Abstract
Objective
To evaluate the ability of progressive optic disc damage detected by assessment of longitudinal stereophotographs to predict future development of functional loss in those with suspected glaucoma.
Methods
The study included 639 eyes of 407 patients with suspected glaucoma followed up for an average of 8.0 years with annual standard automated perimetry visual field and optic disc stereophotographs. All patients had normal and reliable standard automated perimetry results at baseline. Conversion to glaucoma was defined as development of 3 consecutive abnormal visual fields during follow-up. Presence of progressive optic disc damage was evaluated by grading longitudinally acquired simultaneous stereophotographs. Other predictive factors included age, intraocular pressure, central corneal thickness, pattern standard deviation, and baseline stereophotograph grading. Hazard ratios for predicting visual field loss were obtained by extended Cox models, with optic disc progression as a time-dependent covariate. Predictive accuracy was evaluated using a modified R2 index.
Results
Progressive optic disc damage had a hazard ratio of 25.8 (95% confidence interval, 16.0-41.7) and was the most important risk factor for development of visual field loss with an R2 of 79%. The R2s for other predictive factors ranged from 6% to 26%.
Conclusions
Presence of progressive optic disc damage on stereophotographs was a highly predictive factor for future development of functional loss in glaucoma. These findings suggest the importance of careful monitoring of the optic disc appearance and a potential role for longitudinal assessment of the optic disc as an end point in clinical trials and as a reference for evaluation of diagnostic tests in glaucoma.
Glaucoma is a progressive optic neuropathy that is accompanied by typical changes in the visual field.1 Progressive neuroretinal rim thinning, increased excavation, and diffuse and localized loss of the retinal nerve fiber layer are all recognizable features of structural damage in the disease.2 However, their precise relationship with functional deterioration in patients with glaucoma remains largely unclear.3-7
Regulatory agencies throughout the world generally have not approved structural assessment of the optic nerve as a primary end point in clinical trials of glaucoma drugs and devices.8 The Food and Drug Administration has suggested the need to demonstrate that structural measures are predictive of clinically relevant functional outcomes in patients with glaucoma before they can reliably be used as end points in clinical trials. Currently acceptable end points according to the Food and Drug Administration include only intraocular pressure (IOP) and methods for assessment of visual function, such as standard automated perimetry (SAP). However, IOP is only a surrogate for clinically relevant outcomes in glaucoma and its relationship with disease progression is certainly imperfect.9-11 Also, although assessment of visual function is critically important for all patients with glaucoma, there is evidence to suggest that many patients may show evidence of progressive optic disc damage before functional loss is detected by SAP. Both the Ocular Hypertension Treatment Study12 and the European Glaucoma Prevention Study13 demonstrated that a substantial proportion of patients with ocular hypertension who developed glaucoma showed a change first in optic disc photographs. However, despite being included as end points for glaucoma conversion in these studies, progressive optic disc damage has not yet been demonstrated to translate into worse clinically relevant outcomes for these patients.
Previous investigations have shown that cross-sectional baseline structural measurements, either by expert assessment of stereophotographs or objective imaging methods, are predictive of future development of visual field loss in those with suspected glaucoma, suggesting a potential role for these measurements in early detection of disease.14-20 However, measures of predictive ability reported in these studies have generally indicated a low accuracy of cross-sectional structural measures for predicting individual functional outcomes. This is likely due to the wide variation in the appearance of the optic nerve, which makes it difficult to identify early signs of disease at one time. Although detection of progressive optic disc change over time is likely to be a more specific indicator of the presence of structural damage from glaucoma and to correlate better with functional outcomes, the ability of progressive optic disc change in predicting functional outcomes in patients with glaucoma has not been elucidated. The purpose of this study was to evaluate the value of progressive optic disc damage detected by expert assessment of longitudinal stereophotographs in predicting future development of visual field loss in suspected glaucoma.
Methods
Patients from this study were included in a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma (Diagnostic Innovations in Glaucoma Study) conducted at the Hamilton Glaucoma Center, University of California–San Diego. Patients in the Diagnostic Innovations in Glaucoma Study were longitudinally evaluated according to a pre-established protocol that included regular follow-up visits in which patients underwent clinical examination and several other imaging and functional tests. All the data were entered into a computer database. All patients from the Diagnostic Innovations in Glaucoma Study who met the inclusion criteria described below were enrolled in the current study. Informed consent was obtained from all participants. The University of California San Diego Human Subjects Committee approved all protocols, and the methods described adhered to the tenets of the Declaration of Helsinki.
