Abstract
Purpose
Previously we showed that adoptive transfer of in-vivo vaccine-primed and ex-vivo (anti-CD3/anti-CD28) costimulated autologous T cells (ex-T) at day +12 post-transplant increased CD4 and CD8 T cell counts at day +42 and augmented vaccine-specific immune responses in patients with myeloma. Here we investigated the safety and kinetics of T cell recovery after infusing ex-T at day +2 post-transplant.
Experimental Design
In this phase I/II two-arm clinical trial 50 patients with myeloma received autografts after high-dose melphalan followed by infusions of ex-T at day +2 post-transplant. Patients also received pre- and post-transplant immunizations using a pneumococcal conjugate vaccine (PCV) only (Arm B, N = 26) or the PCV plus an HLA-A2-restricted multi-peptide vaccine for HLA–A2 positive patients (Arm A, N = 24).
Results
The mean number of T cells infused was 4.26×1010 (1.59 – 5.0). At day 14 post-transplant, the median CD3, CD4 and CD8 counts were 4198, 1545, and 2858 cells/µl respectively. IL-6 and IL-15 levels increased early post-transplant and IL-15 levels correlated significantly to day 14 T-cell counts. Robust vaccine-specific B and T cell responses were generated. T cell infusions were well-tolerated with no effect on hematopoietic recovery. Eight patients (16%) developed a T cell "engraftment syndrome" characterized by diarrhea and fever that was clinically and histopathologically indistinguishable from grade I-III acute GVHD of the gastrointestinal tract (7 patients) and/or grade I-II cutaneous GVHD (4 patients).
Conclusions
Adoptive T cell transfers achieve robust T cell recovery early post-transplant and induce moderate-to-severe autologous GVHD in a subset of patients.
Keywords: Adoptive T cell transfer, immunotherapy, auto-GVHD, myeloma, gastrointestinal GVHD, interleukin-15
Introduction
High-dose chemotherapy followed by autologous stem cell transplantation (SCT) induces complete responses and extended progression-free survival in about 20–40% of myeloma patients (1–3). However, even after tandem transplants 10-year disease-free survival rates are < 20% and cure rates are probably no better than 10% with long-term follow-up (4). Allogeneic transplants have been performed for myeloma with the rationale that cure rates may be increased through a T cell mediated “graft vs tumor” effect, but increased toxicity and mortality due to graft vs host disease (GVHD) largely offsets the benefit of enhanced disease control (5–8). New approaches are needed to improve upon the results of autologous transplants for myeloma and other blood cancers. Some but not all retrospective studies suggest that more rapid lymphocyte recovery during the early post-transplant period may be associated with better clinical outcomes for myeloma and other hematologic neoplasms (9–11). Furthermore, immune dysfunction following high-dose chemotherapy is clearly associated with an increased risk for serious bacterial and viral infections (12–14). Thus, strategies to augment the recovery and function of autologous T cells post-transplant may be beneficial.
We hypothesized that improved T cell recovery through adoptive transfer of ex-vivo costimulated and expanded autologous T cells might provide a platform for post-transplant immunotherapy of myeloma and other neoplasms and enhance protection from post-transplant infections. Studies in animal models suggest that the early post-transplant period may be suitable for the development of effective antitumor immune responses (15,16). For our studies, autologous T cells were stimulated by coculture with paramagnetic beads to which anti-CD3 and anti-CD28 monoclonal antibodies were conjugated, because signals through CD3 and CD28 can help prevent T cell anergy (17–21). To test this hypothesis, a randomized clinical trial was conducted in which 54 patients with myeloma received infusions of costimulated autologous T cells after autotransplantation, along with immunizations using a 7-valent pneumococcal conjugate vaccine (PCV, Prevnar®) (22). Among the key observations from this study was that infusions of about 1010 ex-vivo costimulated autologous T cells on day 12 post-transplant led to significantly higher CD4 and CD8 T-cell counts at day 42 post-transplant. In addition, combined T cell/vaccine immunotherapy could induce vaccine-specific T cell and antibody immune responses early after transplant. Vaccination prior to collection of T cells for later ex-vivo expansion allowed for the highest levels of vaccine-specific immunity following T cell infusion. A smaller multi-center study of costimulated T cells after autotransplantation for myeloma also showed that infusions of 5–10 × 1010 T-cells on day 3 post-transplant were well-tolerated (23).
Here we present results from a trial which investigated the clinical effects of infusing ex-vivo costimulated autologous T cells at day +2 post-transplant, which is 10 days earlier than in our previous study. The rationale for infusing cells at day +2 was to take advantage of the favorable cytokine milieu induced by severe lymphopenia (e.g. free IL-15, IL-7) that is thought to drive homeostatic lymphocyte expansion. In addition, earlier and more robust T cell recovery might help to promote immune responses to tumor vaccines and protect against post-transplant infections. In the present study, 50 adults who were autografted for myeloma, received up to 5 × 1010 (~109/kg) T cells along with pre and post-transplant immunizations with Prevnar® and for patients who were HLA A2+, a multipeptide tumor antigen vaccine. In this report we have focused on the immune reconstitution that follows adoptive T-cell transfer at day +2. A pronounced schedule-dependent effect of early T cell infusions on T cell recovery was identified. We also report that a subset of patients developed a clinical syndrome consistent with moderate to severe autologous GVHD that was not previously observed in connection with adoptive T cell transfers.
Methods
Trial Design
Study participants were at least 18 years old with symptomatic multiple myeloma that required systemic treatment. All patients had received initial therapy using at least 3 cycles of standard regimens (typically bortezomib, thalidomide or lenalidomide plus dexamethasone) by their referring oncologist prior to study enrollment. Upon study entry, patients were required to have measurable disease (based on serum/urine electrophoresis studies or serum free light chain studies); patients in complete remission (CR) were not eligible unless they had high-risk cytogenetic features (e.g chromosome 13 or 17 deletions, 4;14 or 14;16 translocations, or complex karyotypes). Patients were required to have adequate organ function as defined by serum creatinine levels ≤ 3.0 mg/dl, left ventricular ejection fraction ≥ 45% and lung function parameters ≥ 40% predicted. All participants gave written informed consent upon enrollment in accordance with the Declaration of Helsinki; study approval was obtained from the Institutional Review Boards (IRBs) of the University of Maryland and the University of Pennsylvania as well as the US Food and Drug Administration.
