Abstract
In this article, the role of hypothermia and neuroprotection for neonatal encephalopathy will be discussed. The incidence of encephalopathy due to hypoxia ischemia as well as the pathophysiology will be presented. The diagnosis of encephalopathy in full-term neonates will be discussed. The current management of brain injury that occurs with hypoxia ischemia and the role of hypothermia in preventing brain injury in fetal and neonatal animal models will be reviewed. The current data from randomized control trials of hypothermia as neuroprotection for full-term infants will be presented along with the results of meta-analyses of these trials. Lastly, the status of ongoing neonatal hypothermia trials will be summarized.
Key words: encephalopathy, hypothermia, hypoxia-ischemia, neonate
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) occurs in one to six per 1000 live full-term births. Of affected newborns, 15–20% of affected newborns will die in the postnatal period, and an additional 25% will sustain childhood disabilities (Gunn et al., 2000; Vanucci and Perlman, 1997). The presence of an abnormal neurologic examination in the first few days of life is the single most useful indicator that a brain insult has occurred. Neonates with mild encephalopathy do not have an increased risk of motor or cognitive deficits. Neonates with severe encephalopathy have an increased risk of death and an increased risk of cerebral palsy (CP) and mental retardation amongst survivors (Roberston et al., 1989; Shankaran et al., 1991). Neonates with moderate encephalopathy have significant deficits, memory impairment, visual motor or visual perceptive dysfunction, increased hyperactivity, and delayed school readiness.
The essential criteria suggested as prerequisites to a diagnosis of hypoxic-ischemic insult resulting in moderate or severe encephalopathy in term newborn infants include the following: metabolic acidosis with a cord pH of <7 or a base deficit of ≥12 mmol/L; early onset of encephalopathy; multi-system organ dysfunction; and exclusion of other etiology such as trauma, coagulation disorders, metabolic disorders, and genetic causes (American College of Obstetricians and Gynecologist and American Academy of Pediatrics, 2003).
Pathophysiology of Neonatal Hypoxic-Ischemic Brain Injury
The pathophysiology of brain injury secondary to hypoxia-ischemia has been well studied. Hypoxia-ischemia is associated with two phases of pathologic events that culminate in brain injury. These phases are primary and secondary energy failure based on characteristics of the cerebral energy state used to describe the temporal sequence in newborn animals (Lorek et al., 1994). Primary energy failure is characterized by reductions in cerebral blood flow and O2/substrates (Lorek et al., 1994; Laptook et al., 1992). High-energy phosphorylated compounds such as ATP and phosphocreatine are reduced, and tissue acidosis is prominent. This phase is an essential prerequisite for all deleterious events that follow. Primary energy failure is associated with acute intracellular derangements such as loss of membrane ionic homeostasis, release/blocked reuptake of excitatory neurotransmitters, defective osmoregulation, and inhibition of protein synthesis (Johnston et al., 2001). Excessive stimulation of neurotransmitter receptors and loss of ionic homeostasis mediate an increase in intracellular calcium and osmotic dysregulation. Elevation in intracellular calcium triggers a number of destructive pathways by activating lipases, proteases, and endonucleases (Siesjo and Bengtsson, 1989).
Resolution of hypoxia-ischemia within a specific time interval reverses the fall in high-energy phosphorylated metabolites and intracellular pH, and promotes recycling of neurotransmitters. The duration of time for hypoxia-ischemia to be successfully reversed and promote recovery will be affected by maturation, preconditioning events, substrate availability, body temperature, and simultaneous disease processes. Although recovery of the cerebral energy state may occur following primary energy failure, a second interval of energy failure may occur at a time remote from the initiating event. Secondary energy failure differs from primary energy failure in that declines in phosphocreatine and ATP are not accompanied by brain acidosis (Lorek et al., 1994). The presence and severity of secondary energy failure depends on the extent of primary energy failure. The pathogenesis of secondary energy failure is not as well understood as primary energy failure, but likely involves multiple pathophysiologic processes, including accumulation of excitatory neurotransmitters, oxidative injury, apoptosis, inflammation, and altered growth factors and protein synthesis (Fellman and Raivio, 1997; Liu et al., 1999; Mehmet et al., 1994; Tan et al., 1996; Gluckman et al., 1998).
The interval between primary and secondary energy failure represents a latent phase that corresponds to a therapeutic window. Initiation of therapies during the latent phase in perinatal animals has been successful in reducing brain damage and substantiates the presence of a therapeutic window (Gunn et al., 1997, 1998, 1999). The duration of the therapeutic window is approximately 6 h in near-term fetal sheep based on the neuroprotection associated with brain cooling initiated at varying intervals following brain ischemia. The duration of the therapeutic window can be modified based on the hypothermic regimen: time of initiation of cooling, duration of therapy, and depth of cooling.