Baseline and follow-up annual examinations consisted of a comprehensive ophthalmologic examination that included a medical history review, best-corrected visual acuity, slitlamp biomicroscopy, IOP measurement using Goldmann applanation tonometry, gonioscopy, dilated funduscopic examination using a 78-diopter (D) lens, stereoscopic optic disc photography, and SAP using Full-Threshold or 24-2 Swedish Interactive Threshold Algorithm (Carl Zeiss Meditec Inc, Dublin, California). For each eye, central corneal thickness (CCT) was calculated as the average of 3 measurements obtained during the same visit using an ultrasound pachymeter (Pachette GDH 500; DGH Technology Inc, Philadelphia, Pennsylvania).
To be included, subjects had to have a best-corrected visual acuity of 20/40 or better, spherical refraction within 5.0 D, cylinder correction within 3.0 D, and open angles on gonioscopy. Patients with a history of ocular trauma, other intraocular eye disease, or other diseases possibly affecting visual field (eg, demyelinating diseases, pituitary lesions, or diabetic retinopathy) were excluded. Patients with a history of refractive surgery were also excluded.
A cohort of eyes suspected of having glaucoma was selected from our database. Eyes with suspected glaucoma had a history of elevated IOP (>21 mm Hg) and/or a suspicious or glaucomatous appearance of the optic nerve from cross-sectional evaluation of optic disc stereophotographs obtained at the baseline visit by 2 independent masked graders. Features characteristic of glaucomatous appearance of the optic disc were neuroretinal rim thinning, cupping, and suspicious/abnormal retinal nerve fiber layer defects. A third grader reviewed the photographs in case of disagreement. All eyes had normal and reliable SAP visual fields at baseline. Normal visual field was defined as a mean deviation and pattern standard deviation (PSD) within 95% confidence limits and a glaucoma hemifield test result within normal limits. Eligible subjects were required to have had visual field examinations and optic disc stereophotographs taken within 6 months of each other.
Evaluation of Progressive Optic Disc Damage
The presence of progressive optic disc damage during follow-up was evaluated by masked grading of longitudinally acquired simultaneous stereophotographs of the optic disc (TRC-SS; Topcon Instrument Corp of America, Paramus, New Jersey). Stereoscopic sets of slides were examined using a stereoscopic viewer. Two experienced graders, masked to the subject's identity and other test results, evaluated the photographs. For inclusion, photographs needed to be of adequate quality or better. For each patient, the most recent stereophotograph was compared with the oldest available one to maximize the chance of detecting progressive optic disc change. If the initial grading of the pair of stereophotographs encompassing the longest follow-up period showed progression, the other pairs of stereophotographs were graded in a masked fashion until the earliest date of progression was identified. Each observer was masked to the temporal sequence of the photographs. Definition of change was based on focal or diffuse thinning of the neuroretinal rim, increased excavation, or enlargement of retinal nerve fiber layer defects. Changes in rim color or presence of disc hemorrhage or progressive parapapillary atrophy was not sufficient for characterization of progression. Discrepancies between the 2 graders were resolved by a third experienced grader.
Follow-Up and Determination of End Points
All included eyes were required to have a minimum of 4 reliable visual field tests during follow-up. Reliable tests had 25% or less fixation losses and false-negatives and 15% or less false-positives. Conversion to glaucoma in this study was defined by visual fields alone. Glaucomatous conversion was defined as the development of 3 consecutive abnormal visual field results during follow-up. An abnormal visual field was defined as a PSD with P< .05 and/or a glaucoma hemifield test result outside normal limits. Two experienced glaucoma specialists verified that the visual field defects were consistent with glaucoma based on the repeatability of the defect location and exclusion of artifacts (eyelid, lens rim, and fatigue). Eyes that developed a confirmed visual field defect for SAP were referred to as converters. Eyes that did not develop consecutive abnormal fields were referred to as nonconverters.
For converters, follow-up time was defined as the time between the baseline visit and the date of the first abnormal visual field (the study end point). For nonconverters, follow-up time was defined as the time between the baseline visit and date of last available visual field. During follow-up time, each patient was treated at the discretion of the attending ophthalmologist.
