Abstract
The ability to identify factors responsible for disease in all species depends on the ability to separate those factors which are environmental from those that are intrinsic. This is particularly important for studies on the development of the adaptive immune response of neonates. Studies on laboratory rodents or primates have been ambiguous because neither the effect of environmental nor maternal factors on the newborn can be controlled in mammals that: (i) transmit potential maternal immunoregulatory factors in utero and (ii) are altricial and cannot be reared after birth without their mothers. Employing the newborn piglet model can address each of these concerns. However, it comes at the price of having first to characterize the immune system of swine and its development. This review focuses on the porcine B cell system, especially on the methods used for its characterization in fetal studies and neonatal piglets. Understanding these procedures is important in the interpretation of the data obtained. Studies on neonatal piglets have (a) provided valuable information on the development of the adaptive immune system, (b) lead to important advances in evolutionary biology, (c) aided our understanding of passive immunity and (d) provided opportunities to use swine to address specific issues in veterinary and biomedical research and immunotherapy. This review summarizes the history of the development of the piglet as a model for antibody repertoire development, thus providing a framework to guide future investigators.
Keywords: Swine, Gnotobiotic, Models, Developmental immunology, Review
1. Introduction
1.1. Why the piglet model?
Understanding the intrinsic immunological capability of fetal and neonatal mammals can be compromised by transfer of maternal passive antibodies and other factors in utero (Fig. 1 ). Furthermore, since altricial newborns (rodents and primates) cannot be reared separate from their mothers, it limits the experimental control of external factors that have been shown to impact postnatal development of adaptive immunity (Fig. 2 ). Some reasons why the piglet is a useful model for studies on development of the adaptive immune system are listed in Table 1 .
Fig. 1.
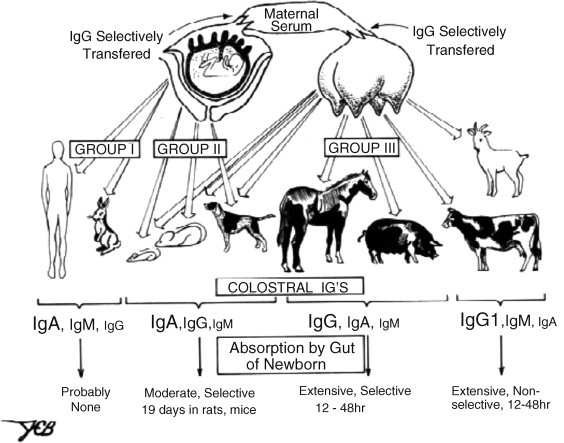
Transfer of immunity from mother to young among common placental mammals. Group I and Group III mammals represent the extremes in which receptor-mediated transport of IgG occurs via the placenta (Grp I) versus the mammary gland (Grp III). This difference is reflected in the Ig constituency of the colostrum; font size depicts relative Ig concentrations in colostrum. Uptake by the suckling neonate differs among mammals. In rodents this is mediated by FcRn which transports IgG while among Grp III mammals it is receptor independent (Butler, 1974).
Fig. 2.
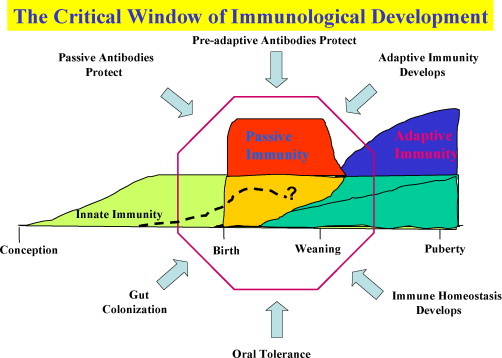
The critical window of immunological development. The period between late gestation and weaning is depicted as a window in which natural and passive antibodies provide protection to the neonate and when colonization drives the development of adaptive immunity including oral tolerance. The dotted line indicates the period in which natural antibodies are considered important. Occurring in an orderly and regulated fashion, the events during this period create a state of immune homeostasis by the time of weaning (Butler et al., 2006, Butler and Sinkora, 2007).
Table 1.
Characteristics of piglets as models for fetal/neonatal studies.
| 1. Large litters of precosial offspring suitable for rearing independently from their mothers. |
| 2. Offspring can be reared on bovine colostrum/milk and milk-based formulae used for infants. |
| 3. Similar nutritional requirements, digestive system, gut flora and respiratory system to that in humans. |
| 4. Similar or identical contagion to those that infect humans. |
| 5. Defense proteins, peptides, cytokines and chemokines homologous to those in other mammals. |
| 6. Light chain repertoire and κ:λ expression similar to humans disparate from that of rodents or other hoofed mammals. |
| 7. TCRVβ and TCRVγδ repertoires and loci arrangements similar to humans. |
| 8. Long gestation offering a large window for studying fetal development. |
| 9. Amenable to in utero inoculations and certain types of fetal manipulations. |
| 10. User-friendly VH system allowing for quantitation of repertoire diversification. |
1.2. Bumps in the road
Choosing an animal model unless it is the species itself, is complicated by diversity among vertebrates in adaptive immunity. Table 2, Table 3 summarize two examples of diversity; diversity among mammals in Ab isotypes and diversity in variable region repertoire.
Table 2.
The C-region repertoire of common mammals.
| Species | IgM | IgD | IgG | IgE | IgA | Cλ | Cκ |
|---|---|---|---|---|---|---|---|
| Human | 1 | 1 | 4 + 1* | 1 + 1* | 2 | 4 + 3 | 1 |
| Mouse | 1 | 1 | 4 | 1 | 1 | 3 + 1* | 1 |
| Rat | 1 | 1 | 4 | 1 | 1 | 1 | ? |
| Rabbit | 1 | 0 | 1 | 1 | 13 | 8 | 2 |
| Bovine | 1 | 1 | 3 | 1 | 1 | 4 | 1 |
| Sheep | 1 | 1 | 2+ | 1 | 1 | >1 | 1 |
| Horse | 1 | 1 | 7 | 1 | 1+ | 4 | 1 |
| Swine | 1 | 1 | 6 | 1 | 1 | ? | 1 |
| Camel | 1 | ? | 3+ | ? | ? | 2 | 1 |
| Cat | 1 | ? | 2+ | 1 | ? | ? | 1 |
| Dog | 1 | 1 | 4 | 1 | 1 | ? | 1 |
| Opossum | 1 | 0 | 1 | 1 | 1 | 6 | 1 |
| Platypus | 1 | 0 | 2 | 1 | 2 | 4 | ? |
* = Pseudogene; ? = identification not confirmed.
Table 3.
Variable region diversity and light chain usage among mammals.
| Species | VH (Fa) | DH | JH | Vλ (Fa) | Jλ | Cλ | % Use | Vκ (Fa) | Jκ | Cκ | % Use |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | 87 (7) | 28 | 9 | 70 (7) | 7 | 7b | 40 | 66 (7) | 5 | 1 | 60 |
| Mouse | >100 (14) | 11 | 4 | 3 (3) | 4 | 4b | 5 | 140 (4) | 4 | 1 | 95 |
| Rat | >100 (11) | 9 | 5 | 15 (4) | 1 | 1 | 9 | 18 (?) | 6 | 9 | |
| Rabbit | >100 (1) | 12 | 6 | ? (?) | 2 | 2 | 10 | >36 | 5 | 2 | 90 |
| Swine | <25 (1) | 3 | 1 | ? (4) | 3? | 3? | 50 | 60 (2) | 5 | 1 | 50 |
| Horse | 10 (2) | 9 | 9 | 25 (3) | 4 | 4 | 93 | 20 (?) | 5 | 1 | 7 |
| Bovine | 15 (2?) | 9 | 2(1) | 20 (?) | 2 | 4 | 98 | ? (?) | 9 | 1 | 2 |
| Sheep | 10 (1) | ? | ? | >100 (?) | 1 | >1 | 98 | 10 (4) | 3? | 1 | 2 |
| Camelid | 42 VHH (1) | ? (?) | ? | 2 | ? | ? (?) | ? | 1 | ? | ||
| 50 VH (1) | 10 | 6 | |||||||||
| Opossum | 12 | ? | ? | 30 (3) | 6 | 6 | ? | 35 (4) | 2+ | 1 | ? |
| Platypus | 25 (1) | >5 | 7 | 15–25 (2) | 6 (3) | 4 | 90 | ? (4+) | ? | ? | 10 |
Number of families (F) of variable region genes.
Jλ–Cλ occurs as duplicons.
If the intent is to use swine as models for primates, the situation regarding IgG and IgA subclasses is different. Since subclass diversification occurred after speciation (Butler, 2006, Butler et al., 2008a) a particular subclass in one species has no homology to one of the same name in another mammal unless closely related (sheep/cattle). Because of this evolutionary divergence, mAb and polyclonal antibodies (pAb) specific for a subclass protein in one species do not specifically recognize (cross-react with) an IgG of the same name in another species. This creates a reagent problem and means that in the case of IgG, this isotype must be characterized for each species and suitable species-specific reagents must be prepared (Section 2.5). Thus, what is learned about the biological role of IgG1 in mice cannot be extrapolated to IgG1 in swine, humans, cattle, horses, guinea pigs, etc. Characterizing a new system and preparing new reagents delays application of any model. Therefore choosing the piglet model has meant confronting a reagent problem which indirectly results from failure to understand the diversity of the adaptive immune system.
A disadvantage in using the piglet as a model is that with one exception, the species is outbred. Therefore the type of in vivo adoptive transfer and in vitro cellular studies that are routine in lab rodents cannot be done in conventional swine. This is somewhat offset because of the size of the species so that in vitro tests can be done using cells from the same animal (autologous; Table 1). Some disadvantages in the use of outbred swine have been overcome: (i) through the use of molecular biology and (ii) by allowing each individual to be treated as a biological unit (similar to humans). Inbred strains are most valuable in identifying traits affecting individuals whereas outbred animals are more likely to identify traits affecting the species.
Regardless of factors that can offset the disadvantages of the piglet model, there is another major disadvantage. Rearing piglets in special facilities, whether SPF autosows or as full-blown germfree or gnotobiotic isolator piglets, is very expensive when compared to the cost of lab rodents although studies with non-human primates are also expensive. Some animal scientists regard the small number of animals used in such studies as statistically irrelevant. This criticism can be challenged because with conventional animals the experimenter has no control over environmental factors that may be responsible for animal variation (Fig. 2). Furthermore, experiments with SPF autosows or isolator piglets are sometimes regarded as “contrived” and unrepresentative of the situation faced by conventionally reared piglets. It must be kept in mind that the purpose of isolator piglet research is to reduce the number of experimental variables so a particular phenomenon can be more easily identified. This review describes the evolution of the various piglet models used in immunological research with special emphasis on those concerning B cells and the antibody repertoire development.
1.3. Use of a new animal model demands characterization and refinement
The concept of using the neonatal piglets model for human infant research was hatched by the lead author in the late 1960s but its feasibility and application to developmental immunology had already been established in the USA (Meyer et al., 1964) and in the Czech Republic (Travnicek et al., 1966, Sterzl and Riha, 1970). However, making fetal and neonatal piglets truly useful models for research in immunology has taken decades of refinement. This review describes the current procedures that have evolved. Initially, few had envisioned the degree of diversity among mammalian immune systems and probably did not appreciate the implications that diversity would have on model building. Investigators using other mammalian and avian models also underestimated the lack of interspecies homology, which is now more clearly recognized and extends not only to Ab structure and diversity but also to B cell development and repertoire diversification (Table 2, Table 3; Butler, 2006; Fig. 3 ; Section 2.2). The piglet has contributed to a number of fundamental discoveries in comparative immunology, the most important of which are summarized in Table 4 .
Fig. 3.
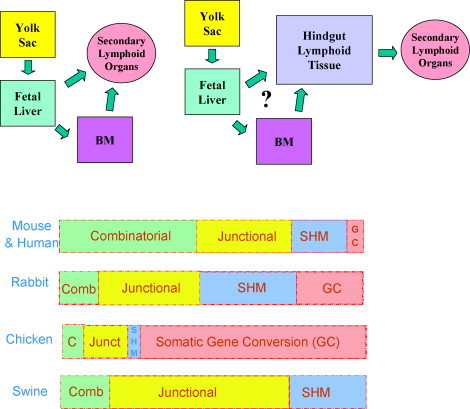
(Top) Diversity in organs and pathways involved in B cell repertoire development among selected vertebrates. Left: mouse and human. Right: chicken, rabbits and perhaps hoofed mammals. (Bottom) The relative importance of mechanisms used by different vertebrates in generating their heavy chain Ab repertoire. SHM = somatic hypermutation; Comb. or C = combinatorial diversity; Junct. or junctional = junctional diversity.
Table 4.
Characterization of the swine immune system has contradicted some established paradigms.
| 1. Class switch recombination can occur in utero without stimulation by environmental antigen (Fig. 4). |
| 2. Extensive combinatorial diversity (e.g., humans and mice) is not necessary for development of the VH repertoire of swine (Fig. 3 Bottom; Table 3). |
| 3. IgG subclasses in different species, regardless of nomenclature, are not homologous and function cannot be extrapolated among mammalian species (Butler et al., 2008a). |
| 4. Phylogeny is unreliable in predicting the features of adaptive immunity in mammals (Table 3). |
| 5. Tdt is active at the first site of VDJ rearrangement (yolk sac) and there is no evidence that activity increases with fetal age (Sinkora et al., 2003, Butler et al., 2000a). |
| 6. The fetal and neonatal thymus is involved in B cell activity (Cukrowska et al., 1998, Butler et al., 2001). |
1.4. Extension of the piglet model
Emphasis to date has been on the development of adaptive immunity during the critical neonatal window (Fig. 2) that impacts all mammals. However, certain features of swine (Table 1) and those that are the result of characterizing the piglet immune system (Table 4) have applications beyond the use of the piglet as a model for developmental immunology. Examples include the use of knock-out pigs and the use of pigs to make humanized antibodies for immunotherapy (Section 5). Isolator piglets can potentially be used to address other medically important issues such as the role of colonization and the transfer of immune modulators from mother to offspring that can be important in addressing the hygiene hypothesis.
2. Characterization of the B cell system
2.1. Experimental approaches
2.1.1. Genomic studies on genes encoding Igs and other B cell genes
Initial genomic studies were based on a porcine lambda phage library cloned using DNA probes prepared from plasmids containing human, rabbit and mouse Cμ, Cδ, Cα, Cɛ and Cγ genes (Dierks, 1988). This process generated lambda clones that would prove useful in later studies. A cosmid library (Clontech) also provided a number of Ig containing clones. Finally, BAC (bacterial artificial chromosome) or PAC (P1-derived artificial chromosome) clones have been used either to analyze the genomic organization of a single Ig gene (by sequencing of subclones or PCR) or to map the order of the genes in a given Ig locus (by sequencing of the whole clones or long distance PCR amplifications). These have proven invaluable in characterizing the porcine Cδ locus (Zhao et al., 2003). A number of Ig containing BAC clones have become important in characterizing genome segments containing CH, Cκ, Cγ and VH (Butler et al., 2004, Butler et al., 2006).
2.1.2. Transcriptional analysis
Transcriptional analyses of isotypes, subisotypes and allotypes have been done in several ways; some are qualitative, those for IgG subclasses are semi-quantitative and those for allotypes are designed as methods for typing. Isotype-specific primers have been applied to cDNA to determine if a particular isotype is expressed in a given sample. For expression studies, primer sets for the five isotypes were designed so that each generated a product of unique length (McAleer et al., 2005, Butler et al., 2001). This system is amenable to adoption for quantitative real-time PCR. The primers generate segments containing parts of CH1 through CH3 (depending on the isotype) and their associated VDJ. Cloning of each product allows the VH usage and VDJs sequence transcribed with each isotype to be analyzed (McAleer et al., 2005, Lemke et al., 2004, Butler et al., 2006, Butler et al., 2008b).
2.1.2.1. IgM and IgD
While evidence for IgM and IgD transcription can be obtained as described above (McAleer et al., 2005) the situation regarding IgD expression is more interesting and will be discussed as part of IgD characterization (Section 2.2.1).
2.1.2.2. IgG subclass expression
PCR is applied to cDNA using the primer set specific for IgG allowing the product to be cloned into pCR2TOPO. The relative number of each subclass or its allotype can be determined by sequential hybridization of individual clones with 32P-DNA probes (Butler and Wertz, 2006, Butler et al., 2008a).