The precise mechanism of neuroprotection by mild hypothermia is still very unclear. Cooling suppresses many of the pathways leading to delayed cell death. Hypothermia also influences cell signaling cascades, which are key factors in the initiation of neuronal injury in the primary phase. Therefore, the therapeutic effects of hypothermia impact both primary injury and the latent phase of hypoxic-ischemic brain injury (Gunn and Thoresen, 2006).
Current Therapies for Neonatal Hypoxic-Ischemic Encephalopathy
The management of neonates with HIE has been limited to supportive intensive care. The latter includes correction of hemodynamic and pulmonary disturbances (hypotension, metabolic acidosis, and hypoventilation), correction of metabolic disturbances (glucose, calcium, magnesium, and electrolytes), treatment of seizures if present, and monitoring for other organ system dysfunction. This management approach does not target any component of the pathophysiological sequence leading to hypoxic-ischemic brain injury and is directed at avoiding injury from secondary events associated with hypoxic ischemia.
Diagnosis of Encephalopathy in Term Infants
A detailed history should be obtained regarding the pregnancy and intrapartum period as the first step in diagnosing encephalopathy. Any event likely to compromise blood or oxygen supply to the fetus should be examined. These events include a history of placental abruption, uterine rupture, amniotic fluid embolism, tight nuchal cord, cord prolapse/avulsion, maternal hemorrhage, trauma or cardio respiratory arrest, severe and sustained fetal bradycardia, and prolonged labor. The majority of infants with encephalopathy do not have an obvious cause for the encephalopathy. There is currently no clear diagnostic test for encephalopathy due to hypoxia-ischemia. A history of maternal elevation of temperature is crucial, as moderate elevation of temperature in the mother increases the risk of neonatal encephalopathy. A history of fetal tachycardia and maternal tachycardia may also raise suspicions of chorioamnionitis. Laboratory evaluations that should be performed include placenta pathology, to evaluate for the presence of placental infection. Elevated biomarkers (elevated cytokines) may improve the ability to predict outcome. All neonates should have a detailed neurologic examination to evaluate the presence of mild, moderate, or severe encephalopathy as per the Sarnat classification.
Cerebral Function Monitoring in the Neonatal Period
Currently, a bedside tool for cerebral function monitoring in term and near term infants is the amplitude-integrated EEG (aEEG). The aEEG records a single channel EEG from biparietal electrodes, and the signal is then filtered, rectified, smoothed, and amplitude-integrated. The aEEG interpretation is based primarily on pattern recognition, and the aEEG correlates well with conventional aEEG. Recent reports indicate that the aEEG predicts neurodevelopmental outcome in term infants with HIE (Spitzmiller et al., 2007). Coupled with an early neurologic examination, the aEEG correlates well with persistent encephalopathy. It has been suggested that aEEG should become part of the initial evaluation of near-term and term infants with HIE. However, caution is raised against using the aEEG for the detection and treatment of neonatal seizures, since the aEEG has not been proven to reliably detect subclinical seizures (Freeman, 2007).
Prevention of Brain injury in Term and Near-Term Infants
Until recently, there were no specific strategies for prevention of brain injury in term and near-term infants. Neuroprotection with brain-specific therapies has been well studied in the preclinical arena over the past 15 years, with the aim to block or dampen the cascade of events triggered by hypoxia and ischemia.
Brain hypothermia is a promising therapy for neuroprotection for encephalopathy presumably due to hypoxic ischemia. A relatively small reduction in brain temperature (1–6°C) of neonatal animals is associated with better maintenance of cerebral energy state during or immediately after ischemia and decrease in release of the excitatory neurotransmitters, caspase-3 activation, and evidence of apoptosis. Other neuroprotective effects of cerebral hypothermia include normalization of a decrease in protein synthesis, reduction in free radicals, and modulation of activation of microglia and cytokine production.
Hypothermia as Neuroprotection: Preclinical Studies
During reperfusion after a timed hypoxic-ischemic insult, there is a period of approximately 30–60 min during which cellular energy metabolism is restored, with progressive resolution of the acute cell swelling secondary to hypoxic depolarization. This is followed by a latent phase, during which oxidative metabolism has normalized (Thoresen, 1995), but there is hyperactivity of glutaminergic receptors, the intracytoplasmic components of the apoptotic pathway are activated, and secondary inflammatory reaction is initiated. This may be followed by secondary deterioration leading to delayed neuronal death after 3 days. Treatment with cerebral hypothermia needs to be initiated as early as clinically feasible in the latent phase before the onset of secondary deterioration and then continued for long-lasting neuroprotection. The therapeutic time window duration decreases with increased severity of cerebral hypoxia-ischemia under normothermia and delayed hypothermia in newborn piglets (Iwata, 2007).
