Abstract
The epithelial–mesenchymal transition is essential in both embryonic development and the progression of carcinomas. Wnt signaling and cadherin-mediated adhesion have been implicated in both processes; clarifying their role will depend on linking them to rearrangements of cellular structure and behavior. β-Catenin is an essential molecule both in cadherin-mediated cell adhesion and in canonical Wnt signaling. Numerous experiments have shown that the loss of cadherin-mediated cell adhesion can promote β-catenin release and signaling; this is accomplished by proteases, protein kinases and other molecules. Cadherin loss can also signal to several other regulatory pathways. Additionally, many target genes of Wnt signaling influence cadherin adhesion. The most conspicuous of these Wnt target genes encode the transcription factors Twist and Slug, which directly inhibit the E-cadherin gene promoter. Other Wnt/β-catenin target genes encode metalloproteases or the cell adhesion molecule L1, which favor the degradation of E-cadherin. These factors provide a mechanism whereby cadherin loss and increased Wnt signaling induce epithelial–mesenchymal transition in both carcinomas and development.
Wnt signaling regulates cadherin-mediated cell adhesion and vice versa. This interplay is important for epithelial-mesenchymal transitions in development and cancer.
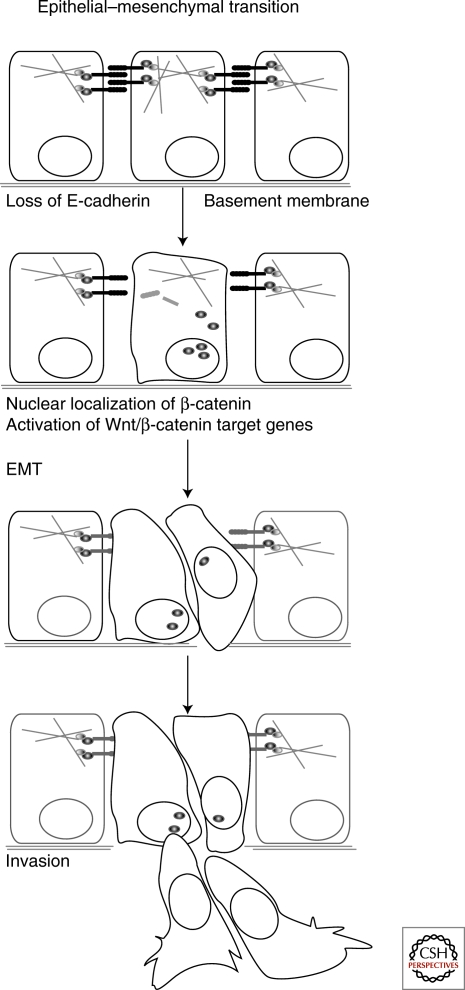
In both normal embryonic development and carcinomas, signals trigger epithelial–mesenchymal transitions (EMT). These biological programs convert polarized, rather immobile epithelial cells to fibroblastoid, highly motile mesenchymal cells (Fig. 1). EMT occurs over and over during embryonic development; for instance, it is crucial in the formation of mesoderm during gastrulation, which produces a three-layered embryo, and formation of the neural crest, the heart, and the craniofacial system (reviewed by Thiery 2002). EMT also occurs in carcinomas, where it promotes invasion and metastasis (reviewed by Thiery 2002; Yang and Weinberg 2008). A thorough understanding of these processes will require mechanistic explanations of how the signaling pathways implicated in EMTs trigger the rearrangements of cellular architecture and changes in behavior that permit it to happen. They should also explain how changes in healthy signaling lead to inappropriate EMTs in carcinomas. After three decades of work, an integrated picture of the connections between these processes is finally coming into focus. The present review centers on the role of cadherin-mediated cell adhesion and its interplay with Wnt/β-catenin signaling, which are both crucial in the process of EMT (see also Nelson and Nusse 2004; Gavert and Ben-Ze’ev 2007).
Figure 1.
Epithelial–mesenchymal transition. EMT can be induced by the loss of E-cadherin and/or by the activation of canonical Wnt signaling. Epithelial cells become more motile and receive a mesenchymal character. The mesenchymal-like cells can pass the basement membrane and become invasive.
In the late 1960s to early 1980s, Hay and collaborators recognized EMT as a distinct biological process in studies with embryos and three-dimensional cell cultures (Trelstad et al. 1967; Greenburg and Hay 1982). Soon afterwards, our group showed that Madin-Darby canine kidney (MDCK) epithelial cells are converted into fibroblastoid cells through the disruption of E-cadherin-mediated cell adhesion with specific antibodies (Imhof et al. 1983; Behrens et al. 1985). Stoker and Gherardi showed that MDCK epithelial cells could be converted into motile fibroblastoid cells by the addition of conditioned medium that contained scatter factor/hepatocyte growth factor, HGF/SF (Stoker et al. 1987; Weidner et al. 1991). Further work showed that MDCK epithelial cells acquired invasive properties, i.e., invaded collagen gels and embryonal heart tissue, following disturbed E-cadherin-mediated cell adhesion (Behrens et al. 1989), or by the addition of HGF/SF (Weidner et al. 1990; Weidner et al. 1991).
The process of EMT is reversible: Invasive human carcinoma cells that had lost E-cadherin expression and epithelial polarity could be made noninvasive by transfection with the E-cadherin cDNA (Frixen et al. 1991; Vleminckx et al. 1991). It could also be shown that poorly differentiated human breast and stomach carcinomas carried inactivating mutations in the E-cadherin gene (Becker et al. 1994; Berx et al. 1995), and inherited germline mutations of E-cadherin were found in early-onset, diffuse-type gastric cancer in human families (Guilford et al. 1998). The loss of E-cadherin expression coincided with the transition from well-differentiated adenoma to invasive carcinoma in a transgenic mouse model of pancreatic carcinogenesis (Perl et al. 1998). In other cases of human carcinomas, down-regulation of E-cadherin occurred by hypermethylation of the E-cadherin gene promoter (Hennig et al. 1995; Strathdee 2002). Beug and collaborators showed that activation of the c-Fos proto-oncogene in mammary gland epithelial cells also induced EMT, e.g., down-regulated E-cadherin, up-regulated several mesenchymal genes, and promoted invasion of the cells into collagen gels (Reichmann et al. 1992). Remarkably, overexpression of stabilized β-catenin also scattered MDCK epithelial cells in tissue culture (Barth et al. 1997). In the last decade, groups have identified several more signaling pathways and specific transcription factors that induce EMT in development and in the invasion and metastasis of carcinomas. These include:
– TGFβ signaling (Oft et al. 1996; Mercado-Pimentel and Runyan 2007; Nguyen and Massague 2007).
– Wnt signaling (Kinzler and Vogelstein 1996; Huelsken et al. 2000; Fodde et al. 2001).
– Tyrosine kinase signaling (the ras pathway; Behrens et al. 1993; Oft et al. 1996; Meiners et al. 1998; Dietrich et al. 1999; Muller et al. 1999; Khoury et al. 2001).
– The transcription factors Snail, Slug, ZEB, E47, and Twist, which suppress E-cadherin expression (Batlle et al. 2000; Cano et al. 2000; Comijn et al. 2001; Peinado et al. 2004b; Yang et al. 2004).
– Tiam1/Rac1 signaling (Habets et al. 1994; Collard et al. 1996).
– Goosecoid and FOXC2 (Hartwell et al. 2006; Mani et al. 2007).
The discovery that cytoplasmic catenins are essential in cadherin-mediated cell adhesion was made in the late 1980s and early 1990s. Kemler’s group reported that the conserved cytoplasmic carboxyl terminus of the cell adhesion molecule E-cadherin binds three proteins with mol.wts. of 102, 88, and 80 kDa, i.e., α-catenin, β-catenin, and plakoglobin (γ-catenin) (Vestweber and Kemler 1984; Ozawa et al. 1989; Nagafuchi and Takeichi 1989). Plakoglobin has been molecularly cloned by Franke and collaborators (Cowin et al. 1986), α-catenin by Takeichi and Tsukita and collaborators (Nagafuchi et al. 1991), and β-catenin by Gumbiner and collaborators (McCrea et al. 1991; Butz et al. 1992). β-Catenin forms a direct link between cadherins and α-catenin, whereas α-catenin also talks with the actin cytoskeleton (Rimm et al. 1995; Drees et al. 2005; Yamada et al. 2005; Abe and Takeichi 2008). This link is essential for strong cadherin-mediated cell adhesion (Nagafuchi and Takeichi 1988; reviewed by Takeichi 1991; Kemler 1993; Gates and Peifer 2005; see Meng and Takeichi 2009 and Shapiro and Weis 2009).
The Wnt/β-catenin signaling pathway has a crucial role in the embryonic development of all animal species, in the regeneration of tissues in adult organisms and in numerous other processes (reviewed by Cadigan and Nusse 1997; Clevers 2006; Klaus and Birchmeier 2008). Mutations or deregulated expression of components of the canonical Wnt pathway can induce disease, most importantly cancer (Bienz and Clevers 2000; Polakis 2000; Moon et al. 2004). Through the Wnt pathway, signals are exchanged between neighboring cells and tissues: Wnt proteins that are secreted from one type of cell interact with surface receptors of neighboring cells. There the signals are passed through the cytoplasm to the nucleus, where gene regulation is modulated. This process controls proliferation, survival, cell migration, differentiation, and patterning in the receiving cells and tissues.
The protein β-catenin plays a critical role in canonical Wnt signaling. Noncanonical Wnt signaling, which does not involve β-catenin, is not discussed here (Veeman et al. 2003). It had been known since the late 1980s that β-catenin (armadillo in Drosophila) is involved in Wnt signaling, but the way this molecule moves from the cytoplasm to the nucleus and interacts with the transcription machinery was unknown (Wieschaus and Riggleman 1987; Riggleman et al. 1990; Peifer et al. 1992). In 1995, an interaction was found between β-catenin and the lymphocyte enhancer binding factor 1 (LEF1). This observation precipitated feverish work, which firmly established that LEF1, and its closest relatives, T cell factors (TCF), are activated by signaling through the Wnt pathway (Behrens et al. 1996; Molenaar et al. 1996; Huber et al. 1996; Riese et al. 1997). LEF1 and TCFs are high mobility group (HMG) proteins and were discovered as DNA-binding proteins that bind to specific sequences in lymphoid enhancers (Travis et al. 1991; van de Wetering et al. 1991; Waterman et al. 1991).
