Abstract
Objective
To describe a receptor-based approach to promote learning about nonsteroidal anti-inflammatory drug (NSAID) chemistry, structure-activity relationships, and therapeutic decision-making.
Design
Three lessons on cyclooxygenase (COX) and NSAID chemistry, and NSAID therapeutic utility, were developed using text-based resources and primary medicinal chemistry and pharmacy practice literature. Learning tools were developed to assist students in content mastery.
Assessment
Student learning was evaluated via performance on quizzes and examinations that measured understanding of COX and NSAID chemistry, and the application of that knowledge to therapeutic problem solving.
Conclusion
Student performance on NSAID-focused quizzes and examinations documented the success of this approach.
Keywords: nonsteroidal antiinflammatory drugs, medicinal chemistry
INTRODUCTION
Nonsteroidal antiinflammatory drugs (NSAIDs) are widely used prescription and nonprescription medications. Their primary mechanism of action is the competitive and reversible inhibition of 2 closely related cyclooxygenase enzymes, COX-1 and COX-2.1 These are the rate-limiting enzymes in the synthesis of the inflammatory prostaglandins PGE2 and PGF2α, the cytoprotective prostaglandin PGE1, and the vasoactive prostanoids thromboxane A2 (TXA2) and prostacyclin (PGI2). In addition to their involvement as mediators of inflammation, prostaglandins have been implicated in diseases such as cancer (specifically lung, breast, and colon), Alzheimer's disease, Parkinson's disease, and cardiovascular diseases including stroke, myocardial infarction, and athlerosclerosis.2
The role of NSAIDs in the prophylaxis and/or treatment of the above-mentioned pathological conditions is emerging and should expand over time, thus justifying the in-depth education of pharmacy students on their scientifically grounded therapeutic use. There are many NSAID products on the US market, and they can be categorized into 5 distinct chemical classes: arylalkanoic acids, salicylates, fenamates, oxicams, and COX-2 selective diarylheteroaromatics. While all NSAIDs contain the common functional groups essential for classification as COX inhibitors, the ability to decipher and analyze often subtle structural differences between them allows practitioners to identify important pharmacologic distinctions that can and should impact therapeutic decision-making and, subsequently, the quality of patient care. A clear understanding of the topography of the active and allosteric binding sites of the COX-1 and COX-2 enzymes, along with a firm grasp of the impact of drug metabolism and SARs on therapeutic and potentially toxic actions, gives pharmacists the scientific edge in predicting NSAID behavior in vivo, and facilitates the design of rationale therapy for individual patients. Only when empowered with this knowledge and armed with these chemically grounded skills can practitioners fulfill their role as the drug experts of the healthcare team.
The 2-semester, 5-credit-hour medicinal chemistry course sequence required of second-year pharmacy students at Creighton University has been described in detail.3,4 The 3-lesson unit on NSAIDS is delivered towards the end of the first semester. The more critical learning objectives are:
Identify the amino acids of the COX active site, and describe the binding interactions between these residues and NSAID functional groups.
Identify important functional and structural differences between the COX-1 and COX-2 isoforms, particularly as they relate to access to the allosteric binding pocket, and the therapeutic consequence of these differences.
Distinguish COX-2 selective inhibitors from non-selective NSAIDs by structure, and describe how they bind to COX-2 allosteric pocket residues.
Diagram the process by which aspirin acetylates the serine (SER) residues of COX-1 and COX-2 enzymes, and the pharmacological and therapeutic implications of this unique mechanism of COX inhibition. Describe the importance of the acetoxy group to aspirin's ability to irreversibly inactivate COX.
Interpret receptor binding affinity, lipophilicity, and metabolic vulnerability of NSAIDs to predict overall nonselective antiinflammatory potency, duration of action, and/or toxicity.
Apply NSAID chemistry, receptor binding, and SAR to therapeutic decision-making.
DESIGN
The 2 Chemical Basis of Drug Action courses take a practice-oriented approach that emphasizes the relevance of chemistry to the contemporary practice of pharmacy. They were purposefully designed to give students the skills necessary to predict biological properties and therapeutic activities of drug molecules.
