Abstract
Objective
To provide students with a comprehensive, integrated presentation on the pharmacology of immuosuppression.
Design
Course content on the pharmacology of immunosuppression relating to organ transplantation and treatment of autoimmune disorders was presented in integrated sequence modules that included content from pharmacology, medicinal chemistry, and therapeutics. Weekly recitation sessions and active-learning exercises were incorporated to allow students to apply the information they learned to integrated patient cases and stimulate involvement and critical thinking. Fundamental material related to the components and functions of the immune system was presented to students early in curriculum with courses such as biochemistry, pathophysiology, and immunology/microbiology.
Assessment
Comprehensive examinations, in-class quizzes, written case submissions, case discussions, review exercises, and group exercises were used to assess student learning.
Conclusion
Students at South University received a comprehensive and detailed understanding of all aspects relating to immunosuppressive therapy. This was accomplished by integrating instruction on immunosuppressive therapy from various disciplines.
Keywords: immunosuppression, pharmacology, organ transplantation, autoimmune disease, immune system
INTRODUCTION
Drugs to suppress human immune response have been used for more than half a century.1 Such agents are essential for treating patients who have received organ transplants or suffer from autoimmune diseases. The main drawback to the early immunosuppressive agents was their lack of specificity. Broad suppression of immune cell function and replication often led to serious toxicities and numerous adverse effects. As understanding of immune system response at the cellular and molecular levels evolved, newer and more specific agents were developed that targeted particular components and elements of the immune response. While these newer immunosuppressive agents are not without potential adverse effects, their efficacy and safety have improved greatly when compared to earlier agents.
Pharmacists can and do play a vital role to ensure the safe and effective use of immunosuppressive agents. Immunosuppressive regimens can be highly complex and often involve a number of different drugs, and pharmacists are uniquely qualified to help both physicians and patients manage these multi-drug regimens. In addition to their expertise in drug selection and interaction, pharmacists also have a detailed understanding of drug pharmacokinetics, which is essential for safe and effective use of organ- and patient-specific immunosuppressive agents. Drug selection often is tailored to the particular tissue being transplanted, since some of the agents can be toxic to transplant organs such as the kidneys, liver, or heart. The risk of rejection also varies from organ to organ and thus influences the level of immunosuppression required. Patient-specific factors like overall health, the presence of comorbidities, and the risk of infection also are factors that govern immunosuppressive drug choices. Pharmacists’ involvement can be vital from the standpoint of predicting and recognizing potential drug interactions and adverse reactions. Transplant recipients who received clinical pharmacy services in addition to traditional patient-care services complied better with immunosuppressant drug regimens than patients who received only traditional patient-care services.2
This manuscript presents an integrated approach to the teaching of immunosuppression pharmacology. The multifaceted nature of immunosuppression provides extensive opportunities for curriculum integration with material from biochemistry, pathophysiology, immunology, pharmacology, medicinal chemistry, pharmacokinetics, pharmaceutics, and infectious disease. Emphasis was placed on active learning and the integration of subject matter from pharmacology, medicinal chemistry, and therapeutics. Student involvement and critical thinking were stimulated in both lecture and recitation sessions through the use of the various pedagogical approaches described below. Essential components in the effective presentation of this topic included good communication among basic science and clinical course coordinators, as well as extensive assessment before, during, and after the course.