Canonical Wnt signaling works in the following fashion (for further detailed discussion of the Wnt pathway, see Cadigan and Pfeifer 2009; McCrea et al. 2009): In the absence of Wnt ligands, cytoplasmic β-catenin is recruited into a destruction complex, in which it interacts with APC and the axins, and is amino-terminally phosphorylated by axin-bound casein kinase 1α (CK1α) and GSK3β (Fig. 2A). Following phosphorylation, β-catenin is targeted for proteasome-dependent degradation involving an interaction with β-TrCP (β-transducin repeat-containing protein), a component of the E3 ubiquitin ligase complex. Therefore, in the absence of Wnt, cytoplasmic β-catenin levels remain low, and the transcription factors LEF1 and TCF interact with Grouchos in the nucleus to repress Wnt-specific target genes (Munemitsu et al. 1995; Aberle et al. 1997; Behrens et al. 1998; Roose et al. 1998; Liu et al. 2002). In the presence of Wnt ligands, LRP5–LRP6 surface receptors are phosphorylated by CK1γ and GSK3β (and possibly further kinases), and Dishevelled is recruited to the plasma membrane, where it interacts with Frizzled receptors and polymerizes with other Dishevelled molecules (Fig. 2B) (Davidson et al. 2005; Bilic et al. 2007; Schwarz-Romond et al. 2007). The inactivation of the destruction complex allows the cytoplasmic stabilization and translocation of β-catenin to the nucleus. Here, β-catenin forms a transcriptionally active complex with LEF1 and TCF transcription factors by displacing Grouchos and interacting with other coactivators (reviewed by Eastman and Grosschedl 1999; Stadeli et al. 2006; Klaus and Birchmeier 2008). This rounds out the picture of how Wnt’s effects on genes are modulated by the activation and repression of β-catenin. What remains to be explained is how the different sets of target genes exert their influence on cell architecture and behavior, which is a major theme of this review.
Figure 2.
Canonical Wnt pathway. (A) In the absence of a Wnt ligand, β-catenin is sequentially phosphorylated in the destruction complex of axin and APC by the kinases CK1α and GSK3β, which results in ubiquitination by β-TRCP and, finally, in the degradation of β-catenin in the proteasom. (B) In the presence of a Wnt ligand, a Fzd/LRP5/6 complex is formed and is bound by Dishevelled, leading to the phosphorylation of the LRP5/6 receptor by GSK3β, CK1γ and other kinases. Phosphorylated LRP5/6 recruits axin to the plasma membrane, which leads to decay of the destruction complex, inhibited phosphorylation, and degradation of β-catenin. Stabilized β-catenin is then translocated to the nucleus, a process that can involve BCl9-2. β-Catenin interacts with TCF/LEF, and the recruitment of cofactors like BCL9, Pygopus, or CBP as well as the replacement of Groucho leads to the activation of target genes.
CROSSTALK BETWEEN CADHERIN-MEDIATED CELL ADHESION AND CANONICAL Wnt SIGNALING THROUGH β-CATENIN
Wnt Signaling and Cadherins Compete for β-Catenin
The concept that canonical Wnt signaling and cadherin-mediated cell adhesion depend on the same pool of β-catenin is based on genetic and overexpression experiments in embryos and cultured cells. Heasman et al. showed in 1994 that overexpression of cadherins in Xenopus embryos inhibited dorsal axis formation, which is a clear function of canonical Wnt signaling (Heasman et al. 1994; see also Fagotto et al. 1996; Torres et al. 1996). Peifer and collaborators showed that armadillo (β-catenin) mutant embryos of Drosophila, which harbor only one E-cadherin allele, showed a less severe segment polarity phenotype than embryos with two cadherin alleles (Cox et al. 1996). Segment polarity is controlled by Wnt signaling (Nüsslein-Volhard et al. 1980; Rijsewijk et al. 1987). Similarly, cadherin overexpression mimicked the wingless (Wnt) phenotype in Drosophila embryos (Sanson et al. 1996). These data from the mid 1990s are thus consistent with a model in which there is crosstalk between β-catenin in two different compartments, the adhesion complex at the plasma membrane and a signaling complex in the nucleus.
Shortly after these findings in model organisms, Geiger and Ben-Ze’ev and collaborators showed an interplay between cadherin-mediated cell adhesion and canonical Wnt signaling in cell culture experiments. In colon cancer cells, expression of N-cadherin or an interleukin receptor-cadherin hybrid (in which the β-catenin binding region of N-cadherin was maintained) triggered a relocation of β-catenin from the nucleus to the plasma membrane and inhibited LEF1-mediated transcription (Sadot et al. 1998; Shtutman et al. 1999; Gottardi et al. 2001; Stockinger et al. 2001). Inducible expression of the Fos proto-oncogene in mammary gland epithelial cells resulted in the loss of E-cadherin and cell polarization, the colocalization of β-catenin with LEF1 in the nucleus, and increased Wnt/β-catenin signaling (Eger et al. 2000). Moreover, the absence of E-cadherin in E-cadherin -/- embryonic stem (ES) cells led to an accumulation of β-catenin with LEF1 in the nucleus and activation of a Wnt reporter (Orsulic et al. 1999). This could be antagonized by expression of E-cadherin.
Behrens and collaborators have recently shown that siRNA-mediated knockdown of E-cadherin augments β-catenin-dependent transcription in colon cancer cells in which the Wnt pathway is active. On the other hand, the same procedure has no effect in nontransformed keratinocytes that do not display Wnt signaling (Kuphal and Behrens 2006). These data indicate that the mere loss of E-cadherin does not activate Wnt signaling—except in cases in which the β-catenin degradation machinery is compromised. These results are consistent with data from breast cell cancer lines showing that the absence of E-cadherin alone does not result in activation of Wnt signaling (van de Wetering et al. 2001). Nor does a loss of E-cadherin function in Rip1Tag2 transgenic mice contribute to Wnt/β-catenin signaling (Herzig et al. 2007). Weinberg and collaborators recently showed that in ras-transformed mammary gland cells (HMLER), shRNA down-regulation of E-cadherin results in translocation of β-catenin from cell–cell junctions to the cytoplasm and nucleus (Onder et al. 2008). This type of β-catenin was nonphosphorylated and thus was not targeted for ubiquitination and degradation. However, in this system, the loss of E-cadherin affected numerous other signaling pathways that have been implicated in metastasis formation.
Gottardi and Gumbiner have performed precise studies to determine what controls β-catenin targeting to cadherin adhesion or to TCF transcriptional complexes (Gottardi and Gumbiner 2004). They showed that Wnt signaling generates a monomeric, intramolecularly folded-back form of β-catenin that binds TCF but not cadherins. In contrast, the cadherin-binding form of β-catenin builds a dimer with α-catenin. X-ray crystallographic studies have shown that cadherin-binding involves all 12 armadillo repeats of β-catenin, whereas TCF-binding requires only the central eight repeats (Graham et al. 2000; Huber and Weis 2001). Thus it is possible that the carboxyl terminus of Wnt-produced β-catenin folds back over armadillo repeats, affecting binding to cadherin but not TCF. The selective binding of β-catenin induced by Wnt could also involve post-translational modifications or the activation of further proteins. Overall, these data suggest that β-catenin’s selectivity between adhesion and transcription are not always coupled; in other words, they might be regulated independently.
BCL9 protein, the product of a human proto-oncogene, also acts in the switch between cadherin cell adhesion and β-catenin signaling. This story has been worked out through work on BCL9 and its ortholog legless, a Drosophila segment polarity gene. Legless, which was isolated in 2002 by the group of Konrad Basler, is required for Wnt signaling in the fly (Kramps et al. 2002). It acts by binding directly to β-catenin. Human BCL9 was discovered in a B-cell lymphoma because of a translocation to the immunoglobulin locus, which caused BCL9 overexpression in the tumors (Willis et al. 1998). Remarkably, human BCL9 could rescue the segment polarity phenotype of the legless mutation (Kramps et al. 2002), indicating functional identity. Vertebrates have a second homolog BCL9-2 (Brembeck et al. 2004; Adachi et al. 2004), which also binds to β-catenin like BCL9. BCL9-2 promotes nuclear location of β-catenin, increased β-catenin signaling, and triggers EMT in vertebrate cells and Zebra fish embryos (Brembeck et al. 2004). BCL9-2 cannot colocalize with the E-cadherin/β-catenin/α-catenin complex at the plasma membrane, but following tyrosine phosphorylation of β-catenin, it is translocated to the nucleus and promotes β-catenin signaling. Thus BCL9 proteins may act in the switch between cadherin-mediated cell adhesion and Wnt signaling (Brembeck et al. 2004; Sampietro et al. 2006; Hoffmans and Basler 2007; de la Roche et al. 2008).
Proteolysis of Cadherins Affects Wnt Signaling
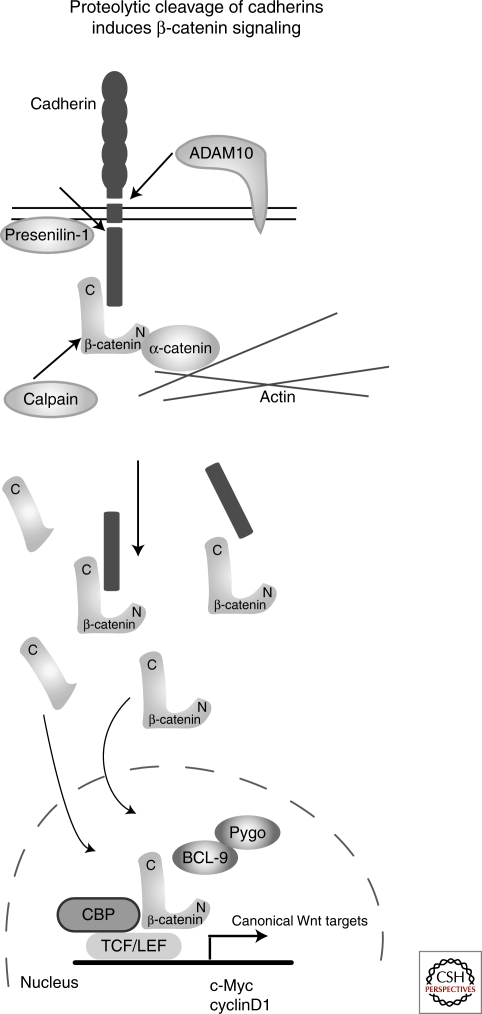
Competition for β-catenin is one mechanism by which cells can modulate Wnt signals. Another is the cleavage of E- and N-cadherin by proteases, which can lead to the release of β-catenin and to up-regulated β-catenin signaling. This occurs in normal development but also in processes such as wound healing, Ca2+-influx, and apoptosis. Cadherins are cleaved intracellularly by proteases like caspase 3 or presenilin, or extracellularily by ADAM10 (Ito et al. 1999; Steinhusen et al. 2001; Marambaud et al. 2002; Maretzky et al. 2005; Shoval et al. 2007). Intracellular cleavage of cadherins releases nearly the entire cytoplasmic domain, whereas extracellular cleavage releases the adhesion domain (Fig. 3). ADAM10 belongs to the family of disintegrins and metalloproteases (ADAM), type I transmembrane proteins that combine cell–cell adhesion and proteinase activity (reviewed by Primakoff and Myles 2000; Seals and Courtneidge 2003). ADAM10 was recently identified as a β-catenin/TFC target gene (Gavert et al. 2007). The group of Saftig has shown that cleavage of E- and N-cadherin by ADAM10 in keratinocytes and neuronal cells, respectively, reduced cell adhesion, increased cell migration and led to translocation of β-catenin to the nucleus. Translocated β-catenin activated Wnt/β-catenin target genes like c-Myc and cyclinD1 (Maretzky et al. 2005; Reiss et al. 2005). These are clear examples of an immediate crosstalk between a loss of cadherin-mediated cell adhesion and Wnt/β-catenin signaling.