Comprehensive and conversational lesson handouts, learning objectives, and lesson summaries entitled Med Chem To Go were provided via the course Web site, and students took a weekly, collaborative open-book quiz over selected lessons prior to class. This allowed the use of class time to delve into topics in greater depth, apply concepts, and solve problems. To assist students in achieving higher order learning goals, several optional worksheets, problem-solving exercises, case studies, and past examinations (with and without answer keys) were made available on the course Web site.
The 3 lesson handouts developed for the NSAIDs series began with an in-depth description of both the common and unique chemical features of the COX isoforms. Key arachidonic acid- and NSAID-binding residues found in the COX active and allosteric sites were identified from the primary literature, and their sidechain chemistry and conformation was described in both text and graphic form (Figure 1). With this essential foundation in receptor chemistry, students were able to explain the chemical mechanisms of non-selective (reversible, irreversible, and time-dependent) COX inhibition, and chemically justify how COX-2 selective inhibitors discriminate between isoforms.
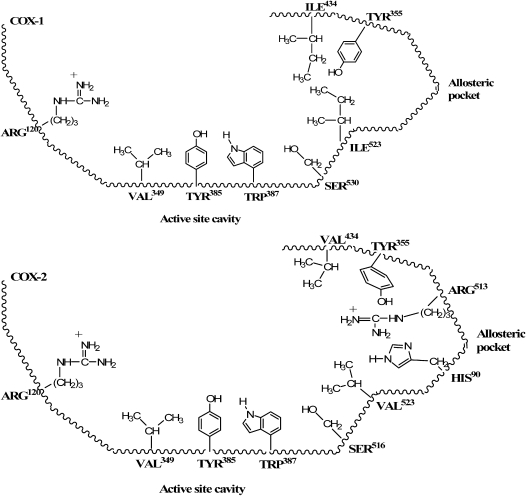
Figure 1.
2D representations of COX-1 and COX-2 NSAID binding sites.
The NSAID lesson handout comprised 53 pages of text and receptor binding and metabolism graphics. The content was divided into 3 sections: (1) Introduction and Receptors, (2) Salicylates, Fenamates, Oxicams, and COX-2 Selective Inhibitors, and (3) Arylalkanoic Acids. All learning materials, including the quiz, examinations, and optional learning exercises, are available from the author. The teaching/learning strategies identified above and described in previous publications allowed each of these sections to be covered in a 50-minute class period.
NSAIDs Introduction and Receptors Lesson
The primary intent of the first NSAIDs lesson was to inculcate an understanding of the chemical topography of COX enzymes at sites where NSAIDs bind, and to demonstrate the chemical similarity between NSAIDs and arachidonic acid, the endogenous COX substrate which looks nothing like NSAIDs to the chemically untrained eye. The elements of this lesson which receive the greatest emphasis are: (1) the amino acid residues that comprise the binding domain of the active site cavity,5-8 (2) the vulnerability of a key SER residue at the edge of the active site to irreversible acetylation by aspirin,6,9 and (3) the structural differences between the allosteric binding area of COX-1 and COX-2, particularly the “gatekeeper” residues that determine access to residues in the allosteric pocket.1,10-11
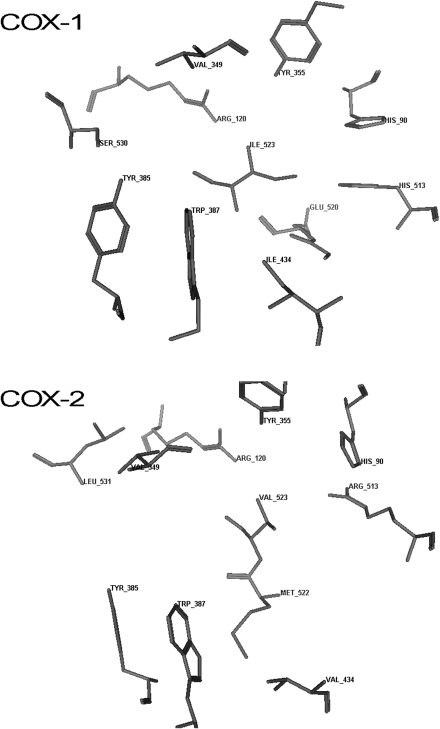
Recognizing that the active site residues involved in binding NSAIDs are identical in the 2 COX isoforms1 (Figure 1) allowed students to appreciate why most marketed NSAID products are non-selective in their action. When treating inflammation, an NSAID should ideally target COX-2 since it is the isoform expressed during pathological conditions.1,12 The ability of most drugs to inhibit COX-1 in addition to COX-2 explains their propensity for use-limiting side effects such as gastrointestinal (GI) distress (a result of COX-1 inhibition in the stomach), hemorrhage (inhibition of COX-1 in the stomach and on platelets), and renal toxicity (the kidney contains COX-1 and COX-2 enzymes). The role of the active site ARG (arginine) in anchoring arachidonic acid and NSAIDs to the active site via electrostatic forces explains why an acidic functional