DESIGN
General educational outcomes and specific learning objectives for the content taught about pharmacology of immunosuppression are listed in Table 1. In order for pharmacy students to understand and apply fundamental concepts related to the pharmacotherapy of immunosuppression, they must have a fundamental knowledge of immune function and the various substances involved in the immune response. Much of this knowledge is presented in the curriculum at South University during quarters 1 through 4 in pathophysiology, biochemistry, and immunology courses (Table 2). In pathophysiology and immunology, students receive a detailed presentation of the immune system, which includes discussions about nonspecific immunity, cell-mediated immunity, and humoral immunity. Mechanisms of immune cell, antibody, and complement function are emphasized with respect to their normal function. Various hypersensitivity reactions are also discussed along with autoimmunity and autoimmune disease. In the pathophysiology courses (quarters 1 and 2), 5 to 7 classroom hours are spent on topics related to the immune system, with another 3 to 5 hours dedicated to this topic in the microbiology/immunology course (quarter 3). In the biochemistry sequence (quarters 1 through 3), students learn about the structure of various antibodies and major histocompatibility complex (MHC) proteins. The molecular mechanism of immune cell and antibody interactions with antigen and MHC is also covered in detail. Three to 4 classroom hours are dedicated to immune system topics within this sequence. The rational for placing these topics within the first year of the curriculum is to provide students with a solid foundation in all aspects related to immune system function. Once students understand the basic components of the human immune system, they are able to more readily grasp the basis of autoimmune diseases and understand the mechanism of action of the various immunosuppressive agents. The pharmacokinetic/pharmaceutics sequence also begins in quarter 2 and provides students with the knowledge they will need to manage the often complex pharmacotherapeutic regimens of patients requiring immuosuppression. The pharmacology, medicinal chemistry, and therapeutics of drugs used for immunosuppression are presented in the integrated sequence modules. These organ-system based modules begin in the first year of the curriculum (quarter 2) and provide students with a highly integrated, sequential presentation of medicinal chemistry, pharmacology, and therapeutics in the areas of inflammation, infectious disease, gastroenterology, nephrology, cardiology, autonomics, central nervous system, endocrinology, oncology, and critical care. The pharmacotherapy of immunosuppression is addressed at a number of points in the integrated sequence modules, with topics such as inflammation, rheumatoid arthritis, inflammatory bowel disease, liver transplant, kidney transplant, and critical care. Each of the integrated sequence modules includes a weekly 3-hour recitation session that focuses on active small-group learning and case studies. These recitations give students an opportunity to apply the fundamental knowledge they learned in lectures to integrated and complex case studies. All integrated sequence modules have 2 course coordinators: 1 from the basic sciences who oversees the medicinal chemistry and pharmacology content and scheduling, and 1 from the pharmacy practice side who oversees the therapeutics content and scheduling. Both coordinators meet prior to the start of the module to agree on the content and drugs to be covered that quarter. Coordinators also meet at the conclusion of the module to evaluate its success and make any necessary revisions to the content or time allotted to each area prior to the next iteration of that module.
Table 1.
General Educational Outcomes and Specific Learning Objectives for the Pharmacology of Immunosuppression
Table 2.
Immunology and Immunosuppression Content in the Doctor of Pharmacy Curriculum at the South University School of Pharmacy
Prevention and management of infectious diseases is an important component of immunosuppressive therapy.3 The students receive infectious disease training relatively early in the curriculum (5-quarter-hours credit in quarter 4), which is advantageous to applying their knowledge of infectious disease in subsequent quarters when transplant pharmacology and pharmacotherapy are discussed. From the teacher's standpoint, the incorporation of infectious disease topics into the teaching of immunosuppression provides another important opportunity for curriculum reinforcement and integration. In addition to integrating subject matter from various other courses into the teaching of immunosuppression, the topic also lends itself to a discussion of pharmacogenomics since the toxicity and metabolism of some important drugs (ie, azathioprine and tacrolimus) can be affected by a number of genetic polymorphisms.4
Pedagogy
In the lectures related to immunology, pathophysiology, and pharmacology, classroom activities were incorporated that targeted various levels of student learning (Table 3). These activities were used in both pathophysiology and integrated sequence lectures. They included mind-mapping exercises, crosswords, and comprehensive interactive reviews, as well as numerous case studies and case vignettes. Mind-mapping exercises were used to enhance student integration of the material and to provide a visual summary of how various topics interrelate. Mind maps were often used after a block of material in a lecture was completed. The map was outlined on the whiteboard at the front of the room and students filled in the key concept points in the map and detailed the connections between them. Crossword puzzles were also used to teach and review pertinent terminology. The definitions of the terms were supplied as the “across” or “down” clues and the students would enter the answers, which were the corresponding terms, in the boxes. Programs are available free online to construct puzzles that lend themselves nicely to review and comprehension check exercises. A free online program, Puzzlemaker (Discovery.com), was used to construct the puzzles. Short review questions were embedded in the lecture as a way of quickly checking class comprehension of material just covered and emphasizing key points in the lecture. Jeopardy-style review exercises were added to lectures as a fun and interactive way of reviewing important material.
Table 3.
Classroom Activities and Critical Thinking
Examinations generally consisted of multiple-choice questions and integrated case questions that drew from immunology, biochemistry, pathophysiology, and pharmacology. Examinations in the integrated sequence included a mix of questions submitted by faculty members teaching pharmacology, medicinal chemistry, and therapeutics. A conscious effort was made to assemble the examinations several days before the test date to allow faculty members and coordinators to review all of the questions. This pre-examination review proved to be effective in enhancing overall examination quality. In addition to providing a thorough proof read, it allowed for the removal of redundant questions and helped ensure complete coverage of all examination topics.