Figure 3.
Proteolytic cleavage of cadherin can release β-catenin and induce canonical Wnt signaling. Under certain cellular conditions, cadherins can be cleaved by proteases like ADAM10 and Presenilin-1. This cleavage leads to loosening of cell adhesion, but also to the release of β-catenin, which is translocated into the nucleus and as a result, activates Wnt/β-catenin target genes. Another protease, calpain, is interfering with the adhesion complex by the amino-terminal cleavage of β-catenin. β-Catenin cleaved by calpain is stabilized and can enter the nucleus and activate target genes.
Presenilin1 (PS1) is an integral membrane protein and a component of the γ-secretase complex, which mediates an ε-cleavage of type I membrane proteins such as the amyloid precursor protein (APP). A mutant form of PS1 (PS1 FAD, familial Alzheimer’s disease) is known to process APP, leading to increased production of the β-amyloid peptide (Aβ42), which is responsible for Alzheimer’s disease (reviewed by Marambaud and Robakis 2005; Selkoe and Wolfe 2007). Mechanistically, PS1 acts in several ways to influence cell adhesion and transcription. PS1 can bind to E-cadherin and β-catenin and promotes their association to the cytoskeleton, and the overexpression of PS1 results in enhanced cadherin-mediated cell adhesion (Georgakopoulos et al. 1999; Baki et al. 2001). Under imbalanced calcium conditions or apoptosis, PS1 does not stabilize cell–cell adhesion but cleaves E-cadherin, which leads to the disassembly of adherens junctions, to the release of a cytoplasmic E-cadherin fragment, and to an increase of soluble β-catenin (Marambaud et al. 2002). It is possible that the release of β-catenin from adherens junctions caused by PS-1 modulates gene expression. It has been shown that the released N-cadherin fragments generated by PS1 favor nuclear localization of β-catenin and promote β-catenin signaling (Uemura et al. 2006). The released fragments of E-cadherin also translocate to the nucleus and affect transcriptional activity (Ferber et al. 2008). In contrast, PS1 can facilitate GSK3β-mediated phosphorylation of β-catenin, thereby favoring the degradation of β-catenin and negatively regulating β-catenin/LEF signaling (Zhang et al. 1998; Kang et al. 1999; Soriano et al. 2001; Kang et al. 2002). This latter type of cross-talk between PS-1 and β-catenin signaling has also been shown by genetic means; tumors with elevated β-catenin signaling developed in the skin of mice with PS-1 deficiency (Xia et al. 2001; Kang et al. 2002). Taken together, this indicates that PS-1 modulates junctional signaling (1) by cleaving cadherins, leading to increased β-catenin signaling and alterations in gene activity, and (2) by stabilizing cell-adhesion and promoting the degradation of β-catenin, thereby decreasing β-catenin mediated transcription.
Recently it has been proposed that the NMDA (N-methyl-d-aspartate) receptor in synapses links cadherin-mediated adhesion and β-catenin signaling (Abe and Takeichi 2007). The NMDA receptor is a glutamate-gated ion channel, which signals through the influx of Ca2+ and induces long-term synaptic plasticity in the nervous system (Carroll and Zukin 2002). N-cadherin has an important function in this process (Arikkath and Reichardt 2008). Triggering the NMDA receptor in neuronal cells activates the proteases ADAM10 and PS-1, which are able to process cadherins (Marambaud et al. 2003; Reiss et al. 2005). Another protease, calpain, has been shown to be critically involved in synaptic plasticity (Staubli et al. 1988). Abe and Takeichi have now shown that the NMDA-dependent activation of calpain cleaves β-catenin at its amino terminus (Fig. 3). As a result, β-catenin is stabilized, accumulates in the nucleus, and induces TCF/LEF1-dependent gene expression (Abe and Takeichi 2007). If it proves to be true that calpain cleaves β-catenin bound to cadherin and that this is essential in synaptic processes, this would establish a direct link between neuronal activity, adhesion between cells at the synapse, and β-catenin signaling.
Phosphorylation and the Control of β-Catenin in Signaling and Adhesion
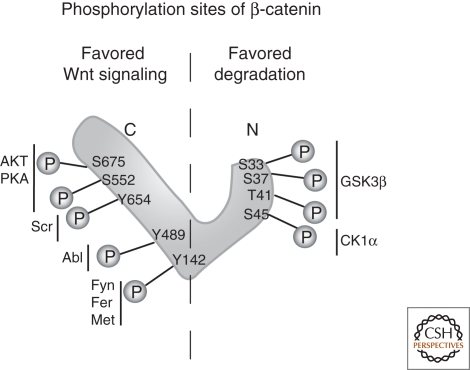
Yet another link between adhesion and Wnt signaling can be found in the phosphorylation of components of the cadherin adhesion system, of the β-catenin degradation complex, or of the TCF transcription system, which regulate the strength of interactions. The action of various serine/threonine and tyrosine kinases therefore influences the function of β-catenin in adhesion and in signaling (reviewed by Daugherty and Gottardi 2007). For instance, the carboxy-terminal phosphorylation of cadherins can strengthen or weaken interactions with β-catenin, depending on the sites of phosphorylation (Choi et al. 2006; Qi et al. 2006). Tyrosine 654 phosphorylation of β-catenin reduces cadherin binding and adhesion function (Fig. 4) (Roura et al. 1999). The phosphorylation of β-catenin at tyrosine 489 or 142 strengthens Wnt signaling (Brembeck et al. 2004; Rhee et al. 2007). The phosphorylation of axin or APC, respectively, on serine or threonine residues can strengthen or weaken interactions with β-catenin, and the phosphorylation of TCFs also influences their binding to β-catenin (Rubinfeld et al. 1996; Willert et al. 1999; Lee et al. 2001; Ha et al. 2004).
Figure 4.
Phosphorylation sites of β-catenin. β-Catenin can be sequentially phosphorylated by different kinases, either promoting the degradation or the signaling activity of β-catenin. Phosphorylation at the amino terminus favors degradation, whereas phosphorylation in the armadillo domain changes the adhesiveness to cadherins and promotes nuclear localization.
Tyrosine kinases and tyrosine phosphatases have been shown to regulate the switch between cadherin-mediated cell adhesion and β-catenin signaling (Warren and Nelson 1987; Behrens et al. 1993; Muller et al. 1999; Roura et al. 1999; reviewed by Daugherty and Gottardi 2007). Insulin-like growth factor II leads to the internalization of E-cadherin/β-catenin and to E-cadherin degradation (Morali et al. 2001). β-Catenin then becomes nuclear, and β-catenin/TCF target genes are activated. HGF/SF action on Met can result in tyrosine 142 phosphorylation of β-catenin and induction of EMT in epithelial cells and axonal growth and branching of neuronal cells (Brembeck et al. 2004; David et al. 2008). A dominant-negative TCF4 can inhibit neural branching. The tyrosine kinase Met is itself a Wnt/β-catenin target gene (Boon et al. 2002). FGF and FGF receptor signaling also affects E-cadherin/β-catenin interaction and β-catenin/TCF signaling (Pai et al. 2008). Hunter and collaborators have shown that EGF receptor signaling induces EMT and caveolin-mediated endocytosis of E-cadherin (Lu et al. 2003). As a result, β-catenin localizes to the nucleus and activates Wnt/β-catenin target genes. EGF treatment also down-regulates caveolin-1, which enhances the transcriptional activity of β-catenin. Caveolin-1 negatively affects canonical Wnt signaling through the recruitment of β-catenin to caveolar membranes (Galbiati et al. 2000). E-cadherin can also be internalized by a different mechanism: Tyrosine phosphorylation of E-cadherin by Src kinase induces the recruitment of the E3 ubiquitin ligase Hakai and favors cell scattering (Fujita et al. 2002). Hakai ubiquitinates E-cadherin and mediates the clathrin-dependent internalization and lysosomal degradation of E-cadherin (Bonazzi et al. 2008; Shen et al. 2008). It is not yet clear whether the action of Hakai influences β-catenin signaling. Recently, the tyrosine phosphatases PTPRK and PCP-2 have been shown to inhibit β-catenin signaling and increase E-cadherin-dependent adhesion (Yan et al. 2006; Novellino et al. 2008). The cumulative evidence suggests that tyrosine phosphorylation-dependent destabilization of cadherin/β-catenin complexes and tyrosine-phosphorylation-dependent activation of β-catenin can both activate signaling and induce EMT.
A novel type of interplay between the loss of N-cadherin-mediated adhesion and transcriptional activity following tyrosine-phosphorylation of β-catenin has recently been discovered in Slit-activated neural retina cells (Rhee et al. 2007). Slit is a secreted axon guidance cue that binds and activates the Robo receptor, and this leads to the inactivation of N-cadherin (Brose et al. 1999). Balsamo and collaborators have shown that a population of N-cadherin-associated β-catenin, which is phosphorylated on tyrosine 489 in response to Slit-activation of the Robo-associated tyrosine kinase abl, is mobilized to the nucleus and becomes transcriptionally active (Rhee et al. 2007). This provides an example in which the down-regulation of cadherin-mediated adhesion in neuronal cells is coordinated with long-term effects on transcription.
The L1 adhesion system has recently also been shown to affect cadherin-mediated cell adhesion and β-catenin signaling (Shtutman et al. 2006). L1 is a transmembrane adhesion molecule with extracellular immunoglobulin-like domains and fibronectin type III repeats. It is up-regulated by Wnt/β-catenin signaling, it is expressed exclusively at the invasive front of colon cancer tissue, and its expression is correlated with tumor progression and metastasis (see also section B; Haspel and Grumet 2003; Gavert et al. 2005; Gavert et al. 2007). It has now been shown that L1 disrupts the formation of adherens junctions by releasing E-cadherin, that it promotes EMT-like transition, and that it induces β-catenin signals (Shtutman et al. 2006).