2.1.2.3. Allotype expression
Expression of the allotypic variants of IgG can be done using the same clone typing procedure described above but using allotype-specific probes. Typing of swine for the IgAa and IgAb variants is done using cDNA or DNA. The cDNA method utilizes a primer set that spans the intron between Cα1 and Cα2 (Brown and Butler, 1994) and because of the mutated splice acceptor site in IgAb, diagnostic allotypic products [106 and 94 bp] are obtained that can be separated on polyacrylamide gels (PAGE; Navarro et al., 2000a). Alternatively, swine may be typed using DNA by taking advantage of a Dde I site in IgAa that is absent in IgAb. The different-sized fragments can be resolved in 2% agarose gels (Navarro et al., 2000b).
2.1.2.4. Analysis of VDJ expression
Since all porcine VH genes belong to a single family (VH3) and swine have a single JH (Sun et al., 1994, Butler et al., 1996) a primer set containing one for the common leader or 5′ FR1 sequence and JH permits PCR recovery of all rearranged VDJs. In the case of isotype-associated VDJ usage, the anti-sense JH primer is replaced with one for the isotype-specific reverse primers. In practice, this procedure generates a first round VDJ-CH product. Thereafter, internal primers consisting of 3′ FR1 and anti-sense JH can be applied to this product to generate isotype-associated VDJs which are cloned. Plasmid DNA from individual clones was (i) sequentially hybridized with various VH gene-specific probes and/or (ii) sequenced (Sun et al., 1998, Butler et al., 2000a, Butler et al., 2006, Sinkora et al., 2003, McAleer et al., 2005).
2.1.2.5. Spectratypic analysis
The CDR3 region of the rearranged heavy chain VDJ (HCDR3) associated with each isotype (see above) is the result of a mixture of (i) exonuclease shortening of VH FR3, DH and JH, (ii) palindromic insertions and (iii) Tdt dependent insertions. Thus the length of HCDR3 becomes a quasi clone marker when the amplified HCDR3 product is separated on PAGE. This procedure is used to determine if: (i) B cell subsets with certain length HCDR3s have been selected and expanded and (ii) peripheral lymphoid tissues show spectratypes indicating the selective immigration of certain B cell clones (Navarro et al., 2000b, Butler et al., 2000a, Butler et al., 2006, Butler et al., 2008b, Butler et al., 2008c, Sinkora et al., 2003, Lemke et al., 2004, McAleer et al., 2005).
2.1.3. Flow cytometry
There is no pan-B cell CD marker for swine other than CD79α which must be detected intracellularly by a cross-reactive anti-human mAb (Jones et al., 1993). Because of this limitation, B cells are detected using anti-μHC which omits most plasma cells and B cell precursors. μHC+ B cells in swine are MHC-II+, CD25lo and CD45RC+ (Sinkora et al., 1998a) while virtually all immature μHC+ B cells are also CD21+ and CD2lo (Sinkora et al., 1998a, Sinkora et al., 1998b). The latter two CD Ags are considered as B-cell differentiation markers in pigs. The expression of CD25, CD79α and SWC3 can be also used for identification of B cell precursors in primary lymphopoietic centers (Sinkora et al., 2002). Cross-reactive CD20 and CD86 were also recently shown to be useful for phenotyping of porcine B cells (Faldyna et al., 2007). However B1 and B2 cells cannot be discriminated by CD5 expression in swine. An anti-CD34 reagents has also been recently described that could prove valuable (Layton et al., 2007).
2.1.4. Immunochemistry
Ouchterlony double diffusion and immunoelectrophoresis were used in the early characterization of porcine Igs (Porter, 1969, Kaltreider and Johnson, 1972, Franek et al., 1974, Vaerman et al., 1970, Cambier and Butler, 1974, Sloan and Butler, 1978, Klobasa et al., 1985a, Klobasa, 1987, Bourne, 1971) and in characterizing the specificity of pAbs to porcine Igs. Once pAbs of proven specificity were available, single radial diffusion became the method of choice for quantitation of Ig levels in adult animals and suckling neonates (Porter, 1969, Bourne and Curtis, 1973, Klobasa et al., 1981, Klobasa et al., 1985a, Klobasa et al., 1985b, Klobasa et al., 1986, Klobasa et al., 1987, Klobasa et al., 1990, Klobasa and Butler, 1987a, Klobasa and Butler, 1987b). The need to quantify these Igs in the ng–μg range, resulted in the development of suitable sandwich ELISAs using pAB and mAb (Butler et al., 2000b, Butler et al., 2002, Butler et al., 2005a, Butler et al., 2008b, Lemke et al., 2004). A number of other ELISA-based immunochemical assays have also been employed such as hapten-inhibition studies (Butler et al., 1985, Dillender, 1990), specific Ab immunoassay (Butler and Hamilton, 1991) and Western blot assays (Lemke, 2006).
2.1.5. Immunohistochemistry
Porter and colleagues were among the first to utilize isotype-specific pAb to identify Ig-containing cells in tissue sections (Allen and Porter, 1970, Atkins et al., 1971). They used enzyme-mediated detection systems on paraffin-embedded tissues (Sainte-Marie, 1961). Results could be visualized with a conventional microscope allowing the tissue architecture to be seen. Various immunoenzymatic detection methods have been used including directly labelled horseradish peroxidase Ab conjugates and the PAP complex (Sternberger et al., 1970, Brown and Bourne, 1976, Butler et al., 1981). The detection of antibodies labelled with alkaline phosphatase using 5-bromo-4-chloro-3-indolyl phosphate as a substrate followed by hematoxylin counterstaining has often been used. Xylene-based media are used with the former and aqueous mounting media with the latter method. Porcine B cells are detected with lower background if unlabeled mAbs versus pAbs are used in the first step (Bianchi et al., 1992). These technologies have been used by many others (Allen and Porter, 1970, Allen and Porter, 1973a, Allen and Porter, 1973b, Chapman et al., 1974, Rothkotter et al., 1991, Cukrowska et al., 1998, Makala et al., 2001). The limit of detection using immunofluorescence is 20 K molecules of Ig bound per cell membrane versus 2–10 K molecules per cell for enzyme-mediated methods. With improvements in frozen-section technology, most immunohistochemistry is now done using cryostat prepared sections. Immunofluorescence is very popular in confocal applications which allow good counterstaining so tissue architecture can also be seen.
2.1.6. Chromatography and protein electrophoresis
Characterization of porcine Igs at the protein level preceded studies based on molecular genetics. These involved size-exclusion, ion-exchange and lectin chromatography. High molecular weight Igs like IgM and polymeric IgA were initially concentrated and characterized by size-exclusion chromatography (Porter, 1969, Porter, 1973, Bourne, 1969, Vaerman, 1970). Fractions recovered were evaluated by PAGE and immunoelectrophoresis (Butler et al., 1985, Klobasa et al., 1985a, Klobasa, 1987, Kaltreider and Johnson, 1972) by reference to Igs of the same isotypes in better-studied species.
Ion-exchange chromatography has proven useful in the separation of IgG subclasses in species were major difference between 2 and 3 subclasses occur. The best example is cattle in which fractions highly enriched in IgG1 and IgG2 can be purified (Butler, 1969, Butler, 1983, Butler and Maxwell, 1972). When applied to swine IgG, the result is a continuum of slightly different IgGs of slightly different charge (Dillender, 1990) that presumably reflects the rather large degree of subclass diversity compared to cattle (see Section 2.2.1) as well as the variable region diversity in polyclonal Igs (Butler et al., 1987).
The use of PAGE in the presence of a disulfide reducing agent allows the polypeptide constituency of porcine Igs to be realized. These studies revealed differences in the size of the heavy and light chains. Unlike camelids (Hamers-Casterman et al., 1993) all porcine Igs appear to be secreted with light chains (Butler et al., 1985, Klobasa, 1987). Polypeptides separated on polyacrylamide gels can be readily transferred to nitrocellulose or functionalized nylon (PVDF; Gershoni and Palade, 1983) and the transferred polypeptides identified using isotype or light chain-specific antibodies in Western blots (Bjerrum and Heegaard, 1988).
2.2. Characterization of Igs, Ig genes and Ig gene expression
2.2.1. Characterization of the major CH Ig isotypes
The nomenclature of the various porcine Igs is available on the CIgW website <http://www.medicine.uiowa.edu/CIgW>. The description of the various isotypes, subisotypes and light chains are described below.
2.2.1.1. IgM and Sμ
A cosmid library was screened with an oligo nucleotide for the single JH of swine (see below) that allowed identification of a clone that also hybridized with a probe specific for porcine Cμ4. This cosmid was mapped and allowed porcine Cμ, two Cμm exons and Sμ to be characterized (Sun and Butler, 1997). The secreted tailpiece and membrane exons were highly conserved (>90% with other species). Homology to other species decreased from 3′ to 5′. Porcine Sμ is 3.2 kb and contains the three major pentameric repeats known to occur in other species. The frequency of these pentamers places the pig phylogenetically closer to humans than mice.
2.2.1.2. IgD
The presence of a Cδ gene in swine was originally overlooked because genomic clones containing Cμ failed to hybridize with Cδ probes for mouse and human IgD (Butler et al., 1996). However, more recent studies identified an Igδ gene in pig (Zhao et al., 2002, Zhao et al., 2003). The pig Cδ gene is located approximately 3.4 kb downstream of the Cμ gene and consists of 7 exons with three of them encoding CH domains, one for the hinge region, one for the secreted tail and the remaining two for transmembrane tail. Like in cattle, the pig δCH1 shows an extremely high similarity (98.7%) to the μCH1 at the DNA level. This apparently resulted from a recent genetic duplication of the μCH1 and its 5′ flanking sequence extending down to the δ gene. This genetic event may have replaced a pre-existing δCH1 exon. Although only a single functional hinge encoding exon is found in pig transcripts, a second putative hinge exon has been observed in the genomic sequence. The inactivity of this hinge exon is due to lack of a normal branch point sequence for RNA splicing. The data obtained so far suggest that like in humans and mice, the pig δ gene can be co-transcribed with Cμ. However, in pigs, a long primary μ-δ RNA transcript can be processed to either a chimeric μCH1-hinge-δCH2-δCH3 or a unique transcript (δCH1-hinge-δCH2-δδCH3), presenting a novel splicing pathway for IgD that has never been observed in other mammals. As the δCH1 is nearly identical to the μCH1, these two kinds of IgD transcripts differ slightly in their sequences and are probably functionally identical. Why the IgD heavy chain in artiodactyls developed a μ-like CH1 domain is unexplained. Duplication of the μCH1 domain extending to the δ gene also involved its 5′ flanking sequence which contains a short, Sμ (switch μ)-like sequence upstream of the δ gene. The short switch region can mediate class switch region recombination (CSR) in cattle (Zhao et al., 2002, Zhao et al., 2003) and may also mediate IgM to IgD switching in pigs.
2.2.1.3. IgA
Porcine IgA had been identified by various investigators in the late 1960s (Porter, 1969, Bourne, 1969, Vaerman et al., 1970). The IgA protein, both monomeric and as SIgA, fulfilled all the criteria for IgA in other species. It has been used by many to prepare IgA-specific mAbs and pAbs (Section 2.4). The gene encoding porcine Cα was recovered from a cDNA library using a probe for rabbit Cα. Compared to human, mouse and rabbit Cα, lowest homology was in Cα1 and highest with Cα3 (Brown and Butler, 1994). The most surprising initial result was the presence of a mutated splice acceptor site in Cα2 requiring use of a downstream AG site. Later this was shown to only be true for the IgAb allotype, not IgAa (Brown et al., 1995). Studies by Navarro et al., 2000a, Navarro et al., 2000b would show that while IgAa and IgAb allotypes were not equally expressed in heterozygotes, no correlation with disease or illness in IgAb homozygotes (with the truncated hinge) could be identified.
2.2.1.4. IgE
Porcine IgE was recovered from the same cDNA library used for cloning Cα. This was done in tandem with the cloning of equine IgE (Navarro et al., 1995). However, the porcine Cɛ sequence was initially present in two separate clones and only later recloned to provide the complete sequence (Wertz and Butler, unpublished). Vernersson et al. (1997) also cloned porcine Cɛ from a cDNA library eventually obtaining the complete sequence in a single clone. Dendrograms show that porcine IgE is homologous to other mammalian IgEs, and is most similar to that of other artiodactyls, but distinct from any Igs in birds. As of yet, the position of Cɛ in the organization of the CH locus has not been established and reagents for monitoring IgE Ab responses have not been developed (Section 2.4).
2.2.1.5. IgG
It was recognized by Metzger and Fougereau in 1967 and confirmed by others using immunochemical methods (Kaltreider and Johnson, 1972, Franek et al., 1974) that swine had antigenic and presumably subclass variants. Studies by Rapacz and Hasler-Rapacz (1982) demonstrated four sets of allotypic variants, perhaps reflecting four subclasses. In 1994 Kacskovics et al., cloned five unique Cγ genes from a cDNA library and directly by PCR; all of these were believed to represent separate subclasses. More recent studies have provided evidence for six putative subclasses in which the original IgG1 and IgG3 of Kacskovics were shown to be the IgG1b and IgG1a alleles of IgG1 (Butler and Wertz, 2006, Butler et al., 2008a). A previously unreported gene, “new IgG3” was shown to be preferentially expressed in the IPP and MLN of newborns (Fig. 4 ). The number of Cγ genes has now grown to eleven, although issues like allelic variants, subclass function and organization in the genome remain unresolved (Butler et al., 2008a). Characterization of porcine IgG has lead to studies on the evolutionary emergence and diversification of genes encoding this “flagship isotype” of mammals. The original claim of Kim et al., 1966, Kim et al., 1968 for a “19S IgG’ have not been confirmed by others. However, the presence of low molecular weight IgG in fetal sera (Franek and Riha, 1964) may have a modern explanation (Butler et al., 2008a).
Fig. 4.
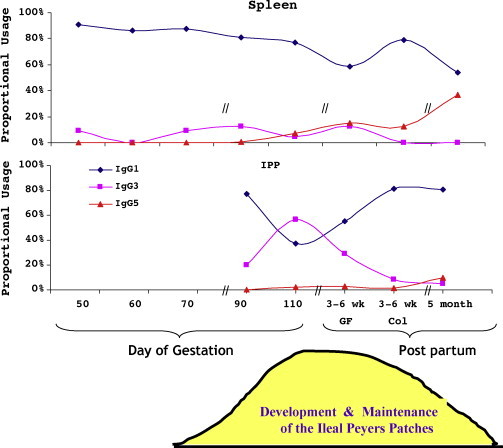
Class switch recombination to IgG occurs during phase 2 of gestation and favors expression of IgG1 throughout fetal life except in the IPP and MLN in late gestation when IgG3 is preferentially expressed in theses organs. IgG3 expression is temporally linked to the time when the IPP is believed to function as a primary lymphoid organ (Butler and Wertz, 2006).
2.2.2. Characterization of porcine light chains
The occurrence of two light chains in swine, kappa and lambda, dates to the work of Hood et al. (1967) based on the N-terminal amino acid sequences. Without the benefit of κ- and λ-specific antibodies or cloned genes, they concluded that swine resemble humans in having nearly equal expression of kappa and lambda. Both Cκ and Cλ were later cloned (Lammers et al., 1991) and the conclusion of Hood et al., was confirmed (Section 3.2.4). Recent studies show that the kappa locus in swine is organized in the same translocon manner as in all other placental mammals (Butler et al., 2004). Data on the organization of the lambda locus remains incomplete although it is clear that tandem Cλ–Jλ repeats are present as in other mammals (Wells et al., unpublished).