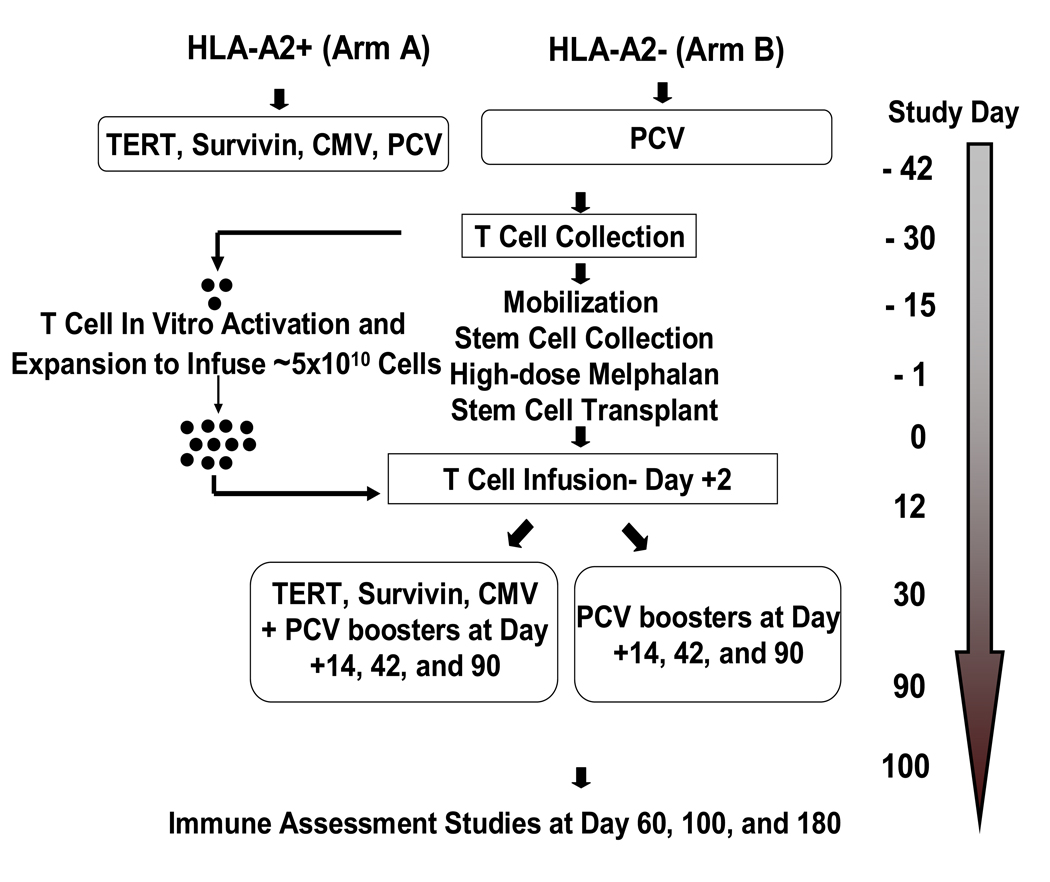
The design of the trial is depicted in Figure 1. Briefly, patients were first tested for HLA A2 status: HLA A2+ patients were assigned to ARM A and A2- patients were assigned to ARM B. Patients who were A2+ (ARM A) received immunizations with 100 µg of each of the following peptides: i) hTERT I540 peptide [ILAKFLHWL] (24); ii) hTERT R572Y peptide [YLFFYRKSV] (25); iii) hTERT D988Y peptide [YLQVNSLQTV] (25); iv) survivin Sur1M2 peptide [LMLGEFLKL] (26); v) CMV control peptide N495 [NLVPMVATV] (27). Vaccinations consisted of aqueous solutions of peptide (each peptide >92% pure and good manufacturing grade; Merck Biosciences AG, Laufelfingen, Switzerland) emulsified in the adjuvant Montanide ISA 51 (Seppic Inc., Paris, France) and delivered subcutaneously in the thigh (right thigh, hTERT I540, hTERT R572Y and hTERT D988Y peptide emulsion; left thigh, Sur1M2 and CMV N495 peptide emulsion). Sargramostim (clinical grade GM-CSF; Berlex Laboratories, Inc., Richmond CA) was also given subcutaneously at each of the two peptide-injection sites (70 µg) per vaccination. Patients in ARM A also received an intramuscular (IM) injection of the pneumococcal conjugate vaccine (PCV, Prevnar®, Wyeth) into the non-dominant deltoid. HLA A2- patients (ARM B), received the PCV immunization only along with one injection of GM-CSF (70 µ g) into each thigh.
Figure 1.
Flow diagram for new myeloma trial.
About 10 days after the first set of immunizations all patients underwent steady-state mononuclear cell collections by apheresis to collect approximately 1 × 108 mononuclear cells per kilogram body weight. Patients proceeded to stem cell mobilization using one of several regimens (most commonly cyclophosphamide at a dose of 1.5 – 4.5 g/m2) followed by subcutaneous injections of G-CSF (10 µg/kg). High-dose therapy consisted of melphalan at a dose of 200 mg/m2 followed by infusions of autologous stem cells (at least 2 × 106 CD34+ cells/kg body weight) at day 0. Costimulated autologous T cells were infused on day +2 (see details below). Standard supportive care measures included antibiotic prophylaxis and administration of G-CSF starting on day +5. Three additional sets of immunizations were given at days +14, +42, and +90 using the same ARM-specific procedure that was used for the first immunization.
T cell Expansion and Adoptive Transfer
The mononuclear cell apheresis product was monocyte-depleted by counter flow centrifugal elutriation (CaridianBCT Elutra™ Cell Separation System) since monocytes may inhibit lymphocyte proliferation. Monocyte-depleted mononuclear cells were then cryopreserved until 9–12 days before the scheduled re-infusion date (day +2 post-transplant). Cells were thawed and co-cultured with Dynal paramagnetic M-450 beads (DynalInvitrogen, Oslo, Norway) coated with anti-CD3 (OKT3, Ortho Biotech, Bridgewater, NJ)/anti-CD28 (clone 9.3) at a ratio of 3 beads per cell first in a Baxter Lifecell flask, and subsequently in the WAVE® bioreactor system (28). The cells were grown in X-VIVO 15™ (Lonza Walkersville, Walkersville, MD) supplemented with 5% pooled human AB serum (Valley Biomedical, Winchester, VA). The cultures were maintained for up to 12 days prior to harvesting and preparation for reinfusion. All infused T cell products were required to meet release criteria specified for T cell phenotype, cell viability, pyrogenicity, and freedom from bead contamination. Culture samples were tested for bacteria and fungi two days prior to harvesting, and from the final cellular product. Testing of the final cellular product for bacterial endotoxin (Endosafe, Charles River) and cell phenotype by flow cytometry were also performed. Cell count, cell viability, and endotoxin testing were done to determine whether the final products met specified release criteria. In addition, the absolute number of cells with a plasma-cell immunophenotype in the preharvest or final product had to be less than or equal to the absolute number of cells of the same population in the post-wash/starting (apheresis) product. Plasma cells were identified by pre-gating on viable cells defined by ViaProbe (Becton Dickinson, San Jose, CA) and then collecting and analyzing CD3−/CD19−/CD38+/CD138+ cells on a Becton Dickinson FACSCalibur with CellQuest software. On the designated infusion date, the T cells were harvested. The beads were removed with a Baxter Fenwal Maxsep® magnetic separator, washed and concentrated with the Baxter Fenwal Harvester System, and resuspended in 100–500 mL 1:1 Plasmalyte A/Dextrose 5%, 0.45% NaCl containing 0.5–1% human serum albumin. The harvested cells were transported by courier from the cell production facility to the patient and infused on the same day (day +2 of transplant). The cells were infused over 20 – 60 minutes without a leukocyte filter, after premedication with acetaminophen and diphenhydramine. Corticosteroids were available at the bedside in the event of an allergic-type reaction. The target number of costimulated T cells for infusion was ~ 5 × 1010 T cells. This target number was 5-fold greater than that used in our previous trial due to the increased expansion efficiency of the WAVE® bioreactor system. Of the 50 expansions, one product failed to meet release criteria due to bacterial contamination; a second expansion was performed and the cells were successfully infused at day 16 post-transplant.
Cytokine Assays
Serum concentrations of cytokines were measured by enzyme linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN, USA) or by the Luminex® bead assay (Invitrogen, Carlsbad, CA, USA) according to the manufacturers’ instructions.
Immunoassays and Phenotyping
Immune responses to the Prevnar ® (pneumococcal conjugate vaccine) were assessed by ELISA binding assays (for antibody responses) and CFSE dilution (for T cell proliferative responses to the CRM 197 carrier protein) as described previously (22) minus CD25 staining. T cell responses to the hTERT and survivin peptides were assessed by in vitro stimulation followed by tetramer analysis as previously described (29) except that all tetramers were obtained from Beckman Coulter. Regulatory T cell subpopulations (CD4+CD127-FOXP3+) were determined by intracellular staining according to the manufacturer’s instructions with anti-FOXP3 (eBiosciences), followed by surface staining with anti-CD127 and anti-CD4 (Beckton Dickinson, BD) for 15 minutes at room temperature. Effector T cell subpopulations (CD3+CD8+) were stained with anti-CD3 and anti-CD8 (BD) for 15 minutes at 4°C.