Students in the integrated sequence modules were also required to read pertinent journal articles from current scientific and clinical literature to supplement lecture material and enrich presentation of the topic. Case studies and case vignettes also were used extensively throughout the curriculum. Brief case vignettes were often embedded in lectures which generally took no more than 5 minutes to complete. These more complex cases were completed by students in small groups during the weekly 3-hour recitations session included with each integrated sequence course. The following is an excerpt of the pharmacology of immunosuppression content taught in the integrated sequence modules. The drugs presented include “classic” immunosuppressive agents as well as newer agents and antibodies. The actual amount of lecture time spent on each agent related to their clinical relevance and also to input from clinical faculty members. Detailed case studies related to immunosuppression were also an effective means of stimulating student application, analysis, and critical thinking.
Pharmacology of Immunosuppression Lecture Content
Immunosuppression for Organ Transplantation.
Drugs that suppress the human immune response are currently used therapeutically both to prevent rejection of transplant organs (alloimmunity) and to treat autoimmune diseases (autoimmunity). Solid organ transplantation most commonly involves the heart, kidney, liver, and lungs.5 The main goals of transplant immunosuppression are: (1) to prevent rejection of the transplant organ; (2) to minimize drug toxicity and side effects; and (3) to minimize the risk of infections. Ideally, these 3 goals would be achieved using the fewest number of drugs and the lowest possible doses that would be effective for both patient and graft survival.
Rejection of transplant tissues can occur in 3 phases: hyperacute, acute, and chronic. Hyperacute rejection involves an immediate (within minutes) response of the recipient's immune system against the transplant tissueand is due mainly to actions of preformed antibodies against the donor HLA (human leukocyte antigen) or ABO (blood type) antigen.6 Destruction of the transplant tissue can be rapid and extensive. Careful matching of donor tissues to the recipient can prevent this type of rejection. Acute rejection is most likely to occur within the first 1 to 3 months posttransplant. Host T-cells are primarily responsible for acute rejection.7 Once activated by foreign antigens on the donor tissue, cytotoxic T-cells infiltrate the donor organ and begin to destroy it by releasing cytotoxic enzymes and proteins (eg, perforins). This form of acute rejection often responds well to treatments that suppress T-cell activity. Although less common, humoral-mediated rejection may also play a role in the acute rejection as host B-cells sensitize to the donor tissue and begin producing antibodies against it. Often the antibody response in acute rejection is directed toward endothelial cells in the host tissue and results in vascular damage. Treatment for this form of acute rejection is not as well defined.
Chronic rejection usually manifests as a slow-developing, long-term process that gradually compromises donor organ integrity and function. Both cell-mediated and humoral processes appear to be involved.8 A key feature of chronic rejection is chronic inflammation of the donor tissue. Activated T-cells release cytokines which in turn recruit and activate macrophages. When these macrophages infiltrate the donor tissue, they attack it by releasing cytolytic enzymes and cytotoxic substances which inflame and injure the transplant tissues. Chronic production of antibodies by activated B-cells and subsequent activation of complement proteins may also contribute to the process of chronic rejection. There are no effective pharmacologic therapies to counter chronic rejection and often the only recourse is a re-transplant.
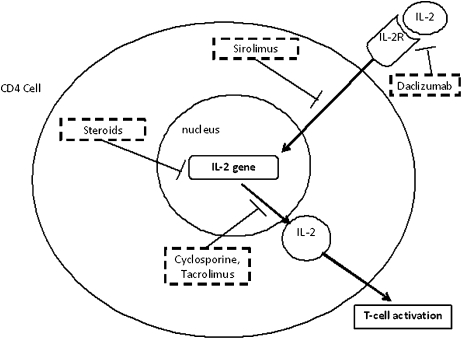
One of the major goals of immunosuppression in transplant patients is to prevent acute rejection in the days immediately following the transplant. To accomplish this, induction therapy is initiated during the actual transplantation and generally lasts 7-10 days posttransplant. Induction therapy usually involves administration of highly potent immunosuppressive antibodies that prevent T-cell activation. Two such agents, daclizumab and basiliximab, are antibodies against the T-cell CD25 (CD = cluster of differentiation) receptor (Table 4). This T-cell receptor is activated with high affinity by interleukin-2 (IL-2) (Figure 1). Since CD25 is only expressed in activated T-cells, these agents are highly specific for T-cells that have already been activated by major histocompatibility complex (MHC) exposure. Daclizumab is a “humanized” antibody that contains 90% human components and is thought to be less antigenic than basiliximab, which is 75% human in origin.