2.2.3. Characterization of the VH and VL repertoire
Characterization of the VH and VL repertoire at the genomic level is incomplete but the gene map for VH-DH-JH region will soon be available (Eguchi et al., submitted) Initially most of what is known about the VH and VL repertoire is based on their expression in cDNA. The porcine VH repertoire is encoded by a single family, the vertebrate VH3 family (Sun et al., 1994, Sun et al., 1998, Butler et al., 2006) often considered the ancestral VH family (Schroeder et al., 1987). More than 20 different VH genes have been described although a number of these could be allelic variants (Butler et al., 2006). Swine use just four VH genes to form 80% of their pre-immune repertoire and seven comprise >95% (Sun et al., 1998, Butler et al., 2006). Furthermore, swine possess only one JH (Butler et al., 1996) and DHA and DHB comprise >98% of their repertoire (Sun and Butler, 1996, Butler et al., 2000a). The expressed porcine Vκ repertoire is comprised of two families; Vκ1 and Vκ2. In the pre-immune repertoire, Vκ2 comprises 90% of the total and Jk2 is used in most rearrangments (Butler et al., 2004). Little is known of the Vλ repertoire of swine although two families corresponding to Vλ3 (IGLV3) and Vλ8 (IGLV8) are used (Butler et al., unpublished).
2.3. Characterization of B cells and Ig containing cells (ACC)
The distribution of ACS in various tissues of swine has been studied by many using immunohistochemistry. These include studies on the intestinal mucosa (Atkins et al., 1971, Allen and Porter, 1973a, Allen and Porter, 1973b, Butler et al., 1981) and many lymphoid tissues (Bianchi et al., 1992, Brown and Bourne, 1976) including thymus (Chapman et al., 1974, Bianchi et al., 1992, Cukrowska et al., 1998). B cell phenotypic studies require cell recovery from lymphoid and other tissues. Procedures for recovery from spleen and lymph nodes are well established while recovery from mucosal tissues typically requires proteolytic enzymes that can result in cell damage and poor recovery. Thus, most flow cytometric studies are done using PBMCs and cells free in body fluids such as BAL. Using flow cytometry and the B cell markers described in Section 2.1.3, it has been shown that CD2 but not CD21 can be re-expressed on the surface of one B cells so that CD21 can be considered as a maturation marker. The function of CD2 on the surface of B cells is not known but CD2 is an adhesin which acts during intercellular interactions so it can be down-regulated by cell-to-cell contact. Once B cells recover from such interactions, CD2 expression on B cells is re-established. CD2 may also be regarded as a developmental marker since porcine B-cell precursors express CD2 earlier than the BCR during B lymphopoiesis (Sinkora et al., 2005). CD5 expression can be low or high, and other markers do not correspond with the CD expression profiles used to identify B1 and B2 cells in mice. If the phenomenon of B1 and B2 cells is applicable to the pig, the discrimination between B1 and B2 cells may have to be done at the molecular level. Interestingly, B cells arising prior to the development of B-cell lymphogenesis in the bone marrow (B1 cells?) contain VDJs that are 100% in-frame on one allele while apparently no rearrangement has taken place on the second while those coming from bone marrow (B2 cells?) display the expected distribution of in-frame and out-of-frame VDJs assuming a random process (Sinkora et al., 2003).
2.4. Cloning, expression and preparation of monoclonal antibodies to some B cell relevant molecules
cDNA clones of swine CD19, CD34, and VpreB were cloned by Sun and Butler using RT-PCR and degenerate primers based on other mammalian cDNA sequences. Based on deduced protein sequences, these proteins showed a high degree of the similarity with those in human, 73% (CD19), 63% (CD34), 60% (VpreB) respectively. Generally, the homology is higher in the 3′ cytoplasmic domain compared with that of the 5′ external domains, reflecting the conservative signaling pathways for these molecules. Two isoforms of CD19 cDNA that differ at the 3′ untranslated region were identified. These could be due to the differential use of poly(A) trapping sites. Functionally, two important Fyn binding motifs (YEND/E), which are also observed in human, were also identified in the cytoplasmic domain of swine CD19. Four glycosylation motifs (NxT/S) were found in the swine CD19 external domain. Like CD19, swine CD34 is a glycoprotein containing eight potential glycosylation sites, which is similar to the nine sites identified in human. On the other hand, there is no protein kinase C phosphorylation site in the swine cytoplasmic domain of CD34 compared with two in humans and one in mouse. Monoclonal antibodies were prepared against swine CD19 and CD34 expressed by bacteria. The specificities of the monoclonal antibodies showed an acceptable reaction with recombinant CD19 protein but poor reaction toward the native CD19 expressed in a B-cell line, L23 (Sun et al., 2004). On the other hand, mAbs again CD34 recognized both recombinant and native CD34 molecules as demonstrated in vitro using swine hematopoietic stem cells (Layton et al., 2007).
Pig VpreB has the typical IgV domain structure. The predicted secondary structure has three major beta sheets in the middle of the sequence flanked by two alpha helixes, which is almost identical with those observed in human Vpre (Fig. 5 ). Swine VpreB appears most similar to that of cattle (70%) but shows only 60% homology to VpreB of mouse and human.
Fig. 5.

The comparative amino acid sequence of VpreB from swine, mouse and human.
2.5. The reagent problem and toolkit issues
2.5.1. Antibodies to Ig isotypes and allotypes
Initially, all anti-Ig reagents used to detect and quantify porcine Igs were pAbs raised in heterologous species, rabbits and goat primarily. These reagents were prepared against highly enriched Ig protein fractions and of course required affinity absorption to remove antibodies to light chains or to contaminating proteins and other Igs present in the immunizing fractions. For example, antisera to IgG were rendered specific using affinity columns containing immobilized F(ab′)2 and IgM and IgA antisera were absorbed on affinity columns containing intact IgG and sometimes fetal serum to remove unwanted antibodies to α2-macroglobulin as was done in the preparation of anti-bovine Igs (Butler and Maxwell, 1972, Butler et al., 1981, Klobasa, 1987). The specificity of these pAb reagents was evaluated by Ouchterlony double diffusion and immunoelectrophoresis when used for single radial diffusion (Klobasa et al., 1985a) or using ELISA technology when used in sandwich ELISAs or SpAbI (Butler, 1988, Butler et al., 2000b).
The preparation of mAb for different isotypes, light chains and subclass and allotypic variants was initiated by Paul et al. (1989), Van Zaane and Hulst (1987) and Henning and Nielsen (1992). The specificity of the resultant products was assessed by ELISA and flow cytometry. Since porcine IgM displays no subclass variation and no demonstrated allotypic variation, IgM-specific mAbs differ primarily in titer and affinity. The initial anti-IgA mAbs were thought to recognize subclasses but this difference in specificity was do to the IgAa and IgAb allotypic variants (Brown et al., 1995, Navarro et al., 2000a, Navarro et al., 2000b). Light chain mAbs were characterized by Sinkora et al. (2001).
The preparation of mAbs to porcine IgG subclasses has been problematic for two reasons. On one hand, purified IgG of each subclass have not been available for use as immunogens and on the other, purified IgG of each subclass were not available for specificity testing. This has lead to confusion in the literature since certain companies market products that they call “anti-IgG1” and “anti-IgG2” without proof of specificity which is complicated by the fact that eleven Cγ genes have been described (Butler and Wertz, 2006, Butler et al., 2008a).
In an effort to address this problem, Muyldermanns, Butler and Lunney through the help of the National Porkboard, have embarked on the preparation of chimeric porcine-camelid heavy chain only antibodies (HCAb) composed of a camelid single variable domain (VHH) and the porcine Fc for most of the eleven described porcine IgGs (Butler and Wertz, 2006, Butler et al., 2008c). These chimeric constructs are cloned into a mammalian expression vector and transformed in NSO cell lines to secrete the monoclonal Ab in the culture medium and then purified using protein A or protein G chromatography. The purified HCAb chimeric IgG for each subclass and allotype isotypes can subsequently be used for preparing isotype specific hybridomas or to use as “gold standards” to assess the specificity of the existing and newly prepared anti-IgG mAbs. To date five chimeric HCAbs have been prepared and these have been used to test the specificity of existing mAb. Results indicate that none of the available mAb are subclass specific and some are highly skewed in the recognition of the IgG1b allotypic variant (Butler et al., 2008c) in the manner reminiscent of antibodies to bovine IgG2 (Butler et al., 1994).
One irony has been the failure to develop IgE-specific mAb even though the porcine IgE sequence and plasmids containing it have been available for a decade. This is unfortunate since swine may serve as an important model for certain types of allergies.
2.5.2. Reagent availability
A number of commercial firms offer anti-swine Ig reagents. None of these have come under any rigid scrutiny so that proof of specificity becomes the responsibility of the user since no FDA-like agency governs the quality of immunological reagents. Commercial suppliers do not describe the criteria used to advertise a reagent they describe as “Fc or subclass-specific”. This is critical since specificity is determined by the assay used (Butler, 1991, Butler, 1988). Presently two mAb that have been widely used and tested are available. These are M160 (ABRM 0521) and M1459 (ABRM 0621). The Center for Veterinary Biologics (CVB) of the USDA has initiated a mAb repository to provide same-source and quality mAb to the research community. The goal is to expand the project to include other well-characterized mAbs specific for other porcine Igs as they become available. Detailed information on these reagents, their original source and how they can be obtained from the CVB is available on the CIgW Website <http://www.medicine.uiowa.edu/CIgW>.
2.5.3. Antibodies to CD markers on B cells
The current status of B cell markers for use in flow cytometry was discussed in Section 2.1.3. A listing of the results of specificity testing is available through the International Swine CD Workshops (Boersma et al., 2001) and from the HLDA8 cross-reactivity studies (Saalmüller et al., 2005, Saalmüller and Aasted, 2007). These studies established a register of mAb reacting with swine B cells. There are also other sources of information on swine CD markers; the US Veterinary Immune Reagent website at www.vetimm.org and USDA-ARS PIN website: http://www.ba.ars.usda.gov/nrfl/nutri-immun-db/cdpin_display_record1.cfm. As indicated in Section 2.4, there are still no mAbs available for such B cell markers as CD19, CD43, VpreB, c-kit and FcγRII but recently an mAb for CD34 has been described (Layton et al., 2007).
3. Fetal piglet studies
3.1. Fetal development and procedures in fetal research
3.1.1. Embryology and placentation
Swine fetuses are relatively large and the gestation is conveniently long allowing changes in development to be followed and for surgical manipulation (Table 1). The 114-day gestation can be divided into three biologically relevant phases. The first phase begins with conception and ends with the beginning of calcification of the skeleton at about 30–35 days of gestation (DG), a point in development when the major internal organs are distinguishable. The second phase involves growth of the fetus and concludes at about 70 DG with the development of the innate immune system that allows responsive to some Ags. The last phase concludes with parturition at which time the newborn piglet has a naïve adaptive immune system, natural antibodies (see below) and is responsive to some pathogens.
3.1.2. Laparotomy, fetal surgery and fetal inoculations
The effect of inhalant anesthetics during surgery seems to have no ill effect on the fetus. Routine surgical procedures can be used to exteriorize the uterus for inoculation of fetuses. From about 35 DG direct inoculation of the fetus (transuterine since the fetus is not exteriorized), or through amniotic fluids is relatively easy as long as there is no need to inoculate a specific organ or site. Relatively small inoculum volumes (<250 μl) can be given safely with a 25 gauge needle. Contamination of the sow from the fetal inoculation is minimized by (a) using a new needle for each fetus or (b) using a double needle method to prevent the needle penetrating the fetus from contacting the uterine wall. If the inoculum is an infectious agent, it puts the sow at risk of infection which can confound the experiment since the maternal infection may spread to fetuses. Inoculations through the uterine wall that target specific organs require additional equipment such as ultrasonic devices.
At the Institute of Microbiology Czech Academy of Science (IMCz) miniature Minnesota-derived gilts between 60 and 100 DG are pretreated by s.c. application of atropine sulphate (0.5 mg per 30 kg of body weight) and anesthetized by halothane mixed with O2 and N2O. Antibiotic pre-treatment of gilts is used if fetuses are given living Escherichia coli. Gilts are positioned in dorsal recumbency, laparotomy is performed and the uterus is exteriorized. Uterotomy is performed if subcutaneous or i.v. application of experimental agents to the fetus is required. Depending on the DG, the uterus and amniotic membrane are incised together or separately and a part of the fetus pulled from the amniotic cavity to apply the agent. Warm and wet (37 °C in non-pyrogenic saline) veils are used to cover parts of the uterus especially during more complicated and time-consuming surgery. Bacteria or sham treated fetuses are then returned to the amniotic cavity and the amniotic membrane and uterine wall are sutured. The abdominal wall is sutured in three layers (peritoneum, muscle and skin). Subsequent surgery to obtain fetuses after the experimental period is performed in a similar manner after treating the gilt with progesterone.
Subcutaneous and intradermal inoculation of fetal piglets with PPD has been described (Trebichavsky et al., 1992). Umbilical vein inoculation has been used for application of inactivated TGEV (Splichal et al., 1997) in experiments with Nocardia delipidated cell mitogen (Rehakova et al., 1998) and in LPS-treated fetuses (Trebichavsky et al., 2002). These procedures require hysterotomy and amniotomy. This and additional manipulation with the umbilical cord increase mortality rates of pig fetuses after the surgery. Mammalian fetuses swallow amniotic fluid in the second part of gestation. Therefore, non-invasive intra-amniotic application can be used for oral administration of Ag to fetuses. This route of inoculation was successfully used for LPS (Trebichavsky et al., 2002) and for induction of inflammatory cytokines by different strains of E. coli (Splichalova et al., 2004, Splichalova et al., 2005). Intrauterine breathing movements probably explain why intra-amniotic applied E. coli bacteria can be found in the fetal airways (Splichal et al., 2002).
Due to the diffuse placentation in swine, it is very difficult to collect blood from the umbilical cord or exteriorize the umbilical cord. Typically, fetuses in one uterine horn will receive a treatment and fetuses in the other uterine horn will receive a sham treatment. The serosal surface of the uterus can be marked with suture to identify an injection site so that at the time of necropsy of the sow, one can identify a specific fetus based on uterine position. Upon euthanasia of the sow, the gravid uterus should be removed from the sow as quickly as possible to access the fetuses. Whole blood can be collected via puncture of the umbilical vein or heart. If the experimental design requires the inoculated fetus to be born alive, it is possible to identify fetuses with an intradermal injection of indelible dye. However, this method is not fail-proof, and like all intrauterine injections, sterility is absolutely necessary since any microbial contamination can result in fetal death and loss of the pregnancy.
3.2. Fetal development of the B cell repertoire
3.2.1. B cell lymphogenesis
B cell lymphogenesis begins in the yolk sac at DG20 as determined by VDJ rearrangement. However, there is little or no transcription at this site (Sinkora et al., 2003). With the gradual disappearance of yolk sac, both VDJ rearrangements and VDJ transcripts are seen in fetal liver at DG30 (Sinkora et al., 2003, Butler et al., 2000a). Light chains are first transcribed at DG40 in fetal liver and Cλ is favored 20:1 although this may reflect λ5 transcription (Butler et al., 2005b). IgM containing cells (ACS) are present by DG45 (Sinkora et al., 2005) and protein and Cμ transcripts are present in BM and spleen from DG60-110 (Butler et al., 2000a). Cμ transcripts and IgM ACC appear in thymus at DG90 along with IgG and IgA ACC (Butler et al., 2001, Cukrowska et al., 1998, Bianchi et al., 1992). Cμ transcripts are not seen in the ileal Peyers patches (IPP) until DG110, nor are those for IgA and IgG, although no tests were conducted with probes for IgE or IgD. The IgM and IgA repertoires at DG110 in the IPP is totally unselected (Navarro et al., 2000b). However, IgD and IgE transcripts are widespread at birth including expression of IgE in thymus (McAleer et al., 2005). IgG transcripts, especially IgG1, are present in fetal spleen as early as DG50 (Butler and Wertz, 2006; Fig. 4). B cell lymphogenesis follows the pattern described in mice and humans with two notable exceptions: (i) CSR occurs early in fetal life in the absence of environmental Ag and somatic hypermutation (SHM) and (ii) the presence of B cells expressing all isotypes are present in thymus. The former may reflect a stochastic event (Deenick et al., 1999) and also indicate that activation induced cytosine deaminase (AID) may alone not explain CSR and SHM. Occurrence of B cell-associated transcripts in thymus may suggest that abortive B cell lymphogenesis occurs in this organ. The latter is consistent with the apparently large numbers of cells that have accumulated in the subcapsular region that display unselected VDJ rearrangements but do not express surface Ig (Butler et al., 2000a, Sinkora et al., 2005). Modern studies and those of Chapman et al. (1974) and Binns and Symons (1974) supersede studies indicating that fetal piglets have no ACS or serum Igs (Kim et al., 1966, Kim et al., 1968, Kim, 1975).