group capable of becoming anionic at physiologic pH is an essential NSAID structural feature. Carboxylic acid moieties are by far the most common, although an acidic enol fulfills the anionic moiety requirement in oxicams. A trio of aromatic and/or lipophilic active site residues explains how 2 aromatic NSAID moieties enhance antiinflammatory potency, both by augmenting COX receptor affinity through hydrophobic or π electron-requiring van der Waals interactions, and by providing the lipophilicity that propels the drug across biological membranes to reach those receptors. While not obvious in Figure 1, the 3-dimensional models of COX active and allosteric site residues found in Figure 2 document that these amino acids are out of plane with one another, which demands a non-coplanar orientation of NSAID aromatic moieties for optimal binding affinity.13-16
Figure 2.
3D model of the COX-1 and COX-2 NSAID binding site residues.
While not a component of the active site residue repertoire, COX-1 SER530 is accessible to NSAIDs.6,9 If the chemistry is right (as it is in aspirin due to the unique acetoxy ester moiety) the oxygen atom of SER can launch a nucleophilic attack at the electrophilic carbonyl carbon of the ester, which hydrolyzes the NSAID and transfers an acetyl group to the SER. This irreversible transacetylation reaction (diagrammed in a stepwise fashion in the lesson handout) leads to a potent and long-lasting inhibition of COX-1. Since aspirin is the only NSAID with a hydrolysable acetoxy (or any other) ester, it is the only drug product capable of participating in this reaction. This leads to its well-earned reputation for GI bleeding and attenuated platelet aggregation secondary to irreversible inhibition of COX-1 in the stomach and in platelets, respectively. While the analogous SER516 residue of COX-2 can also be acetylated by aspirin, the larger active site cavity of this isoform still permits endogenous substrate binding. However acetylated COX-2 becomes a lipogenase, synthesizing leukotrienes rather than prostaglandins.6,9
In low doses (81 mg), the ability of aspirin to selectively inhibit platelet COX-1 as described above explains its widespread use to decrease the risk of myocardial infarction and stroke. Students understand that COX-1 selectivity is lost when doses are increased, and that the inhibition of COX-2 in the vessel wall that occurs with higher doses can negate the cardiovascular protecting action of low-dose aspirin17 by inhibiting the production of the antiaggretatory prostanoid PGI2. Students also recognize that, to prevent excessive platelet aggregation, aspirin must have full access to the COX-1 active site. Thus, through understanding the chemical mechanism of aspirin's irreversible inhibition of platelet COX-1, students can justify the importance of counseling patients using aspirin for cardiovascular health, to (1) keep doses low, and (2) separate administration of aspirin and other reversible NSAID being used to treat pain or inflammation (eg, ibuprofen, naproxen) by several hours.17
Class time was also spent discussing the important exchange of isoleucine (ILE) for valine (VAL) at the “lip” of the allosteric pocket of COX-1 and COX-2, respectively (see Figure 1).1,10,16 Coupled with a distinctly different orientation of tyrosine (TYR) 355,11 the smaller VAL residues (particularly VAL523) open up the COX-2 allosteric pocket for therapeutic manipulation. In contrast, the bulkier ILE residues and the uninviting conformation of TYR355 in COX-1 prohibit binding (and, therefore, enzyme inhibition) of diarylheteroaromatic NSAIDs such as celocoxib (see Figure 1).