Table 4.
Immunosuppressive Drugs Used for Solid Organ Transplant
aAll agents listed can increase a patients risk for infections.
Figure 1.
Immunosuppressive drugs affecting IL-2.
Two polyclonal antithymocyte globulins are also available for both induction therapy and the treatment of acute rejection. One antibody, Atgam, is equine-derived, while the second (thymoglobulin) is rabbit-derived. Both bind to a wide range of lymphocyte CD receptors. Once bound, both antithymocyte globulins trigger complement-mediated lysis of T-cells with their subsequent depletion. While both agents are highly potent immunosuppressants, their broad mechanism of effects can predispose transplant patients to a wide range of infections. Binding of these globulins can also trigger the release of cytokines from T-cells. The resulting “cytokine release syndrome” can cause significant headache, fever, chills, and nausea in patients.
A third form of immunosuppressive antibody is the murine-derived monoclonal murononmab (OKT3). This globulin is directed against the T-cell CD3 cell surface receptor, a receptor that is involved in activation of T-cells. Since OKT3 is murine in origin, patients can produce antibodies against the mouse-specific portions of the molecule. Patients should be tested for the development of such antibodies since they can interfere with efficacy of the drug.
After the initial phase of induction therapy, transplant patients are shifted to a life-long maintenance regimen. The main goal of this phase of immunosuppression is to prevent further episodes of acute rejection to ensure long-term survival of both the donor organ and recipient. Drug and dose selection must be carefully weighed in order to maximize therapeutic benefit while minimizing the risk of toxicity. Three main classes of drugs are currently used for maintenance therapy: antimetabolites; lymphocyte signaling inhibitors; and corticosteroids.
Antimetabolite immunosuppressants include older agents such as azathioprine and methotrexate, as well as the newer agents, mycophenylate mofetil and leflunomide. All of these drugs interfere with key metabolic pathways in various immune cells, which in turn can inhibit their proliferation and potentially induce apoptosis.9 Azathioprine was the first such agent used for immuosuppression related to organ transplants. It is a prodrug of mercaptopurine, a drug that interferes with purine nucleic acid metabolism and thus lymphoid cell replication. One major drawback to the use of older agents, such as azathioprine, is their lack of specificity and potential for inhibiting replication in other highly proliferative tissues such as bone marrow and gut (Table 4). Significant increases in blood levels of azathioprine are observed if used in conjunction with allopurinol.10
The newer antimetabolites, mycophenolic acid (MPA) and mycophenolate mofetil (MMF), have gradually replaced azathioprine since their mechanism of action makes them more specific inhibitors of T-cells.9 Both MPA and MMF inhibit the enzyme inosine monophosphate dehydrogenase (IMPDH), which is essential in the formation of guanosine. The result is both cytostatic and apoptotic. Lymphocytes are highly susceptible to blockade of this enzyme, since they synthesize purines de novo. In addition, both agents preferentially inhibit the type II isoform of IMPDH, which is found primarily in lymphocytes. This high degree of specificity significantly reduces the toxic effect of in other tissues. MMF is administered as a prodrug with high oral bioavailability; it is hydrolyzed to MPA.
A second mechanism by which immunosuppressive agents act is by inhibiting the signaling of cytokines which are involved in activation of lymphocytes (Figure 1).11,12 Cyclosporine has been used clinically for several decades and was the first agent successfully used in heart transplantation. Cyclosporine inhibits the release of interleukin-2 (IL-2) from activated T-cells. IL-2 is essential for the activation and proliferation of other T-cells. At the cellular level, activation of T-cells involves a cytoplasmic phosphatase called calcineurin and Ca2+-calmodulin. In activated T-cells, cytoplasmic calcineurin and Ca2+-calmodulin form a complex which activates (by dephosphorylation) the cytoplasmic transcription factor NFAT (nuclear factor of activated T-cells) and enhances transcription of IL-2. Cyclosporine enters the cytoplasm of T-cells and binds to a protein called cyclophilin. The cyclosporine-cyclophilin complex now interacts with calcineurin to prevent its activity and thus subsequent activation of IL-2 transcription. Although cyclosporine is approved for use in both organ transplantation and rheumatoid arthritis, it exhibits numerous toxicities including nephrotoxicity which can be irreversible with chronic exposure (Table 4).