3.2.2. Natural or pre-adaptive antibodies
Fetal piglets especially in the last phase of gestation (DG70+) have low levels (10–27 μg/ml) of IgM, IgA and IgG in blood; the latter accounting for >80% of total Ig (Butler et al., 2001). Transcripts encoding these Igs contain non-mutated VDJs, i.e., germline VDJs. These comprise the natural antibodies that in better-studied species have broad specificity, high connectivity, and low affinity; they also harbor autoreactive antibodies (Dighiero et al., 1985). These natural antibodies are considered part of the innate immune system (Ochsenbein and Zinkernagel, 2000) and their broad specificity allows recognition of most common pathogens.
3.2.3. VH repertoire development
From the earliest point of VDJ rearrangement, four major VH genes dominate the pre-immune repertoire and this dominance remains unchanged throughout fetal life (Sun et al., 1998). These have been designated VHA, VHB, VHC, VHE and VHF and account for ∼80% of the VH repertoire; by including VHF, VHX and VHZ >95% of the repertoire can be accounted for (Butler et al., 2006). As described in Section 2, swine have only a single JH and DHA and DHB account for nearly all DH usage in fetal and adult animals. TdT is active from the onset of VDJ rearrangement at DG20 in yolk sac and continues at apparently the same level of activity through fetal life (Butler et al., 2000a, Sinkora et al., 2003). The mean length of HCDR3 remains at 41.5 ± 9.2 bp through fetal life. Thus, fetal VH repertoire development in piglets differs from that in mice and humans because (i) Tdt is active throughout B cell lymphogenesis and (ii) combinatorial diversity is limited to a few VH genes, two DH segments and one JH.
3.2.4. The Vκ and Vλ repertoire
Light chain transcripts are present in fetal liver at DG40 with a surprising 20:1 dominance of Cλ which persists in BM throughout fetal life. The prominent Cλ usage in fetal liver and BM probably reflects transcription of λ5, part of the surrogate light chain complex. This complex is believed to exist in swine since the gene encoding VpreB has been cloned (Section 2.1.2; Fig. 5). However the Cκ:Cλ ratio is 1:1 in spleen during midgestation, ∼2:1 in thymus (Butler et al., 2005b) and ∼3:2 in adult peripheral B cells (Hood et al., 1967).
Ninety percent of the fetal and newborn pre-immune Vκ repertoire is comprised of the IGKV2 family (Vκ2); the remainder is contributed by IGKV1 (Vκ1; Butler et al., 2004). As discussed in Section 2, both families are highly homologous to their human counterparts. Of the five recognized Jκ genes, Jκ2 accounts for 90% of the pre-immune repertoire. Much less is known about the Vλ repertoire in fetal life, although IGLV3 and IGLV8 are preferentially used (Butler et al., unpublished).
While studies on Cλ usage are incomplete, two features of light chain usage are notable: (i) the restricted pre-immune Vκ repertoire resembles the restricted VH repertoire of this species and (ii) the nearly equal usage of Cκ and Cλ resembles humans. The latter is disparate from rodents and rabbits that favor Cκ and other hoofed mammals that strongly favor Cλ (Table 2).
4. Studies on newborn piglets
4.1. Survey of models and procedures
4.1.1. Derivation of germfree and gnotobiotic piglets
Pasteur first questioned whether animals could live free of bacteria and reasoned that they could not, but suggested that the concept be tested (Levenson et al., 1959). About a decade later, Nuttal and Thierfelder (1895–1896) addressed the issue and raised a guinea pig germ-free for 13 days. Others followed, raising animals of several species bacteria-free, including chickens, goats and rodents (Glimstedt, 1936, Wostmann, 1959). By the mid-1940s experience and technology had allowed researchers to not only maintain animals germ-free for long periods of time, but to also breed them under those conditions (Levenson et al., 1959). Germfree animals are those reared without exposure to environmental bacteria, fungi or known viruses. Gnotobiotic refers to animals that have been provided some type of known microbe such benign E. coli. The pig is an attractive model for postpartum immunological studies because the six-layer epitheliochorial placenta is a barrier between maternal and fetal circulation preventing translocation of Igs. Newborn piglets must acquire protective antibodies from colostrum (Fig. 1) so colostrum-deprived piglets are not able to survive in conventional conditions and readily succumb to bacterial sepsis. However, colostrum-deprived sterile-derived piglets (CDCD) survive well in isolators. Piglets can be delivered from full-term pregnant sows by hysterectomy or hysterotomy (caesarian section). Pregnant sows must be obtained from farms free of porcine reproductive and respiratory syndrome virus (PRRSV), and vaccinated against parvovirus and porcine circovirus (PCV2). Infection of the sow with any of these agents may result in stillborn piglets, or the infection of piglets in utero or during caesarian section. Hysterectomy is performed on a sow hoisted into a vertical position by the hind legs and anesthetized by CO2 gas. The uterus is quickly removed through a midline incision and placed in a stainless-steel isolator through a germicidal trap containing chlorine bleach. Piglets are extracted from the uterus and placed in attached piglet rearing isolators, which are later sealed off from the surgical isolator and detached. Hysterotomy is performed by attaching a flexible film isolator to the skin on the flank of an anesthetized sow and working through gloves in the isolator, making an incision using an electric cautery through the plastic film and the integument of the sow. After the uterus is exposed and opened, piglets are removed and passed into an adjacent isolator for cleaning and umbilical cord clamping and trimming. Miniats and Jol (1978) compared the hysterectomy and hysterotomy methods of germ-free piglet derivation for efficiency and freedom from contamination. Hysterectomy under general anesthesia requires fewer personnel hours; four hrs verus seven hrs for hysterotomy. Much of the greater surgery time demand of hysterotomy is associated with suturing the sow, which is rehabilitated. By contrast, hysterectomized sows are euthanized. Piglet survival was similar for the two methods (84–90%), but bacterial contamination is greater (23% versus 9%) following the hysterotomy method.
The precise derivation process, construction of isolators, distribution of piglets to isolators and maintenance procedures differ somewhat at various facilities. This review describes the procedures used at the National Animal Disease Center (NADC), South Dakota State University (SDSU) and IMCz. Investigations at the NADC over the years have shown that successful derivation of germ-free piglets is dependent on several criteria: (i) sterilization of pig isolators, (ii) aseptic removal of the piglet from the uterus, (iii) transfer into the isolator and (iv) maintenance of a sterile environment for the pig. Achieving these criteria involves the following steps. The isolator units must be cleaned with sodium hypochlorite, soap, and water. Once clean, the isolator is loaded with all of the diet and supplies (syringe, needles, blood tubes, etc.) needed for the duration of the experiment. The isolator is then sterilized with ethylene oxide and vented prior to use. Intake and exhaust air is HEPA-filtered. Piglets are aseptically derived from the sow by caesarian section at 113 days of gestation and then immediately passed into a sterile isolator through a dip tank filled with an iodine-based solution. Piglets do not develop a breathing reflex for at least 10 s after removal from the uterus which may be related to the use of an inhalant anesthesia. Piglets that do not quickly initiate breathing can be given doxapram hydrochloride. Aseptic techniques must be used to transfer piglets into additional isolators as needed and isolators are maintained at an ambient temperature of 32 °C and gradually decreased to a maintenance temperature of 27 °C.
The hysterotomy method of piglet delivery into germ-free isolators is employed SDSU. The pregnant sow is brought to the large animal surgery facility on gestation day 112, and the hair on her right flank is removed by clipping. The sow is walked into the surgery suite and strapped to a hydraulic surgery table placed in vertical position. Experience at the Institute of Animal Husbandry at Mariensee showed that endocrine stress could be reduced by walking the sow daily to the surgical suite for 4 days prior to surgery. Once on the table, skin above the epidural space in the lumbosacral area is surgically prepared and 5 cc of lidocaine hydrochloride (2%) is instilled under the skin. A spinal needle is placed into the epidural space and 20 cc of lidocaine hydrochloride is injected. After 5–10 min, when the hindquarters of the sow are relaxed due to the epidural anesthesia, the animal is placed in left recumbency by tilting the surgery table to its horizontal position and the animal secured onto the table surface. The right flank of the sow is scrubbed three times with povadone iodine surgical scrub after cleansing with a strong disinfectant such as 1-Stroke Environ™ (Steris Inc., St. Louis, MO). The povadone iodine scrub solution is removed by washing with sterile phosphate-buffered saline solution, and the skin surface is dehydrated by a final wash with 95% ETOH. The alcohol is evaporated to leave a dry skin surface using a hand-held hair dryer. The dry skin surface is then coated with an iodine povacrylex-containing surgical skin preparation solution (DuraPrep, 3 M, St. Paul, MN) and dried with assistance of the hair dryer to form a thin plastic film. The skin of the sow's right flank is then coated with a thin film of livestock identification tag cement (Ruscoe Company, Akron, OH) applied with the use of a tongue depressor. The cement is cured to a tacky condition by application of heat from the hair dryer for approximately 4 min. Simultaneous to the surgical preparation of the sow, a similar coat of tag cement is applied after alcohol cleansing to the surface of the surgical bubble (see below) and allowed to cure for about 10 min. The surgical bubble is then adhered to the right side of the sow such that the cement on the skin of the sow, and that on the surface of the bubble bond together. The area covered by tag cement is about 25 cm × 25 cm, sufficient to secure the surgical field and retain bubble inflation after surgical incision. At SDSU, piglets are delivered into the isolators through a polyethylene flexible film tube (52 in. circumference; Home Plastics, Inc., Des Moines, IA) that is fitted to the port of an isolator. Both ends of the tube are sealed with adhesive tape, but an opening is cut in one end of sufficient size to tape the bubble to the isolator port. Pairs of circular holes are cut through either side of the tubing to accommodate shoulder-length surgical gloves (QRP Gloves, Inc., Tuscan, AZ), which are attached to the tubing using rings of PCV drain pipe taped to the tubing, with gloves attached to the PCV pipe with metal ring clamps. Surgical supplies, cloth towels for drying piglets, and navel clamps, all steam sterilized in wrapping paper, plus piglet feed and animal care supplies are imported into the isolator before attachment of the polyethylene tubing that will serve as sterile surgical bubble (Fig. 6C). The interior of the isolator and surgical tube bubble are sterilized with formaldehyde vapor dispersed by placing 40% formaldehyde in a metal can containing potassium permanganate which oxidizes in the presence of water, vaporizing the formaldehyde. Formaldehyde is purged from the isolator and surgical bubble through an exhaust hose attached to the exit filter, after 24 h of sterilization. Due to impregnation of towels, sponges, and sterilization wrapping paper with formaldehyde, purging may require 7–10 days. Once the surgical bubble is secured to the sow, a right flank incision is made through the side of the bubble adhered to the sow, and the uterus is exteriorized into the inflated bubble. Longitudinal incisions (typically one per piglet) are made in the uterus, and the piglets are removed. An umbilical clamp is placed on the umbilicus of each piglet and the cord is cut. Each piglet is passed through the isolator port into a sterile gnotobiotic unit and cleaned with a sterile towel. If at anytime during the surgery, the sow appears sensitive to surgical manipulation, isoflurane anesthesia with nitrous oxide and oxygen is given by mask from an anesthetic machine (SurgiVet, Waukesha, WI). However, this is to be avoided when possible, as piglets extracted subsequent to the use of gas anesthesia exhibit substantial lethargy, and require substantial manual manipulation before they begin to breathe spontaneously. After all piglets are delivered, the sow is euthanized by electrocution, following sedation with Telazol™ (Ft. Dodge, IA).
Fig. 6.

Gnotobiotic and autosow facilities. (A) Gnotobiotic isolator at SDSU with the surgery bubble attached to isolator entry port. (B) Gnotobiotic isolators shown from port end. The exterior port cap is taped in place to prevent loss due to interior pressure when the interior port cap is removed. The interior port cap is visible through the isolator canopy in the first isolator. (C) View from the top of a gnotobiotic the isolator through isolator canopy. A stainless steel wire mesh rack is fixed to the top of the isolator pen dividers and is used for storage of milk replacer and other supplies. The can in the port contains potassium permanganate used as a catalyst to boil and vaporize the formaldehyde sterilizing solution. The yellow band around the lip of the isolator is a motorcycle clamp used to prevent internal air pressure from pealing tape away from isolator. (D) The autosow facilities at the Institute of Animal Husbandry, Mariensee, Germany. Each animal is housed separately so that an accurate measurement of the amount of milk or milk replacer consumed can be determined. Feeding time is computer controlled. The mixing and warming chamber for the milk is visible just below the thermostatically controlled heat lamps. (E) Back side of the autosow feeding device showing the system used to measure the amount of milk consumed and the chambers containing the wash and rinse fluids of the automatic feeder washing system.
Piglets initially delivered into a single isolator are distributed among several isolators by means of a flexible transfer sleeve installed between isolator ports and fogged with peracetic acid solution after closing off port cylinders with inside caps. The piglets placed individually in isolator compartments more readily learn to feed from a bowl than piglets group housed, because they are not distracted by each other and the natural tendency to nurse on each other's umbilical stumps. This is also true for piglets prepared for entry into an autosow (see below).
4.1.2. Maintenance of isolator piglets
Once established in isolators at the NADC, piglets are fed a commercially available pasteurized milk diet twice daily for the duration of the experiment with increasing quantities to a maximum of 370 ml per feeding. At SDSU piglets are fed milk replacer (Esbilac; PetAg, Inc., Hampshire, IL) three times daily, and the amount of milk replacer is gradually increased to accommodate the growing piglet's nutritional needs. A typical feeding schedule is 8:00 a.m.; noon and 5:00 p.m. Night feeding is not required. At SDSU the average daily consumption of condensed cow's milk when given free choice was 0–9 days: 300 ml; 10–13 days: 1125 ml; 14–23 days: 1350 ml; 24–33 days: 1575 ml and 34–43 days: 1800 ml. Piglets given milk replacer free choice became obese, with clinical signs of cardiac insufficiency. In the autosow system (see later) the feeding cycle and dosage is computer programmed and driven. Isolators are designed (see below) to have one or more ports that can be decontaminated with atomized chlorine dioxide. The ports are used to pass into the isolator challenge inoculum and to pass out samples and pigs. In addition, isolator design takes into account the storage of liquid and solid wastes for the duration of experiment, or there is a disposal system that prevents contamination of the isolator. The isolator room temperature should be about 92 °F (33 °C) for newborn piglets and allowed to be lowered to 27 °C for maintenance. Several days after delivery, piglets are tested for contamination by rectal culture.
4.1.3. Construction of germ-free Isolators
Early germ-free animal studies were conducted using rigid isolator units which were expensive to manufacture and difficult to transport and maintain. The use of flexible film isolators for rearing germ-free animals was first reported by Trexler and Reynolds in 1957. However, due to damage caused to vinyl or polyethylene fabric by heat sterilization, chemical methods of sterilization had to be devised. Peracetic acid in 2% solution, with 0.1% sodium alkylarylsulfonate was used. The peracetic acid was very corrosive, and materials such as plastics, glass and stainless steel had to be employed in isolator equipment. Both rigid and flexible film isolators utilized rubber gloves attached with ring clamps to manipulate materials and animals within isolator chambers. Plastic film joints were sealed with adhesive tape. Air filters were constructed of stainless steel mesh cylinders covered with glass wool and shrouded with plastic. At SDSU piglet isolators are custom manufactured stainless steel tanks that are four feet long, two feet wide and two feet high (Fig. 6). Each is divided with three removable partitions providing for four piglet pens suitable for neonatal piglets when all partitions are in place. For studies requiring accommodation of piglets beyond 2 weeks of age, it is recommended that two of the partitions be removed and only two pigs be housed in an isolator. As isolators become soiled over time it is recommended that for experiments lasting 4–6 weeks that piglets be housed four at a time for the first 2 weeks, then transferred to fresh 2-pen isolators every 2 weeks thereafter. Isolators of the type described are not suitable for conventional piglets older than 6 weeks of age although mini-pigs could be held longer. Each pen contains a perforated stainless steel floor insert that raises the pen 2 in. above the base of the isolator unit. The insert is fitted with slotted plastic mat, for piglet comfort, improved traction, and to facilitate waste drainage. A metal feed pan is bolted to either side of each partition. A rigid stainless steel mesh platform used for feed and equipment storage is secured to the top of the isolator pens, and provides a place to hold piglets for treatment. Each isolator unit is mounted on a stainless steel cart fitted with a cylindrical isolator port, an inlet and outlet filter, and a blower unit attached to the inlet filter. A flexible film canopy (Standard Safety Equipment Company, McHenry, IL) fitted with one pair of sleeves on each side is attached with adhesive tape (Vinyl #471; 3 M, St. Paul, MN; Fig. 6A) to the stainless steel isolator tank, entry port and filters. Rubber gloves (Lab Safety Supply, Inc., Janesville, WI) are fitted to each of the sleeves with a plastic collar and attached with adhesive tape. The canopy rises 24 in. above the isolator and is inflated by the differential pressure between the inlet filter and outlet filter. Attachment of the canopy to the isolator port is near its linear center to allow for attachment of a flexible film cap to each end of the port cylinder. Internal air pressure secures the internal port, but the external port must be secured with adhesive tape. The external cap is fitted with two cylindrical fittings through which aerosolised peracetic acid can be injected with a pressure sprayer as surface sterilizing agent for items such as canned piglet formula or packaged specimens passed into or out of the isolator (sterilization time is 20 min). When not in use, the fittings are plugged with rubber corks to preserve port sterility (Fig. 6B).