Statistical Methods
The observation times were day 14, day 60, day 100, and day 180. Since the observation times were slightly different between the current trial and the previous trial and some observations were missing, to compare CD3, CD4, and CD8 counts between the two trials, the generalized t-test was used based on the expectation-maximization algorithm (30). The nonlinear longitudinal data model was employed for analyzing the absolute lymphocyte counts (ALC) curve relative to time post-transplant. A paired Mann-Whitney (Wilcoxon) test was used to evaluate differences in serum cytokine concentrations between enrollment and post-transplant timepoints. Pearson’s correlation coefficient was used for correlation analyses.
Results
From December 2006 to December 2008, 52 patients were enrolled at the two participating institutions. Table 1 shows the major clinical characteristics for the study patients. After initial immunization, two patients did not mobilize adequate stem cells to proceed to transplant and therefore 50 (26 ARM A and 24 ARM B) patients were autografted. To analyze post-transplant T cell recovery, both ARM A and ARM B patients were pooled together.
Table 1.
Patient Characteristics
| Characteristic | Value | |
|---|---|---|
| Total Number | 52 | |
| Median Age | 54 (range 37–68) | |
| Gender | ||
| Female | 26 (50%) | |
| Male | 26 (50%) | |
| Ethnicity | ||
| African-American | 22 (42%) | |
| Caucasian | 29 (56%) | |
| Asian | 1 (2%) | |
| Prior Therapy | ||
| Median Number | 1 (range, 1–3) | |
| % Bortezomib-treated | 28% | |
| % Thal/Rev-treated | 74% | |
| % Received XRT | 14% | |
| Cytogenetics | ||
| Abnormal | 41% | |
| Normal | 59% | |
| Myeloma Subtypes | ||
| IgA | 27% | |
| IgG | 68% | |
| Light Chain | 5% | |
Clinical Effects of Day +2 T cell Transfers
The mean and median numbers of T cells infused were 4.26×1010 and 4.54 × 1010 respectively, (range, 1.59 – 5.0). The mean CD4/CD8 ratio was 2.48 (range, 0.62–11.09). The day +2 T cell transfers were well-tolerated with common adverse effects being chills/rigors (67%), nausea (21%) and low grade fevers (18%) (Supplementary Table 1). All early infusion-related toxicities were grade I/II. Later toxicities which were possibly, probably, or definitely considered to be related to the T cell infusions are also tabulated (Supplementary Table 1). These toxicities, also mainly grade I/II, included mild maculopapular rashes (typically involving the face/scalp/neck/upper chest, 51%), fevers (21%), arthralgias (15%), myalgias (10%) and conjunctivitis (8%) which was readily responsive to glucocorticoid eyedrops. One patient had incomplete platelet recovery (grade III) (~ 40–50,000/µl at 6 months post-transplant) considered to be possibly related to the T-cell infusion. There were no documented cases of autoantibody formation (e.g. ANA, thyroid antibodies) nor autoimmune endocrinopathies.
T cell Recovery
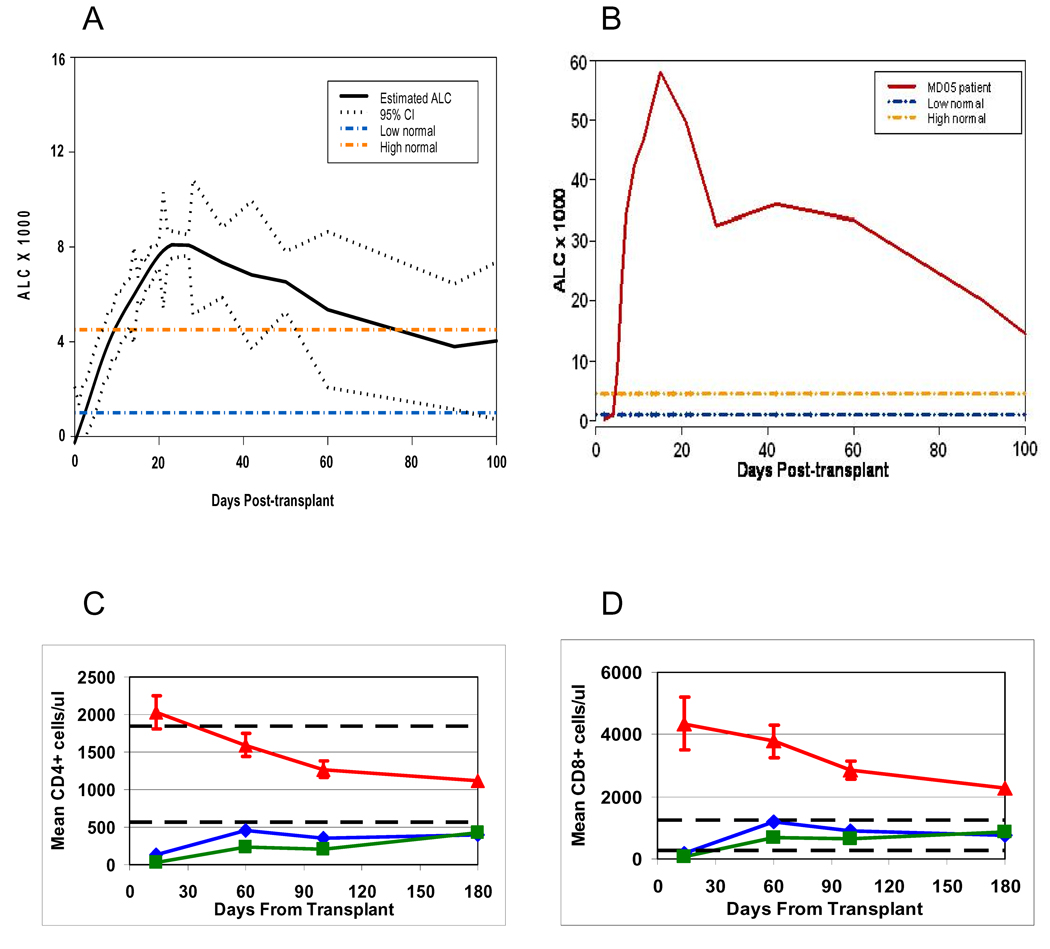
Lymphocyte recovery was rapid and robust following day +2 T cell transfers. At about day +5 post-stem cell transplant, a relative and absolute lymphocytosis developed which persisted throughout the transplant period. Hematopoietic recovery occurred simultaneously with the lymphocytosis which “peaked” at about 4 weeks post-transplant. Thereafter the lymphocytosis gradually subsided but the ALC (absolute lymphocyte count) remained at or above the upper limit of the normal range until at least day +100 post-transplant. Figure 2A shows a statistically modeled ALC curve with 95% confidence interval for all the patients. There was significant variability in the magnitude of the CD4/CD8/CD3 lymphocyte counts which were observed at each timepoint (Supplementary Table 2). Figure 2B shows the most dramatic example of post-transplant T-cell lymphocytosis (MD05). The number of CD4 cells infused was significantly and positively correlated to the day 14 and day 100 CD4 counts (Pearson’s correlation coefficient r = 0.285, P = 0.0318 and r = 0.316, P = 0.0284, respectively).
Figure 2.
A: Statistically modeled absolute lymphocyte count (ALC) vs time following stem cell transplantation for all myeloma study patients (black line) with 95% confidence interval. Dashed orange and blue lines show the upper and lower limits of normal lymphocyte counts (for healthy adults) for reference.
B: Shown is the ALC curve for a single patient (MD05) who exhibited the most dramatic lymphocyte recovery. Dashed yellow and blue lines show the upper and lower limits of normal lymphocyte counts for reference.