Cooling to a Depth of 4–6°C versus Control in the Animal Model of Hypoxia-Ischemia
There is established evidence in fetal and neonatal models, and across species, that cooling by 4–6°C versus controls has been neuroprotective while being well tolerated in animal models (Bona et al., 1998; Busto et al., 1987; Carroll and Beek, 1992; Colbourne and Corbett, 1994; Gunn et al., 1997, 1998; O'Brien et al., 2006; Sirimanne et al., 1996; Thoresen et al., 1996, 2001; Tooley et al., 2002, 2003, 2005; Yager and Asselin, 1996). The duration of cooling in these studies varied from 3 to 72 h, and each study compared a specific depth of cooling to controls. The depth of cooling achieved in each of these studies was a rectal temperature of 28–33°C (Bona et al., 1998; Carroll and Beek, 1992; O'Brien et al., 2006; Thoresen et al., 2001). Scalp temperatures achieved in other studies were 21.3–23.9°C (Tooley et al., 2003). Extradural brain temperatures in studies by Gunn et al. (1998, 1999) are reported as low as 30°C. Actual brain temperature studies document temperatures of 30–32.2°C (Tooley et al., 2002), 31.1°C (Tooley et al., 2005), and 32°C (Colbourne and Corbett, 1994). None of these studies comparing a specific depth of hypothermia to controls report any adverse effects except one report of a piglet shivering during the cooling (Tooley et al., 2005).
Cooling to Temperatures (up to Depth of 4–6°C) versus Control in the Animal Model
The neuroprotective pattern of therapy with hypothermia is temperature specific. There is suggestive data that optimal neuroprotection appears to occur at different temperatures in the cortical and deep gray matter. Neuroprotection with hypothermia by 4–6°C has been documented by many modalities, including a decrease in brain energy utilization measured by magnet resonance spectroscopy (Laptook et al., 2005), reduction of infarct size (Taylor et al., 2002), decrease in neuronal cell loss (Gunn et al., 1998), retention of sensory motor function (Bona et al., 1998), preservation of hippocampal structure (Carroll and Beek, 1992; Colbourne and Corbett, 1994), and recovery of electroencephalographic activity (Gunn et al., 1998). Hypothermia initiated immediately post-reperfusion was protective, with progressively increased protection with increasing depth of temperature, noted in studies that compared different depths of hypothermia versus control. Covey and Oorschot (2007) noted hypothermia of 5°C administered post-insult for 6 h offered better neuroprotection for striatal neurons than 2°C. Iwata et al. (2005) have demonstrated that cooling at two different regimens (rectal temperatures of 35°C and 33°C, compared to normothermia of 38.5–39.0°C) for 48 h demonstrated progressive increase in neuronal viability in gray matter. Laptook et al. (1995) has demonstrated a linear relationship between brain energy utilization rate and brain temperature over the range of temperatures of 27.6–41°C, with a 1°C reduction in brain temperature leading to 5.3% reduction in brain energy utilization rate. Taylor et al. (2002) looked at infarct size with cooling to 33°C and 30°C compared to normothermia, and found smaller infarct size at both depths compared to normothermia. Williams et al. (1997) evaluated cerebral energy metabolism during hypoxia-ischemia, and demonstrated that when compared to controls, NMR metabolites were preserved at 31°C and 34°C. None of these studies comparing differing depths of temperature to controls documents adverse effects of hypothermia at theses temperatures. In addition, adjusting brain temperatures from 28°C and 41°C did not alter any systemic variable in the piglet model except for heart rate, which directly correlated with brain temperature (Laptook et al., 1995).
Hypothermia as Neuroprotection: Clinical Studies
To date, two large randomized controlled trails and one large pilot study have been completed evaluating hypothermia as neuroprotection for term and near term infants with HIE. The multicenter Cool Cap Study involved 243 infants with moderate or severe encephalopathy and an abnormal aEEG, who were either cooled to a temperature of 34–35°C for 72 h or treated with temperature maintenance in normothermia range with conventional care (Gluckman et al., 2005) The primary outcome of the study was death or disability at 18 months. Cooling was provided by selective head cooling with mild systemic cooling. Death or severe disability occurred in 66% of infants randomized to conventional care and 55% randomized to the cooled group, odds ratio (OR, 95% CI) of 06.1 (0.34–1.09), p = 0.10. The effect of head cooling for infants with the most severe aEEG changes was not protective; on the other hand, the effect of head cooling for infants with less severe aEEG changes (n = 172) was protective with OR 0.42 (0.22–0.80; p = 0.009).