At the IMCz the isolators for breeding of pigs are fabricated mainly from flexible and transparent material (these isolators require an inner cage), fiberglass or stainless steel (both types are simultaneously the cages). Integral parts of the isolator are an entry part (enabling the transport of materials into and out of the isolator and their sterilization with 2% peracetic acid, respectively), input and output filters and gloves for the manipulation inside the isolator. Unlike the studies at SDSU and the NADC, miniature pigs (e.g., Minnesota, Hanford, Göttingen, Yucatan) are used for breeding in conditions of space-limited isolators. In general, conventional piglets can be maintained a maximum of 6 weeks in the isolators while minipigs can be housed for 8–9 weeks. In comparison with laboratory rodents, the piglets have at least two advantages: large newborns and discontinuity of the breeding. Both save money and labor. The miniature Minnesota-derived piglets are derived from pregnant gilts (less than 30 kg of body weight) that are i.m. treated with A and D vitamins on the 80th day of gestation and with synthetic progesterone on the 105th day of gestation to keep the uterine cervix closed. They were treated s.c. with 0.5 mg atrophine sulphate on the 112th day of gestation, and hysterectomy was performed under halothane anesthesia (1.5–2.5% and 50:50 oxygen and nitrous oxide combination) in dorsal recumbency. Laparotomy was performed in the middle part of abdomen and the uterus was exteriorized, cut and moved through 6% chloramine solution into a sterile birthing isolator where the piglets were removed from the uterus. It was necessary to apply iron and vitamin K to piglets as soon as possible to cover requirements of fast growth and later vitamins B group too (Mandel and Travnicek, 1987). The sterility of germ-free piglets was tested by rectal swabs obtained twice a week and cultivated for aerobes and anaerobes.
4.1.4. Conventional piglets and early weaning
Compared to germ-free pigs, conventional pigs, i.e., pigs that are derived the “old-fashion way”, are much easier to produce. However, vertical transmission of pathogens and exposure to undefined Ags are major confounding factors when studying the immune response. Natural birth of the pigs, or farrowing, produces pigs that are exposed to microbes and have ingested colostrum. Both of these events are important variables; e.g., microbial exposure may involve ingestion of pathogenic and/or non-pathogenic microflora in differing quantities (Fig. 2). Likewise, one pig may ingest more or less colostrum when compared to a littermate (Section 4.2.2). Moreover, the character of colostrum can vary among sows from the same farm, and the “normal” farm-specific microflora is highly variable among farms. In the case of IgG, levels in serum and colostrum can vary 2-fold, with first litter gilts having the lowest levels (Klobasa et al., 1985b) and older sows the highest IgA levels (Klobasa and Butler, 1987a). The protocol for deriving and maintaining “clean” conventional pigs involves early weaning of pigs to reduce the potential for vertical transmission of pathogens. First, the sow is placed into a clean farrowing crate in a clean room at least a few days prior to parturition. Up to the time of farrowing the sow, crate, and room are kept as clean as possible. About day 112 of gestation (114 days is normal gestation length for sow), an additional heat source is supplied to the crate (heat lamps) to provide a warm space for the neonatal pigs. Second, after farrowing cleaning of the crate is minimized for a couple of days to avoid soaking the newborn pigs. Third, at 1–2 days of age supplemental milk (a commercially available milk powder that is mixed with water) is provided in small amounts. Since pigs are very precosial, they can be weaned at 2–3 days of age. This early weaning reduces environmental influences but also requires significant attention to animal care and feeding.
4.1.5. Caesarian-derived colostrum-deprived piglets (CDCD)
Like germ-free pigs, CDCD piglets are aseptically derived from the sow by caesarian section, and have no contact with their mother. Since CDCD pigs are not raised in a sterile environment and they have no passively acquired protection, they are very susceptible to microbes that would be non-pathogenic in a conventional pig. Thus, raising CDCD pigs can be more of an art than a science. Although CDCD pigs may be vulnerable to normal microflora, they should be free of most pig pathogens making this type of pig a relatively cheap alternative to germ-free pigs in certain circumstances. At the NADC surgically derived pigs are transferred to suitable “clean rooms”. This housing may use individual cages or a raised-deck that accommodates a group of CDCD pigs. CDCD pigs are fed commercially available pasteurized milk or a neonatal livestock milk replacer.
4.1.6. SPF autosow piglets
Jimmy Leece designed special controlled feeding devices to be used to rear CDCD piglets and/or those collected directly from the disinfected birth canal into plastic bags (Leece, 1969). While the latter does not assure lack of contamination it (i) is adequate for physiological and nutritional studies and (ii) avoids laparotomy and often the sacrifice of the mother. Rather than expensive milk replacers, autosow piglets are reared on bovine colostrum and milk, which although not sterile, provides nutrients for good growth. This procedure reached its highest development at the Institute for Animal Husbandry in Mariensee, Germany. These studies involved elegantly designed cages in which the amount of cow's milk delivered and consumed was computer controlled and measured for each cage (Fig. 6D). The feeding cycle and dosage is programmed to simulate normal nursing intake and intervals, including a sleep cycle.
4.1.7. Immunization and inoculation of newborn piglets
4.1.7.1. Virus
Immunization and inoculation of newborn piglets can be accomplished through drenching of the oral and nasal cavities and by injections (subcutaneous, i.m., intradermal, intravascular). Inocula must be sterile from adventitious agents and physiologically compatible with the animal, e.g., pH is about 7.4. Intravenous injection can be via ear vein, internal jugular vein or cranial vena cava. Studies investigating the respiratory mucosal immune response are accomplished by dribbling the inoculum into each nostril while the pig is restrained with its snout held up. This procedure in a conscious pig results in inoculation of the oropharyngeal cavity, and may lead to inhalation, ingestion of inoculum and some loss by sneezing. Injections are typically followed by an antiseptic solution applied to the injection site.
4.1.7.2. Model Ags
Model Ags have been developed in studies of rodents and rabbits in which the epitope is chemically defined and the macromolecular “carrier” has well-defined properties. For example, macromolecules like bacterial LPS that display repeating epitopes are thymus-independent (TI) which means that an Ab response does not require T cells and in some cases the immunogen behaves like a mitogen (TI-1). Some weak T cell help may be needed in responses to large carbohydrate Ags (TI-2). The third category are those immunogens that are T cell dependent (TD) and lead to pronounced secondary responses, affinity maturation and CSR. Well described models for each include: LPS = TI-1, polymerized dextran (Ficoll) = TI-2 and essentially all foreign protein = TD. TI-2 immunogens represent the common polysaccharide Ags of bacterial capsules. The vast majority of foreign Ags, plasma proteins, viral glycoproteins, are of the TD variety. With this background it is possible to chemically construct model Ags for the TI-2 and TD categories by conjugating a defined chemical group such as phosphorylcholine (PC), dinitrophenyl (DNP), 4-hydroxy-3-nitrophenyl acetyl (NP), and arsanilic acid (ARS) to immunogenic carriers like foreign IgG, albumin, ovalbumin or Ficoll. The advantage of such model Ags is that the defined epitope is available as a hapten and responsiveness exclusively to the epitope can then be measured or it can be used as a competitive inhibitor (Butler et al., 1985, Dillender, 1990). In fetal and neonatal piglet studies, conjugates of PC-Brucella (TI-2), PC-ovalbumin (TD), TNP-Ficoll (TI-2), fluorescein-Ficoll (TI-2), fluorescein-KLH (TD or TNP-KLH (TD) have been used (Butler et al., 1985, Butler et al., 2000b, Butler et al., 2002, Butler et al., 2005a).
4.2. Results of studies on isolator, CDCD and autosow piglets
4.2.1. The critical window of neonatal development
Fig. 2 describes the critical window concept and the factors that influence development during this period. These represent factors that are experimenter-controlled in the various models described above. For example, all piglets that are deprived of colostrum are models for evaluating its effect on development. The autosow system is most amenable since piglets can be administered computer-controlled dosages of milk/colostrum. This is cumbersome in a simple CDCD system in which animals are reared in clean rooms and ingestion by individual animals is difficult to control. Moreover, both CDCD and autosow piglet studies can be compromised by inadequate control of bacteria. While bacterial control in germfree or gnotobiotic facilities can be obtained, controlled administration of milk and colostrum to each animal is labor intensive and at risk for contamination. Regardless of the weaknesses of each system, all have contributed valuable information for understanding immunological events during the “critical window”.
4.2.2. Data obtained using autosow piglets
Autosow studies have enhanced our understanding of events during the “critical window” in several ways. These include the (i) greater understanding of intestinal uptake of maternal Igs from milk and colostrum by suckling piglets and (ii) regulatory effects of absorbed Igs on de novo synthesis of Igs by neonates.
Conventional piglets absorbing only 10–50% of the average for littermates fail to survive. Using the autosow piglets, the 2–8-fold differences in uptake was nearly eliminated suggesting that environmental factors that cannot be controlled in the conventional setting, rather than genetic differences, were responsible for differential uptake in conventionally reared piglets and consequently survival (Klobasa et al., 1981). Neither studies with conventional animals nor autosow piglets provide evidence for selective uptake of one isotype such as in the case of FcRn-mediated uptake of IgG in rodents (Guyer et al., 1976, Borthistle et al., 1977). Both polymers like polyvinyl pyrrolidone (Leece and Morgan, 1962) and homologous and heterologous Igs of all isotypes (Werhahn et al., 1981, Klobasa et al., 1990) are taken up indiscriminately by the neonatal gut in the period immediately after birth. Thus uptake does not appear to be receptor-mediated. However, the non-specific uptake of IgG, IgM and IgA by the gut epithelium is indirectly proportional to the time after birth when animals first suckled. In conventional piglets this uptake was reduced 6–8-fold when feeding was delayed for 24 h. Autosow studies showed that the decreased uptake was due to the progressively lower amount of Ig available as the transition from colostrum to mature milk occurred (Klobasa et al., 1981, Klobasa and Butler, 1987b). Studies show a consistent 1:2.5 to 1:3 ratio of ingested Ig (all isotypes) to Ig appearing in blood (Werhahn et al., 1981).
Autosow studies also showed that glucose ingestion could block intestinal uptake (Leece, 1966, Werhahn et al., 1981) and that feeding heterologous protein results in similar “closure”. When 125I porcine IgG was given 3 hr after birth, intact IgG appeared in blood whereas if bovine colostrum was fed and 125I-IgG first given 72 h later, only low molecular weight peptides appear in blood (Werhahn et al., 1981). This result suggests that early ingestion, followed by a day long delay in suckling, could explain the poor survival of some conventional piglets (Klobasa et al., 1981).
Autosow studies combined with conventional studies also revealed that the de novo production of Igs by suckling newborn was indirectly proportional to the amount of Ig they had absorbed into blood (Klobasa et al., 1981). It was also shown that “experienced sows” had a greater impact on this “immunosuppression” than first litter gilts (Klobasa et al., 1985b) and that bovine colostrum produced the same suppression (Klobasa et al., 1990). This lead to two discrete hypotheses that remains untested. First, piglets absorbing less maternal Ig from colostrum may be less protected and more likely to respond to environmental Ags with elevated Ig levels. Alternatively, maternal colostrum may contain a variety of immunoregulatory factors that may include anti-idiotypic antibodies (Wikler et al., 1980, Rodkey and Adler, 1983) that form immune complexes and suppress B cells through the FcgRIIβ pathway (Phillips and Parker, 1983) or provide immunoregulatory peptides (Janusz et al., 1987; Butler, 1998, 2005). Suppression was lost upon storage of bovine colostrum, suggesting a labile regulatory factor (Klobasa et al., 1990). This is an “under-researched” area in immunobiology that is reviewed by Butler and Kehrle which has implications for understanding events during the “critical window” (Fig. 2).
4.2.3. Data obtained using CDCD piglets
CDCD pigs are used to study infectious disease, and to develop monovalent antisera against a specific Ag or pathogen. Proper derivation of CDCD piglets should provide animals free of swine pathogens except for those that can be transmitted in utero. Thus, the CDCD pig model can be used to study the direct effect of infection with single or multiple pathogens, and the host response is not confounded by extraneous factors. CDCD pigs are a “middle model” between germ-free pigs that are highly susceptible to infection, and conventional piglets protected by colostrum/milk and perhaps the positive influence of normal gut flora. The increased susceptibility of CDCD pigs enables virulent pathogens to be more easily identified since clinical disease in conventional pigs may be masked. For example, clinical symptoms are observed in CDCD pigs infected with Bordetella bronchiseptica but not in conventional pigs (Brockmeier et al., 2002a, Harms et al., 2001, Bolin et al., 2001) However, this is not always the case (Van Reeth et al., 2001) suggesting many factors come into play when studying the host/pathogen interaction. Although the same is true for germ-free pigs, CDCD pigs are much easier and cheaper to maintain for such infectious disease studies. Although the susceptibility of CDCD pigs to pathogens makes them a good model for pathogenesis studies, many pathogens can be too potent resulting in acute mortality that provides little insight into the pathogenesis of the agent under study.
4.2.4. The isolator piglet and the role of gut colonization
It has generally accepted that normal gut flora influences the development of the neonatal immune system (Cebra et al., 1998) and this is supported by early studies in rodents (Gustafsson and Laurell, 1959, Thorbecke, 1959). Since in the piglet model there is no transfer of environmental Ags and maternal IgG in utero (Fig. 1), newborn piglets offer a “blank slate”. This system was utilized to quantify the effect of normal gut flora on serum Ig levels and VH gene usage. This study resulted in a remarkable 10–30-fold elevation of serum Ig levels in 6 weeks, with preferential elevation of serum IgA (Butler et al., 2000b). Furthermore, repertoire diversification included changes in VH usage and somatic hypermutation (SHM) that was associated with mucosal tissue but not spleen or PBMCs. Monoassociation with benign E. coli alone elevated Ig levels 10-fold and most importantly, allowed piglets to be immunresponsive to both TD and II-2 immunogens (Butler et al., 2002; Fig. 7 ). This adjuvant effect of colonization could be ascribed to PAMPs recognized by TLR9, TLR4 and NOD2 in studies using purified ligands for these innate immune receptors (Butler et al., 2005a).
Fig. 7.
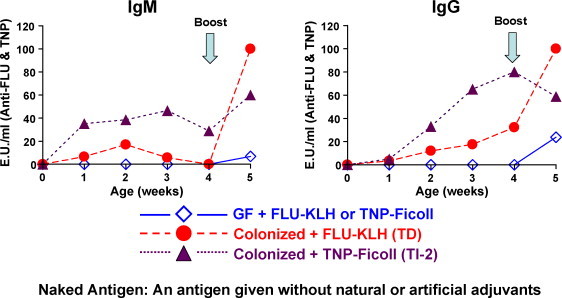
The IgM and IgG Ab response to naked TD (FLU-KLH) and TI-2 (TNP-Ficoll) antigens by germfree piglets (GF) and piglets monoassociated with benign Escherichia coli (colonized). Naked Antigen = an antigen given without natural or artificial adjuvants.
