber AL 2007. The Sugar Model: Autocatalytic activity of the triose-ammonia. Orig Life Evol Biospheres 37:105–111 [DOI] [PubMed] [Google Scholar]
- Weber AL 2008. Sugar-driven prebiotic synthesis of 3,5(6)-dimethylpyrazin-2-one: A possible nucleobase of a primitive replication process. Orig Life Evol Biospheres 38:279–292 [DOI] [PubMed] [Google Scholar]
- Westheimer FH 1987. Why nature chose phosphates. Science 235:1173–1178 [DOI] [PubMed] [Google Scholar]
- Wieland T, Bokelmann E, Bauer L, Lang HU 1953. Polypeptide syntheses. 8. Formation of sulfur containing peptides by the intramolecular Liebigs. Ann Chem 582:129–149 [Google Scholar]
- Zepik HH, Rajamani S, Maurel MC, Deamer D 2007. Oligomerization of thioglutamic acid: encapsulated reactions and lipid catalysis. Orig Life Evol Biospheres 37:495–505 [DOI] [PubMed] [Google Scholar]