Tacrolimus is a second immunosuppressive agent that exerts its effects via inhibition of calcineurin and IL-2 release. It is a macrolide antibiotic derived from bacteria. In the cytoplasm, tacrolimus inhibits calcineurin through interaction with the protein FKBP (Figure 1). The potency of tacrolimus is significantly greater than that of cyclosporine. While tacrolimus exhibits similar tissues toxicities to cyclosporine (ie, renal, liver) the profile of other side effects differs significantly. The metabolism of both cyclosporine and tacrolimus may be affected by genetic polymorphisms in CYP3A4 & 5 (CYP=cytochrome P450), as well as by variations in the intestinal p-glycoprotein drug transporter. Genotyping of patients for CYP3A and ABCB1 (the gene that codes for p-glycoprotein) may one day be used to further enhance individualization of therapy with these agents.4
A third agent that can also affect signaling of T-cells via IL-2 is sirolimus (also known as rapamycin). The mechanism of sirolimus is similar to that of tacrolimus in that it binds to cytosolic FKBP, however, it does not cause blockade of calcineurin.13 Rather, the sirolimus-FKBP complex blocks a cytosolic protein called mTOR (molecular target of rapamycin). The mTOR protein is a phosphorylase that is activated by IL-2 and which regulates several proteins involved in T-cell proliferation. The administration of sirolimus does not block synthesis of IL-2 but rather blocks T-cell activation by IL-2. Sirolimus is also a potent inhibitor of B-cell proliferation and antibody production. However, unlike cyclosporine and tacrolimus, sirolimus is not nephrotoxic. Sirolimus does, however, carry a black box warning for liver and lung transplants. A longer half-life analog, Everolimus, is approved for use in Europe but is still under study in the United States. The antiproliferative effects of sirolimus have also been used in drug-eluting coronary stents to inhibit overgrowth of vascular smooth muscle and coronary re-occlusion.
The antiinflammatory and immune-modulating effects of the glucocorticoids have been known for many years. Both endogenous and synthetic glucocorticoids exert their cellular effects via glucocorticoid receptors (GR). Many of the classic metabolic effects (and unwanted side-effects) of the glucocorticoids are mediated by transcriptional activation, while their antiinflammatory actions appear to be more dependent upon the repression of gene transcription. This finding suggests that one day glucocorticoid derivatives might be synthesized that are more selective for their antiinflammatory actions. Several additional mechanisms of action have been proposed for the glucocorticoids including interaction with other transcription factors such as nuclear factor-κB (NF-κB) and modulation of various second messenger signaling pathways.14
Glucocorticoids are first-line immunosuppressive agents for both solid-organ and hematopoietic transplants. The actions of glucocorticoids on inflammation and immune response are numerous. Glucocorticoid administration decreases the number of circulating lymphocytes, monocytes, eosinophils, and basophils. The release of various inflammatory cytokines from macrophages and monocytes is also markedly reduced by glucocorticoid administration. Glucocorticoids can also inhibit the synthesis of prostaglandins through several mechanisms including inhibition of cyclooxygenase 2 transcription and activation of proteins (lipocortin-1 and MAPK phosphatase), which inhibit cytosolic phospholipase A2.
The potent clinical antiinflammatory and immune-suppressing effects of the glucocorticoids must be carefully weighed against their potentially significant adverse metabolic and endocrine effects (Table 5). The adverse effects observed with glucocorticoid use are both time- and dose-dependent.
Table 5.
Immunosuppressive Drugs Used to Treat Autoimmune Disease
While individual drugs may have specific toxicities to consider, all patients receiving immunosuppressive for organ transplantation are at increased risk for infections.3 These infections can be caused by opportunist organisms such as pneumocystis, cytomegalovirus, and various fungi, which are often difficult to treat. Antimicrobials are used universally in transplant patients for both prophylaxis and treatment of infectious organisms.