C: Mean CD4+ T cell counts for current trial (red triangles) plus standard error bars vs. previous trial (blue diamonds = day +12, green squares = day +100); P<0.0001 for current trial curve vs. previous trial curves. Dashed lines show the upper and lower limits of normal CD4+ lymphocyte counts (for healthy adults) for reference.
D: Mean CD8+ T cell counts for current trial (red triangles) plus standard error bars vs. previous trial (blue diamonds = day +12, green squares = day +100); P<0.0001 for current trial curve vs. previous trial curves. Dashed lines show the upper and lower limits of normal CD8+ lymphocyte counts (for healthy adults) for reference.
Figures 2C and 2D depict the patterns of CD4+ and CD8+ T cell recovery in the current myeloma trial versus the previous trial (22). In this previous study patients were randomized to receive costimulated T cells at either day +12 (blue lines, diamonds) or day +100 (green lines, squares). These plots demonstrate that the CD4+ and CD8+ T cell counts were significantly higher in the current study at all the timepoints examined including days +14, +60, +100 and +180 (P≤ 0.001).
Hematopoietic Recovery
To determine whether the early lymphocyte recovery which followed day +2 adoptive T cell transfer had an effect on hematopoietic recovery, we compared the times to absolute neutrophil counts (ANC) ≥ 500/µl for 2 consecutive days for the current study patients with an historical control population of 102 myeloma patients who had standard autografts without additional T cells (31). The median number of days to neutrophil recovery in the current trial was 12 days [range 10–18] versus 12 days in the historical cohort [P = 0.49]. Similarly, the median days to an unsupported platelet count ≥ 20,000/µl was similar for the two populations: 14 days [range 0–28] in the current trial versus 14.5 days in the historical cohort [P = 0.78].
T cell Engraftment Syndrome
Seven patients (MD03, MD11, MD24, MD30, UP07, UP23, UP26) developed a post-transplant T cell “engraftment syndrome” characterized by watery diarrhea (up to 2000 cc/day), abdominal pain, and fever (in six of the patients). In six patients (MD03, MD11, MD24, MD30, UP23, UP26) this syndrome developed at a median of 14 days post-transplant (range 9–17 days) while in one patient (UP07) it occurred around day 60 post-transplant. Two patients including UP-23 and an eighth patient, UP-25, developed early bright facial rashes at about day +9 post-transplant. The mean day 14 CD4 count for the 7 patients who developed early GVHD-like T cell engraftment syndromes was 2443 (range 863 – 4049) while the mean day 14 CD8 count for this subset of patients was 5822 (range 671 – 11571). There were no significant differences in the CD4 and CD8 counts for the subset of patients who developed early “GVHD” versus the group of patients who did not. Since phenotypic changes in the T cell compartment could also contribute to development of GVHD, the balance of effector and regulatory T cells was examined before and after T cell transfers. Compared to the time of enrollment (baseline measurement) a significant increase in the ratio of effector to regulatory T cells (Teff/Treg) was observed at day +14 post-transplant: Mean log (Teff/Treg) = 2.23 at day +14 vs 1.08 at enrollment, P < 0.0001. However, perhaps due to its small size, the subgroup of patients who developed clinical GVHD did not appear to exhibit a significantly higher Teff/Treg ratio at this timepoint compared to the rest of the patients.
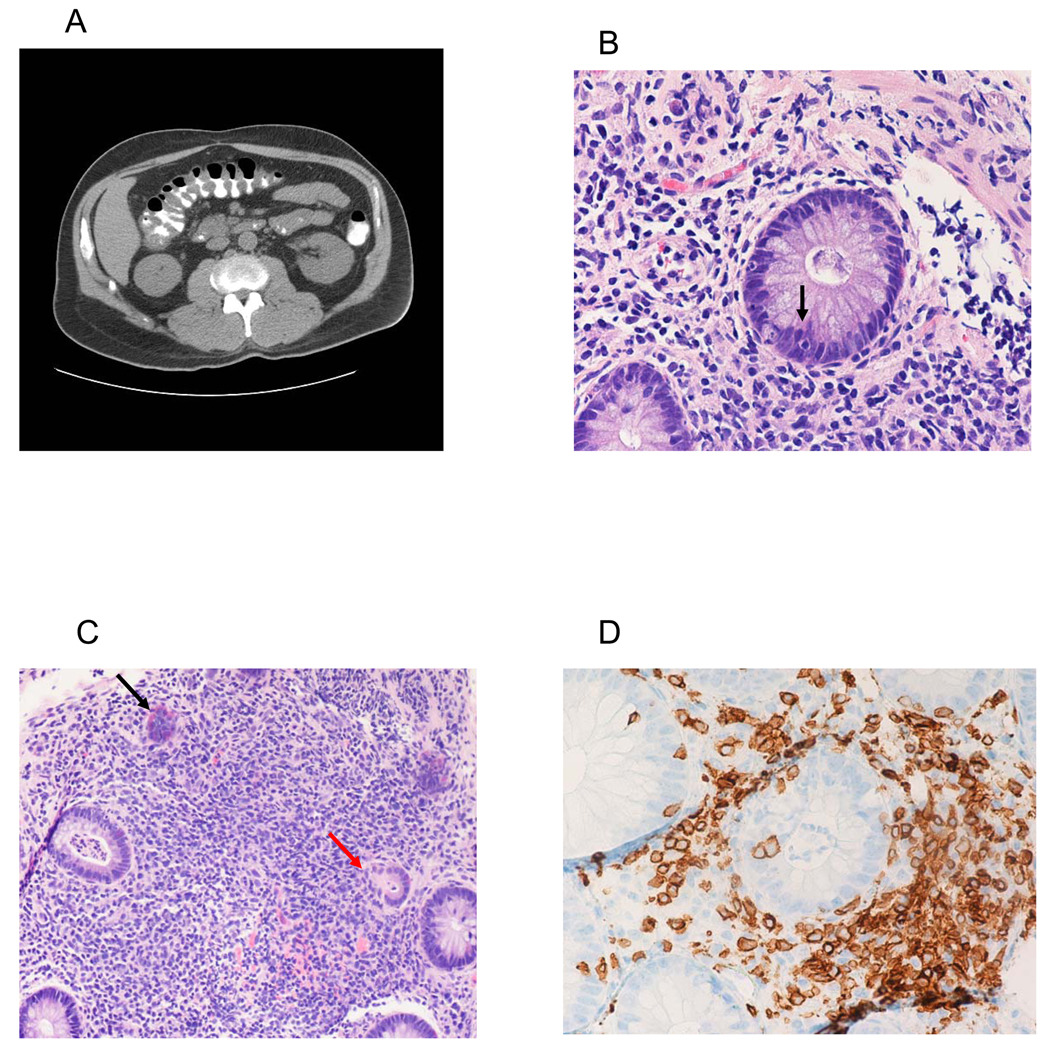
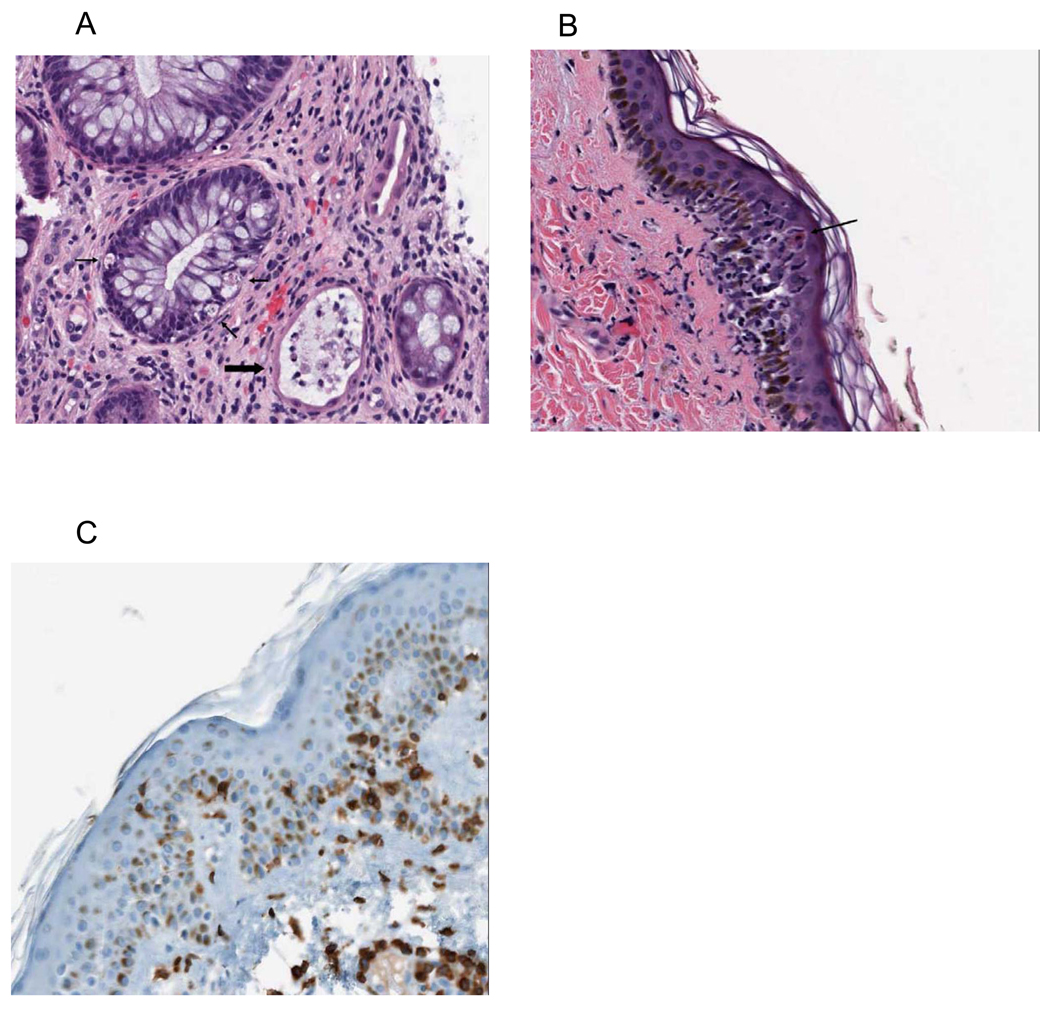
All 7 patients with apparent “GI-GVHD” had colonoscopic biopsies at a median of 17 days post-transplant (range, 15–81): In 6 patients (MD03, MD11, MD24, MD30, UP23, UP26) the biopsies were interpreted to show histopathologic grade II-III GVHD of the gut. The biopsy for the patient with late onset symptoms (UP07) was considered to be non-specific, although intraepithelial lymphocytosis and rare apoptotic bodies were observed. Figure 3A depicts a representative CT scan of the abdomen taken at the time of symptom onset for MD11 which shows bowel wall thickening consistent with GVHD. Figure 3B shows a representative colon biopsy from MD11 which demonstrates crypt cell apoptosis while