The large randomized controlled pilot study preformed at seven centers with 65 infants involved moderate systemic whole body hypothermia to 33°C for 48 h compared to normothermia maintained at 37°C (Eicher et al., 2005). The safety report of this pilot study documented that infants in the hypothermia group had more significant bradycardia, longer dependence on pressor medications, higher prothrombin times, more seizures, and need for more plasma and platelets transfusions. At 12 months of age, death or severe motor scores occurred in 52% of hypothermia group compared to 84% of normothermia group (p = 0.02). In a sub-group analysis, out-born infants were more likely to die than inborn infants, with an OR of 10.7 (1.3–90.0).
The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network trial evaluated 102 infants randomized to hypothermia with whole body cooling to 33.5°C for 72 h compared to 106 control infants randomized to conventional care (Shankaran et al., 2005). The primary outcome was death or moderate/severe disability at 18 months of age. The infants in the hypothermia group had significantly lower heart rates than the infants in the control group throughout the 72-h intervention period. There was no significant difference in systolic or diastolic blood pressure between groups. The frequency of adverse events during study intervention was low: one infant in each group had arrhythmia, two infants in the hypothermia group had acidosis, three infants in the hypothermia group and two control group infants had bleeding, and four cooled infants had altered skin integrity. The primary outcome was noted in 44% of infants in the hypothermia group compared to 62% of infants in the control group, with a risk ratio of 0.72 (0.54–0.95). There was a trend for cooling to benefit infants in both moderate and severe encephalopathy groups.
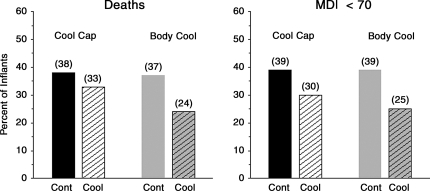
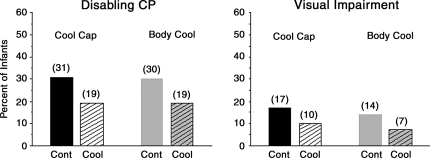
There are important differences between the Cool Cap and the Whole Body Hypothermia Trial. The two trials use different entry criteria distinguished primarily by the use of the aEEG in the Cool Cap trail. The mode of cooling used in each trial was different, and it is unknown if one cooling regimen is superior to the other. The primary outcomes were not defined in a similar manner in the two trials. Although not powered to evaluate moderate or severe encephalopathy separately, decrease in death and moderate to severe disability was seen in the whole body cooling trial in both moderate and severe encephalopathy (Table 1). As the primary outcome of each trial was the combined end point of death or disability, it is important to ascertain that the therapies did not salvage infants with severe disabilities who would have otherwise died in the absence of the intervention. As shown in Figures 1 and 2, the results provide assurance that this did not occur. As can be seen in the figures, the mortality rate and percent of infants with Mental Development Index (MDI) of <70 were reduced (Fig. 1). The rate of disabling CP was reduced from 31% in the control group to 18% in the Cool Cap Study, and from 30% in the control to 19% in the Whole Body Cooling Trial of the NICHD Network (Fig. 2).
Table 1.
Hypoxic-Ischemic Encephalopathy (HIE)
FIG. 1.
Comparison of the percent of infants who died and survivors with a Mental Development Index (MDI, Bayley scores) of <70 at 18–22 months in the CoolCap and Body Cooling trials. The number over each column represents the actual percent. Cont, control group; Cool, cooled group.
FIG. 2.
Comparison of the percent of infants with disabling cerebral palsy (CP) and visual impairment (blindness) at 18–22 months in the CoolCap and Body Cooling trials. Disabling CP is based upon a Gross Motor Performance Score of 3–5 in the Body Cooling Trial and a classification of neuromotor disability in the CoolCap Trial. The number over each column represents the actual percent. Cont, control group; Cool, cooled group.