4.2.5. The use of isolator piglets for studies on neonatal pathogens
At the IMCz, isolators piglets have been experimentally infected only after the closure of intestinal barrier for large molecules (later than 48 h after hysterectomy) with 106 to 108 CFU of bacteria cells as described elsewhere for E. coli (Kozakova et al., 2006) or Salmonella (Trebichavsky et al., 2006) infections. Gnotobiotic piglets have been used for both virological (Krakowka et al., 2007) and bacteriological (Shirkey et al., 2006) studies. Inoculation of germfree piglets with highly pathogenic strains of enteric bacteria is usually fatal. In the case of E coli, there is another component involved since some piglets lack the adhesion receptor for enterotoxigenic E. coli (Francis et al., 1998). Therefore, germfree piglets have sometimes been used to determine the degree of pathogen virulence. Germ-free piglets have been employed in research at SDSU for several decades and have been used in a variety of experiments including documenting virulence (Collins et al., 1989, Francis et al., 1986), establishing the nature of pathogenesis of infectious agents (Francis et al., 1989, McKee et al., 1995, Zhang et al., 2006, Dykstra et al., 1993, Christopher-Hennings et al., 1993, Collins et al., 1991, Rossow et al., 1995), determining the consequence of multiple agent infections (Benfield et al., 1988), determining the time course of infection (Rossow et al., 1996), assessing the virulence of a pathogen expressing a variety of virulence-associated genes (McKee et al., 1995, Zhang et al., 2006, Francis et al., 1989), the establishment of the etiology of disease (Francis et al., 1986) and determining host susceptibility factors (Francis et al., 1998).
Isolator piglets are also susceptible to fatal viral infections such as virulent forms of porcine circovirus (PCV2). However, germfree piglets given swine influenza (SIV) become ill, develops lung lesions and make anti-viral antibodies but usually resolve the infection in 3 weeks. This is noteworthy since it indicates either that (i) PAMPs are not required for anti-viral responses or (ii) viral PAMPs (RNA and DNA) are provided by SIV.
Other viral infections have marked immunoregulatory effects. This group may also involve PCV2 but more work is needed. Especially porcine reproductive and respiratory syndrome virus (PRRSV) has this effect (Lemke et al., 2004). PRRSV given to isolator piglets, either monoassociated with benign E. coli or free of gut flora, undergo pronounced immune dysregulation characterized by hypergammaglobulinemia, lymphoid adenopathy and autoimmune disease (Lemke et al., 2004). These animals are characterized by expansion of undiversified pre-immune repertoire, especially B cells with hydrophobic HCDR3s (Butler et al., 2007, Butler et al., 2008b). Since it is well known that the pre-immune repertoire harbors many autoantibodies (Dighiero et al., 1985, Wardmann et al., 2003; see Section 3.2.2) the appearance of autoimmunity and deposition of immune complexes in the kidney is not surprising. Noteworthy is that conventional piglets infected with the same virus develop less pronounced immune dysregulation suggesting that either passively transferred maternal factors or the effect of a full normal gut flora work to restore immune homeostasis (Fig. 2; Table 5 ).
Table 5.
Immune dysregulation in conventional (conv) versus isolator piglets.
| Treatment | Ig levels (μg/ml)a |
Fold increaseb |
Autoantibody titer |
Deposition in kidney | |||||
|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgA | IgM | IgG | IgA | Golgi | dsDNA | ||
| Control-conv (21 dpi)c | 180 ± 20 | 4,020 ± 53 | 50 ± 10 | – | – | – | |||
| PRRSV-conv (21 dpi) | 920 ± 100 | 11,500 ± 30 | 102 ± 8d | ||||||
| Germfree Isolator (28 dpi) | 24 ± 4 | <50 | <5 | ND | ND | – | |||
| PRRSV isolator | 1600 ± 102 | 16,020 ± 900 | 230 ± 45 | ||||||
Data are mean ± S.D. Differences are obvious by inspection.
Fold increase compared to corresponding controls.
dpi = days post-inoculation.
Conventional piglets have ingested maternal colostrum so one-fifth of the IgM, one-third of the IgG and one-half of the IgA is of maternal origin.
5. Pigs and piglets in medical research
5.1. Fully human polyclonal antibodies from genetically modified pigs
Recent advances in gene knockout and cloning technology in pigs, developed by the scientific team at Revivicor Inc. (Polejaeva et al., 2000, Phelps et al., 2003, Mendicino et al., 2008), make it possible for the first time to delete the pig antibody producing (immunoglobulin or Ig) genes and to replace them with human equivalents such that these animals will produce fully human polyclonal antibodies for human biomedical applications. These human antibodies can then be harvested, purified, and delivered clinically, as a therapeutic or as a prophylactic. The products as antibodies would address a significant clinical need in prevention of infectious diseases, including for patients that are immunosuppressed (such as transplant patients), antibiotic-resistant infections, viral infections, and autoimmunity. Furthermore, this technology has far reaching clinical applicability both in the general healthcare sector and for military biowarfare countermeasure applications (i.e., anthrax and Ebola). This strategy provides the ability to produce high avidity polyclonal antibodies including IVIG or as an anti-infective with many advantages over current antibiotics. It provides solutions for viral infections including HIV, hepatitis and influenza and for toxins. In addition, it offers high volume, scalable production in farm animals. The technology provides broad-based therapeutic opportunities beyond infectious diseases including anti-Rh factor, autoimmune disease, diagnostics, and cancer. A similar program is being developed in cattle (Kuroiwa et al., 2002). However, unlike in the cattle system where a mixture of human and cattle antibodies is produced, Revivicor's strategy aims at knocking out all the pig Ig genes (heavy-chain, kappa and lambda light-chain genes), such that no chimeric antibodies can be generated. The pig model offers advantages over that of the cattle in regards to shorter gestation time, large litters, better defined Ig loci and minimal risk from BSE.
The characterization of the three porcine Ig genes (heavy-chain, lambda light chain, and kappa light chain) allows construction of gene knockout vectors for inactivation. Each porcine Ig gene locus is disrupted by homologous recombination (gene knockout) and replaced with megabase-sized, functional human counterpart Ig gene loci. This is first done in pig fibroblast cells, which are subsequently used for somatic cell nuclear transfer (cloning) to produce the product animals that harbor each of the modifications. Two of the porcine Ig genes have already been targeted and inactivated (heavy-chain and kappa) and a small herd of pigs has been established that harbor these genetic modifications. Vectors for all three human-counterpart Ig genes are being constructed. Eventually, fully human antibodies raised against predetermined, specific antigens (i.e., targets like HIV, hepatitis, CMV, Rh factor, or anthrax coat protein) can be produced following immunization of these genetically-engineered pigs.
5.2. The pig model in cystic fibrosis (CF)
CF is the most common lethal genetic disease among people of Caucasian decent. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene cause this autosomal recessive disease (Davis, 2006). Murine CFTR gene knockout and knockins of human mutations are disappointing in their recapitulation of the human lung disease phenotype, and there is a great need for alternative animal models.
Compared to rodents, the pig lung is anatomically and physiologically more similar to humans (Maina and van Gils, 2001, Pabst and Binns, 1994; Table 1) and has been studied extensively in xenotransplantation (Sachs et al., 2001). The prenatal maturation of the pig lung is similar to humans and includes extensive alveolarization (Ackermann, 2001). Pigs are vulnerable to a number of respiratory Gram positive and negative bacterial diseases (Haemophilus parasuis, Streptococcus suis, type II, Pasteurella multocida, Actinobacillus pleuropneumoniae and Mycoplasma hyopneumoniae) and viral infections (swine influenza, coronaviruses, circoviruses, porcine parvovirus, pseudorabies virus, porcine reproductive and respiratory syndrome) (Brockmeier et al., 2002b, Soerensen et al., 2005). These diseases are well characterized and include documented cellular, immunologic, and chemokine/cytokine responses to both spontaneous and experimental infections (Thacker, 2006, Van Reeth et al., 1999).
Section 1 described the precosial nature of piglets and their suitability for use in germfree and gnotobiotic conditions. Table 1, Table 4 summarize features of swine that make them of significant interest for potential studies of a porcine CF model since the onset of infection and inflammation in early lung disease in humans is difficult to study. This has lead to controversies regarding the primary and secondary lung disease manifestations. Recently Michael Welsh and colleagues used gene targeting and somatic cell nuclear transfer to produce heterozygous CFTR knockout pigs. Male fetal fibroblasts were generated that were positive for CFTR-targeting at one allele as detected by multiple PCR screens. Genomic Southern blot analysis confirmed proper gene targeting and revealed that most clones were free of random integration events. In collaboration with Randy Prather's group at the University of Missouri-Columbia, somatic cell nuclear transfer was subsequently performed to generate CFTR knockout piglets. Initial studies suggest that these animals display many of the characteristic of CF in humans (Rogers et al., 2008).
There is little doubt that the energy and resources that will be applied to the CFTR pig model will as a bi-product, advance our understanding of the porcine immune system and its development, especially the respiratory tract. Eventually, CFTR knockouts will be reared in isolator facilities which can provide insight into how an epithelial cell gene defect can effect development of immune homeostasis during the critical window (Fig. 2). Furthermore, the CFTR pig model may provide the reasons and resources for further characterization of (i) the porcine cytokine system, (ii) innate immune factors like defensins and (iii) the role of alveolar macrophages in antigen-driven B cell repertoire diversification and antibody responses.
Conflict of interest
None.
Acknowledgements
The IUIS/Pfizer Distinguished Veterinary Immunologist award to the first author was made possible by the efforts of the team comprised of the co-authors and those acknowledged below.
This review recognizes the major investigators who have and are contributing to the development of piglet and swine models for use in developmental and comparative immunology and in addressing diseases of swine as well as humans. We also acknowledge those who did not contribute data for the review but who were instrumental in obtaining important data. Drs. Ronald Christenson, Midwest Animal Research Center, USDA, Clay Center, NE; Steven Ford, Department of Animal Science, University of Wyoming, Laramie; Einhardt Werhahn, Institut Fuer Tierzucht und Tierenverhalten, Mariensee, Germany; Margret Dillender, Pfizer Animal Research, Groton, Mass; Klaus Petzoldt (formerly) and Burkart Franz, both from the Institute of Microbiology, Hannover Veterinary School, Hannover, Germany. We also acknowledge the NIH Fogarty International Center, NIH-HL, NIH-AIAID, NSF-CMB, NSF-Int’l, USDA-NRI, National Porkboard, Max Planck Society, Bundes Forschung fuer Landwirtschaft, Deutsche Forschungs Gemeinshaft and Czech Academy of Science for their support in developing the piglet model.
References
- Ackermann M. Respiratory tract. In: Pond J.E., Mersmann H.J., editors. Biology of the Domestic Pig. Cornell University Press; New York: 2001. pp. 502–532. [Google Scholar]
- Allen W.D., Porter P. The demonstration of immunoglobulins in porcine intestinal tissue by immunofluorescence with observations on the effect of fixation. Immunology. 1970;18:799–806. [PMC free article] [PubMed] [Google Scholar]
- Allen W.D., Porter P. The relative distribution of IgM and IgG cells in intestinal ucosa and lymphoid tissue of the young unweaned pig and their significance in ontogenesis of secretory immunity. Immunology. 1973;24:493–501. [PMC free article] [PubMed] [Google Scholar]
- Allen W.D., Porter P. Localization of immunofluorescence of secretory component and IgA in intestinal mucosa of the young pig. Immunology. 1973;24:365–374. [PMC free article] [PubMed] [Google Scholar]
- Atkins A.M., Schofield G.C., Reeders T. Studies on the structure and distribution of immunoglobulin A containing cells in the gut of the pig. J. Anat. 1971;109:385–395. [PMC free article] [PubMed] [Google Scholar]
- Benfield D.A., Francis D.H., McAdaragh J.P. Combined rotavirus and K99 Escherichia coli infection in gnotobiotic pigs. Am. J. Vet. Res. 1988;49:330–337. [PubMed] [Google Scholar]
- Bianchi A.T.J., Ywart R.J., Jeurissen S.H.M., Moonen-Leusen H.W.M. Development of the B- and T-cell compartments in porcine lymphoid organs from birth to adult life: an immunohistological approach. Vet. Immunol. Immunopathol. 1992;33:201–221. doi: 10.1016/0165-2427(92)90182-p. [DOI] [PubMed] [Google Scholar]
- Binns R.M., Symons D.B.A. The ontogeny of lymphocyte immunoglobulin determinants and their relationship to antibody production in fetal pigs. Res. Vet. Sci. 1974;16:260–262. [PubMed] [Google Scholar]
- Bjerrum O.J., Heegaard N.H.H., editors. Vol. 1. CRC Press; Boca Raton, FL: 1988. p. 265. (Handbook of Immunoblotting of Proteins). [Google Scholar]
- Boersma W.J., Zwart R.J., Sinkora J., Rehakova Z., Haverson K., Bianchi A.T. Summary of workshop findings for porcine B-cell markers. Vet. Immunol. Immunopathol. 2001;80:63–78. doi: 10.1016/s0165-2427(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Bolin S.R., Stoffregen W.C., Nayar G.P., Hamel A.L. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J. Vet. Diagn. Invest. 2001;13:185–194. doi: 10.1177/104063870101300301. [DOI] [PubMed] [Google Scholar]
- Borthistle B.K., Kubo R.T., Brown W.R., Grey H.M. Studies on receptors for IgG on epithelial cells of the rat intestine. J. Immunol. 1977;119:471–476. [PubMed] [Google Scholar]
- Bourne J. IgA immunoglobulin from porcine serum. Biochim. Biophys. Acta Res. Commun. 1969;36:138–145. doi: 10.1016/0006-291x(69)90660-3. [DOI] [PubMed] [Google Scholar]
- Bourne, F.J., 1971. Pig immunoglobulins. PhD Thesis, University of Bristol, UK.
- Bourne F.J., Curtis J. The transfer of immunoglobulins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology. 1973;24:157–162. [PMC free article] [PubMed] [Google Scholar]
- Brockmeier S.L., Register K.B., Magyar T., Lax A.J., Pullinger G.D., Kunkle R.A. Role of the dermonecrotic toxin of Bordetella bronchiseptica in the pathogenesis of respiratory disease in swine. Infect. Immun. 2002;70:481–490. doi: 10.1128/IAI.70.2.481-490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeier S.L., Halbur P.G., Thacker E.L. Porcine respiratory disease complex. In: Brogden K., Guthmiller J., editors. Polymicrobial Diseases. American Society for Microbiology; Washington, DC: 2002. pp. 231–258. [Google Scholar]
- Brown P.J., Bourne F.J. Development of immunoglobulin-containing cell populations in intestine, spleen and mesenteric lymph node of the young pig, as demonstrated by peroxidase-conjugated antiserum. Am. J. Vet. Res. 1976;37:1309–1314. [PubMed] [Google Scholar]
- Brown W.R., Butler J.E. Characterization of the single Cα gene of swine. Mol. Immunol. 1994;31:633–642. doi: 10.1016/0161-5890(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Brown W.R., Kacskovics I., Amendt B., Shinde R., Blackmore N., Rothschild M., Butler J.E. The hinge deletion variant of porcine IgA results from a mutation at the splice acceptor site in the first Cα intron. J. Immunol. 1995;154:3836–3842. [PubMed] [Google Scholar]
- Butler J.E. Bovine immunoglobulins. J. Dairy Sci. 1969;52:1895–1909. [Google Scholar]
- Butler J.E. Immunoglobulins of the mammary secretions. In: Larson B.L., Smith V., editors. vol. III. Academic Press; New York: 1974. pp. 217–255. (Lactation, a Comprehensive Treatise). Chapter V. [Google Scholar]
- Butler J.E. Bovine immunoglobulins: an augmented review. Vet. Immunol. Immunopathol. (Adv. Vet. Immunol.) 1983;4:43–152. doi: 10.1016/0165-2427(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Butler J.E. The immunochemistry of sandwich ELISAs. Principles and applications for the quantitative determination of immunoglobulins. In: Kemeny D.M., Challacombe S.J., editors. ELISA and Other Solid-Phase Immunoassays. John Wiley & Son Ltd.; 1988. pp. 155–180. [Google Scholar]
- Butler J.E. Perspectives configurations and principles. In: Butler J.E., editor. Immunochemistry of Solid-phase Immunoassay. CRC Press; 1991. pp. 3–26. Chapter 1. [Google Scholar]
- Butler, J.E., 2006. Preface: why I agreed to do this, in: Butler J.E. (Guest Ed.), Antibody Repertoire Development. Developmental and Comparative immunology Special Edition 30, pp. 1–17.