Figure 3C shows crypt dropout (red arrow) and neuroendocrine hyperplasia (black arrow), typical features of gut GVHD. Immunohistochemical staining for T cell subsets (Figure 3D, Supplementary Figure 1) shows a prominent lymphocytic infiltrate along with intraepithelial localization of CD3+, CD4+, and CD8+ T cells. Figure 4A also shows the grade III GVHD histopathology for MD30 – the patient with the most severe clinical syndrome that was observed. All 7 patients were initially treated with 1–2 mg/kg of methylprednisolone and/or oral budesonide followed by a rapid taper over about 1 month and exhibited rapid and complete clinical responses.
Figure 3.
A: CT scan of abdomen showing thickened bowel wall (MD11).
B: Crypt cell apoptosis (MD11).
C: Features of GVHD including crypt lysis (red arrow) and neuroendocrine cell hyperplasia (black arrow) (MD11).
D: Intraepithelial CD3+ T cells (MD11).
Figure 4.
A: Grade III GVHD of gut (MD30) showing apoptotic crypt cells (thin arrows) and complete crypt dropout (thick arrow).
B: Grade II GVHD of skin (MD30) with apoptotic keratinocytes (black arrow) and basal vacuolization and degeneration.
C: Intraepidermal & intradermal CD3+ T cells (MD30).
Four patients including MD30, UP23 and UP26 (who also had GI GVHD) and UP25 (no GI symptoms), developed early skin rashes including generalized erythroderma (MD30) or bright facial rashes (UP23, UP25, UP26) at about day +9 post-transplant. A biopsy of one of these rashes (MD30) showed typical grade II skin GVHD with apoptotic keratinocytes, basal vacuolization and CD3+ T cell infiltration of the dermis and epidermis (Figures 4B, 4C) while the skin biopsy of a second patient with facial rash alone (UP25) showed a follicular and eccrine duct-centric infiltration of lymphocytes in the superficial dermis (data not shown). These rashes resolved in about 1 week with (MD30) or without (UP23, UP25 and UP26) systemic glucocorticoids.
Mechanisms of T-cell Recovery
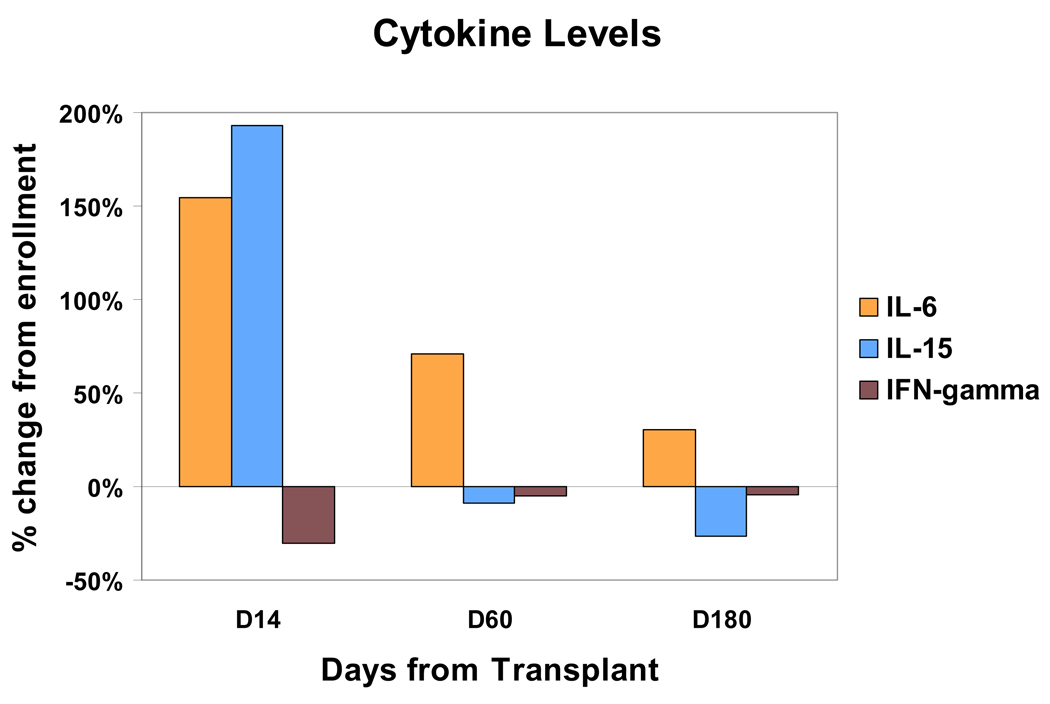
To explore the mechanisms that may contribute to early post-transplant recovery and expansion of transferred T cells, serum levels of various cytokines were measured serially before and after transplant. Serum levels of IL-2, IL-4, IL-6, IL-7, IL-12, IL-17 and interferon gamma were measured for 21 consecutive patients at enrollment, T cell collection, day +14 post-transplant, day +60, day +100 and day +180, using the Luminex ® multiplex cytokine assay system. By this method, only IL-6 levels showed a significant increase (P < 0.03) during the early post-transplant period, while IFN-gamma levels showed a modest but significant decrease at day +14 compared to enrollment (P = 0.03); the levels of IL-2, IL-4, IL-7, IL-12 and IL-17 did not significantly change between these timepoints. Figure 5 depicts the cytokine level changes for IL-6 and IFN-gamma in terms of % change compared to enrollment, although the statistical analysis was based on the serum cytokine concentrations. No significant correlation between IL-6 levels and T-cell recovery parameters could be established. Thirty (30) consecutive sets of serum samples were also assayed for IL-15 levels using a standard ELISA assay, and a statistically significant increase (~2-fold) was observed at day +14 compared to enrollment (P< 0.0001, Figure 5). In addition, highly significant inverse correlations were found between the day 14 IL-15 levels and the day 14 ALC (r = −0.452, P = 0.0054), day 14 CD3 count (r = −0.399, P = 0.0177), day 14 CD4 count (r= −0.467, P = 0.0071) and day 14 CD8 count (r= −0.386, P = 0.0234). About midway through, the study was amended to allow collection of serum samples at day +2 post-transplant (just prior to T cell transfers) and thus a limited number of patients could also be assayed for day +2 IL-15 levels. The mean IL-15 level at day +2 was more than 5 times higher than at enrollment (P < 0.0001). Furthermore, a positive correlation was found between day +2 IL-15 levels and day +14 CD8 counts (Pearson’s correlation coefficient r = 0.6583, P = 0.038). Positive correlations between day +2 IL-15 levels and day +14 CD3 and CD4 counts were marginally or non-significant (CD3: r = 0.548, P = 0.059; CD4: r = 0.5168, P = 0.095) perhaps due to the small numbers of patients who were tested.