Immunosuppression for Autoimmune Disease
Another application of immunosuppressive drugs is in the treatment of autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease (Table 5). Autoimmunity is defined as a condition in which the patient's own immune system begins to attack and destroy tissues that are native to the body. Autoimmunity appears to develop when immune surveillance fails and the patient's own cells are mistaken for foreign cells.15 While the mechanism by which autoimmunity develops is uncertain, it appears more frequently with expression of certain MHC subtypes. Autoimmunity can involve the production of autoantibodies from B-cells against specific tissues (eg, the glomerular basement membrane in Goodpasture's disease) or the activation of cytotoxic T-cells against a specific tissue (eg, pancreatic beta cells in type I diabetes). Unlike the treatment of transplant rejection, which is often highly specific, pharmacotherapy for autoimmunity often involves broad immunosuppression.
In addition to glucocorticoids, other important agents for the treatment of autoimmune diseases include antiproliferatives such as methotrexate (an inhibitor of T-cell folate metabolism) and leflunomide (an inhibitor of T & B-cell purine synthesis). In addition to its effect on immune cells, methotrexate also exerts significant antiinflammatory actions. Both agents are approved for treatment of rheumatoid arthritis and both are considered disease modifiers since they not only improve symptoms but also slow progression of the disease.
Several anticytokine antibodies are also used clinically for treatment of rheumatoid arthritis and inflammatory bowel disease. One cytokine in particular, tumor necrosis factor-alpha (TNF-α), appears to be an important factor in the activation of immune cells (macrophages and activated T-cells) and their subsequent inflammatory response. Three anti-TNF-α drugs are currently available: adalimumab (a fully humanized anti- TNF-α antibody); infliximab (partially humanized TNF-α antibody); and etanercept (a TNF-α receptor dimer). While treatment with these agents appears to reduce symptoms of the disease, they do not appear to modify the eventual course of the disease. Since they are protein drugs they must be administered parenterally.
EVALUATION AND ASSESSMENT
Educational studies have suggested that most students have a 15-20 minute attention span, with this in mind various exercises were interspersed into lectures at 20-30 minute intervals. Based on the authors’ impressions of the student response in class and from numerous student comments on course evaluations, these exercises have a very positive impact on the lectures.
Assessment of student learning related to immunosuppression was made using a number of instruments including in-class tests, unannounced quizzes, formal case study submissions, and a comprehensive final examination. Examinations contained multiplechoice questions, modified K-type questions, case-based questions, matching diagrams, and table fill-ins. Examinations were designed to contain a balance of questions that addressed all levels of Bloom's taxonomy.16,17 Average examination grades were in the upper 70% to lower 80% range and tended to improve somewhat over the course of the quarter. Final examinations were cumulative in nature and designed to assess the overall comprehension of students to course material. Students tended to score well on questions related to basic immunology and pathophysiology (82% average on the most recent pathophysiology examination related to the immune system). The class average for written case recitations on related material in the integrated sequence modules was generally above 80%. Students in required courses who scored below 75% on an examination were required to attend a 3-hour examination remediation session in which they worked independently to correct wrong answers on their examinations and provide a brief written explanation as to why their original answer was incorrect. Directed classroom discussions and lecture-embedded review questions were also used to acutely assess student comprehension and areas of potential weakness. Student evaluations at the end of the course were generally positive. Students’ average course evaluation ratings for pathophysiology was 4.8 out of 5 and 4.5 out of 5.0 for the integrated sequence module in which the pharmacology of immunosuppression was mainly taught. Student response rates for pathophysiology ranged from 80.8% - 90.7%, while those for the relevant integrated sequence modules were from 52.0% - 73.6%. Response rates for course evaluations at SUSOP were high for the first-year class (ie, pathophysiology), but dropped off somewhat in years 2 and 3 of the didactic curriculum (ie, later integrated sequence modules). Numerous students commented on the usefulness of case studies and in-class exercises on their course evaluations.
DISCUSSION
Although significant strides have been made in immunosuppressive therapy, many challenges remain regarding reducing drug toxicity, enhancing target specificity, and improving overall outcomes. Current pharmacologic research is focused on identifying new and more specific targets for immunosuppression, as well as improving the pharmacokinetic parameters of the agents that are currently used. The latter may be facilitated by the application of pharmacogenomics and pharmacogenetic testing.18
The pharmacology and therapeutics of immunosuppression is an interesting yet challenging topic to teach. Students need to be competent and clear in their understanding of the human immune response before they can truly comprehend the mechanisms of immunosuppressive drug actions and toxicity. A concise review of the function of the various immune cells and their mechanism of activation was helpful prior to a discussion of the actual drugs. Previous pathophysiology and immunology lectures were posted on student forums or E-Companion for students to review on their own prior to beginning the pharmacology of immunosuppression. Faculty members teaching this topic likewise need to be confident in their understanding of the human immune response. Active review of the most current literature and clinical studies in this area are essential not only for clinical faculty members but for those teaching in the basic sciences as well. Material for lecture presentation was drawn from several sources including pharmacology texts19,20 and therapeutics texts,21,22 as well as from numerous clinical and basic science review articles were particularly useful. Students’ scores on questions related to basic immunology and pathophysiology indicated a solid comprehension of the fundamental concepts needed to understand the pharmacotherapy of immunosuppression.