While COX-2 selective inhibitors have an inherently lower risk of inducing GI distress and hemorrhage (COX-1 mediated activities), the risk of negative cardiovascular events11 secondary to inhibiting PGI2 in the vessel wall (a COX-2 mediated action) is significantly greater, particularly without the balancing effect of decreasing TXA2 levels through platelet COX-1 inhibition. It was the enhanced cardiovascular risk that prompted the removal of 2 COX-2 selective NSAIDs (rofecoxib and valdecoxib) from the US market. A television commercial for the remaining COX-2 selective NSAID celocoxib aired several times during the semester the class was being taught, and the instructor used this to illustrate how the therapeutic benefits and toxicity risks claimed or disclosed in the Celebrex advertisement could be fully explained through an understanding of this receptor-focused chemistry.
NSAIDs Therapeutic Agents Lessons
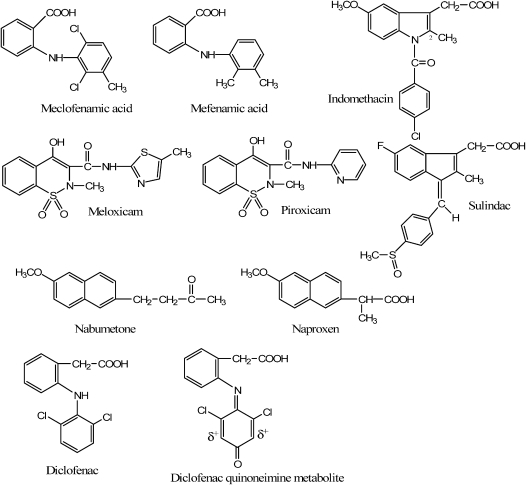
In addition to reinforcing the general COX receptor-binding and isoform selectivity principles emphasized in the Introduction and Receptors lesson, more subtle nuances of COX binding, drug metabolism, and SAR influences on activity profile that impact therapeutic decision-making were introduced, such as the following (structures shown in Figure 3):
The 25-fold anti-inflammatory activity enhancement of meclofenamic acid over mefenamic acid, due to a more consistently non-coplanar orientation of aromatic rings and enhanced lipophilicity provided by the 2 large ortho-chlorine atoms. The non-coplanar orientation of rings is important for optimal binding to the trio of aromatic/aliphatic COX active-site residues.13
The preferential COX-2 inhibiting action of meloxicam compared to the COX-1 preferring piroxicam,18 presumably due to the former's thioether-containing aromatic moiety. The sulfur atom of meloxicam's aromatic thiazole ring is one of the few features that distinguish it from the pyridine-containing piroxicam. Sulfur atoms associated with primary sulfonamide or methylsulfone moieties are commonly found on COX-2 selective inhibitors.
Indomethacin's time-dependent pseudoirreversible inhibition of COX-1, attributed to the insertion of the 2-CH3 group into a hydrophobic pocket of the enzyme with a resultant change in enzyme conformation.19
The prolonged duration of action and mechanistically predicted attenuated GI distress risk of sulindac (compared to indomethacin) and nabumetone (compared to naproxen), attributed to their prodrug status. As sulindac and nabumetone are both inactive until they reach activating enzymes in the liver, neither inhibits COX-1 enzymes in the gut after oral administration.
The anticipated enhanced margin of GI safety of nabumetone compared to sulindac, due to the lack of an acidic functional group on the parent nabumetone structure. The lack of an acidic functional group while in the gut decreases the risk of physical irritation of the gastrointestinal mucosa.
The potential for diclofenac-induced hepatotoxicity in vulnerable patients, the result of the formation of an electrophilic quinoneimine metabolite.20 The 2 o-chlorine atoms increase the electrophilic character of the m positions of the halogen-containing aromatic ring, making the quinoneimine (the oxidized form of the CYP2C9 and 3A4-generated phenolic metabolite) vulnerable to attack by nucleophilic hepatocyte CYS residues.
Figure 3.
NSAID structures.