Hyperthermia in Infants with Hypoxic-Ischemic Encephalopathy
The NICHD trial of whole body hypothermia demonstrated the occurrence of elevated core body temperature in the control group infants when temperatures were measured in a consistent manner in the 76 h of intervention and re-warming phase (Shankaran et al., 2005). Of the 102 infants randomized to the usual care group, 50 infants had a maximum esophageal temperature of ≥38°C. Higher core temperatures were associated with significant increases in risk of death or impairment in the control group (Laptook et al., 2008). In a secondary analysis of the Cool Cap trial, investigators also noted an association between elevated temperatures in the control group and increased risk of death or disability (Wyatt et al., 2007). Hyperthermia after brain injury adds to the risk of more severe neurologic damage, and studies in adults and pediatric subjects consistently support association between higher core temperatures and worse outcome (Dietrich and Bramlett, 2007; Bramlett and Dietrich, 2007). In the animal model, seizures associated with a hypoxic-ischemic insult result in aggravation of neuronal cell death, specifically within the hippocampus (Yager et al., 2004). The damage to the hippocampus occurs in the setting of spontaneously occurring hyperthermia of 1.5°C; rat pups in whom hyperthermia was prevented during seizures displayed significant reduction in brain damage compared to controls. In another study, neonatal rats subjected to hypoxic-ischemic injury were noted to have selective and long-lasting learning and memory impairments during behavioral tasks, and hypothermia to 27°C significantly reduced the attentional deficit in behavioral tasks, whereas hyperthermia aggravated the behavioral deficit and the brain injury (Mishima et al., 2004).
Four secondary analyses have been published from the NICHD RCT. In one study examining the relationship of elevated temperature after HIE, 22% of esophageal core temperatures measured among the control group infants were higher than 37.5°C. The odds of death or disability were increased 3.6–4- fold for each centigrade increase in the highest quartile of temperature in the control group. These results may reflect underlying brain injury and/or adverse effects of temperature on outcome (Laptook et al., 2008). Another study evaluating predictors of outcome has revealed that the classification and regression tree model, but not the scoring system developed from identified variables and odds ratios, was superior to the early neurologic examination in predicting death/disability in this study (Ambalavanan et al., 2006). A secondary analysis involving spot urine samples collected in 58 study participants revealed that a high urinary lactate/creatinine ratio was associated with death or disability (Oh et al., 2008). Lastly, detailed analysis of the randomized controlled trial data revealed safety of hypothermia during the intervention period and during follow-up to 18–22 months (Shankaran et al., 2008).
Status of Ongoing Hypothermia Trials
The European “neo.nEuro.network” (n.n.n.) Trial of whole body cooling to 33.5°C was closed to recruitment after 129 infants were enrolled as investigators felt that current evidence of benefits of hypothermia do not justify randomization of subjects; follow-up is ongoing. The U.K. Total Body Hypothermia (TOBY) Trial of total whole body cooling to 33.5°C for 72 h has completed enrollment of the planned 325 infants, and follow-up of infants is ongoing. Lastly, the Australian Infant Cooling Evaluation (ICE) Trial (33.5°C for 72 h) closed recruitment after 218 of a planned 300 infants due to lack of equipoise; follow-up is ongoing.
Meta-Analyses of Trials
Three independent systematic reviews recently published have concluded that therapeutic hypothermia (1) significantly reduces both death and disability after perinatal encephalopathy (Table 2); (2) is safe; (3) produces outcomes that are homogeneous both within and between trials (Jacob et al., 2007; Schulzke 2007; Shah et al., 2007).
Table 2.
Meta-Analysis of Hypothermia for Term Infants with Hypoxic-Ischemic Encephalopathy (HIE)
| Outcome | Relative risk (95% CI) |
|---|---|
| Death or moderate severe disability | |
| Shah et al., 2007 | 0.76 (0.65–0.88) |
| Schulzke et al., 2007 | 0.78 (0.66–0.92) |
| Jacob et al., 2007 | 0.76 (0.65–0.89) |
| Mortality | |
| Schulzke et al., 2007 | 0.75 (0.59–0.96) |
| Jacob et al., 2007 | 0.74 (0.58–0.94) |
| Moderate severe disability | |
| Schulzke et al., 2007 | 0.72 (0.53–0.98) |
| Jacob et al., 2007 | 0.68 (0.51–0.92) |
Need for Longer Follow-Up of Infants Receiving Hypothermia
The age of follow-up for infants enrolled in trials of neuroprotection therapy for HIE is a critical issue in evaluating efficacy of therapy. All the current published trials have evaluated hypothermia as a neuroprotective strategy with the primary outcome of death or disability at 18 months of age. This is the earliest age at which major disability can be ruled out with a high level of confidence. To assess effects beyond 18–22 months, it is necessary to evaluate the relationship of intervention to early childhood outcome, because hypothermia may influence not only major motor and cognitive deficits detected at 18 months but also more subtle effects of brain injury in childhood. These include behavior, learning, fine motor development, executive function, attention, and psychosocial outcome. Both the current trials (Cool Cap and NICHD Network Trial) are evaluating follow-up of the infants at early childhood.