Logistical issues must be considered by both course coordinators and faculty members when presenting a topic this complex and broad. One of those issues is ensuring complete and consistent coverage of topics since aspects of immunosuppression are taught at several different points in the curriculum with regards to various diseases and treatments. A second issue is coordinating coverage of immunosuppressive drugs from the standpoints of pharmacology, medicinal chemistry, and therapeutics in order to minimize redundancy and provide information that is complementary. Detailed discussions of course content among clinical and basic science faculty members can greatly facilitate the effectiveness with which an integrated course is delivered. Each of the integrated sequence modules at South University has both a clinical and basic sciences coordinator. Faculty members worked together closely to ensure thorough coverage and integration of the pharmacology, medicinal chemistry, and therapeutics content in each area. Faculty members from various disciplines often sat in on related lectures given by faulty members from other disciplines. For example, pharmaceutical science faculty members teaching in the integrated sequence modules were required to attend all of the other lectures given by other faculty members in the particular integrated sequence in which they taught. This strategy broadened the author's perspectives on the topics taught, and provided valuable clinical insights that significantly enhanced the quality of the author's pharmacology presentations.
Despite its innate complexity, the teaching of immunosuppression pharmacology can also be highly rewarding. The integrative nature of the topics allows instructors to draw on material from biochemistry, molecular biology, pathophysiology, and immunology. Mechanisms of drug action are complex and require discussion of multiple cellular signaling pathways as well as gene transcription. The medicinal chemistry of many of these agents is likewise interesting and complex. The number of agents available for clinical use had grown significantly in recent years and now includes a number of monoclonal and polyclonal antibodies. By their nature, antibody-based drugs have unique pharmacologic actions and properties which make them interesting topics of discussion. Immunosuppression therapy for transplant patients is often highly specific with regards to both the organ being transplanted and the profile of the patient, and this can make for an interesting contrast with immunosuppressive drugs used for treatment of autoimmune diseases, which generally tend to be less specific in their actions. Likewise, mechanisms of drug toxicity can often be related directly back to the specificity of the agents and their mechanism of action.
When first faced with the prospect of teaching the pharmacology of immunosuppression, one might be overwhelmed with the scope and detail of the topic. The author personally struggled with the urge to go back and do comprehensive reviews of immunology and biochemistry, but quickly realized that a brief review of key elements such as T- and B-cells, antibodies, and cytokines was all that time allowed. Another difficulty faced when first teaching this topic was clarifying the role that each of various immunosuppressive agents played in the overall therapeutic scheme. For example, which drugs were most suited for induction therapy? Which agents were currently used as mainstays for long-term maintenance therapy? Which drugs were first-line agents for treating acute episodes of rejection? To resolve these uncertainties before teaching this topic, a great deal of time was spent reading both current pharmacology and therapeutics chapters on the subjects as well as reviewing pertinent clinical literature. The author was also fortunate enough to sit through a number of therapeutics lectures given by clinical faculty members from various institutions that specialized in transplant pharmacy. Making a clear distinction between immunosuppression used for the treatment of autoimmune diseases versus immunosuppression for organ transplantation was also quite helpful. A solid understanding of the topic from all aspects gave the author the confidence needed to go into the classroom and tackle this topic with students. When teaching, feedback was sought from students through multiple mechanisms, including short quizzes, review questions, case vignettes, classroom discussion, and course evaluations to ensure that delivery of the material was clear and effective. Based on student feedback, there were several changes made to improve delivery of the material. One of these changes included posting of the previous pathophysiology notes related to the immune system on the current course forum so students would have access to detailed notes and lectures regarding normal immune system function. A second revision focused on the sequence in which the material regarding the pharmacology of immunosuppression was presented. Initially, presentation of immunosuppressive agents was based mainly on their historical order of clinical implementation. However, student comprehension appeared to significantly improve when drugs were presented according to their specific target(s) of action, ie, calcineurin inhibitors, antimetabolites, etc. Third, the amount of time spent presenting and reviewing actual drug mechanisms of action was increased since this proved to be an area in which students had initial difficulty based on their examination performance on related questions. A number of excellent diagrams regarding drug mechanisms of action were also incorporated into the lectures. With continued self-assessment and fine-tuning, the author can honestly say that the pharmacology of immunosuppression has become one of his favorite (but not easiest!) topics to teach.