The Catenin p120 Relieves Kaiso-Mediated Repression of Wnt Target Genes
The turnover of cadherins is another crucial element of cell adhesion. It is critically dependent on the catenin p120, which binds to the highly conserved juxtamembrane domain of cadherins and increases their stability at the cell membrane. Depletion of p120 leads to an immediate turnover of E-cadherin and to a weakening of cell–cell adhesion (reviewed by Reynolds and Roczniak-Ferguson 2004). McCrea and collaborators have shown that p120 also influences the Wnt pathway through an interaction with the transcription factor Kaiso (Kim et al. 2004; Park et al. 2005; see also McCrea et al. 2009). Kaiso belongs to the BTB/POZ (zinc finger and broad-complex, tramtrack and bric-a-brac/poxvirus) protein family. The zinc-finger of Kaiso binds both CpG methylated sequences and a defined promoter consensus motif (Daniel et al. 2002; Kim et al. 2004). The association of Kaiso to promoters has been shown to repress Wnt target genes in Xenopus embryos and in mammalian cell lines by interfering with the binding of β-catenin to TCF/LEF (Kim et al. 2004; Park et al. 2005; Spring et al. 2005). It was recently suggested that Kaiso function may be mediated by its interaction with the DNA binding domain of TCF, leading to the displacement of TCF from promoters (Ruzov et al. 2009). The interaction of p120 with Kaiso influences the repressor function of Kaiso either by sequestrating Kaiso in the cytoplasm or by replacing it from the promoter (Park et al. 2005). It is likely that p120 affects the nuclear shuttle of Kaiso, since the NLS of p120 is crucial to the inhibition of Kaiso-mediated transcriptional repression (Kelly et al. 2004a; Kelly et al. 2004b). A functional interplay between p120, Kaiso, and Wnt target genes has also been observed in human cancer (reviewed by van Roy and McCrea 2005). The Wnt pathway also converges with the p120/Kaiso pathway further upstream, since p120 is stabilized by Frodo, a Dsh interacting partner. The interaction of Dsh with Frodo leads to stabilization of p120, which in turn enhances the displacement of Kaiso from target genes (Park et al. 2006). This means that the cell adhesion-modulating catenin p120 enables a further cross talk of cell junctions and the Wnt pathway in a β-catenin-independent fashion.
Adhesion and Wnt/β-Catenin Signaling in Cardiomyopathy
Mutations in genes that encode desmosomal proteins, including desmosomal cadherins, are the cause of arrhythmogenic right ventricular cardiomyopathies (ARVC) in humans. In this disease, muscle tissue of the right ventricle of the heart is replaced by fibrofatty tissue, resulting in arrhythmias and sudden death (reviewed by Sen-Chowdhry et al. 2004; MacRae et al. 2006). The mutated genes include all types of components of desmosomes: The cadherins desmocollins and desmogleins, the catenins plakoglobin and plakophilin2, and the linker protein to intermediate filaments, desmoplakin (MacRae et al. 2006; see also Heuser et al. 2006). Genetic experiments in mice have corroborated the essential role of desmosomal proteins in heart structure and function (see for instance Ruiz et al. 1996; Grossmann et al. 2004). Previously it had been firmly believed that the primary cause of ARVC is the weakening of desmosomal adhesion between cardiomyocytes in the diseased heart. However, the work of Garcia-Gras et al. has introduced a new twist in concepts: The cardiac-specific ablation of desmoplakin in mice is sufficient to cause nuclear translocation of plakoglobin (γ-catenin), which then inhibits Wnt/β-catenin signaling (Zhurinsky et al. 2000; Garcia-Gras et al. 2006). It is known that Wnt/β-catenin signals supports cardiomyogenesis, whereas inhibition promotes adipogenesis (Ross et al. 2000). This could explain the reduced production of cardiomyocytes and the increased production of adipocytes, which is observed in ARVC. These data nicely show how a switch from cadherin-mediated cell-adhesion to modulated Wnt signaling might result in the complex properties of a human disease.
General Remarks
The studies described above show how a loss of E-cadherin-mediated cell adhesion induces β-catenin release and signaling. These functions cause biological responses such as EMT, invasion and metastasis (Fig. 1). In other cases, loss of E-cadherin induces EMT without promoting β-catenin mobilization, but it affects other signaling pathways (see also Perrais et al. 2007; Onder et al. 2008). A compromised β-catenin degradation machinery appears to be the prerequisite for β-catenin signaling, which follows the loss of cadherins. β-Catenin occupies different functional niches in the cells, and the flow between these niches and the subsequent effects on cell adhesion and signaling are regulated by further events such as phosphorylation. Taken together, the data show intricate networks of both interacting and separate pathways that control cell adhesion and signaling, and their contribution to EMT needs to be unraveled in each case.
CANONICAL Wnt SIGNALING AFFECTS CADHERIN-MEDIATED CELL-ADHESION
So far, our focus has mainly been on events within the cytoplasm that affect the cell by modulating β-catenin’s functions in cell adhesion and its availability as a transcription factor in combination with other molecules. This section summarizes the effects of Wnt signaling at the gene level, showing its impact on cell–cell adhesion through the regulation of the expression of diverse cell adhesion-modulating molecules. For a frequently updated overview of Wnt target genes see Roel Nusse’s webpage (www.stanford.edu/∼rnusse/wntwindow.html).
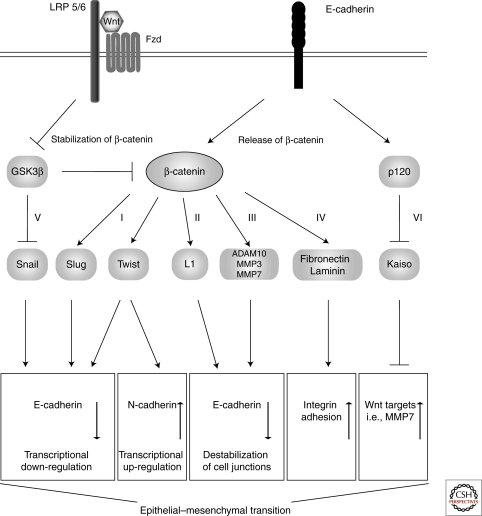
Some Wnt target genes encode for components of cell junctions such as L1-CAM (Gavert et al. 2005), Nr-CAM (Conacci-Sorrell et al. 2002) and connexin 43 (van der Heyden et al. 1998). E-cadherin is negatively regulated by Wnt signaling (Huber et al. 1996; Jamora et al. 2003). Other Wnt targets encode transcription factors that negatively control the expression of cadherins like Twist (Howe et al. 2003) and Slug (Vallin et al. 2001; Conacci-Sorrell et al. 2003). Additionally, Wnt targets also encode enzymes that are involved in regulating the stability of cell adhesions, such as Tiam1 (Malliri et al. 2006), Matrilysin (Brabletz et al. 1999; Crawford et al. 1999) and Stromelysin (Prieve and Moon 2003). Wnt/β-catenin signaling also directly controls the expression of the mesenchymal genes fibronectin and laminin-5γ2 (Gradl et al. 1999; Hlubek et al. 2004). Wnt target genes that affect cell motility also include fascin (Vignjevic et al. 2007), EphB/Ephrin (Batlle et al. 2005), CD44 (Wielenga et al. 1999), and S100/A4-metastasin (Stein et al. 2006). Overall, Wnt signaling appears to modulate target genes that cause a switch from primarily epithelial to more mesenchymal cells (Fig. 5).
Figure 5.
Canonical Wnt signaling modulates cell adhesions. Activated Wnt signaling leads to stabilization and nuclear localization of β-catenin, which induces the expression of Wnt target genes: I) The transcription factors Slug and Twist both repress the expression of E-cadherin, and Twist activates the expression of N-cadherin. II) The cell adhesion molecule L1 interferes with E-cadherin-mediated cell adhesion. III) The proteases MMP3 and MMP7 modulate the extracellular matrix, and MMP3, MMP7, and ADAM10 cleave E-cadherin. IV) The cell matrix molecules fibronectin and laminin favor cell-substrate adhesion. V) Wnt signaling inactivates GSK3β, leading to stabilization of Snail, which also inhibits the expression of E-cadherin. VI) p120 relieves the repressor Kaiso from Wnt target genes.
Wnt Targeted Transcription Factors Regulate Cadherin Expression—Twist, Snail and Slug
Twist is a bHLH transcription factor that was first identified in Drosophila as an inducer of mesoderm formation (Nüsslein-Volhard et al. 1984; Simpson 1983; Thisse et al. 1988). Twist is also expressed in mesodermal cells of vertebrates (Wolf et al. 1991; Fuchtbauer 1995; Wang et al. 1997). Wnt1 regulates Twist expression in Drosophila and murine cells (Bate and Rushton 1993; Howe et al. 2003), and Wnt/β-catenin signaling regulates Twist expression in migrating cells in Xenopus (Borchers et al. 2001). Gene ablation experiments in the mouse show that Twist is important for cell migration and cell localization during cranial neural tube morphogenesis (Chen and Behringer 1995; Soo et al. 2002). Twist down-regulates the expression of E-cadherin by binding to E-box elements of the promotor (Ip et al. 1992; Rohwedel et al. 1995; Lee et al. 1997; Yin et al. 1997; Kophengnavong et al. 2000), and it induces EMT and invasion and metastasis of mammary gland cells (Yang et al. 2004). In contrast, Twist is also involved in the up-regulation of N-cadherin (Oda et al. 1998; Yang et al. 2004; Alexander et al. 2006). N-cadherin can act as a proinvasive adhesion molecule that is produced in carcinomas following a so-called cadherin switch (Wheelock and Johnson 2003; Hazan et al. 2004). In spite of its opposing regulatory functions on the promoters of E- and N-cadherin, both of these functions of Twist promote EMT. The same type of duality has been observed in tumors and cancer cell lines (Hsu et al. 1996; Hazan et al. 1997; Sandig et al. 1997; Tomita et al. 2000; Rosivatz et al. 2002; Gravdal et al. 2007). Because transfections of E-cadherin cannot fully correct EMT in mammary gland cells, it is suggested that Twist affects additional EMT-inducing genes (Yang et al. 2004). These data reveal a mechanism by which Wnt signaling can promote EMT, tumor progression, and metastasis via the regulation of Twist.
Like Twist, the transcription repressors Snail and Slug are involved in the regulation of EMT (Nieto et al. 1994; Sefton et al. 1998; Batlle et al. 2000; Cano et al. 2000; Veltmaat et al. 2000; ten Berge et al. 2008). Snail was first identified in Drosophila (Nüsslein-Volhard et al. 1984; Grau et al. 1984) and its homolog Slug in the chick (Nieto et al. 1994). Both Snail and Slug bind to the E-box motive of the E-cadherin promoter through a carboxy-terminal zink-finger domain and suppress the expression of E-cadherin (Batlle et al. 2000; Cano et al. 2000; Hajra et al. 2002; Bolos et al. 2003). This is mediated by the amino-terminal SNAG domain of Snail, which recruits a histone deacetylase (Batlle et al. 2000; Peinado et al. 2004a). Slug is not able to repress the expression of E-cadherin in rat bladder carcinoma cells, but disrupts desmosomes (Savagner et al. 1997).