Application of Content to Therapeutic Decision-Making
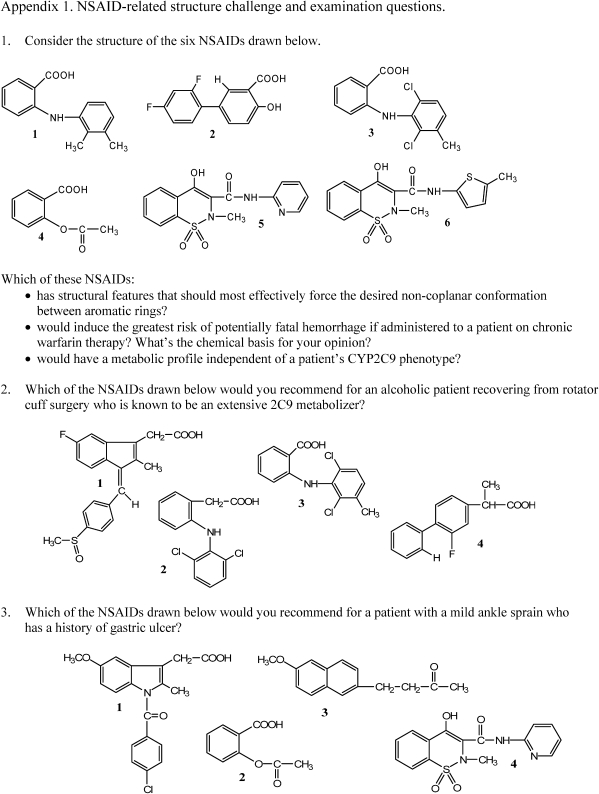
Class sessions were used not only to reinforce the content provided in the lesson handout and augmented by readings from the recommended textbook, but also to practice therapeutic application skills. Patient care-related questions that can be answered through an analysis of structure were posed to students throughout the lecture, and structure challenge exercises were regularly incorporated to encourage analytical thinking and collaborative problem-solving. Examples of an NSAID structure challenge and recent problem-based NSAID examination questions are provided in Appendix 1.
EVALUATION AND ASSESSMENT
The level at which students mastered NSAIDs content and achieved learning outcomes was measured through performance on the NSAIDs pre-lecture reading comprehension quiz and the NSAIDs component of the closed-book examination. While the quiz content was exclusively focused on NSAID chemistry, NSAIDs content represented only 25%-35% of the examination most commonly administered during the last week of the semester, and often competed for students’ attention with topics such as opioid analgetics, proton pump inhibitors,3 antihyperlipidemic agents,4 and antihistamines.
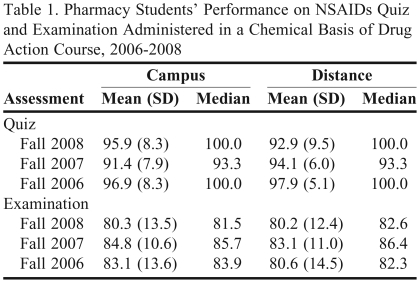
The performance of Chemical Basis of Drug Action students on NSAID quizzes and examinations from 2006 through 2008 is provided in Table 1. Performance averages document content mastery at a high level.
Table 1.
Pharmacy Students’ Performance on NSAIDs Quiz and Examination Administered in a Chemical Basis of Drug Action Course, 2006-2008
DISCUSSION
The assessment data show that Chemical Basis of Drug Action students consistently performed at an average B to B+ level on the NSAID component of examinations, and an A level on the NSAIDs quizzes. In addition to the consistent B to B+ performance on the NSAIDs component of examinations, the level of performance partity between students attending class on campus and those enrolled in the school's entry level (first professional degree) distance PharmD pathway was noteworthy. Clearly, students are meeting performance expectations under the challenge of high stakes examinations often given at a point in the semester when focus and intellectual energy can be difficult to sustain.
The Chemical Basis of Drug Action courses have at their core the education of practitioners who are grounded and conversant in the science that underpins the profession. In our program, pharmacy students are prepared to analyze drug action at the molecular level through an introductory series of chemical basis lectures that stress the fundamentals and clinical application of acid-base chemistry,21 receptor chemistry (including a review of amino acid structure and properties), and drug metabolism.
By the time the NSAIDs are covered in the Chemical Basics of Drug Action course, students can readily predict the preferred intermolecular interactions of active site residues, and can envision the nature of drug functional groups that will be required to bind to them. Recognizing what receptors demand for stabilizing drug occupancy helps minimize the need for blind memorization of SAR rules. For a given receptor protein, students do need to recognize which of a limited number of functionally similar residues is present within an active site (eg, the cationic anchoring residue in COX is ARG rather than LYS), but that level of memorization is comparatively minimal and, once mastered, allows them to essentially develop their own accurate roster of SAR rules that define optimal activity (or at least more readily comprehend the rules outlined in the lesson handout and textbook).