Statistical Analysis
The primary purpose of the study was to determine whether progressive optic disc damage identified on stereophotographs was predictive of future development of SAP visual field loss (conversion). Other variables analyzed as potential risk factors for development of glaucomatous visual field loss were age, baseline IOP, CCT, and the baseline SAP visual field index PSD. Hazard ratios (HRs) for the association between progressive optic disc damage and the development of glaucomatous SAP visual field loss were obtained by extended Cox models with time-dependent covariates. Progressive optic disc damage on stereophotographs was entered as a time-dependent covariate with a value of 0 if no progression had occurred up to a particular point and 1 when progression occurred at that point. Similar models have been used in the literature for the investigation of the relationship between a binary nonreversible time-dependent covariate and survival.21,22 We report HRs from univariable models, which do not adjust for the presence of other factors as well as adjusted HRs from multivariable Cox models. For the multivariable models, we report HRs after adjustment for age, baseline IOP, CCT, and SAP PSD. These variables have been reported to be significantly associated with the risk of developing glaucomatous visual field loss among patients with ocular hypertension or suspected glaucoma.10,15,23,24
We also evaluated the ability of baseline subjective stereophotograph evaluation (grading and vertical cup-disc ratio) in predicting development of visual field abnormalities. Univariable HRs were reported for stereophotograph grading (glaucoma vs normal) as well as for vertical cup-disc ratio. Adjusted HRs were also reported for these variables after adjustment for age, baseline IOP, CCT, and SAP PSD. We compared the performance of baseline grading with optic disc progression grading in predicting future development of visual field loss.
To assess and compare the importance of variables in determining the outcome, we used an index proposed by O'Quigley et al25 and modified by Royston.26 This index is equivalent to the Nagelkerke coefficient of determination of a linear model and measures the amount of variation in the outcome (survival time) explained by the predictor or, in other words, the strength of the relationship between the predictor and the outcome in a survival model. Confidence intervals (CIs) for the R2 were obtained by bootstrapping, with 1000 replications.
To adjust for the correlation between both eyes of the same individual, we used a shared frailty model, with frailty assumed to follow a gamma distribution with a mean of 1 and variance of θ estimated from the data. Adding shared frailty to a survival model is analogous to adding a random effect to a linear regression as a way to account for the correlation between clusters of observations.27 Frailties enter multiplicatively on the hazard function and are used to model within-group correlation (in this application, correlation between both eyes of the same patient). Observations within a group share the same frailty, and the extent of the correlation is measured by θ. We assumed that both eyes of the same individual were correlated because some individuals would inherently be more frail than others, that is, some subjects would inherently be more prone to develop glaucoma than others.
Statistical analyses were performed using Stata, version 9.0 (Stata Corp, College Station, Texas). The α level (type I error) was set at .05.
Results
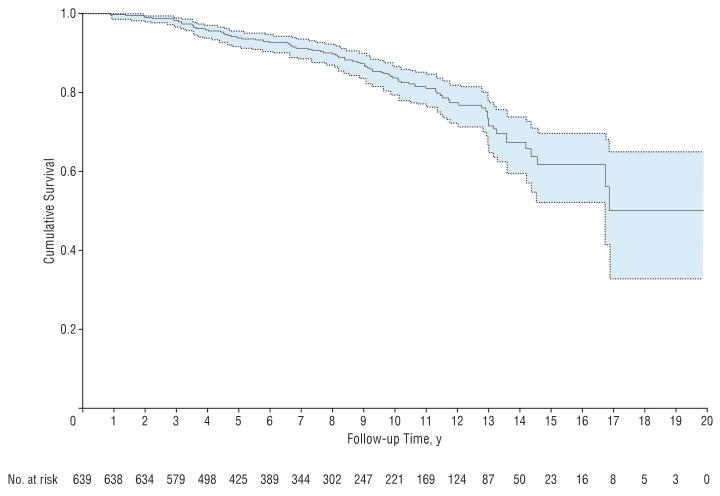
The study included 639 eyes of 407 patients with a mean age of 58 years (SD, 13 years). Two hundred forty-one patients (59%) were female. Three hundred thirty-two patients were white (82%), 61 were black (15%), and 14 were of Asian descent (3%). Average follow-up time for all patients was 8.0 years (median, 7.4 years; first quartile, 4.2 years; third quartile, 11.1 years). Ninety-five eyes (15%) showed conversion from a normal SAP visual field to a repeatable visual field defect. Table 1 presents baseline clinical and demographic characteristics of converters and nonconverters. Figure 1 illustrates the Kaplan-Meier estimated cumulative probability of developing visual field loss in the study.
Table 1. Baseline Demographic and Clinical Characteristics for Converters and Nonconverters.
| Mean(SD) | ||
|---|---|---|
| Characteristic | Convertersa (n=95) |
Nonconvertersb (n=544) |
| Age, y | 63 (11) | 56 (12) |
| Female sex, % | 60 | 55 |
| Race, % | ||
| White | 86 | 82 |
| Black | 13 | 14 |
| Asian | 1 | 4 |
| Baseline IOP, mm Hg | 30.1 (9.7) | 25.3 (6.5) |
| Central corneal thickness, μm | 556 (40) | 563 (37) |
| Baseline PSD, dB | 2.05 (0.38) | 1.68 (0.37) |
| Baseline vertical cup-disc ratio | 0.64 (0.18) | 0.55 (0.19) |
| Baseline glaucomatous optic disc grading, % | 65 | 35 |
| Evidence of optic disc progression during follow-up, % | 66 | 6 |
Abbreviations: IOP, intraocular pressure; PSD, pattern standard deviation.