References
- Ambalavanan N. Carlo W.A. Shankaran S. Bann C.M. Emrich S.L. Higgins R.D. Tyson J.E. O'Shea T.M. Laptook A.R. Ehrenkranz R.A. Donovan E.F. Walsh M.C. Goldberg R.N. Das A. the National Institute of Child Health Human Development (NICHD) Neonatal Research Network. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–2093. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologist and American Academy of Pediatrics. Neonatal encephalopathy and cerebral palsy. Defining the pathogenesis and pathophysiology. Library of Congress. 2003:1–93. [Google Scholar]
- Bona E. Hagberg H. Løberg E.M. Bågenholm R. Thoresen M. Protective effect of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr. Res. 1998;43:738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Progressive damage after brain and spinal cord injury; pathomechanisms and treatment strategies. Prog. Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Busto R. Dietrich W.D. Globus M.Y.T. Valdes I. Scheinberg P. Ginsberg M.D. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J. Cereb. Blood Flow Metab. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- Carroll M. Beek O. Protection against hippocampal CA cell loss by post-ischemic hypothermia is dependent of delay of initiation and duration. Metab. Brain Dis. 1992;7:45–50. doi: 10.1007/BF01000440. [DOI] [PubMed] [Google Scholar]
- Colbourne F. Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;656:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Covey M.V. Oorschot D.E. Effect of hypothermic post-treatment on hypoxic-ischemic striatal injury, and normal striatal development, in neonatal rats: a stereological study. Pediatr. Res. 2007;62:646–651. doi: 10.1203/PDR.0b013e318157d1fe. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Bramlett H.M. Hyperthermia and central nervous system injury. Prog. Brain Res. 2007;162:201–217. doi: 10.1016/S0079-6123(06)62011-6. [DOI] [PubMed] [Google Scholar]
- Eicher D.J. Wagner C.L. Katikaneni L.P. Hulsey T.C. Bass W.T. Kaufman D.A. Horgan M.J. Languani S. Bhatia J. Givelichian L.M. Sankaran K. Yager J.Y. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. J. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Fellman V. Raivio K.O. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr. Res. 1997;41:599–606. doi: 10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Freeman J.M. The use of amplitude-integrated electroencephalography: beware of its unintended consequences. Pediatrics. 2007;119:615–617. doi: 10.1542/peds.2006-3650. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D. Guan J. Williams C. Scheepens A. Zhang R. Bennet L. Gunn A. Asphyxial brain injury–the role of the IGF system. Mol. Cell Endocrinol. 1998;140:95–99. doi: 10.1016/s0303-7207(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D. Wyatt J. Azzopardi D.V. Ballard R. Edwards A.D. Ferriero D.M. Polin R.A. Robertson C.M. Thoresen M. Whitelaw A. Gunn A.J. on behalf of the Cool Cap Study Group. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomized trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Gunn A.J. Gunn T.R. de Haan H.H. Williams C.E. Gluckman P.D. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn A.J. Gunn T.R. Gunning M.I. Williams C.E. Gluckman P.D. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–1106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- Gunn A.J. Bennet L. Gunning M.I. Gluckman P.D. Gunn T.R. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr. Res. 1999;46:274–280. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Gunn A.J. Cerebral hypothermia for prevention of brain injury following perinatal asphyxia. Curr. Opin. Pediatr. 2000;12:111–115. doi: 10.1097/00008480-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Gunn A.J. Throresen M. Hypothermic neuroprotection. NeuroRx. 2006;3:154–169. doi: 10.1016/j.nurx.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata O. Thornton J.S. Sellwood M.W. Iwata S. Sakata Y. Noone M.A. O'Brien P.F. Bainbridge A. De Vita E. Ravich G. Peebles D. Scaravalli F. Cady E.B. Ordidge R. Wyatt J.S. Robertson N.J. Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann. Neurol. 2005;58:75–87. doi: 10.1002/ana.20528. [DOI] [PubMed] [Google Scholar]