Conformational aspects of receptor chemistry are shared where known, as in the non-coplanar orientation of aromatic/lipophilic residues in the COX active site and the sterically restricted allosteric pocket of the COX-1 isoform. Of course, students must also be aware of structural requirements for effective distribution to sites of action, and most have a firm grasp of functional groups that augment or attenuate distribution-promoting lipophilicity. Likewise, by this point in the course, students can predict metabolically vulnerable drug functional groups and anticipate metabolites, but time must be spent learning which of the many potential biotransformations actually do occur to an appreciable extent, what enzyme(s) (including specific CYP isoforms) catalyze them, and whether the resultant metabolites are active or inactive.
The understanding of drug action gained through this receptor-grounded approach to NSAID SAR is most valuable to pharmacy students when they can reliably apply it to solve patient-centered therapeutic challenges. Because students can now more confidently reason their way through the SAR rules and analyze the pharmacodynamic pros and cons of various NSAID structures, more class time can be spent developing chemistry-based problem-solving and therapeutic decision-making skills. In addition to the collaborative structure challenges conducted in class, students have access to study questions, chemistry-focused therapeutic case studies, and a complex and chemically comprehensive NSAIDs computerized case study based on characters from The Godfather trilogy.22 While not all students take advantage of the optional pen-and-paper exercises, both summative course and focused case study evaluations consistently document that they do run the computerized cases. Students claim these electronic learning tools facilitate understanding of drug chemistry, hone therapeutic application and decision-making skills, and make studying enjoyable.
Students invest a significant amount of individual study time reading lesson handouts and exploring the optional learning exercises available on the course Web site. With that level of student commitment, however, only three 50-minute class sessions are needed to gain the knowledge and experience needed to competently analyze structure and predict the pharmacological behavior and therapeutic utility of a wide variety of marketed NSAID molecules. This high ratio between learning and time invested is achieved by combining motivated students with scientifically rigorous and clinically relevant information, providing a variety of resources that promote mastery of concepts and principles, and giving students opportunities to practice required content application skills both in and outside of the classroom.
Student ratings of the Chemical Basis of Drug Action courses and the author's teaching strategies document a sustained student-perceived high level of meaningful learning. Most students recognized the value of understanding receptor topography in order to develop and/or interpret SAR, and many have expressed both excitement and pride about their ability to apply their unique knowledge of drug chemistry to answer therapeutic-related problems in the workplace.
CONCLUSIONS
The receptor-grounded approach to the instruction of NSAID chemistry and SAR is an effective strategy to foster meaningful learning of this class of widely used therapeutic agents. The approach described can be generalized and applied across all classes of drugs that employ receptor-dependent mechanisms to translate chemical structure into pharmacological action.
ACKNOWLEDGEMENT
The author gratefully acknowledges Dr. Brian Henriksen, Creighton University School of Pharmacy and Health Professions, for the generation of the COX enzymes graphic that appears as Figure 2.