Snail and Slug not only play a role in cell adhesion, but also their functions are affected by the Wnt pathway. The Slug promoter harbors a LEF binding site and is responsive to β-catenin/TCF signaling, which leads to the expression of Slug and in turn to the down-regulation of E-cadherin (Vallin et al. 2001; Conacci-Sorrell et al. 2003). The Snail promoter is not directly responsive to β-catenin/TCF signaling (Conacci-Sorrell et al. 2003), but Wnt signaling leads to increased levels of Snail by inhibiting GSK3β. Blocking GSK3β activity increases the transcriptional level of Snail (Bachelder et al. 2005) and also increases its stability; Snail is a substrate of GSK3β, and phosphorylation marks Snail for degradation (Zhou et al. 2004; Yook et al. 2005). Additionally, Snail interacts directly with β-catenin and enhances Wnt target gene expression, and is believed to stimulate the Wnt pathway in a positive feedback loop (Stemmer et al. 2008). These data indicate that Slug and Snail are important regulators of cadherin-mediated cell adhesion and that the Wnt pathway affects cell–cell adhesion via regulation of these transcriptional regulators (Fig. 5).
Two other aspects of these transcription factors need to be mentioned here. First, Snail, Slug, and Twist are regulated by many signaling pathways, of which Wnt is only one. It has been shown that the cellular context determines which pathways crosstalk to Snail, Slug, and Twist and regulate the expression of E-cadherin (reviewed by Nieto 2002; Huber et al. 2005). Next to the E-boxes, the E-cadherin promoter harbors other regulatory elements, such as cis-regulatory elements (Stemmler et al. 2005), and can be regulated by other transcription factors like δEF1/ZEB1, Sip-1/ZEB2, and E12/E47 (Grooteclaes and Frisch 2000; Comijn et al. 2001; Perez-Moreno et al. 2001).
Second, EMT plays a crucial role in gastrulation, a process in which the regulation of E-cadherin has an important function (Burdsal et al. 1993). During gastrulation, epiblast cells form the primitive streak from which endoderm and mesoderm are generated. The importance of canonical Wnt signaling can be seen from the fact that Wnt3, β-catenin, and LRP5/LRP6-deficient mouse epiblasts fail to form the primitive streak (Liu et al. 1999; Huelsken et al. 2000; Kelly et al. 2004c). The down-regulation of E-cadherin in mouse gastrulation is not controlled by Twist, as Twist is not expressed in the primitive streak (Chen and Behringer 1995). However, Snail is expressed in the primitive streak in the mouse (Smith et al. 1992), and ablation of Snail leads to the continued expression of E-cadherin transcripts in the mesoderm. It also causes defects in the generated mesoderm cell layer (Carver et al. 2001). FGF signaling has an important role during mouse gastrulation, and a deficiency in FGFR1 prevents the expression of Snail, in turn disturbing the down-regulation of E-cadherin and EMT (Deng et al. 1994; Yamaguchi et al. 1994; Ciruna et al. 1997; Deng et al. 1997; Ciruna and Rossant 2001). So the down-regulation of E-cadherin does not seem to be controlled by the Wnt pathway in mouse gastrulation, but further data indicate a cross talk of Wnt and FGF signaling. In FGFR1-deficient epiblasts, the expression of the Wnt target Brachyury is negatively affected, but lowering E-cadherin levels rescues Brachyury expression in the primitive streak (Ciruna and Rossant 2001). Therefore, increased E-cadherin levels are thought to sequester β-catenin and negatively affect canonical Wnt signaling.
Wnt Signaling Induces Expression of Proteases that Decrease Cell–Cell Adhesion
EMT and metastasis depend on modifications of the extracellular matrix and cell–cell adhesion through the cleavage of E-cadherin. This is achieved by proteases, which are regulated by Wnt signaling. A direct canonical Wnt target is the metalloproteinase Matrilysin (MMP7; Crawford et al. 1999). Stromelysin-1 (MMP3) expression is regulated by Wnt5a in a β-catenin-independent manner, indicating that Stromelysin-1 expression is controlled by the noncanonical Wnt pathway (Prieve and Moon 2003). Both Stromelysin-1 and Matrilysin degrade components of the extracellular matrix and are up-regulated in colorectal cancers, and MMP7 has been shown to promote metastasis (reviewed by Wagenaar-Miller et al. 2004). Both proteases also cleave E-cadherin, which results in the shedding of the E-cadherin ectodomain and causes cells to become motile and invasive (Fig. 5) (Lochter et al. 1997; Noe et al. 2001).
The Wnt Target Gene Tiam1 and IQGAP1 Modulate Cell–Cell Adhesion
Wnt has additional effects on cell–cell adhesion through its regulation of target genes. Tiam1 (T-cell lymphoma invasion and metastasis 1) is a nucleotide exchange factor that selectively activates Rac1 (Michiels et al. 1995) and is required for cadherin-based cell adhesions and suppression of epithelial motility (Hordijk et al. 1997; Malliri et al. 2004). Tiam1 modulates the dynamics of cell–cell adhesion by regulating tight junction assembly via activation of the Par complex (reviewed by Mertens et al. 2006) and by stabilizing the β-catenin/E-cadherin complex (Fukata et al. 1999). Tiam1 is a Wnt target gene, as shown by the fact that its expression can be induced by Wnt1 and correlates with active Wnt signaling in human colon adenoma, adenomatous polyps of Min/+ mice, and in the crypts of the small intestine (Liu et al. 2005; Malliri et al. 2006; Minard et al. 2006). Min/+ mice deficient in Tiam1 develop a reduced number of intestinal tumors and an impaired formation of mammary tumors. The developing tumors are however more aggressive and invade the submucosa in larger fractions (Malliri et al. 2006). These data indicate that Wnt signaling interferes with Tiam1 activity, which regulates cell growth, cell migration, and adherens junction formation.
A further mechanism is seen in the case of IQGAP1 (IQ motif containing GTPase-activating protein 1), which is involved in cytoskeletal dynamics and other cellular functions (reviewed by Briggs and Sacks 2003; Brandt and Grosse 2007). IQGAP1 regulates the dynamics of E-cadherin-based adherens junctions (Izumi et al. 2004) and interferes with β-catenin–α-catenin complex formation by binding the amino terminus of β-catenin, leading to reduced E-cadherin-mediated cell adhesion (Kuroda et al. 1998). It has recently been shown that IQGAP1 is up-regulated in colorectal carcinomas and promotes the invasiveness of colon cancer cells (Hayashi et al. 2009). GTP-bound Rac1 inhibits the interaction between IQGAP1 and β-catenin and stabilizes cell–cell adhesion (Fukata et al. 1999). As IQGAP1 acts downstream of Rac1, one likely possibility is that Tiam1/Rac1 positively regulates cell adhesion by inhibiting IQGAP1. Therefore, it is plausible that Wnt signaling affects cell adhesion via Tiam1/Rac1, which balances the activity of IQGAP1. Alongside its function in cell adhesion, IQGAP1 influences Wnt signaling, because in cells with stabilized β-catenin, IQGAP1 enhances the transcriptional coactivator function of β-catenin (Briggs et al. 2002). These results are an example of a cross-talk of canonical Wnt and Rac signaling including Tiam1 and IQGAP1 in the regulation of cell–cell adhesion.
General Remarks
The work described in this section shows several ways in which canonical Wnt signaling affects cadherin-mediated cell adhesion: (1) it down-regulates E-cadherin expression via the transcription factors Twist and Slug, (2) it up-regulates adhesion molecules that favor cell motility, such as N-cadherin and L1, and (3) it induces proteases and other EMT promoters. Wnt signaling can therefore induce a cadherin switch and weaken cell–cell adhesion (Fig. 5). Wnt/β-catenin signaling is up-regulated in many developmental processes and by mutations in the progression of tumors (reviewed by Clevers 2006; Grigoryan et al. 2008). Overall, cadherin loss and Wnt/β-catenin signaling can thus cooperate to promote EMT, invasion and metastasis.
Until recently there have been considerable gaps in our ability to link signals to the architectural and behavioral changes in cells which lead to EMT and metastases. But as this review shows, the Wnt pathway and the regulation of cell adhesion are strongly linked by a number of complementary mechanisms. This suggests a model that integrates a number of fundamental processes that underlie development and disease.
ACKNOWLEDGMENTS
We thank Russ Hodge (Berlin) for helpful discussions and improvements on the text. Our work was funded by the Deutsche Forschungsgemeinschaft (DFG), the Mildred-Scheel-Stiftung für Krebsforschung (Deutsche Krebshilfe), the European Union, and the Federal Ministry of Research and Technology of Germany (BMFT).