- Iwata O. Iwata S. Thornton J.S. De Vita E. Bainbridge A. Herbert L. Scaravilli F. Peebles D. Wyatt J.S. Cady E.B. Robertson N.J. Therapeutic time window duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;1154:173–180. doi: 10.1016/j.brainres.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Jacob S. Hunt R. Tarnow-Mordi W. Inder T. Davis P. Cooling for newborns with hypoxic-ischemic encephalopathy. Cochrane Database Syst. Rev. 2007;4:1–46. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- Johnston M.V. Trescher W.H. Ishida A. Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr. Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Laptook A.R. Corbett R.J. Arencibia-Mireles O. Ruley J. Glucose-associated alterations in ischemic brain metabolism of neonatal piglets. Stroke. 1992;23:1504–1511. doi: 10.1161/01.str.23.10.1504. [DOI] [PubMed] [Google Scholar]
- Laptook A.R. Corbett R.J. Sterett R. Garcia D. Tollefsbol G. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using P and H magnetic resonance spectroscopy. Pediatr. Res. 1995;38:919. doi: 10.1203/00006450-199512000-00015. [DOI] [PubMed] [Google Scholar]
- Laptook A. Tyson J. Shankaran S. McDonald S. Ehrenkranz R. Fanaroff A. Donovan E. Goldberg R. O'Shea T.M. Higgins R.D. Poole W.K. National Institute of Child Health and Human Development Neonatal Research Network. Elevated temperature after hypoxic-ischemic encephalopathy: a risk factor for adverse outcome. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.H. Kwon D. Schielke G.P. Yang G.Y. Silverstein F.S. Barks J.D. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J. Cereb. Blood Flow Metab. 1999;19:1099–1108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- Lorek A. Takei Y. Cady E.B. Wyatt J.S. Penrice J. Edwards A.D. Peebles D. Wylezinska M. Owen-Reece H. Kirkbride V. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr. Res. 1994;36:699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Mehmet H. Yue X. Squier M.V. Lorek A. Cady E. Penrice J. Sarraf C. Wylezinska M. Kirkbride V. Cooper C. Increased apoptosis in the cingulate sulcus of newborn piglets following transient hypoxia-ischaemia is related to the degree of high energy phosphate depletion during the insult. Neurosci. Lett. 1994;181:121–125. doi: 10.1016/0304-3940(94)90574-6. [DOI] [PubMed] [Google Scholar]
- Mishima K. Ikeda T. Yoshikawa T. Aoo N. Egashira N. Xia Y.X. Ikenoue T. Iwasaki K. Fugiwara M. Effects of hypothermia and hyperthermia on attention and spatial learning deficits following neonatal hypoxia-ischemic insult in rats. Behav. Brain Res. 2004;151:209–217. doi: 10.1016/j.bbr.2003.08.018. [DOI] [PubMed] [Google Scholar]
- O'Brien F.E. Iwata O. Thornton J.S. De Vita E. Sellwood M.W. Iwata S. Sakata Y.S. Charman S. Ordidge R. Cady E.B. Wyatt J.S. Robertson N.J. Delayed whole body cooling to 33 or 35°C and the development of impaired energy generation consequential to transient cerebral hypoxia-ischemia in the newborn piglet. Pediatrics. 2006;117:1549–1558. doi: 10.1542/peds.2005-1649. [DOI] [PubMed] [Google Scholar]
- Oh W. Perritt R. Shankaran S. Merritts M. Donovan E.F. Ehrenkranz R.A. O'Shea T.M. Tyson J.E. Laptook A.R. Das A. Higgins R. NICHD Neonatal Research Network. Association between urinary lactate to creatinine ratio and neurodevelopmental outcome in term infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2008;153:375–378. doi: 10.1016/j.jpeds.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberston C.M.T. Finer N.N. Grace M.G.A. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J. Pediatr. 1989;114:753–760. doi: 10.1016/s0022-3476(89)80132-5. [DOI] [PubMed] [Google Scholar]
- Schulzke S.M. Rao S. Patole S.K. A systematic review of cooling for neuroprotection with hypoxic-ischemic encephalopathy—are we there yet? BMC Pediatr. 2007;7:1–30. doi: 10.1186/1471-2431-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P.S. Ohlsson A. Perlman A. Hypothermia to treat neonatal hypoxic ischemic encephalopathy. Arch. Pediatr. Adolesc. Med. 2007;161:951–958. doi: 10.1001/archpedi.161.10.951. [DOI] [PubMed] [Google Scholar]
- Shankaran S. Laptook A.R. Ehrenkranz R.A. Tyson J.E. McDonald S.A. Donovan E.F. Fanaroff A.A. Poole W.K. Wright L.L. Higgins R.D. Finer N. Carlo W.A. Duara S. Oh W. Cotton C.M. Stevenson D.K. Stoll B.J. Lemons J.A. Guillet R. Jobe A.H. the NICHD Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shankaran S. Woldt E. Koepke T. Bedard M.P. Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum. Dev. 1991;25:135–148. doi: 10.1016/0378-3782(91)90191-5. [DOI] [PubMed] [Google Scholar]