- Butler J.E., Maxwell C.F. Preparation of bovine immunoglobulins and free secretory component and their specific antisera. J. Dairy Sci. 1972;55:151–164. doi: 10.3168/jds.S0022-0302(72)85453-5. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Hamilton R.G. Quantitation of specific antibodies: methods of expression, standards solid-phase considerations and specific applications. In: Butler J.E., editor. Immunochemistry of Solid-phase Immunoassay. CRC Press; 1991. pp. 173–198. Chapter 9. [Google Scholar]
- Butler, J.E., Kehrle, M E.J., 2005. Immunocytes and immunoglobulins in milk, in: Mestecky, J., Lamm, M.E., Strober, W., McGhee, J.R., Mayer, L, Bienenstock, J. (Eds.), Mucosal Immunology, 3rd edition, Academic Press, NY. pp. 1763–1793.
- Butler J.E., Wertz N. Antibody repertoire development in fetal and neonatal piglets. XVII. IgG subclass transcription revisited with emphasis on new IgG3. J. Immunol. 2006;177:5480–5489. doi: 10.4049/jimmunol.177.8.5480. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Klobasa F., Werhahn E. The differential localization of IgA, IgM, and IgG in the gut of suckled neonatal piglets. Vet. Immunol. Immunopathol. 1981;2:53–65. doi: 10.1016/0165-2427(81)90038-6. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Cambier J.C., Klobasa F. The characterization of a TEPC-15, hapten modifiable idiotypic system in swine. Mol. Immunol. 1985;22:1159–1168. doi: 10.1016/0161-5890(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Borca M.V., Heyermann H., Dillender M., Bielecka M. The heterogeneity of bovine IgG2. III. The basis of the ion-exchange heterogeneity of bovine IgG2a is the result of VH-region variation. Mol. Immunol. 1987;24:1317–1326. doi: 10.1016/0161-5890(87)90127-1. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Navarro P., Heyermann H. The heterogeneity of bovine IgG2. VI. The comparative specificity of monoclonal and polyclonal capture antibodies for IgG2a (A1) and IgG2a (A2) Vet. Immunol. Immunopathol. 1994;40:119–133. doi: 10.1016/0165-2427(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Sun J., Navarro P. The swine immunoglobulin heavy chain locus has a single JH and no identifiable IgD. Int. Immunol. 1996;8:1897–1904. doi: 10.1093/intimm/8.12.1897. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Weber P., Sinkora M., Sun J., Ford S.J., Christenson R. Antibody repertoire development in fetal and neonatal piglets. II. Characterization of heavy chain CDR3 diversity in the developing fetus. J. Immunol. 2000;165:6999–7011. doi: 10.4049/jimmunol.165.12.6999. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Sun J., Weber P., Francis D. Antibody repertoire development in fetal and neonatal piglets. III. Colonization of the gastrointestinal tracts results in preferential diversification of the pre-immune mucosal B-cell repertoire. Immunology (British) 2000;100:119–130. doi: 10.1046/j.1365-2567.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.E., Sun J., Weber P., Ford S.P., Rehakova Z., Sinkora J., Lager K. Antibody repertoire development in fetal and neonatal piglets. IV. Switch recombination, primarily in fetal thymus occurs independent of environmental antigen and is only weakly associated with repertoire diversification. J. Immunol. 2001;167:3239–3249. doi: 10.4049/jimmunol.167.6.3239. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Weber P., Sinkora M., Baker D., Schoenherr A., Mayer B., Francis D. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J. Immunol. 2002;169:6822–6830. doi: 10.4049/jimmunol.169.12.6822. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Wertz N., Wang H., Sun J., Chardon P., Piumi F., Wells K. Antibody repertoire in fetal and neonatal pigs. VII. Characterization of the pre-immune kappa light chain repertoire. J. Immunol. 2004;173:6794–6805. doi: 10.4049/jimmunol.173.11.6794. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Francis D., Freeling J., Weber P., Sun J., Krieg A.M. Antibody repertoire development in fetal and neonatal piglets. IX. Three PAMPs act synergistically to allow germfree piglets to respond to TI-2 and TD antigens. J. Immunol. 2005;175:6772–6785. doi: 10.4049/jimmunol.175.10.6772. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Wertz N., Sun J., Wang H., Lemke C., Chardon P., Puimi F., Wells K. The pre-immune variable kappa repertoire of swine is selectively generated from certain subfamilies of Vk2 and one Jk gene. Vet. Immunol. Immunopathol. 2005;108:127–137. doi: 10.1016/j.vetimm.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Weber P., Wertz N. Antibody repertoire development in fetal and neonatal pigs. XIII. “Hybrid VH genes” and the pre-immune repertoire revisited. J. Immunol. 2006;177:5459–5470. doi: 10.4049/jimmunol.177.8.5459. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Sinkora M. The isolater piglet: A model for studying the development of adaptive immunity. Immunol. Res. 2007;39:33–51. doi: 10.1007/s12026-007-0062-7. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Lemke C.D., Weber P., Sinkora M., Lager K.D. Antibody repertoire development in fetal and neonatal piglets. XIX. Undiversified B cells with hydrophobic HCDR3s preferentially proliferate in PRRS. J. Immunol. 2007;178:6320–6331. doi: 10.4049/jimmunol.178.10.6320. [DOI] [PubMed] [Google Scholar]
- Butler, J.E., Wertz, N., Deschacht, N., Kacskovics, I., 2008a. Porcine IgG: Structure, genetics and evolution. Immunogenics (in press). [DOI] [PubMed]
- Butler J.E., Weber P., Wertz N., Lager K.M. Porcine reproductive and respiratory syndrome virus (PRRSV) subverts normal development of adaptive immunity by proliferation of germline-encoded B cells with hydrophobic HCDR3s. J. Immunol. 2007;178:6320–6331. [Google Scholar]
- Butler, J.E., Muyldermanns, S., Boyd, P., Wertz, N., Lunney, J.K., 2008c. The use of heavy chain chimeric antibodies to study IgG subclass proteins from species in which subclasses cannot be biochemically purified. J. Immunol. Methods.
- Cambier J.C., Butler J.E. A rapid method for the purification of immunoglobulin M (IgM) from the sera of certain mammalian species. Prep. Biochem. 1974;4:31–46. doi: 10.1080/00327487408068184. [DOI] [PubMed] [Google Scholar]
- Cebra J.J., Jian H.Q., Sterzl J., Tlaskalova-Hogenova H. The role of mucosal microbiota in the development and maintenance of the mucosal immune system. In: Ogra P.O., Mestecky J., Lamm M.E., Strober W., McGhee J.R., Bienenstock J., editors. Mucosal Immunology. Academic Press; 1998. pp. 267–280. (Chapter 17) [Google Scholar]
- Chapman H.A., Johnson J.S., Cooper M.D. Ontogeny of Peyer's patches and immunoglobulin-containing cells in pigs. J. Immunol. 1974;112:555–563. [PubMed] [Google Scholar]
- Christopher-Hennings J., Willgohs J.A., Francis D.H., Raman U.A., Moxley R.A., Hurley D.J. Immunocompromise in gnotobiotic pigs by verotoxin producing Escherichia coli (0111.NM) Infect. Immun. 1993;61:2304–2308. doi: 10.1128/iai.61.6.2304-2308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.E., Bergeland M.E., Bouley D., Ducommun A.L., Francis D.H., Yeske P. Diarrhea associated with Clostrium perfringens type A enterotoxin in neonatal piglets. J. Vet. Diagn. Invest. 1989;1:351–353. doi: 10.1177/104063878900100414. [DOI] [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Pijoan C. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1991;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Cukrowska B., Trebichavsky I., Rossmann P., Rehakova Z., Sinkora J., Haverson K., Lodinova-Zadnikova R., Tlaskalova-Hogenova H. Antigenic stimuli do not influence thymic B lymphocytes: a morphological and functional study of germ-free and conventionally reared piglets. Dev. Immunol. 1998;6:171–178. doi: 10.1155/1998/57820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P.B. Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 2006;17:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- Deenick E.K., Hasbold J., Hodgkins P.D. Switching to IgG3, IgG2b and IgA is division linked and independent revealing a stochastic framework for describing differentiation. J. Immunol. 1999;163:4707–4717. [PubMed] [Google Scholar]
- Dierks, S.E., 1988. Construction and characterization of hapten-binding chimeric IgE molecules. PhD Thesis, University of Iowa.
- Dighiero G.P., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal mice. J. Immunol. 1985;134:765–771. [PubMed] [Google Scholar]
- Dillender, M., 1990. The immune response of swine to phosphorylcholine. PhD Thesis, University of Iowa.
- Dykstra S.A., Moxley R.A., Janke B.H., Nelson E.A., Francis D.H. Clinical signs and lesions in gnotobiotic pigs inoculated with Shiga-like toxin I from Escherichia coli. Vet. Pathol. 1993;30:410–417. doi: 10.1177/030098589303000502. [DOI] [PubMed] [Google Scholar]
- Eguchi, T., Wertz,N., Puimi,F., Uenishi, H., Sun X-Z., Wells, K., Chardon, P., Butler, J.E., Antibody repertoire development in fetal and neonatal piglets. XI. The relationship of VDJ usage and the genomic organization of the VH locus (submitted). [DOI] [PubMed]
- Faldyna M., Samankova P., Leva L., Cerny J., Oujezdska J., Rehakova Z., Sinkora J. Cross-reactive anti-human monoclonal antibodies as a tool for B-cell identification in dogs and pigs. Vet. Immunol. Immunopathol. 2007;119:56–62. doi: 10.1016/j.vetimm.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Francis D.H., Collins J.E., Duimstra J.R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect. Immun. 1986;51:953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D.H., Moxley R.A., Andraos C.Y. Edema disease-like brain lesions in gnotobiotic piglets infected with Escherichia coli serotype 0157:H7. Infect. Immun. 1989;57:1339–1342. doi: 10.1128/iai.57.4.1339-1342.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D.H., Grange P.A., Zeman D.H., Baker D.R., Sun R., Erickson A.K. Expression of mucin-type glycoprotein K88 receptors strongly correlates with piglet susceptibility to K88+ enterotoxigenic Escherichia coli, but adhesion of this bacterium to brush borders does not. Infect. Immun. 1998;66:4050–4055. doi: 10.1128/iai.66.9.4050-4055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franek F., Riha I. Purification and structural characterization of 5Sγ-globulin of newborn pigs. Immunochemistry. 1964;1:49–63. doi: 10.1016/0019-2791(64)90056-4. [DOI] [PubMed] [Google Scholar]
- Franek F., Doskocil J., Simek L. Different types of precipitating antibodies in early and late porcine anti-dinitrophenyl sera. Immunochemistry. 1974;11:803–809. doi: 10.1016/0019-2791(74)90301-2. [DOI] [PubMed] [Google Scholar]
- Gershoni J.M., Palade G.E. Protein blotting: principles and applications. Anal. Biochem. 1983;131:1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Glimstedt G. Bakterien freie Meerschweinchen Afzucht, Lebesfahigheit und Wachstum, neist Untersuchungen uber lymphatische Gewebe. Acta Physiol. Scand. Suppl. 1936;30:1. [Google Scholar]
- Gustafsson B.E., Laurell C.B. Gammaglobulin production in germfree rats after bacterial contamination. J. Exp. Med. 1959;110:675–684. doi: 10.1084/jem.110.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer R.L., Koshland M.E., Knopf P.M. Immunoglobulins binding by mouse intestinal epithelial cells receptors. J. Immunol. 1976;117:587–593. [PubMed] [Google Scholar]
- Hamers-Casterman C., Atarhouch T., Muyldermanns S., Robinson G., Haines C., Bajyana S.E. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- Harms P.A., Sorden S.D., Halbur P.G., Bolin S.R., Lager K.M., Morozov I., Paul P.S. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol. 2001;38:528–539. doi: 10.1354/vp.38-5-528. [DOI] [PubMed] [Google Scholar]
- Henning M.D., Nielsen K.H. Cross-reactivity of monoclonal antibodies to bovine immunoglobulins with immunoglobulins of other species. Vet. Immunol. Immunopathol. 1992;34:235–243. doi: 10.1016/0165-2427(92)90167-o. [DOI] [PubMed] [Google Scholar]
- Hood L., Gray W.R., Sanders B.G., Dreyer W.J. Light chain evolution. Cold Spring Harb. Symp. Quant. Biol. 1967;32:133–146. [Google Scholar]
- Janusz M., Wieczorek Z., Spiegel K. Immunoregulatory properties of synthetic peptides, fragments of a proline-rich polypeptide (PRP) from ovine colostrum. Mol. Immunol. 1987;24:1029–1031. doi: 10.1016/0161-5890(87)90069-1. [DOI] [PubMed] [Google Scholar]
- Jones M., Cordell J.L., Beyers A.D., Tse A.G.D., Mason D.Y. Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. J. Immunol. 1993;150:5429–5435. [PubMed] [Google Scholar]
- Kacskovics I., Sun J., Butler J.E. Five subclasses of swine IgG identified from the cDNA sequences of a single animal. J. Immunol. 1994;153:3566–3573. [PubMed] [Google Scholar]
- Kaltreider H.B., Johnson J.S. Porcine immunoglobulins. I. Identification of subclasses and preparation of specific antisera. J. Immunol. 1972;109:992–998. [PubMed] [Google Scholar]
- Kim Y.B., Bradley G., Watson D.W. Ontogeny of the immune response. II. Characterization of the 19 S γG and 7 S γG immunoglobulins in the true primary and secondary responses of piglets. J. Immunol. 1966;97:189–195. [PubMed] [Google Scholar]
- Kim Y.B., Bradley S.G., Watson D.W. Ontogeny of the immune response. V. Further characterization of the 19 S γG and 7 S γG immunoglobulins in the true primary immune responses in germfree colostrum-deprived piglets. J. Immunol. 1968;101:224–236. [PubMed] [Google Scholar]
- Kim, Y.B., 1975. Developmental immunology in the piglet, in: Bergsma, D., Good, R.A., Finstad, J. (Eds.), Immunodeficiency Diseases in Man and Animals. Birth Defects. Original article series. The National Foundation for March of Dimes, Sinauer Assoc., Sunderland, MA. pp.549–557.