Figure 5.
% Change in mean serum cytokine levels at various timepoints after stem cell transplantation. P = 0.03 for day +14 vs enrollment for IL-6; P = 0.03 for day +14 vs enrollment for IFN-gamma; P < 0.0001 for day +14 vs. enrollment levels for IL-15. (Note: The statistical analysis was based on cytokine concentrations)
Functional Immune Studies
One purpose of the current study was to confirm and perhaps extend the observations of robust and early recovery of B and T cell function that were made in the previous study of combined vaccine and T cell immunotherapy (22). Based on 29 patients analyzed to date, the estimated mean total pneumococcal antibody response (sum of titers for serotypes 6B, 14, 19F and 23F) at day 100 post-transplant was 98.7 µg/ml in the current trial vs 138.6 µg/ml in the earlier trial (P = 0.45). At day 100 post-transplant, the mean % of CD4+/CD25+ T cells which proliferated (CFSEdim) in response to the CRM-197 carrier protein was 6.0% (N=22) in the current trial vs 7.1% at day 114 (N = 8) in the previous trial (P not significant). Based on 15 total ARM A (HLA A2 positive) patients analyzed to date, 6 patients (40%) had positive tetramer responses at one or more timepoints after immunization, defined as tetramer staining by flow cytometry > 0.1% (and ≥ 3-fold increase vs enrollment/baseline).
Discussion
High-dose therapy in conjunction with autologous stem cell rescue can achieve a minimal disease state and improve disease-free survival for patients with myeloma and other hematologic malignancies. However, relapses are common due to the lack of an effective antitumor immune response to eliminate residual disease. In principle, the period following high-dose therapy should be conducive to the application of immunotherapy given the low tumor burden and the potential reduction in T-regulatory populations. Unfortunately, following standard autologous transplants the immune system is typically characterized by immune cell depletion and impaired immune-cell function which may persist for up to 4–10 years (32,33).
In a previous randomized study, we found that a single infusion of about 8 × 109 costimulated autologous T cells at day +12 post-transplant accelerated the numerical and functional recovery of both CD4+ and CD8+ T cells and could provide help for vaccination during the early post-transplant period (22). Passenger lymphocytes in the autologous peripheral blood stem cell products alone were insufficient for these purposes.
We hypothesized that adoptive transfer of costimulated T cells at day +2 post-transplant might take greater advantage of early post-transplant homeostatic lymphocyte expansion mechanisms, since lymphopoietic cytokine levels peak very soon after lymphodepleting chemotherapy (34). Indeed results from the current study showed that robust CD4+ and CD8+ T cell counts developed as early as day 14 post-transplant. T cell counts were significantly higher at days 14, 60, 100 and 180 post-transplant than in the previous study when costimulated T cells were infused at day +12 or day +100 post-transplant. These data indicate that T cell recovery was augmented by earlier T cell transfer, although the 5-fold greater number of T cells which were infused in the present study may also have contributed to the enhanced recovery which was observed, particularly at later timepoints.
The mechanisms responsible for the post-transplant T cell recovery are unclear but may include T cell growth stimulation by host-derived cytokines. Interleukin-7 and interleukin-15 play important roles in lymphocyte homeostasis, and in animal models of allogeneic transplantation IL-7 and IL-15 administration improved post-transplant immune reconstitution (34–36). Of the cytokines assayed, only IL-6 and IL-15 showed significant increases between the baseline/enrollment timepoint and day 14. IL-6 is known to increase early after allogeneic stem cell transplantation in conjunction with infections or GVHD (37,38). To further analyze whether the increased IL-6 or IL-15 levels might contribute to the early T cell recovery, a correlation analysis was performed between day 14 IL-6 or IL-15 levels and day 14 T cell counts. Notably, IL-6 levels did not correlate to any T cell recovery parameter, while IL-15 levels correlated significantly but inversely to day 14 ALC, CD3, CD4 and CD8 counts. The inverse correlation between IL-15 levels and T cell levels is consistent with the model that IL-15 activity is regulated through binding to circulating T cells so that large numbers of T cells act like a cytokine “sink” for IL-15. Thus, IL-15 levels which rise within days after completion of high-dose therapy, drive T cell expansion but fall quickly by day 14 as the T cells recover (39,40). Indeed the lowest value which was measured for IL-15 at day 14 post-transplant (1.0 pg/ml) occurred in the patient MD05 who had the most rapid and robust T cell recovery (Figure 2B). In keeping with this model, studies of IL-15 levels at day + 2 post-transplant (just prior to T cell transfers) showed a significant positive correlation with day +14 CD8 counts.
One potential concern regarding the day +2 transfers of costimulated T cells was the impact on hematopoietic recovery. However, a comparison of neutrophil and platelet recovery in the current study with a large cohort of myeloma patients who received autografts without costimulated T cells showed no significant differences in recovery kinetics.
An unexpected observation was that a subset of patients (16%) developed an early T cell engraftment syndrome characterized by watery diarrhea and fever that was clinically and histopathologically indistinguishable from moderate to severe acute GVHD of the gastrointestinal tract and mild to moderate GVHD of the skin. All patients with diarrhea responded rapidly and durably to courses of moderate-dose systemic or nonabsorbable glucocorticoids. Autologous GVHD of the skin has been previously recognized in autograft recipients, especially among those who received post-transplant courses of immunomodulatory agents like cyclosporine A (CSA) or IL-2 (41–44). In addition to the four (8%) early cases of skin GVHD, grade I skin rashes were also observed later in about 50% of patients in the current study which chiefly involved the face, scalp, neck and chest.