- Shankaran S. Pappas A. Laptook A.R. McDonald S.A. Ehrenkranz R.A. Tyson J.E. Walsh M. Goldberg R.N. Higgins R.D. Das A. NICHD Neonatal Research Network. Outcomes of safety and effectiveness in a multicenter randomized controlled trial of whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:791–798. doi: 10.1542/peds.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo B.K. Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J. Cereb. Blood Flow Metab. 1989;9:127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- Sirimanne E.S. Blumberg R.M. Bossano D. Gunning M. Edwards A.D. Gluckman P.D. Williams C. The effect of prolonged modification of cerebral temperature on outcome after hypoxia-ischemic brain injury in the infant rat. Pediatr. Res. 1996;39:591–597. doi: 10.1203/00006450-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Spitzmiller R.E. Phillips T. Meinzen-Derr J. Hoath S.B. Amplitude-integrated EEG is useful in predicting neurodevelopment outcome in full-term infants with hypoxic-ischemic encephalopathy: a meta-analysis. J. Child Neurol. 2007;22:1069–1078. doi: 10.1177/0883073807306258. [DOI] [PubMed] [Google Scholar]
- Tan W.K. Williams C.E. During M.J. Mallard C.E. Gunning M.I. Gunn A.J. Gluckman P.D. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr. Res. 1996;39:791–797. doi: 10.1203/00006450-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Taylor D.L. Mehmet H. Cady E.B. Edwards A.D. Improved neuroprotection with hypothermia delayed by 6 hours following cerebral hypoxia-ischemia in the 14-day-old rat. Pediatr. Res. 2002;51:13–19. doi: 10.1203/00006450-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Thoresen M. Bågenholm R. Løberg E.M. Apricena F. Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch. Dis. Child. 1996;74:F3–F9. doi: 10.1136/fn.74.1.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen M. Penrice J. Lorek A. Cady E.B. Wylezinski M. Kirkbride V. Cooper C.E. Brown G.C. Edwards A.D. Wyatt J.S. Reynolds E.O.R. Mild hypothermia following severe transient hypoxia-ischemic ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr. Res. 1995;5:667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- Thoresen M. Simmonds M. Satas S. Tooley J. Silver I.A. Effective selective head cooling during posthypoxic hypothermia in newborn piglets. Pediatr. Res. 2001;49:594–599. doi: 10.1203/00006450-200104000-00024. [DOI] [PubMed] [Google Scholar]
- Tooley J. Satas S. Eagle R. Silver I.A. Thoresen M. Significant selective head cooling can be maintained long-term after global hypoxia ischemia in newborn piglets. Pediatrics. 2002;109:643–649. doi: 10.1542/peds.109.4.643. [DOI] [PubMed] [Google Scholar]
- Tooley J. Satas S. Porter H. Silver I.A. Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets in neuroprotection. Ann. Neurol. 2003;53:65–72. doi: 10.1002/ana.10402. [DOI] [PubMed] [Google Scholar]
- Tooley J.R. Eagle R.C. Satas S. Thoresen M. Significant head cooling can be achieved while maintaining normothermia in the newborn piglet. Arch. Dis. Child. Fetal Neonatal Ed. 2005;90:F262–F266. doi: 10.1136/adc.2003.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanucci R.C. Perlman J.M. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997;100:1004–1014. doi: 10.1542/peds.100.6.1004. [DOI] [PubMed] [Google Scholar]
- Williams G. Dardzinski B.J. Buckalew A.R. Smith M.B. Modest hypothermia preserves cerebral energy metabolism during hypoxia-ischemia and correlates with brain damage: a P nuclear magnet resonance study in unanesthetized neonatal rats. Pediatr. Res. 1997;42:700–708. doi: 10.1203/00006450-199711000-00024. [DOI] [PubMed] [Google Scholar]
- Wyatt J.S. Gluckman P.D. Liu P.Y. Azzopardi D. Ballard R. Edwards A.D. Ferreiro D.M. Polin R.A. Robertson C.M. Thoresen M. Whitelaw A. Gunn A.J. for the Cool Cap Study Group. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- Yager J.Y. Asselin J. Effects of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke. 1996;27:919–926. doi: 10.1161/01.str.27.5.919. [DOI] [PubMed] [Google Scholar]
- Yager J.Y. Armstrong E.A. Jaharus C. Saucier D.M. Wirrell E.C. Preventing hypothermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]