Eyes that developed visual field loss during follow-up.
Eyes that did not develop visual field loss during follow-up.
Figure 1.
Kaplan-Meier curve illustrating the cumulative probability of survival during the study. The end point was defined as development of repeatable visual field loss. Dotted lines show the 95% confidence limits.
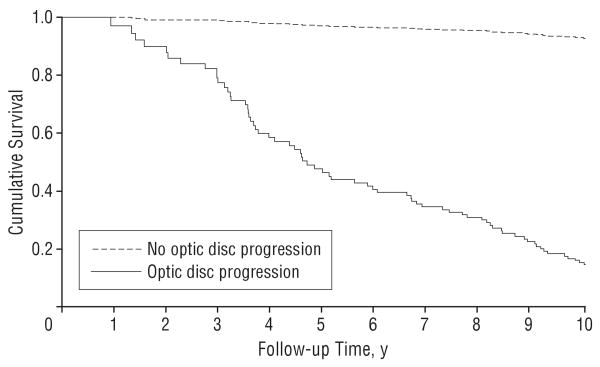
Ninety-six eyes (15%) had progressive optic disc damage on stereophotographs during the follow-up period. From these eyes, 63 (66%) developed visual field conversion during follow-up. From the 543 eyes that showed no evidence of progressive optic disc damage during follow-up, only 32 (6%) developed visual field loss during follow-up. Table 2 presents the results of the Cox model that included optic disc progression as a time-dependent covariate. Eyes that had progressive optic disc damage had an almost 26 times higher chance of developing visual field loss during follow-up (HR, 25.8; 95% CI, 16.0-41.7). The estimated frailty variance θ was 0.513 and it was significantly different from 0 according to a likelihood ratio test (P=.02), indicating the presence of significant correlation between both eyes from the same individual. Figure 2 shows estimated survival curves for development of visual field loss for an eye that had progressive optic disc damage during follow-up vs an eye that did not develop optic disc changes during the entire follow-up period.
Table 2. Results of the Univariable and Multivariable Cox Models Evaluating the Relationship Between Predictors and Risk of Development of Visual Field Loss.
| Predictor | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Optic disc progression | 25.8 (16.0-41.7) | <.001 | 16.0 (9.8-25.9) | <.001 |
| Baseline glaucomatous optic disc grading | 3.76 (2.04-6.94) | <.001 | 2.47 (1.35-4.52) | .003 |
| Baseline vertical cup-disc ratio, per 0.1 larger | 1.45 (1.22-1.71) | <.001 | 1.35 (1.15-1.59) | <.001 |
| Baseline IOP, per 1 mm Hg higher | 1.06 (1.02-1.11) | .003 | ||
| Central corneal thickness, per 40 μm thinner | 1.87 (1.28-2.72) | <.001 | ||
| Baseline PSD, per 0.1 dB higher | 1.25 (1.16-1.35) | <.001 | ||
| Age, per decade older | 2.24 (1.67-3.01) | <.001 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; IOP, intraocular pressure; PSD, pattern standard deviation.
Figure 2.
Survival curves illustrating the cumulative probability of developing visual field loss during the study for eyes that showed optic disc progression on stereophotographs compared with eyes that did not show evidence of optic disc progression during follow-up.
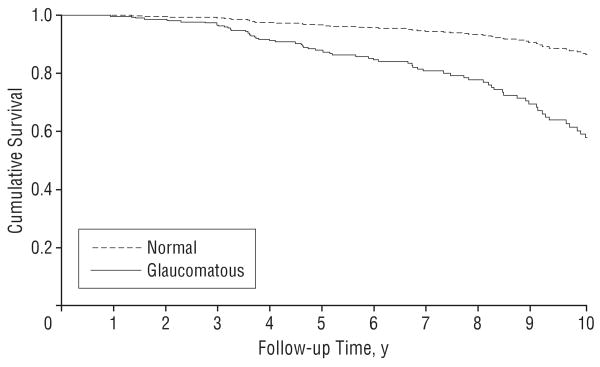
In univariable analysis, older age, higher baseline IOP, thinner corneas, and larger values of baseline PSD were also significantly associated with risk of conversion (Table 2). A baseline (cross-sectional) grading of optic disc photographs indicating glaucomatous damage resulted in an HR of 3.76 (95% CI, 2.04-6.94). A larger vertical cup-disc ratio on the baseline optic disc stereophotograph was also significantly associated with conversion (HR, 1.44 per 0.1 larger; 95% CI, 1.22-1.71). Figure 3 shows estimated survival curves for development of visual field loss for eyes that had a baseline glaucomatous grading on stereophotographs vs eyes that had a normal grading.