Autologous GVHD of the GI tract is much rarer than autologous GVHD of the skin. In one prospective report of 97 autotransplant patients who received CSA, only 4 (4%) developed GI GVHD; all cases were mild (grade I) and only 2 cases were confirmed by biopsy (45). A larger retrospective series of 681 autograft recipients reported a frequency of GI GVHD of 13% but most of these cases involved the upper GI tract causing nausea and vomiting; classical symptoms of diarrhea occurred in only 5% of patients and were mild in all cases (46). In addition, biopsy confirmation of GI GVHD in this series occurred at mean of 42 days post-transplant. This previously published experience contrasts with the present study in which biopsy confirmation of GI GVHD occurred at a median of 17 days post-transplant. In addition, the cases of GI GVHD that we observed were more severe both clinically and pathologically and preferentially involved the lower GI tract similar to the pattern seen in allogeneic transplants. It is also noteworthy that we did not observe any cases of clinical GI GVHD in the earlier study in which 54 patients received costimulated T cells at day +12 or day +100 post-transplant (22). In contrast to the histopathologic features of GI GVHD which typically occur in allogeneic transplant patients, the changes which were observed in the present study included more prominent lymphocytic infiltration relative to the degree of crypt cell or keratinocyte apoptosis. The histopathology of allogeneic cutaneous GVHD typically includes basal vacuolopathy and degeneration with or without lymphocytic infiltration, but eccrine gland involvement and destruction (as observed in the skin biopsy from one of our study patients) has also been reported especially in the chronic phase of allogeneic GHVD (47). A pattern of eccrine gland GVHD was described previously in patients who received an autologous transplant for CML followed by linomide, an immunomodulatory agent (48).
Another form of immunotherapy which can induce enterocolitis in cancer patients is the administration of anti-CTLA4 antibodies (Ipilimumab ®, Medarex-BMS) to block downregulation of T-cell function (49,50). The enterocolitis which was observed in about 21% of anti-CTLA4 treated patients appears to exhibit a distinct histopathology. Ipilimumab-induced enterocolitis had features of inflammatory bowel disease with absence of GVHD-like features of crypt cell apoptosis and crypt dropout and presence of neutrophilic rather than lymphocytic inflammation. Notably, the occurrence of enterocolitis and other autoimmune events in anti-CTLA4 antibody treated patients was highly significantly associated with tumor regression (50).
The mechanisms for the GVHD-like T cell engraftment syndrome which we observed are under investigation. These may involve transient loss of self-tolerance due to suppression of T regulatory populations after high-dose chemotherapy in combination with activation and expansion of effector T cells by ex-vivo costimulation, leading to an increase in the ratio of effector to regulatory T cells. Indeed, a significant increase in the Teff/Treg ratio was identified as early as day +14 post-transplant, coincident with the onset of GVHD, but we could not demonstrate a significant difference in this ratio between patients who developed GVHD and those who did not. Initial immunoassays for patients in this study confirmed in a larger number of patients the robust and early antibody and T cell proliferative responses to the Prevnar® (pneumococcal conjugate) vaccine that were reported earlier (22). In addition, initial tetramer studies suggest that the strategy of early T cell transfer and pre- and post-transplant vaccinations can elicit immune responses to the putative tumor antigen peptides (hTERT and survivin) that were used in this study. Additional followup will be needed to clarify whether the robust immune recovery after day +2 adoptive T cell transfers and the diminution of self-tolerance that appears to occur in a subset of patients will be associated with better clinical outcomes.
Statement of Translational Relevance
Immune cell depletion after high-dose chemotherapy for hematological malignancies may be profound and long-lasting. This form of immunodeficiency may contribute to post-transplant infections (e.g. varicella-zoster and pneumococcal sepsis) and compromise the ability to generate anti-tumor immunity through vaccinations. In this paper, we describe a novel strategy that involves early post-transplant infusions of costimulated autologous T cells. This strategy is safe and leads to rapid, robust and durable CD4 and CD8 T cell recovery without impeding hematopoietic reconstitution. Importantly, a significant proportion of patients develop an early GVHD-like syndrome likely due to transient suppression of self-tolerance – a mechanism which may be necessary for effective cancer immunotherapy. The combination of enhanced immune cell recovery and reduced self-tolerance following high-dose therapy and adoptive transfer of activated autologous T cells may also promote the development of clinically significant immune responses to cancer vaccines.
Supplementary Material
A: Intraepithelial CD4+ T cells (MD11).
B: Intraepithelial CD8+ T cells (MD11).
Acknowledgments
We thank the apheresis centers and nurses of the BMT programs of the University of Maryland Greenebaum Cancer Center and the Abramson Cancer Center for outstanding clinical care provided to our patients. We are grateful for outstanding research support from John Cottrell, Mel Rohall and Marian Williams (University of Maryland) and superb technical support from Lori Landgrebe, Linda Cheung, and Dawn Maier of the Clinical Cell and Vaccine Production Facility at the University of Pennsylvania. We also thank Drs. William Twaddell, Olga Ioffe, Daisy Alapat and Charles Salley (University of Maryland) for help with the histopathology.
Research Support: This work was supported by the National Institutes of Health (5R21CA130293-02[A.P.R.]), the Leukemia and Lymphoma Society (7414-07 [C.H.J., B.L.L.,R.H.V.]),the Beckman Foundation [R.H.V.], and Elsie Hull and James Sprague MD
Footnotes
Parts of this study were presented at the 2007 meeting of the American Society of Hematology, Atlanta, GA and the 2008 AACR Translational Cancer Medicine meeting, Jerusalem, Israel.
Authorship Statement: A.P. Rapoport designed the research, performed the research, analyzed the data and wrote the paper. E.A. Stadtmauer designed the research, performed the research and helped write the paper. N.Aqui performed the research and helped write the paper. D. Vogl performed the research. A. Chew analyzed data. H-B. Fang performed statistical analysis. S. Janofsky performed research. K. Yager performed the research. E. Veloso performed research. Z. Zheng performed research. T. Milliron performed research. S. Westphal performed research. J. Cotte performed research. H. Huynh performed research. A. Cannon performed research. S. Yanovich performed research. G. Akpek performed research. M. Tan designed the research and analyzed data. K. Virts performed research. K. Ruehle performed research. C. Harris performed research. R.H. Vonderheide designed research, performed research, contributed vital new reagents and helped write the paper. B.L. Levine designed research, performed research, contributed vital reagents, analyzed data and helped write the paper. C.H. June designed the research, contributed vital new reagents, analyzed the data and helped write the paper.
Conflict of Interest Statement: A.P. Rapoport (corresponding author) has no conflicts of interest to declare. E.A. Stadtmauer has no conflicts of interest to declare. N.Aqui has no conflicts of interest to declare. D. Vogl has no conflicts of interest to declare. A. Chew has no conflicts of interest to declare. H-B. Fang has no conflicts of interest to declare. S. Janofsky has no conflicts of interest to declare. K. Yager has no conflicts of interest to declare. E. Veloso has no conflicts of interest to declare. Z. Zheng has no conflicts of interest to declare. T. Milliron has no conflicts of interest to declare. S. Westphal has no conflicts of interest to declare. J. Cotte has no conflicts of interest to declare. H. Huynh has no conflicts of interest to declare. A. Cannon has no conflicts of interest to declare. S. Yanovich has no conflicts of interest to declare. G. Akpek has no conflicts of interest to declare. M. Tan has no conflicts of interest to declare. K. Virts has no conflicts of interest to declare. K. Ruehle has no conflicts of interest to declare. C. Harris has no conflicts of interest to declare. R.H. Vonderheide declares a potential financial conflict of interest related to inventorship on a patent regarding hTERT as a tumor-associated antigen for cancer immunotherapy. B.L. Levine has no conflicts of interest to declare. C.H. June has no conflicts of interest to declare.