Footnotes
Editors: W. James Nelson and Elaine Fuchs
Additional Perspectives on Cell Junctions available at www.cshperspectives.org
REFERENCES
- Abe K, Takeichi M 2007. NMDA-receptor activation induces calpain-mediated β-catenin cleavages for triggering gene expression. Neuron 53:387–397 [DOI] [PubMed] [Google Scholar]
- Abe K, Takeichi M 2008. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci 105:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R 1997. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16:3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Jigami T, Yasui T, Nakano T, Ohwada S, Omori Y, Sugano S, Ohkawara B, Shibuya H, Nakamura T, et al. 2004. Role of a BCL9-related β-catenin-binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res 64:8496–8501 [DOI] [PubMed] [Google Scholar]
- Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, Heimark RL 2006. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res 66:3365–3369 [DOI] [PubMed] [Google Scholar]
- Arikkath J, Reichardt LF 2008. Cadherins and catenins at synapses: Roles in synaptogenesis and synaptic plasticity. Trends Neurosci 31:487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM 2005. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: Implications for the epithelial-mesenchymal transition. J Cell Biol 168:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Marambaud P, Efthimiopoulos S, Georgakopoulos A, Wen P, Cui W, Shioi J, Koo E, Ozawa M, Friedrich VL Jr, et al. 2001. Presenilin-1 binds cytoplasmic epithelial cadherin, inhibits cadherin/p120 association, and regulates stability and function of the cadherin/catenin adhesion complex. Proc Natl Acad Sci 98:2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ 1997. NH2-terminal deletion of β-catenin results in stable colocalization of mutant β-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol 136:693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate M, Rushton E 1993. Myogenesis and muscle patterning in Drosophila. Comptes rendus de l’Academie des sciences 316:1047–1061 [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature Cell Biol 2:84–89 [DOI] [PubMed] [Google Scholar]
- Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. 2005. EphB receptor activity suppresses colorectal cancer progression. Nature 435:1126–1130 [DOI] [PubMed] [Google Scholar]
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H 1994. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 54:3845–3852 [PubMed] [Google Scholar]
- Behrens J, Birchmeier W, Goodman SL, Imhof BA 1985. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: Mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol 101:1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382:638–642 [DOI] [PubMed] [Google Scholar]
- Behrens J, Mareel MM, Van Roy FM, Birchmeier W 1989. Dissecting tumor cell invasion: Epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol 108:2435–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W 1998. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280:596–599 [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120:757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F 1995. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. The EMBO J 14:6107–6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M, Clevers H 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311–320 [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C 2007. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316:1619–1622 [DOI] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J Cell Sci 116:499–511 [DOI] [PubMed] [Google Scholar]
- Bonazzi M, Veiga E, Cerda JP, Cossart P 2008. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalisation of Listeria monocytogenes. Cell Microbiol 10:2208–2222 [DOI] [PubMed] [Google Scholar]
- Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST 2002. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 62:5126–5128 [PubMed] [Google Scholar]
- Borchers A, David R, Wedlich D 2001. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 128:3049–3060 [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T 1999. β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am Am J Pathol 155:1033–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt DT, Grosse R 2007. Get to grips: Steering local actin dynamics with IQGAPs. EMBO Rep 8:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W 2004. Essential role of BCL9-2 in the switch between β-catenin’s adhesive and transcriptional functions. Genes Develop 18:2225–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MW, Sacks DB 2003. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep 4:571–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MW, Li Z, Sacks DB 2002. IQGAP1-mediated stimulation of transcriptional co-activation by β-catenin is modulated by calmodulin. J Biolog Chem 277:7453–7465 [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T 1999. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96:795–806 [DOI] [PubMed] [Google Scholar]
- Burdsal CA, Damsky CH, Pedersen RA 1993. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development 118:829–844 [DOI] [PubMed] [Google Scholar]
- Butz S, Stappert J, Weissig H, Kemler R 1992. Plakoglobin and β-catenin: Distinct but closely related. Science 257:1142–1144 [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R 1997. Wnt signaling: A common theme in animal development. Genes Develop 11:3286–3305 [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Pfeifer M 2009. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol 1:a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83 [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS 2002. NMDA-receptor trafficking and targeting: Implications for synaptic transmission and plasticity. Trends Neurosci 25:571–577 [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T 2001. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol 21:8184–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR 1995. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Develop 9:686–699 [DOI] [PubMed] [Google Scholar]
- Choi HJ, Huber AH, Weis WI 2006. Thermodynamics of β-catenin-ligand interactions: The roles of the N- and C-terminal tails in modulating binding affinity. J Biolog Chem 281:1027–1038 [DOI] [PubMed] [Google Scholar]
- Ciruna B, Rossant J 2001. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Develop Cell 1:37–49 [DOI] [PubMed] [Google Scholar]
- Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J 1997. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: A role for FGFR1 in morphogenetic movement through the primitive streak. Development 124:2829–2841 [DOI] [PubMed] [Google Scholar]
- Clevers H 2006. Wnt/β-catenin signaling in development and disease. Cell 127:469–480 [DOI] [PubMed] [Google Scholar]
- Collard JG, Habets GG, Michiels F, Stam J, van der Kammen RA, van Leeuwen F 1996. Role of Tiam 1 in Rac-mediated signal transduction pathways. Current Topics Microbiol Immunol 213:253–265 [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F 2001. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7:1267–1278 [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell ME, Ben-Yedidia T, Shtutman M, Feinstein E, Einat P, Ben-Ze’ev A 2002. Nr-CAM is a target gene of the β-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Develop 16:2058–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A 2003. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: The roles of β-catenin signaling, Slug, and MAPK. J Cell Biol 163:847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO 1986. Plakoglobin: A protein common to different kinds of intercellular adhering junctions. Cell 46:1063–1073 [DOI] [PubMed] [Google Scholar]
- Cox RT, Kirkpatrick C, Peifer M 1996. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol 134:133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM 1999. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 18:2883–2891 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Spring CM, Crawford HC, Reynolds AB, Baig A 2002. The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res 30:2911–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty RL, Gottardi CJ 2007. Phospho-regulation of β-catenin adhesion and signaling functions. Physiology 22:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David MD, Yeramian A, Dunach M, Llovera M, Canti C, de Herreros AG, Comella JX, Herreros J 2008. Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential β-catenin phosphorylation. J Cell Sci 121:2718–2730 [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C 2005. Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438:867–872 [DOI] [PubMed] [Google Scholar]
- de la Roche M, Worm J, Bienz M 2008. The function of BCL9 in Wnt/β-catenin signaling and colorectal cancer cells. BMC Cancer 8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P 1994. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Develop 8:3045–3057 [DOI] [PubMed] [Google Scholar]
- Deng C, Bedford M, Li C, Xu X, Yang X, Dunmore J, Leder P 1997. Fibroblast growth factor receptor-1 (FGFR-1) is essential for normal neural tube and limb development. Develop Biol 185:42–54 [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C 1999. The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126:1621–1629 [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI 2005. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman Q, Grosschedl R 1999. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Current Opinion Cell Biol 11:233–240 [DOI] [PubMed] [Google Scholar]
- Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R 2000. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of β-catenin and upregulation of β-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol 148:173–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Gluck U, Gumbiner BM 1996. Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. J Cell Biol 132:1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H, Daniel J, Fujita Y 2008. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem 283:12691–12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R, Smits R, Clevers H 2001. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 1:55–67 [DOI] [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W 1991. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 113:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchtbauer EM 1995. Expression of M-twist during postimplantation development of the mouse. Dev Dyn 204:316–322 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W 2002. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4:222–231 [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, et al. 1999. Cdc42 and Rac1 regulate the interaction of IQGAP1 with β-catenin. J Biol Chem 274:26044–26050 [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Brown AM, Weinstein DE, Ben-Ze’ev A, Pestell RG, Lisanti MP 2000. Caveolin-1 expression inhibits Wnt/β-catenin/Lef-1 signaling by recruiting β-catenin to caveolae membrane domains. J Biol Chem 275:23368–23377 [DOI] [PubMed] [Google Scholar]
- Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ 2006. Suppression of canonical Wnt/β-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest 116:2012–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J, Peifer M 2005. Can 1000 reviews be wrong? Actin, α-Catenin, and adherens junctions. Cell 123:769–772 [DOI] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze’ev A 2007. β-Catenin signaling in biological control and cancer. J Cell Biochem 102:820–828 [DOI] [PubMed] [Google Scholar]
- Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, Ben-Ze’ev A 2005. L1, a novel target of β-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 168:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Sheffer M, Raveh S, Spaderna S, Shtutman M, Brabletz T, Barany F, Paty P, Notterman D, Domany E, et al. 2007. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res 67:7703–7712 [DOI] [PubMed] [Google Scholar]
- Georgakopoulos A, Marambaud P, Efthimiopoulos S, Shioi J, Cui W, Li HC, Schutte M, Gordon R, Holstein GR, Martinelli G, et al. 1999. Presenilin-1 forms complexes with the cadherin/catenin cell-cell adhesion system and is recruited to intercellular and synaptic contacts. Mol cell 4:893–902 [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM 2004. Distinct molecular forms of β-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167:339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM 2001. E-cadherin suppresses cellular transformation by inhibiting β-catenin signaling in an adhesion-independent manner. J Cell Biol 153:1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradl D, Kuhl M, Wedlich D 1999. The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Mol Cell Biol 19:5576–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TA, Weaver C, Mao F, Kimelman D, Xu W 2000. Crystal structure of a β-catenin/Tcf complex. Cell 103:885–896 [DOI] [PubMed] [Google Scholar]
- Grau Y, Carteret C, Simpson P 1984. Mutations and chromosomal rearrangements affecting the expression of snail, a gene involved in embryonic patterning in Drosophila melanogaster. Genetics 108:347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA 2007. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res 13:7003–7011 [DOI] [PubMed] [Google Scholar]
- Greenburg G, Hay ED 1982. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol 95:333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W 2008. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of β-catenin in mice. Genes Develop 22:2308–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooteclaes ML, Frisch SM 2000. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene 19:3823–3828 [DOI] [PubMed] [Google Scholar]
- Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W 2004. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol 167:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE 1998. E-cadherin germline mutations in familial gastric cancer. Nature 392:402–405 [DOI] [PubMed] [Google Scholar]
- Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI 2004. Mechanism of phosphorylation-dependent binding of APC to β-catenin and its role in β-catenin degradation. Mol Cell 15:511–521 [DOI] [PubMed] [Google Scholar]
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG 1994. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77:537–549 [DOI] [PubMed] [Google Scholar]
- Hajra KM, Chen DY, Fearon ER 2002. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res 62:1613–1618 [PubMed] [Google Scholar]
- Hartwell KA, Muir B, Reinhardt F, Carpenter AE, Sgroi DC, Weinberg RA 2006. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci 103:18969–18974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel J, Grumet M 2003. The L1CAM extracellular region: A multi-domain protein with modular and cooperative binding modes. Front Biosci 8:s1210–1225 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Nabeshima K, Aoki M, Hamasaki M, Enatsu S, Yamauchi Y, Yamashita Y, Iwasaki H 2009. Overexpression of IQGAP1 in advanced colorectal cancer correlates with poor prognosis. Dysregulation of E-cadherin-based cell-to-cell adhesion. Int J Cancer (in press) [DOI] [PubMed] [Google Scholar]