Figure 3.
Survival curves illustrating the cumulative probability of developing visual field loss during the study for eyes that had a baseline glaucomatous grading on optic disc stereophotographs vs eyes that had a normal grading.
Presence of optic disc progression during follow-up was the most important risk factor for development of visual field conversion (R2, 79%; 95% CI, 65%-87%) (Table 3). That is, 79% of the variation in survival times could be explained by this variable. Baseline cross-sectional glaucoma grading on stereophotographs had a much lower R2 of 21% (95% CI, 9%-37%). R2 values for the other factors ranged from 6% to 26% (Table 3).
Table 3. Predictive Capability of Each Factor as Measured by the R2.
| Predictor | R2 (95% CI) |
|---|---|
| Optic disc progression | 79 (65-87) |
| Baseline grading | 21 (9-37) |
| Baseline vertical cup-disc ratio | 21 (8-37) |
| Baseline IOP | 10 (2-22) |
| Central corneal thickness | 6 (1-15) |
| Baseline PSD | 26 (15-40) |
| Age | 23 (11-39) |
Abbreviations: CI, confidence interval; IOP, intraocular pressure; PSD, pattern standard deviation.
In multivariable analysis adjusting for age, IOP, CCT, and PSD, progressive optic disc damage was still highly predictive of development of functional loss, with adjusted HR of 16.0 (95% CI, 9.8-25.9) (Table 2). Baseline glaucomatous grading had an adjusted HR of 2.47 (95% CI, 1.35-4.52). We also built a multivariable Cox model that included age, IOP, CCT, PSD, baseline grading, and optic disc progression. This model revealed an adjusted HR of 13.5 (95% CI, 8.0-22.6) for optic disc progression.
Comment
In the current study, we found that evidence of progressive optic disc damage on longitudinal stereophotographs was highly predictive of development of functional loss in glaucoma. Patients who showed optic disc progression during follow-up were much more likely to develop visual field defects compared with patients in whom no change could be identified on the appearance of the optic disc. These findings may have significant implications for early detection of disease by ways of monitoring the optic nerve and adjusting therapy to avoid development of visual function loss with potential impairment in vision-related quality of life. Also, they suggest that optic nerve change over time is a valid surrogate for development of functional loss in glaucoma and therefore could potentially be used as an end point in glaucoma clinical trials.
Patients suspected of having glaucoma at baseline and who had progressive optic disc change on stereophotographs had an almost 26 times higher chance of developing a visual field defect (HR, 25.8; 95% CI, 16.0-41.7) during follow-up. Presence of optic disc progression was the most important predictive factor for conversion, with an R2 of 79%, well above that of any other known risk factor for development of glaucoma, such as IOP and corneal thickness. This is not surprising if we consider that progressive structural deterioration is indicative of the disease itself, rather than a risk factor.
Although IOP has traditionally been used as an end point in clinical trials, it is an imperfect surrogate for the clinical outcomes of the disease. Many patients' diseases can progress despite low IOP levels and others remain stable despite having IOP measurements that are considered high.12,13,28 Furthermore, IOP is not a suitable end point for clinical trials investigating certain treatment modalities for glaucoma, such as neuroprotective therapies. The use of visual fields as the sole end point in glaucoma trials is potentially limited by the need for large samples, long-term follow-up, variability of results, and inconsistency in the available methods to define visual field progression.29 Being a valid surrogate for development of functional loss, progressive optic disc damage could be used as an end point in glaucoma clinical trials with a number of advantages, including faster acquisition of a sufficient number of end points with reduction in sample size requirements, enabling shorter and less expensive trials.
Detection of progressive structural damage in glaucoma has significant clinical implications for patients. Recent analysis of population-based data has suggested that even mild visual field loss in patients with glaucoma already carries a significant negative impact in vision-related quality of life measures.30 Also, assuming conservative treatment efficacy, more than 10% of patients with glaucoma who have diagnosed early visual field damage and were followed up under treatment will still develop significant visual impairment or blindness from the disease during their lifetime.31 This evidence collectively points to the need for early detection and treatment of glaucoma before significant visual field loss has developed. Therefore, monitoring of the optic nerve appearance for detection of change before substantial visual field damage occurs could potentially decrease rates of functional impairment associated with the disease.