References
- 1.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 2.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Barlogie B, Tricot GJ, van Rhee F, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006;135:158–164. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- 5.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. [PubMed] [Google Scholar]
- 6.Alyea E, Weller E, Schlossman R, et al. T-cell—depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001;98:934–939. doi: 10.1182/blood.v98.4.934. [DOI] [PubMed] [Google Scholar]
- 7.Lokhorst HM, Wu K, Verdonck LF, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4364. doi: 10.1182/blood-2003-11-3862. [DOI] [PubMed] [Google Scholar]
- 8.Garban F, Attal M, Michallet M, et al. on behalf of the IFM group. Prospective comparison of autologous stem cell transplantation followed by a dose-related allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04) trial in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 9.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 10.Porrata LF, Markovic SN. Timely reconstitution of immune competence affects clinical outcome following autologous stem cell transplantation. Clin Exp Med. 2004;4:78–85. doi: 10.1007/s10238-004-0041-4. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari D, Gao F, Hidalgo J, et al. Prognostic significance of early lymphocyte recovery after post-autografting administration of GM-CSF in non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2007;40:671–675. doi: 10.1038/sj.bmt.1705795. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport AP. Immunity for tumors and microbes after autotransplantation: if you build it, they will (not) come. Bone Marrow Transplant. 2006;37:239–247. doi: 10.1038/sj.bmt.1705242. [DOI] [PubMed] [Google Scholar]
- 13.Youssef S, Rodriguez G, Rolston KV, Champlin RE, Raad II, Safdar A. Streptococcus pneumoniae infections in 47 hematopoietic stem cell transplantation recipients: clinical characteristics of infections and vaccine-breakthrough infections, 1989–2005. Medicine. 2007;86:69–77. doi: 10.1097/md.0b013e31803eb176. [DOI] [PubMed] [Google Scholar]
- 14.Frère P, Pereira M, Fillet G, Beguin Y. Infections after CD34-selected or unmanipulated autologous hematopoietic stem cell transplantation. Eur J Haematol. 2006;76:102–108. doi: 10.1111/j.1600-0609.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 15.Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Lu G, Levisky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2006;95:3011–3019. [PubMed] [Google Scholar]
- 16.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Yee C, Beavo JA. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 18.Boussiotis VA, Freeman GJ, Taylor PA, et al. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat Med. 2000;6:290–297. doi: 10.1038/73144. [DOI] [PubMed] [Google Scholar]
- 19.Levine BL, Ueda Y, Craighead N, Huang ML, June CH. CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. International Immunology. 1995;7:891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- 20.Levine BL, Bernstein WB, Connors M, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 21.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 22.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of Immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 23.Vij R, Borello I, Martin T, et al. A phase I/II study of xcellerated T cells™ after autologous peripheral blood stem cell transplantation in patients with multiple myeloma [abstract] Blood. 2003;102:#139. [Google Scholar]
- 24.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 25.Scardino A, Gross DA, Alves P, et al. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- 26.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 27.McLaughlin-Taylor E, Pande H, Forman SJ, et al. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 28.Hami LS, Green C, Leshinsky N, Markham E, Miller K, Craig S. GMP production and testing of Xcellerated T cells for the treatment of patients with CLL. Cytotherapy. 2005;6:554–562. doi: 10.1080/14653240410005348. [DOI] [PubMed] [Google Scholar]
- 29.Domchek SM, Recio A, Mick R, et al. Telomerase-specific T-cell immunity in breast cancer: Effect of vaccination on tumor immunosurveillance. Cancer Res. 2007;67:10546–10555. doi: 10.1158/0008-5472.CAN-07-2765. [DOI] [PubMed] [Google Scholar]
- 30.Tan M, Fang HB, Tian GL, Houghton PJ. Small-sample inference for incomplete longitudinal data with truncation and censoring in tumor xenograft models. Biometrics. 2002;58:612–620. doi: 10.1111/j.0006-341x.2002.00612.x. [DOI] [PubMed] [Google Scholar]
- 31.Gojo I, Meisenberg B, Guo C, et al. Autologous stem cell transplantation followed by consolidation chemotherapy for patients with multiple myeloma. Bone Marrow Transplant. 2006;37:65–72. doi: 10.1038/sj.bmt.1705192. [DOI] [PubMed] [Google Scholar]
- 32.Guillaume T, Rubinstein DB, Symann M. Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood. 1998;92:1471–1490. [PubMed] [Google Scholar]
- 33.Nordoy T, Husebekk A, Aaberge IS, et al. Humoral immunity to viral and bacterial antigens in lymphoma patients 4–10 years after high-dose therapy with aBMT. Serological responses to revaccinations according to EBMT guidelines. Bone Marrow Transplant. 2001;28:681–687. doi: 10.1038/sj.bmt.1703228. [DOI] [PubMed] [Google Scholar]
- 34.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alpdogan O, Schmaltz C, Muriglan SJ, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 36.Alpdogan O, Eng JM, Muriglan SJ, et al. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005;105:865–873. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 37.Imamura M, Hashino S, Kobayashi H, et al. Serum cytokine levels in bone marrow transplantation: synergistic interaction of interleukin-6, interferon-gamma and tumor necrosis factor-alpha in graft-versus-host disease. Bone Marrow Transplant. 1994;13:745–751. [PubMed] [Google Scholar]
- 38.Liem LM, van Houwelingen HC, Goulmy E. Serum cytokine levels after HLA-identical bone marrow transplantation. Transplantation. 1998;66:863–871. doi: 10.1097/00007890-199810150-00009. [DOI] [PubMed] [Google Scholar]
- 39.Williams K, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooley S, June CH, Schoenberger SP, Miller JS. Adoptive Therapy with T cells/NK cells. Biol Blood Marrow Transplant. 2007;13:33–42. [Google Scholar]
- 41.Jones RJ, Vogelsang GB, Hess AD, et al. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet. 1989;333:754–757. doi: 10.1016/s0140-6736(89)92575-0. [DOI] [PubMed] [Google Scholar]
- 42.Yeager AM, Vogelsang GB, Jones RJ, et al. Induction of cutaneous graft-versus-host disease by administration of cyclosporine to patients undergoing autologous bone marrow transplantation for acute myeloid leukemia. Blood. 1992;79:3031–3035. [PubMed] [Google Scholar]
- 43.Marin GH, Porto A, Prates V, Napal J, Etchegogen O, Rubens L, et al. Graft versus host disease in autologous stem cell transplantation. J Exp Clin Cancer Res. 1999;18:201–208. [PubMed] [Google Scholar]
- 44.Massumoto C, Benyunes MC, Sale G, et al. Close stimulation of acute graft-versus-host disease by interleukin 2 administered after autologous bone marrow transplantation for hematologic malignancy. Bone Marrow Transplant. 1996;17:351–356. [PubMed] [Google Scholar]
- 45.Baron F, Gothot A, Simon J-P, et al. Clinical course and predictive factors for cyclosporine-induced autologous graft-versus-host disease after autologous haematopoietic stem cell transplantation. Br J Haematol. 2000;111:745–753. [PubMed] [Google Scholar]
- 46.Holmberg L, Kikuchi K, Gooley TA, et al. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: Incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2005;12:226–234. doi: 10.1016/j.bbmt.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Akosa AB, Lampert IA. The sweat gland in graft versus host disease. J Pathol. 1990;161:261–266. doi: 10.1002/path.1711610314. [DOI] [PubMed] [Google Scholar]
- 48.Rowe JM, Rapoport AP, Ryan DH, et al. Treatment of chronic myelogenous leukemia with autologous bone marrow transplantation followed by roquinimex. Bone Marrow Transplant. 1999;24:1057–1063. doi: 10.1038/sj.bmt.1702037. [DOI] [PubMed] [Google Scholar]
- 49.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Intraepithelial CD4+ T cells (MD11).
B: Intraepithelial CD8+ T cells (MD11).