REFERENCES
- 1.Vane JR, Bakhle S, Botting RM. Cyclooxygenase 1 and 2. Ann Rev Pharmacol Toxicol. 1998;38(April):97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Chubb AJ, Fitzgerald DJ, Nolan KB, Moman E. The productive conformation of prostaglandin G2 at peroxidase site of prostaglandin endoperoide H synthase: docking, molecular dynamics, and site-directed mutagenesis studies. Biochemistry. 2006;45(3):811–820. doi: 10.1021/bi051973k. [DOI] [PubMed] [Google Scholar]
- 3.Roche VF. The chemically elegant proton pump inhibitors: A self-contained, clinically relevant medicinal chemistry lesson. Am J Pharm Educ. 2006;70(5) doi: 10.5688/aj7005101. Article 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roche VF. Antihyperlipidemic statins: a self-contained, clinically relevant medicinal chemistry lesson. Am J Pharm Educ. 2005;69(4) Article 77. [Google Scholar]
- 5.Thuresson ED, Lakkides KM, Rieke CJ, et al. Prostaglandin endoperoxide H synthase-1. J Biol Chem. 2001;276(13):10347–10359. doi: 10.1074/jbc.M009377200. [DOI] [PubMed] [Google Scholar]
- 6.Furse KE, Pratt DA, Porter NA, Lybrand TP. Molecular dynamics simulations of arachidonic acid complexes with COX-1 and COX-2: insights into equilibrium behavior. Biochemistry. 2006;45(10):3198–3205. doi: 10.1021/bi052337p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palomer A, Perez JJ, Navea S, Llorens O, Pascual J, Garcia L, Mauleon D. Modeling cyclooxygenase inhibition: implication of active site hydration on the selectivity of ketoprofen analogues. J Med Chem. 2000;43(11):2280–2284. doi: 10.1021/jm9906217. [DOI] [PubMed] [Google Scholar]
- 8.Kiefer JR, Pawlitz JL, Moreland KT, et al. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature. 2000;405:97–101. doi: 10.1038/35011103. [DOI] [PubMed] [Google Scholar]
- 9.Rowlinson SW, Crews BC, Goodwin DC, Schneider C, Gierse JK, Marnett LJ. Spatial requirements for 15-(R)-hydroxy-5Z-8Z-11Z-13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2. Why acetylated COX-1 does not synthesize 15-(R)-HETE. J Biol Chem. 2000;275(9):6586–6591. doi: 10.1074/jbc.275.9.6586. [DOI] [PubMed] [Google Scholar]
- 10.Kurumbail RG, Stevens AM, Gierse JK, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 11.Anana R, Rap PNP, Chen Q-H, Knaus EE. Synthesis and biological evaluation of linear phenylethynylbenzenesulfonamide regioisomers as cyclooxygenase-1/-2 (COX-1/-2) inhibitors. Bioorganic Med Chem. 2006;14:5259–5265. doi: 10.1016/j.bmc.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Riendeau D, Percival MD, Brideau C, et al. Etoricoxib (MK-0662):Preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exper Ther. 2001;296(2):558–566. [PubMed] [Google Scholar]
- 13.Borne R, Levi M, Wilson N. Nonsteroidal anti-inflammatory drugs. In: Williams DA, Lemke TL, editors. Foye's Principles of Medicinal Chemistry. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2008. p. 970. [Google Scholar]
- 14.Harman CA, Turman MV, Kozak KR, et al. Structural basis of enantioselective inhibition of cyclooxygenase-1 by S-alpha-substituted indomethacin ethanolamides. J Biol Chem. 2007;282:28096–28105. doi: 10.1074/jbc.M701335200. [DOI] [PubMed] [Google Scholar]
- 15.Rowlinson SW, Kiefer JR, Prusakiewicz JJ, et al. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J Biol Chem. 2003;278:45763–45769. doi: 10.1074/jbc.M305481200. [DOI] [PubMed] [Google Scholar]
- 16. RCSB Protein Data Bank. http://www.rcsb.org/pdb/static.do?p=search/index.html. Accessed September 8, 2009.
- 17. Postmarket Drug Safety Information for Patients and Providers. Food and Drug Administration Web site. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/default.htm Accessed November 24, 2009.
- 18.Vane JR, Gotting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(3A):2S–8S. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- 19.Prusakiewicz JJ, Felts AS, Mackenzie BS, Marnett LJ. Molecular basis of the time-dependent inhibition of cyclooxygenase by indomethacin. Biochemistry. 2005;43:15439–15445. doi: 10.1021/bi048534q. [DOI] [PubMed] [Google Scholar]
- 20.Takakusa H, Masumoto H, Mitsuru A, Okazaki O, Sudo K. Markers of electrophilic stress caused by chemically reactive metabolites in human hepatocytes. Drug Metab Dispos. 2008;36(5):816–823. doi: 10.1124/dmd.107.018002. [DOI] [PubMed] [Google Scholar]
- 21.Roche VF. Improving pharmacy students’ understanding and long-term retention of acid-base chemistry. Am J Pharm Educ. 2007;71(6) doi: 10.5688/aj7106122. Article 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roche VF, Zito SW. Development and evaluation of a new computerized medicinal chemistry case study on NSAID chemistry and therapeutics: the Drugfather [abstract]. Meeting Abstracts. 109th Annual Meeting of the American Association of Colleges of Pharmacy, Chicago, Illinois, July 19-23, 2008. Am J Pharm Educ. 2008;72(3) Article 72. [Google Scholar]