- Hazan RB, Kang L, Whooley BP, Borgen PI 1997. N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun 4:399–411 [DOI] [PubMed] [Google Scholar]
- Hazan RB, Qiao R, Keren R, Badano I, Suyama K 2004. Cadherin switch in tumor progression. Ann NY Acad Sci 1014:155–163 [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C 1994. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79:791–803 [DOI] [PubMed] [Google Scholar]
- Hennig G, Behrens J, Truss M, Frisch S, Reichmann E, Birchmeier W 1995. Progression of carcinoma cells is associated with alterations in chromatin structure and factor binding at the E-cadherin promoter in vivo. Oncogene 11:475–484 [PubMed] [Google Scholar]
- Herzig M, Savarese F, Novatchkova M, Semb H, Christofori G 2007. Tumor progression induced by the loss of E-cadherin independent of β-catenin/Tcf-mediated Wnt signaling. Oncogene 26:2290–2298 [DOI] [PubMed] [Google Scholar]
- Heuser A, Plovie ER, Ellinor PT, Grossmann KS, Shin JT, Wichter T, Basson CT, Lerman BB, Sasse-Klaassen S, Thierfelder L, et al. 2006. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Human Gen 79:1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T 2004. β-catenin activates a coordinated expression of the proinvasive factors laminin-5 γ2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer 108:321–326 [DOI] [PubMed] [Google Scholar]
- Hoffmans R, Basler K 2007. BCL9-2 binds Arm/β-catenin in a Tyr142-independent manner and requires Pygopus for its function in Wg/Wnt signaling. Mech Develop 124:59–67 [DOI] [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG 1997. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 278:1464–1466 [DOI] [PubMed] [Google Scholar]
- Howe LR, Watanabe O, Leonard J, Brown AM 2003. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res 63:1906–1913 [PubMed] [Google Scholar]
- Hsu MY, Wheelock MJ, Johnson KR, Herlyn M 1996. Shifts in cadherin profiles between human normal melanocytes and melanomas. J Invest Dermatol Symp 1:188–194 [PubMed] [Google Scholar]
- Huber AH, Weis WI 2001. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell 105:391–402 [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R 1996. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Develop 59:3–10 [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H 2005. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Current Opinion Cell Biol 17:548–558 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W 2000. Requirement for β-catenin in anterior-posterior axis formation in mice. J Cell Biol 148:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof BA, Vollmers HP, Goodman SL, Birchmeier W 1983. Cell-cell interaction and polarity of epithelial cells: Specific perturbation using a monoclonal antibody. Cell 35:667–675 [DOI] [PubMed] [Google Scholar]
- Ip YT, Park RE, Kosman D, Bier E, Levine M 1992. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Develop 6:1728–1739 [DOI] [PubMed] [Google Scholar]
- Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S, Tomita K, Mimori T, Saya H 1999. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of β-catenin from cell-cell contacts. Oncogene 18:7080–7090 [DOI] [PubMed] [Google Scholar]
- Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y 2004. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol 166:237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E 2003. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH 1999. Presenilin 1 facilitates the constitutive turnover of β-catenin: Differential activity of Alzheimer’s disease-linked PS1 mutants in the β-catenin-signaling pathway. J Neurosci 19:4229–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH 2002. Presenilin couples the paired phosphorylation of β-catenin independent of axin: Implications for β-catenin activation in tumorigenesis. Cell 110:751–762 [DOI] [PubMed] [Google Scholar]
- Kelly KF, Otchere AA, Graham M, Daniel JM 2004a. Nuclear import of the BTB/POZ transcriptional regulator Kaiso. J Cell Sci 117:6143–6152 [DOI] [PubMed] [Google Scholar]
- Kelly KF, Spring CM, Otchere AA, Daniel JM 2004b. NLS-dependent nuclear localization of p120ctn is necessary to relieve Kaiso-mediated transcriptional repression. J Cell Sci 117:2675–2686 [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC 2004c. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development 131:2803–2815 [DOI] [PubMed] [Google Scholar]
- Kemler R 1993. From cadherins to catenins: Cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 9:317–321 [DOI] [PubMed] [Google Scholar]
- Khoury H, Dankort DL, Sadekova S, Naujokas MA, Muller WJ, Park M 2001. Distinct tyrosine autophosphorylation sites mediate induction of epithelial mesenchymal like transition by an activated ErbB-2/Neu receptor. Oncogene 20:788–799 [DOI] [PubMed] [Google Scholar]
- Kim SW, Park JI, Spring CM, Sater AK, Ji H, Otchere AA, Daniel JM, McCrea PD 2004. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat Cell Biol 6:1212–1220 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B 1996. Lessons from hereditary colorectal cancer. Cell 87:159–170 [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W 2008. Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8:387–398 [DOI] [PubMed] [Google Scholar]
- Kophengnavong T, Michnowicz JE, Blackwell TK 2000. Establishment of distinct MyoD, E2A, and twist DNA binding specificities by different basic region-DNA conformations. Mol Cell Biol 20:261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K 2002. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin-TCF complex. Cell 109:47–60 [DOI] [PubMed] [Google Scholar]
- Kuphal F, Behrens J 2006. E-cadherin modulates Wnt-dependent transcription in colorectal cancer cells but does not alter Wnt-independent gene expression in fibroblasts. Exp Cell Res 312:457–467 [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, et al. 1998. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science 281:832–835 [DOI] [PubMed] [Google Scholar]
- Lee YM, Park T, Schulz RA, Kim Y 1997. Twist-mediated activation of the NK-4 homeobox gene in the visceral mesoderm of Drosophila requires two distinct clusters of E-box regulatory elements. J Biol Chem 272:17531–17541 [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW 2001. Physiological regulation of [β]-catenin stability by Tcf3 and CK1epsilon. J Cell Biol 154:983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A 1999. Requirement for Wnt3 in vertebrate axis formation. Nat Gen 22:361–365 [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837–847 [DOI] [PubMed] [Google Scholar]
- Liu L, Wu DH, Ding YQ 2005. Tiam1 gene expression and its significance in colorectal carcinoma. World J Gastroenterol 11:705–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ 1997. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T 2003. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer cell 4:499–515 [DOI] [PubMed] [Google Scholar]
- MacRae CA, Birchmeier W, Thierfelder L 2006. Arrhythmogenic right ventricular cardiomyopathy: Moving toward mechanism. J Clin Invest 116:1825–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliri A, Rygiel TP, van der Kammen RA, Song JY, Engers R, Hurlstone AF, Clevers H, Collard JG 2006. The rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J Biol Chem 281:543–548 [DOI] [PubMed] [Google Scholar]
- Malliri A, van Es S, Huveneers S, Collard JG 2004. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem 279:30092–30098 [DOI] [PubMed] [Google Scholar]
- Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA 2007. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci 104:10069–10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Robakis NK 2005. Genetic and molecular aspects of Alzheimer’s disease shed light on new mechanisms of transcriptional regulation. Genes, Brain, Behavior 4:134–146 [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, et al. 2002. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J 21:1948–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK 2003. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114:635–645 [DOI] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P 2005. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci 102:9182–9187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B 1991. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254:1359–1361 [DOI] [PubMed] [Google Scholar]
- McCrea PD, Gu D, Balda M 2009. Junctional music that the nucleus hears. Cold Spring Harb Perspect Biol 1:a002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners S, Brinkmann V, Naundorf H, Birchmeier W 1998. Role of morphogenetic factors in metastasis of mammary carcinoma cells. Oncogene 16:9–20 [DOI] [PubMed] [Google Scholar]
- Meng W, Takeichi M 2009. Adherens junction: Molecular architecture and regulation. Cold Spring Harb Perspect Biol 1:a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Runyan RB 2007. Multiple transforming growth factor-β isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells, Tissues, Organs 185:146–156 [DOI] [PubMed] [Google Scholar]
- Mertens AE, Pegtel DM, Collard JG 2006. Tiam1 takes PARt in cell polarity. Trends Cell Biol 16:308–316 [DOI] [PubMed] [Google Scholar]
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG 1995. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375:338–340 [DOI] [PubMed] [Google Scholar]
- Minard ME, Ellis LM, Gallick GE 2006. Tiam1 regulates cell adhesion, migration and apoptosis in colon tumor cells. Clin Exp Metas 23:301–313 [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86:391–399 [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A 2004. WNT and β-catenin signalling: Diseases and therapies. Nat Rev Genet 5:691–701 [DOI] [PubMed] [Google Scholar]
- Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L 2001. IGF-II induces rapid β-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene 20:4942–4950 [DOI] [PubMed] [Google Scholar]
- Muller T, Choidas A, Reichmann E, Ullrich A 1999. Phosphorylation and free pool of β-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem 274:10173–10183 [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P 1995. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci 92:3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M 1988. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J 7:3679–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M 1989. Transmembrane control of cadherin-mediated cell adhesion: A 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Reg 1:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S 1991. The 102 kd cadherin-associated protein: Similarity to vinculin and posttranscriptional regulation of expression. Cell 65:849–857 [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R 2004. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303:1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Massague J 2007. Genetic determinants of cancer metastasis. Nat Rev Genet 8:341–352 [DOI] [PubMed] [Google Scholar]
- Nieto MA 2002. The snail superfamily of zinc-finger transcription factors. Nat Rev 3:155–166 [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J 1994. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264:835–839 [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M 2001. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114:111–118 [DOI] [PubMed] [Google Scholar]
- Novellino L, De Filippo A, Deho P, Perrone F, Pilotti S, Parmiani G, Castelli C 2008. PTPRK negatively regulates transcriptional activity of wild type and mutated oncogenic β-catenin and affects membrane distribution of β-catenin/E-cadherin complexes in cancer cells. Cell Signal 20:872–883 [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Lohs-Schardin M, Sander K, Cremer C 1980. A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant of Drosophila. Nature 283:474–476 [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E, Kluding H 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Roux’s Arch Develop Biol 193:267–282 [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S, Takeichi M 1998. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Develop Biol 203:435–450 [DOI] [PubMed] [Google Scholar]
- Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E 1996. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Develop 10:2462–2477 [DOI] [PubMed] [Google Scholar]
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA 2008. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 68:3645–3654 [DOI] [PubMed] [Google Scholar]
- Orsulic S, Huber O, Aberle H, Arnold S, Kemler R 1999. E-cadherin binding prevents β-catenin nuclear localization and β-catenin/LEF-1-mediated transactivation. J Cell Sci 112:1237–1245 [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R 1989. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 8:1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai R, Dunlap D, Qing J, Mohtashemi I, Hotzel K, French DM 2008. Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating β-catenin signaling. Cancer Res 68:5086–5095 [DOI] [PubMed] [Google Scholar]
- Park JI, Kim SW, Lyons JP, Ji H, Nguyen TT, Cho K, Barton MC, Deroo T, Vleminckx K, Moon RT, et al. 2005. Kaiso/p120-catenin and TCF/β-catenin complexes coordinately regulate canonical Wnt gene targets. Develop Cell 8:843–854 [DOI] [PubMed] [Google Scholar]
- Park JI, Ji H, Jun S, Gu D, Hikasa H, Li L, Sokol SY, McCrea PD 2006. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: Distinct catenin subfamilies promote Wnt signals. Develop Cell 11:683–695 [DOI] [PubMed] [Google Scholar]
- Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM 1992. The vertebrate adhesive junction proteins β-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol 118:681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Ballestar E, Esteller M, Cano A 2004a. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24:306–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Marin F, Cubillo E, Stark HJ, Fusenig N, Nieto MA, Cano A 2004b. Snail and E47 repressors of E-cadherin induce distinct invasive and angiogenic properties in vivo. J Cell Sci 117:2827–2839 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A 2001. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem 276:27424–27431 [DOI] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G 1998. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392:190–193 [DOI] [PubMed] [Google Scholar]
- Perrais M, Chen X, Perez-Moreno M, Gumbiner BM 2007. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell 18:2013–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P 2000. Wnt signaling and cancer. Genes Develop 14:1837–1851 [PubMed] [Google Scholar]
- Prieve MG, Moon RT 2003. Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57 mg mouse mammary epithelial cells. BMC Develop Biol 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P, Myles DG 2000. The ADAM gene family: Surface proteins with adhesion and protease activity. Trends Genet 16:83–87 [DOI] [PubMed] [Google Scholar]