Previous investigations have shown that baseline structural measurements predict future development of visual field loss in suspected glaucoma. Such evidence comes from studies using cross-sectional grading of optic disc photographs and imaging methods for structural evaluation in glaucoma, including confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry.14-20 In the present study, we also found that a baseline glaucomatous appearance of the optic nerve was predictive of conversion, with an HR of 3.76 (95% CI, 2.04-6.94), similar to that in other studies. However, the predictive ability of baseline grading, as measured by the R2 of 21% was weak. This is in agreement with other studies, such as the confocal scanning laser ophthalmoscopy ancillary study to the Ocular Hypertension Treatment Study. In that study, the positive predictive value of an abnormal result in the Moorfields regression analysis was only 14.1%, demonstrating the low accuracy of baseline measures to predict future individual outcomes.16 This is not surprising; due to the wide variability of the optic nerve appearance in the normal population, a single optic disc examination is frequently nondiagnostic in the early stages of glaucoma. Evidence of progressive damage seems to be a more robust indicator of the presence of damage and therefore is more associated with development of functional loss. Progressive disc damage performed significantly better than cross-sectional evaluation of the optic disc appearance in predicting development of visual field defects, as indicated by the coefficient of determination. The findings from our study support the use of progressive optic disc damage as a reference standard for evaluation of diagnostic tests in glaucoma. This approach has been suggested by Medeiros et al32 and presents a number of advantages over the use of cross-sectional evaluation of the optic disc appearance and visual fields in certain situations.33,34
A recent work by Chauhan et al35 evaluated whether progressive optic disc changes measured by the Topographic Change Analysis software of the Heidelberg Retina Tomograph (Heidelberg Engineering GmbH, Dossenheim, Germany) were predictive of functional loss in a cohort of 81 patients. Among the many different criteria the authors evaluated, only a conservative criterion was able to significantly predict future functional deterioration with a positive likelihood ratio of only 3.02, which according to evidence-based medicine classification36 would indicate a minimal impact in changing the probability of the outcome. Calculation of a positive likelihood ratio for progressive optic disc change in our study revealed a much better value of 16.5, indicating a strong effect in changing probability of functional loss. This comparison suggests that progressive structural changes identified by optic disc stereophotographs seem to carry greater clinical impact than progression identified by the Topographic Change Analysis. However, additional studies comparing the 2 methods in the same population are required to substantiate this conclusion. Also, different methods to evaluate progression using Heidelberg Retina Tomograph data and different imaging instruments used for detection of structural change may perform better than the Topographic Change Analysis.
It should be noted that 6% of the eyes, corresponding to 34% of all converters in our study, developed visual field defects without showing any evidence of progressive optic disc damage during follow-up. This figure is very similar to that of the Ocular Hypertension Treatment Study, in which 35% of patients with ocular hypertension followed up over time first developed a visual field abnormality with no evidence of optic disc change.12 Our study differed from the Ocular Hypertension Treatment Study, as the former also included patients with suspicious or abnormal optic nerves at baseline. However, when only the sample with normal-appearing optic discs at baseline had been analyzed (386 eyes or 60% of the total sample), the HR for progressive optic disc change in predicting visual field loss was still 24.9 (95% CI, 10.5-59.0; P<.001). It remains unclear why some patients seem to first develop a structural change while others first change in function, but this could be related to the accuracy of the methods used to evaluate structural and functional changes as well as individual morphology and factors governing susceptibility to damage. Whatever the reasons might be, these findings highlight the importance of monitoring both structure and function in patients suspected of having glaucoma. Other methods have been proposed to evaluate visual field progression, and there is considerable disagreement among the different methods. We also reanalyzed our data using the glaucoma change probability maps as used in the Early Manifest Glaucoma Trial to define visual field conversion. Progressive optic disc change had a similar HR to predict functional loss (HR, 20.3; 95% CI, 11.9-34.6). Our findings also confirmed the previous reports that older age, higher IOP, and thinner corneas are significant risk factors for conversion to glaucoma in patients suspected of having the disease. However, progressive optic disc damage remained a highly significant predictive variable even after adjustment for all these other risk factors.