SUMMARY
A comprehensive, integrated presentation on the pharmacology of immuosuppression was presented in integrated sequence modules that included content from pharmacology, medicinal chemistry, and therapeutics. Grades on comprehensive examinations and in-class quizzes indicated the students had achieved a thorough understanding of all aspects relating to immunosuppressive therapy.
REFERENCES
- 1.Allison AC. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacol. 2000;47(5):63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 2.Chisholm MA, Mulloy LL, Jagadeesan M, DiPiro JT. Impact of clinical pharmacy services on renal transplant patients’ compliance with immunosuppressive medications. Clin Transplant. 2001;15(2):330–336. doi: 10.1034/j.1399-0012.2001.150505.x. [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338(24):1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 4.Hesselink DA, vanGelder T, van Schalk RHN. The pharmacogenetics of calcineurin inhibitors: one step closer toward individualized immunosuppression. Pharmacogenomics. 2005;6(4):323–337. doi: 10.1517/14622416.6.4.323. [DOI] [PubMed] [Google Scholar]
- 5. United Network for Organ Sharing. http://www.unos.org. Accessed October 21, 2009.
- 6.Rifle G, Mousson C, Laurent M, Guignier F, Kais H. Donor-specific antibodies in allograft rejection: clinical and experimental data. Transplantation. 2005;79(3):S14–S18. doi: 10.1097/01.tp.0000153292.49621.60. [DOI] [PubMed] [Google Scholar]
- 7.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3(5):525–533. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14(4):387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 9.Allison AC, Eugui E. Mechanisms of action of mycophenylate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80(2S):S181–S190. doi: 10.1097/01.tp.0000186390.10150.66. [DOI] [PubMed] [Google Scholar]
- 10.Brooks RJ, Dorr RT, Durie BG. Interaction of allopurinol with 6-mercaptopurine and azathioprine. Biomed Pharmacother. 1982;36(4):217–222. [PubMed] [Google Scholar]
- 11.Marshall SE, Welsh KI. The role of cytokine polymorphisms in rejection after solid organ transplantation. Genes Immun. 2001;2(6):297–303. doi: 10.1038/sj.gene.6363795. [DOI] [PubMed] [Google Scholar]
- 12.Kapturczak MH, Meier-Kriesche HU, Kaplan B. Pharmacology of calcineurin antagonists. Transplant Proc. 2004;36(2):25–31. doi: 10.1016/j.transproceed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal SN. Sirolimus: its discovery, biological properties and mechanism of action. Transplant Proc. 2003;35(3):7–14. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 14.Rhen T, Cidlowski JA. Antiinflammatory actions of glucocorticoids – new mechanism for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 15.Davidson A, Diamond B. Autoimmunity. N Engl J Med. 2001;345(5):340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 16.Bloom BS. New York: David McKay Co, Inc; 1956. Taxonomy of Educational Objectives. Handbook 1: Cognitive Domain. [Google Scholar]
- 17.Wongwiwatthananukit S, Popovich NG, Bennett DE. Assessing pharmacy student knowledge on multiple-choice examinations using partial-credit scoring of combined-response multiple-choice items. Am J Pharm Educ. 2000;64(2):1–10. [Google Scholar]
- 18.Regazzi MB, Alessiani M, Rinaldi M. New Strategies in Immunosuppression. Transplant Proc. 2005;37(6):2675–2678. doi: 10.1016/j.transproceed.2005.06.104. [DOI] [PubMed] [Google Scholar]
- 19.Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy.of Drug Therapy. 2nd Edition. Philadelphia, Penn: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. p. 795. [Google Scholar]
- 20.Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 11th ed. New York: McGraw Hill; 2009. p. 963. [Google Scholar]
- 21.Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York: McGraw Hill; 2009. p. 1459. [Google Scholar]
- 22.Koda-Kimble MA, Kradjan WA, Guglielmo B, Young LL. Applied Therapeutics: The Clinical Use of Drugs. 7th Edition. Philadelphia, Penn: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. p. 33. [Google Scholar]