- Qi J, Wang J, Romanyuk O, Siu CH 2006. Involvement of Src family kinases in N-cadherin phosphorylation and β-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell 17:1261–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J, Busslinger M, Beug H 1992. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell 71:1103–1116 [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P 2005. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and β-catenin nuclear signalling. EMBO J 24:742–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Roczniak-Ferguson A 2004. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 23:7947–7956 [DOI] [PubMed] [Google Scholar]
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J 2007. Cables links Robo-bound Abl kinase to N-cadherin-bound β-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol 9:883–892 [DOI] [PubMed] [Google Scholar]
- Riese J, Yu X, Munnerlyn A, Eresh S, Hsu SC, Grosschedl R, Bienz M 1997. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell 88:777–787 [DOI] [PubMed] [Google Scholar]
- Riggleman B, Schedl P, Wieschaus E 1990. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell 63:549–560 [DOI] [PubMed] [Google Scholar]
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R 1987. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 50:649–657 [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS 1995. α 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci 92:8813–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwedel J, Horak V, Hebrok M, Fuchtbauer EM, Wobus AM 1995. M-twist expression inhibits mouse embryonic stem cell-derived myogenic differentiation in vitro. Exp Cell Res 220:92–100 [DOI] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H 1998. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395:608–612 [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF 2002. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol 161:1881–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA 2000. Inhibition of adipogenesis by Wnt signaling. Science 289:950–953 [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M 1999. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem 274:36734–36740 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P 1996. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272:1023–1026 [DOI] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke WW, et al. 1996. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol 135:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzov A, Hackett JA, Prokhortchouk A, Reddington JP, Madej MJ, Dunican DS, Prokhortchouk E, Pennings S, Meehan RR 2009. The interaction of xKaiso with xTcf3: A revised model for integration of epigenetic and Wnt signalling pathways. Development 136:723–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B 1998. Inhibition of β-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci 95:15339–15344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W 2006. Crystal structure of a β-catenin/BCL9/Tcf4 complex. Mol Cell 24:293–300 [DOI] [PubMed] [Google Scholar]
- Sandig M, Voura EB, Kalnins VI, Siu CH 1997. Role of cadherins in the transendothelial migration of melanoma cells in culture. Cell Motil Cytoskel 38:351–364 [DOI] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP 1996. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383:627–630 [DOI] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP 1997. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol 137:1403–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M 2007. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Bioll 14:484–492 [DOI] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA 2003. The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes Develop 17:7–30 [DOI] [PubMed] [Google Scholar]
- Sefton M, Sanchez S, Nieto MA 1998. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 125:3111–3121 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS 2007. Presenilin: Running with scissors in the membrane. Cell 131:215–221 [DOI] [PubMed] [Google Scholar]
- Sen-Chowdhry S, Lowe MD, Sporton SC, McKenna WJ 2004. Arrhythmogenic right ventricular cardiomyopathy: Clinical presentation, diagnosis, and management. Am J Med Am J 117:685–695 [DOI] [PubMed] [Google Scholar]
- Shapiro L, Weis WI 2009. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol 1:a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Hirsch DS, Sasiela CA, Wu WJ 2008. Cdc42 regulates E-cadherin ubiquitination and degradation through an epidermal growth factor receptor to Src-mediated pathway. J Biol Chem 283:5127–5137 [DOI] [PubMed] [Google Scholar]
- Shoval I, Ludwig A, Kalcheim C 2007. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development 134:491–501 [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A 1999. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci 96:5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB 2006. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res 66:11370–11380 [DOI] [PubMed] [Google Scholar]
- Simpson P 1983. Maternal-Zygotic Gene Interactions during Formation of the Dorsoventral Pattern in Drosophila Embryos. Genetics 105:615–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Franco del Amo F, Gridley T 1992. Isolation of Sna, a mouse gene homologous to the Drosophila genes snail and escargot: Its expression pattern suggests multiple roles during postimplantation development. Development 116:1033–1039 [DOI] [PubMed] [Google Scholar]
- Soo K, O’Rourke MP, Khoo PL, Steiner KA, Wong N, Behringer RR, Tam PP 2002. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Develop Biol 247:251–270 [DOI] [PubMed] [Google Scholar]
- Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH 2001. Presenilin 1 negatively regulates β-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of β-amyloid precursor protein and notch processing. J Cell Biol 152:785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring CM, Kelly KF, O’Kelly I, Graham M, Crawford HC, Daniel JM 2005. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the β-catenin/TCF target gene matrilysin. Exp Cell Res 305:253–265 [DOI] [PubMed] [Google Scholar]
- Stadeli R, Hoffmans R, Basler K 2006. Transcription under the control of nuclear Arm/β-catenin. Curr Biol 16:R378–385 [DOI] [PubMed] [Google Scholar]
- Staubli U, Larson J, Thibault O, Baudry M, Lynch G 1988. Chronic administration of a thiol-proteinase inhibitor blocks long-term potentiation of synaptic responses. Brain Res 444:153–158 [DOI] [PubMed] [Google Scholar]
- Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W, et al. 2006. The metastasis-associated gene S100A4 is a novel target of β-catenin/T-cell factor signaling in colon cancer. Gastroenterology 131:1486–1500 [DOI] [PubMed] [Google Scholar]
- Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O 2001. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem 276:4972–4980 [DOI] [PubMed] [Google Scholar]
- Stemmer V, de Craene B, Berx G, Behrens J 2008. Snail promotes Wnt target gene expression and interacts with β-catenin. Oncogene 27:5075–5080 [DOI] [PubMed] [Google Scholar]
- Stemmler MP, Hecht A, Kemler R 2005. E-cadherin intron 2 contains cis-regulatory elements essential for gene expression. Development 132:965–976 [DOI] [PubMed] [Google Scholar]
- Stockinger A, Eger A, Wolf J, Beug H, Foisner R 2001. E-cadherin regulates cell growth by modulating proliferation-dependent β-catenin transcriptional activity. J Cell Biol 154:1185–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M, Gherardi E, Perryman M, Gray J 1987. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327:239–242 [DOI] [PubMed] [Google Scholar]
- Strathdee G 2002. Epigenetic versus genetic alterations in the inactivation of E-cadherin. Sem Cancer Biol 12:373–379 [DOI] [PubMed] [Google Scholar]
- Takeichi M 1991. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1455 [DOI] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R 2008. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem cell 3:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP 2002. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454 [DOI] [PubMed] [Google Scholar]
- Thisse B, Stoetzel C, Gorostiza-Thisse C, Perrin-Schmitt F 1988. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J 7:2175–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, Bussemakers MJ, Schalken JA 2000. Cadherin switching in human prostate cancer progression. Cancer Res 60:3650–3654 [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT 1996. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol 133:1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis A, Amsterdam A, Belanger C, Grosschedl R 1991. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor α enhancer function [corrected]. Genes Develop 5:880–894 [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Hay ED, Revel JD 1967. Cell contact during early morphogenesis in the chick embryo. Develop Biol 16:78–106 [DOI] [PubMed] [Google Scholar]
- Uemura K, Kihara T, Kuzuya A, Okawa K, Nishimoto T, Bito H, Ninomiya H, Sugimoto H, Kinoshita A, Shimohama S 2006. Activity-dependent regulation of β-catenin via epsilon-cleavage of N-cadherin. Biochem Biophys Res Comm 345:951–958 [DOI] [PubMed] [Google Scholar]
- Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F 2001. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/β-catenin signaling. J Biol Chem 276:30350–30358 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Oosterwegel M, Dooijes D, Clevers H 1991. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J 10:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Barker N, Harkes IC, van der Heyden M, Dijk NJ, Hollestelle A, Klijn JG, Clevers H, Schutte M 2001. Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res 61:278–284 [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH 1998. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci 111:1741–1749 [DOI] [PubMed] [Google Scholar]
- van Roy FM, McCrea PD 2005. A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer 5:956–964 [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT 2003. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Develop Cell 5:367–377 [DOI] [PubMed] [Google Scholar]
- Veltmaat JM, Orelio CC, Ward-Van Oostwaard D, Van Rooijen MA, Mummery CL, Defize LH 2000. Snail is an immediate early target gene of parathyroid hormone related peptide signaling in parietal endoderm formation. Int J Develop Biol 44:297–307 [PubMed] [Google Scholar]
- Vestweber D, Kemler R 1984. Some structural and functional aspects of the cell adhesion molecule uvomorulin. Cell Different 15:269–273 [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, Louvard D, Ben-Ze’ev A, Robine S 2007. Fascin, a novel target of β-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res 67:6844–6853 [DOI] [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F 1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66:107–119 [DOI] [PubMed] [Google Scholar]
- Wagenaar-Miller RA, Gorden L, Matrisian LM 2004. Matrix metalloproteinases in colorectal cancer: Is it worth talking about? Cancer Metast Rev 23:119–135 [DOI] [PubMed] [Google Scholar]
- Wang SM, Coljee VW, Pignolo RJ, Rotenberg MO, Cristofalo VJ, Sierra F 1997. Cloning of the human twist gene: Its expression is retained in adult mesodermally-derived tissues. Gene 187:83–92 [PubMed] [Google Scholar]
- Warren SL, Nelson WJ 1987. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol Cell Biol 7:1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman ML, Fischer WH, Jones KA 1991. A thymus-specific member of the HMG protein family regulates the human T cell receptor C α enhancer. Genes Develop 5:656–669 [DOI] [PubMed] [Google Scholar]
- Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W 1990. Scatter factor: Molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol 111:2097–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, et al. 1991. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci 88:7001–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR 2003. Cadherins as modulators of cellular phenotype. Ann Rev Cell Develop Biol 19:207–235 [DOI] [PubMed] [Google Scholar]
- Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST 1999. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 154:515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E, Riggleman R 1987. Autonomous requirements for the segment polarity gene armadillo during Drosophila embryogenesis. Cell 49:177–184 [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R 1999. Wnt-induced dephosphorylation of axin releases β-catenin from the axin complex. Genes Develop 13:1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis TG, Zalcberg IR, Coignet LJ, Wlodarska I, Stul M, Jadayel DM, Bastard C, Treleaven JG, Catovsky D, Silva ML, et al. 1998. Molecular cloning of translocation t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21. Blood 91:1873–1881 [PubMed] [Google Scholar]
- Wolf C, Thisse C, Stoetzel C, Thisse B, Gerlinger P, Perrin-Schmitt F 1991. The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Develop Biol 143:363–373 [DOI] [PubMed] [Google Scholar]
- Xia X, Qian S, Soriano S, Wu Y, Fletcher AM, Wang XJ, Koo EH, Wu X, Zheng H 2001. Loss of presenilin 1 is associated with enhanced β-catenin signaling and skin tumorigenesis. Proc Natl Acad Sci 98:10863–10868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ 2005. Deconstructing the cadherin-catenin-actin complex. Cell 123:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J 1994. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Develop 8:3032–3044 [DOI] [PubMed] [Google Scholar]
- Yan HX, Yang W, Zhang R, Chen L, Tang L, Zhai B, Liu SQ, Cao HF, Man XB, Wu HP, et al. 2006. Protein-tyrosine phosphatase PCP-2 inhibits β-catenin signaling and increases E-cadherin-dependent cell adhesion. J Biol Chem 281:15423–15433 [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA 2008. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Develop Cell 14:818–829 [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA 2004. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939 [DOI] [PubMed] [Google Scholar]
- Yin Z, Xu XL, Frasch M 1997. Regulation of the twist target gene tinman by modular cis-regulatory elements during early mesoderm development. Development 124:4971–4982 [DOI] [PubMed] [Google Scholar]
- Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ 2005. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem 280:11740–11748 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, et al. 1998. Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395:698–702 [DOI] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC 2004. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6:931–940 [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze’ev A 2000. Plakoglobin and β-catenin: Protein interactions, regulation and biological roles. J Cell Sci 113:3127–3139 [DOI] [PubMed] [Google Scholar]