Our study has limitations. It was not a randomized clinical trial and patients were treated at the discretion of the attending ophthalmologist during follow-up. Therefore, it is possible that the finding of progressive optic disc damage during regular follow-up examinations may have triggered an increase in treatment to prevent further progression. This may have prevented some patients with progressive optic disc damage from developing functional loss or may have increased the survival time until visual field conversion. However, if present, this effect would actually have caused an underestimation of the already high predictive accuracy of progressive optic disc damage. Another potential limitation is related to the subjective component of optic disc assessment, which may result in suboptimal reproducibility of optic disc stereophotograph grading for progression.37,38 In our study, we used highly trained graders to evaluate the presence of progressive optic disc damage, which increases reproducibility but may not be transferable to other settings, such as when general ophthalmologists grade optic disc photographs for progression. This could potentially limit the use of longitudinal evaluation of optic disc stereophotographs in clinical practice. It is possible that objective structural evaluation by imaging instruments may help improve reproducibility of detecting optic disc change over time.9,39,40 However, these limitations are also present with visual fields owing to their subjective nature and lack of standardized methods for evaluation of change. Also, such limitations should not impair the use of progressive optic disc damage as end points in clinical trials as long as the evaluation is performed in a standardized way by specialized optic disc reading centers with highly trained graders.
In conclusion, the presence of progressive optic disc damage on stereophotographs was a highly predictive factor for future development of functional loss in glaucoma. Our findings suggest the importance of careful monitoring of the optic disc appearance in patients with glaucoma and subjects suspected of having the disease. Also, they suggest a potential role for longitudinal assessment of the optic disc as an end point in clinical trials and as a reference for evaluating diagnostic tests in glaucoma.
Acknowledgments
Funding/Support: This work was supported in part by grants EY11008 (Dr Zangwill) and EY08208 (Dr Sample) from the National Eye Institute. Participants received retention incentive grants in the form of glaucoma medications at no cost from Alcon Laboratories Inc, Allergan, Pfizer Inc, and SANTEN Inc.
Footnotes
Financial Disclosure: Drs Medeiros, Zangwill, Sample, and Weinreb have received research support from Carl-Zeiss Meditec.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43(4):293–320. doi: 10.1016/s0039-6257(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Hernandez M, Pablo LE, Armas-Domingue K, Rodriguez de la Vega R, Ferreras A, Gonzalez de la Rosa M. Structure-function relationship depends on glaucoma severity. Br J Ophthalmol. 2009 doi: 10.1136/bjo.2008.154815. [DOI] [PubMed] [Google Scholar]
- 4.Racette L, Medeiros FA, Bowd C, Zangwill LM, Weinreb RN, Sample PA. The impact of the perimetric measurement scale, sample composition, and statistical method on the structure-function relationship in glaucoma. J Glaucoma. 2007;16(8):676–684. doi: 10.1097/IJG.0b013e31804d23c2. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CA, Cioffi GA, Liebmann JR, Sample PA, Zangwill LM, Weinreb RN. The relationship between structural and functional alterations in glaucoma: a review. Semin Ophthalmol. 2000;15(4):221–233. doi: 10.3109/08820530009037873. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner SK, Johnson CA, Cioffi GA. Evaluation of the structure-function relationship in glaucoma. Invest Ophthalmol Vis Sci. 2005;46(10):3712–3717. doi: 10.1167/iovs.05-0266. [DOI] [PubMed] [Google Scholar]
- 7.Harwerth RS, Carter-Dawson L, Smith EL, III, Crawford ML. Scaling the structure– function relationship for clinical perimetry. Acta Ophthalmol Scand. 2005;83(4):448–455. doi: 10.1111/j.1395-3907.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- 8.Weinreb RN, Kaufman PL. The glaucoma research community and FDA look to the future: a report from the NEI/FDA CDER Glaucoma Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2009;50(4):1497–1505. doi: 10.1167/iovs.08-2843. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros FA, Alencar LM, Zangwill LM, et al. The relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116(6):1125.e1-3–1133.e1-3. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. discussion 829-830. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DR, Normal Tension Glaucoma Study Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14(2):86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. discussion 829-830. [DOI] [PubMed] [Google Scholar]
- 13.Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I, European Glaucoma Prevention Study (EGPS) Group Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112(3):366–375. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006;142(4):576–582. doi: 10.1016/j.ajo.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123(10):1351–1360. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 16.Zangwill LM, Weinreb RN, Beiser JA, et al. Baseline topographic optic disc measurements are associated with the development of primary open-angle glaucoma: the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123(9):1188–1197. doi: 10.1001/archopht.123.9.1188. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi K, Bowd C, Weinreb RN, Medeiros FA, Sample PA, Zangwill LM. Retinal nerve fiber layer thickness measurements with scanning laser polarimetry predict glaucomatous visual field loss. Am J Ophthalmol. 2004;138(4):592–601. doi: 10.1016/j.ajo.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 18.Alencar LM, Bowd C, Weinreb RN, Zangwill LM, Sample PA, Medeiros FA. Comparison of HRT-3 glaucoma probability score and subjective stereophotograph assessment for prediction of progression in glaucoma. Invest Ophthalmol Vis Sci. 2008;49(5):1898–1906. doi: 10.1167/iovs.07-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medeiros FA, Zangwill LM, Bowd C, Vasile C, Sample PA, Weinreb RN. Agreement between stereophotographic and confocal scanning laser ophthalmoscopy measurements of cup/disc ratio: effect on a predictive model for glaucoma development. J Glaucoma. 2007;16(2):209–214. doi: 10.1097/IJG.0b013e31802d695c. [DOI] [PubMed] [Google Scholar]
- 20.Gordon MO, Torri V, Miglior S, et al. Ocular Hypertension Treatment Study Group. European Glaucoma Prevention Study Group Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114(1):10–19. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen PK. Repeated assessment of risk factors in survival analysis. Stat Methods Med Res. 1992;1(3):297–315. doi: 10.1177/096228029200100305. [DOI] [PubMed] [Google Scholar]
- 22.Feuer EJ, Hankey BF, Gaynor JJ, Wesley MN, Baker SG, Meyer JS. Graphical representation of survival curves associated with a binary non-reversible time dependent covariate. Stat Med. 1992;11(4):455–474. doi: 10.1002/sim.4780110408. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros FA, Sample PA, Zangwill LM, Bowd C, Aihara M, Weinreb RN. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136(5):805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 24.Miglior S, Pfeiffer N, Torri V, Zeyen T, Cunha-Vaz J, Adamsons I, European Glaucoma Prevention Study (EGPS) Group Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114(1):3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 25.O'Quigley J, Xu R, Stare J. Explained randomness in proportional hazards models. Stat Med. 2005;24(3):479–489. doi: 10.1002/sim.1946. [DOI] [PubMed] [Google Scholar]
- 26.Royston P. Explained variation for survival models. Stata J. 2006;6(1):83–96. [Google Scholar]
- 27.Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1(3):255–273. doi: 10.1007/BF00985760. [DOI] [PubMed] [Google Scholar]
- 28.Drance SM. The Collaborative Normal-Tension Glaucoma Study and some of its lessons. Can J Ophthalmol. 1999;34(1):1–6. [PubMed] [Google Scholar]
- 29.Katz J, Congdon N, Friedman DS. Methodological variations in estimating apparent progressive visual field loss in clinical trials of glaucoma treatment. Arch Ophthalmol. 1999;117(9):1137–1142. doi: 10.1001/archopht.117.9.1137. [DOI] [PubMed] [Google Scholar]
- 30.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP, Los Angeles Latino Eye Study Group Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143(6):1013–1023. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. 2009;116(5):823–832. doi: 10.1016/j.ophtha.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 32.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Use of progressive glaucomatous optic disk change as the reference standard for evaluation of diagnostic tests in glaucoma. Am J Ophthalmol. 2005;139(6):1010–1018. doi: 10.1016/j.ajo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Medeiros FA, Ng D, Zangwill LM, Sample PA, Bowd C, Weinreb RN. The effects of study design and spectrum bias on the evaluation of diagnostic accuracy of confocal scanning laser ophthalmoscopy in glaucoma. Invest Ophthalmol Vis Sci. 2007;48(1):214–222. doi: 10.1167/iovs.06-0618. [DOI] [PubMed] [Google Scholar]
- 34.Medeiros FA, Vizzeri G, Zangwill LM, Alencar LM, Sample PA, Weinreb RN. Comparison of retinal nerve fiber layer and optic disc imaging for diagnosing glaucoma in patients suspected of having the disease. Ophthalmology. 2008;115(8):1340–1346. doi: 10.1016/j.ophtha.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauhan BC, Nicolela MT, Artes PH. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmology. doi: 10.1016/j.ophtha.2009.04.031. published online June 4, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Jaeschke R, Guyatt GH, Sackett DL, The Evidence-Based Medicine Working Group Users' Guides to the Medical Literature, III: how to use an article about a diagnostic test, B: what are the results and will they help me in caring for my patients? JAMA. 1994;271(9):703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 37.Jampel HD, Friedman D, Quigley H, et al. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol. 2009;147(1):39–44. e1. doi: 10.1016/j.ajo.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parrish RK, II, Schiffman JC, Feuer WJ, et al. Ocular Hypertension Treatment Study Group Test-retest reproducibility of optic disk deterioration detected from stereophotographs by masked graders. Am J Ophthalmol. 2005;140(4):762–764. doi: 10.1016/j.ajo.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50(4):1675–1681. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fayers T, Strouthidis NG, Garway-Heath DF. Monitoring glaucomatous progression using a novel Heidelberg Retina Tomograph event analysis. Ophthalmology. 2007;114(11):1973–1980. doi: 10.1016/j.ophtha.2007.01.035. [DOI] [PubMed] [Google Scholar]