- Klobasa F. Methodische und geratetechnische Probleme im Erfahrungen bei Anwendung immunologische Verfahren in der Tierzuchtforschung. Landbauforsch. Volk. 1987;37:1–17. [Google Scholar]
- Klobasa F., Butler J.E. The influence of lactation number, animal and gland variations on the absolute and relative concentrations of IgG, IgM and IgA and albumin in the lacteal secretions of sows throughout lactation. Am. J. Vet. Res. 1987;48:176–182. [PubMed] [Google Scholar]
- Klobasa F., Butler J.E. Absolute and relative concentrations of immunoglobulin G, M and A and albumin in the lacteal secretions of sows of different lactation numbers. Am. J. Vet. Res. 1987;48:176–182. [PubMed] [Google Scholar]
- Klobasa F., Werhahn E., Butler J.E. Regulation of humoral immunity in the piglet by immunoglobulins of maternal origin. Res. Vet. Sci. 1981;31:195–206. [PubMed] [Google Scholar]
- Klobasa F., Habe F., Werhahn E., Butler J.E. Changes in the concentration of serum IgG, IgA, and IgM of sows throughout the reproductive cycle. Vet. Immunol. Immunopathol. 1985;10:341–353. doi: 10.1016/0165-2427(85)90023-6. [DOI] [PubMed] [Google Scholar]
- Klobasa F., Habe F., Werhahn E., Butler J.E. Influences of breed and gestation number on the concentration of serum IgG, IgA and IgM of sows throughout the reproductive cycle. Vet. Immunol. Immunopathol. 1985;10:355–366. doi: 10.1016/0165-2427(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Klobasa F., Butler J.E., Werhahn E., Habe F. Maternal-neonatal immunoregulation in swine. II. Influence of multi-parity on de novo synthesis by piglets. Vet. Immunol. Immunopathol. 1986;11:149–159. doi: 10.1016/0165-2427(86)90094-2. [DOI] [PubMed] [Google Scholar]
- Klobasa F., Werhahn E., Butler J.E. The composition of sow milk during lactation. J. Anim. Sci. 1987;64:1458–1466. doi: 10.2527/jas1987.6451458x. [DOI] [PubMed] [Google Scholar]
- Klobasa F., Butler J.E., Habe F. Maternal-neonatal immunoregulation: suppression of de novo immunoglobulin synthesis of IgG and IgA, but not IgM, in neonatal piglets by bovine colostrum, is lost upon storage. Am. J. Vet. Res. 1990;51:1407–1412. [PubMed] [Google Scholar]
- Kozakova H., Kolinska J., Lojda Z., Rehakova Z., Sinkora J., Zakostelecka M., Splichal I., Tlaskalova-Hogenova H. Effect of bacterial monoassociation on brush-border enzyme activities in ex-germ-free piglets: comparison of commensal and pathogenic Escherichia coli strains. Microbes Infect. 2006;8:2629–2639. doi: 10.1016/j.micinf.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Ellis J., McNeilly F., Waldner C., Rings D.M., Allan G. Mycoplasma hyopneumoniae bacterins and porcine circovirus type 2 (PCV2) infection: induction of postweaning multisystemic wasting syndrome (PMWS) in the gnotobiotic swine model of PCV2-associated disease. Can. Vet. J. 2007;48:716–724. [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa Y., Kasinathan P., Choi Y.J., Naeem R., Tomizuka K., Sullivan E.J., Knott J.G., Duteau A., Goldsby R.A., Osborne A., Ishida I., Robl J.M. Cloned transchromosomic calves producing human immunoglobulin. Nat. Biotechnol. 2002;20:890–894. doi: 10.1038/nbt727. [DOI] [PubMed] [Google Scholar]
- Lammers B.M., Beaman K.D., Kim Y.B. Sequence analysis of porcine light chain cDNAs. Mol. Immunol. 1991;28:877. doi: 10.1016/0161-5890(91)90051-k. [DOI] [PubMed] [Google Scholar]
- Layton D.S., Strom A.D.G., O’Neil T.E., Broadway M.M., Stephenson G.L., Morris K.R., Muralitharan M., Sandrin M.S., Lerino F.L., Bean A.G.D. Development of an anti-porcine CD34 monoclonal antibody that identifies hemopoietic cells. Exp. Hematol. 2007;35:171–178. doi: 10.1016/j.exphem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Leece J.G. Glucose milliequivalents eaten by the neonatal pig and cessation of intestinal absorption of large molecules (closure) J. Nutr. 1966;90:240–244. doi: 10.1093/jn/90.3.240. [DOI] [PubMed] [Google Scholar]
- Leece J.G. Rearing colostrum-free pigs in an automatic feeding device. J. Anim. Sci. 1969;2:27–33. doi: 10.2527/jas1976.423622x. [DOI] [PubMed] [Google Scholar]
- Leece J.G., Morgan D.O. Effect of dietary regimen on cessation of intestinal absorption of large molecules (closure) in the neonatal pig and lamb. J. Nutr. 1962;78:263–268. doi: 10.1093/jn/78.3.263. [DOI] [PubMed] [Google Scholar]
- Lemke, C.D., 2006. PRRSV infection of neonatal piglets induces immune dysregulation and modulation. PhD Thesis, University of Iowa.
- Lemke C.D., Haynes J.S., Spaete R., Adolphson D., Vorwald A., Lager K., Butler J.E. Lymphoid hyperplasia resulting in immune dysregulation is caused by PRRSV infection in pigs. J. Immunol. 2004;172:1916–1925. doi: 10.4049/jimmunol.172.3.1916. [DOI] [PubMed] [Google Scholar]
- Levenson S.M., Mason R.P., Huber T.E., Malm O.L., Horowitz R.E., Einheber A. Germfree animals in surgical research. Ann. Surg. 1959;150:713–730. doi: 10.1097/00000658-195910000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina J.N., van Gils P. Morphometric characterization of the airway and vascular systems of the lung of the domestic pig, Sus scrofa: comparison of the airway, arterial and venous systems. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2001;130:781–798. doi: 10.1016/s1095-6433(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Makala L.H., Kamada T., Nagasawa H., Igarashi I., Fujisaki K., Suzuki N., Mikami T., Haverson K., Bailey M., Stokes C.R., Bland P.W. Ontogeny of pig discrete Peyer's patches: expression of surface antigens. J. Vet. Med. Sci. 2001;63:625–636. doi: 10.1292/jvms.63.625. [DOI] [PubMed] [Google Scholar]
- Mandel L., Travnicek J. The minipig as a model in gnotobiology. Nahrung. 1987;V31(5–6):613–618. doi: 10.1002/food.19870310580. [DOI] [PubMed] [Google Scholar]
- McAleer J., Weber P., Sun J., Butler J.E. Antibody repertoire development in fetal and neonatal piglets. XI. The thymic B cell repertoire develops independently from that in blood and mesenteric lymph nodes. Immunology. 2005;114:171–183. doi: 10.1111/j.1365-2567.2004.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee M.L., Melton-Celsa A.R., Moxley R.A., Francis D.H., O’Brien A.D. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 Cells. Infect. Immun. 1995;62:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendicino, M., Ramsoondar, J., Phelps, C., Vaught, T., Ball, S., Dai, Y., LeRoith, T., Mohahan, J., Chen, S., Dandro, A., Boone, J., Jobst, P., Vance, A., Wertz, N., Polejaeva, I., Butler, J.E., Ayares, D., Wells, K., 2008. Targeted disruption of the porcine immunoglobulin heavy chain locus produces a B cell null phenotype. Nature Biotechnology (submitted).
- Metzger J.J., Fougereau M. Characterization of two subclasses of γG immunoglobulin in swine. CR Hebd. Seances Acad. Sci. Ser. P. Sci. Nat. 1967;265:724–727. [PubMed] [Google Scholar]
- Meyer R.C., Bohl E.H., Kohler E.M. Procurement and maintenance of germ-free swine for microbiological investigation. Appl. Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniats O.P., Jol D. Gnotobiotic pigs-derivation and rearing. Can. J. Comp. Med. 1978;42:428–437. [PMC free article] [PubMed] [Google Scholar]
- Navarro P., Barbis D.P., Antczak D., Butler J.E. The complete cDNA and deduced amino acid sequence of an equine IgE. Mol. Immunol. 1995;32:1–8. doi: 10.1016/0161-5890(94)00143-o. [DOI] [PubMed] [Google Scholar]
- Navarro P., Christenson R., Ekhardt G., Lunney J.K., Rothschild M., Bosworth B., Lemke J., Butler J.E. Genetic differences in the frequency of the hinge variants of porcine IgA is breed dependent. Vet. Immunol. Immunopathol. 2000;73:287–295. doi: 10.1016/s0165-2427(00)00150-1. [DOI] [PubMed] [Google Scholar]
- Navarro P., Christenson R., Weber P., Rothschild M., Ekhard G., Butler J.E. Porcine IgA allotypes are not equally transcribed or expressed in heterozygous swine. Mol. Immunol. 2000;37:653–664. doi: 10.1016/s0161-5890(00)00086-9. [DOI] [PubMed] [Google Scholar]
- Nuttal, G.H.F., Thierfelder, H., 1895–1896. Thieresches Lebon ohne Bakterien im Verdauungskanal. Z. Physiol. Chem. 21, 109, 22: 62.
- Ochsenbein A.F., Zinkernagel R. Natural antibodies and complement link innate and acquired immunity. Immunol. Today. 2000;1:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- Pabst R., Binns R.M. The immune system of the respiratory tract in pigs. Vet. Immunol. Immunopathol. 1994;43:151–156. doi: 10.1016/0165-2427(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Paul P., Mengeling W.L., Malstrom C.E., van Deusen R.A. Production and characterization of monoclonal antibodies to porcine immunoglobulin gamma, alpha and light chains. Am. J. Vet. Res. 1989;50:471–479. [PubMed] [Google Scholar]
- Phelps C., Koike C., Vaught T., Boone J., Wells K., Chen S., Ball S., Specht S., Polejaeva I., Monahan J., Jobst P., Sharma S., Lamborn A., Garst A., Moore M., Demetris A., Rudert W., Bottino R., Bertera S., Trucco M., Starzl T., Dai Y., Ayares D. Production of alpha-1,3-galactosyltransferase deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N.E., Parker D.C. Fc-dependent inhibition of mouse B cell activation by whole anti-μ antibodies. J. Immunol. 1983;130:602–608. [PubMed] [Google Scholar]
- Polejaeva I., Chen S., Vaught T., Page R., Mullins J., Ball S., Dai Y., Boone J., Walker S., Ayares D., Colman A., Campbell K. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- Porter P. Transfer of immunoglobulins IgG, IgA and IgM to lacteal secretions in the parturent sow and their absorption by the neonatal piglets. Biochim. Biophys. Acta. 1969;181:381–392. doi: 10.1016/0005-2795(69)90271-2. [DOI] [PubMed] [Google Scholar]
- Porter P. Studies of porcine secretory IgA and its component chains in relation to intestinal absorption of colostral immunoglobulins by the neonatal pig. Immunology. 1973;24:163–176. [PMC free article] [PubMed] [Google Scholar]
- Rapacz J., Hasler-Rapacz J. Immunogenetic studies on polymorphism, postnatal passive acquisition and development of immunoglobulin gamma (IgG) in swine. Proceedings of the 2nd International Congress Gen. and Appl. Livestock Production, vol. III; Editorial Garsi, Madrid; 1982. [Google Scholar]
- Rehakova Z., Sinkora J., Sinkora M., Kozakova H., Splichal I., Barot-Ciorbaru R., Trebichavsky I. Effect of controlled antigenic stimulation on lymphocyte subsets in pigs and pig fetuses. Folia Microbiol. (Praha) 1998;43(5):513–516. doi: 10.1007/BF02820808. [DOI] [PubMed] [Google Scholar]
- Rodkey L.S., Adler F.L. Regulation of natural anti-allotype antibody responses by idiotype network induced auto-anti idiotypic antibodies. Exp. Med. 1983;157:1920–1931. doi: 10.1084/jem.157.6.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C.S., Stoltz D.A., Meyerholz D.K., Ostedgaard L.S., Rokhlina T., Taft P.J., Rogan M.P., Itani O.A., Kabel A.C., Wohlford-Lenane C.L., Davis G.J., Hanfland R.A., Smith T.L., Samuel M., Wax D., Murpy C.N., Rieke A., Whitworth K., Us A., Sterner T.D., Brogden K.A., Shilyansky J., McCray P.B., Zabner J., Prather R.S., Welsch M.J. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow K., Collins J.E., Goyal S.M., Nelson E.A., Christopher-Hennings J., Benfield D.A. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in gnotobiotic pigs. Vet. Pathol. 1995;32:361–373. doi: 10.1177/030098589503200404. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Collins J.E., Goyal S.M., Nelson E.A., Christopher-Hennings J., Benfield D.A. Chronological immunohistochemical detection and localization of porcine reproductive and respiratory syndrome virus in gnotobiotic pigs. Vet. Pathol. 1996;33:551–556. doi: 10.1177/030098589603300510. [DOI] [PubMed] [Google Scholar]
- Rothkotter H.J., Ulbrich H., Pabst R. The postnatal development of gut lamina propria lymphocytes: number, proliferation, and T and B cell subsets in conventional and germ-free pigs. Pediatr. Res. 1991;29:237–242. doi: 10.1203/00006450-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Aasted B. Summary of the animal homologogue section of HLDA8. Vet. Immunol. Immunopathol. 2007;19(1–2):2–13. doi: 10.1016/j.vetimm.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Lunney J.K., Daubenberger C., Davis W., Fischer U., Gobel T.W., Griebel P., Hollemweguer E., Lasco T., Meister R., Schuberth H.-J., Sestak K., Sopp P., Steinbach F., Xiao-Wei W., Aasted B. Summary of the animal homologue section of HLDA8. Cell. Immunol. 2005;236:51–58. doi: 10.1016/j.cellimm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Sachs D.H., Sykes M., Robson S.C., Cooper D.K. Xenotransplantation. Adv. Immunol. 2001;79:129–223. doi: 10.1016/s0065-2776(01)79004-9. [DOI] [PubMed] [Google Scholar]
- Sainte-Marie G. A paraffin embedding technique for studies employing immunofluorescence. J. Histochem. Cytochem. 1961;10:250–256. [Google Scholar]
- Schroeder H.W., Jr., Hillson J.L., Perlmutter R.M. Early restriction of the human antibody repertoire. Science. 1987;238:791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Shirkey T.W., Siggers R.H., Goldade B.G., Marshall J.K., Drew M.D., Laarveld B., Van Kessel A.G. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp. Biol. Med. (Maywood) 2006;231(8):1333–1345. doi: 10.1177/153537020623100807. [DOI] [PubMed] [Google Scholar]
- Sinkora M., Sinkora J., Rehakova Z., Splichal I., Yang H., Parkhouse R.M.E., Trebichavsky I. Prenatal ontogeny of lymphocyte subpopulations in pigs. Immunology. 1998;95:595–603. doi: 10.1046/j.1365-2567.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkora J., Rehakova Z., Sinkora M., Cukrowska B., Tlaskalova-Hogenova H., Bianchi A.T., De Geus B. Expression of CD2 on porcine B lymphocytes. Immunology. 1998;95:443–449. doi: 10.1046/j.1365-2567.1998.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkora J., Rehakova Z., Samankova L., Haverson K., Butler J.E., Zwart R., Boersma W. Characterization of monoclonal antibodies recognizing immunoglobulin κ and λ chains in pigs by flow cytometry. Vet. Immunol. Immunopathol. 2001;80:79–91. doi: 10.1016/s0165-2427(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Sinkora J., Rehakova Z., Sinkora M., Cukrowska B., Tlaskalova-Hogenova H. Early development of immune system in pigs. Vet. Immunol. Immunopathol. 2002;87:301–306. doi: 10.1016/s0165-2427(02)00056-9. [DOI] [PubMed] [Google Scholar]
- Sinkora M., Sun J., Sinkorova J., Christenson R.K., Ford S.P., Butler J.E. Antibody repertoire development in fetal and neonatal piglets. VI. B cell lymphogenesis occurs in multiple sites with differences in the frequency of in-frame rearrangements. J. Immunol. 2003;170:1781–1788. doi: 10.4049/jimmunol.170.4.1781. [DOI] [PubMed] [Google Scholar]
- Sinkora M., Butler J.E., Holtmeier W., Sinkorova J. Lymphocyte development in fetal piglets: facts and surprises. Vet. Immunol. Immunopathol. 2005;108:177–184. doi: 10.1016/j.vetimm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Sloan G.J., Butler J.E. Incomplete inhibition of the Fc-binding ability of nitrated protein A of Staphylococcus aureus. Infect. Immun. 1978;21:659–662. doi: 10.1128/iai.21.2.659-662.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen C.M., Holmskov U., Aalbaek B., Boye M., Heegaard P.M., Nielsen O.L. Pulmonary infections in swine induce altered porcine surfactant protein D expression and localization to dendritic cells in bronchial-associated lymphoid tissue. Immunology. 2005;115:526–535. doi: 10.1111/j.1365-2567.2005.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splichal I., Rehakova Z., Sinkora M., Sinkora J., Trebichavsky I., Laude H., Charley B. In vivo study of interferon-alpha-secreting cells in pig foetal lymphohaematopoietic organs following in utero TGEV coronavirus injection. Res. Immunol. 1997;148:247–256. doi: 10.1016/S0923-2494(97)80866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splichal I., Trebichavsky I., Splichalova A., Ditetova L., Zahradnickova M. Escherichia coli administered into pig amniotic cavity appear in fetal airways and attract macrophages into fetal lungs. Physiol. Res. 2002;51:523–528. [PubMed] [Google Scholar]
- Splichalova A., Splichal I., Trebichavsky I., Hojna H. Expression of inflammatory markers in pig amnion after intraamniotic infection with nonpathogenic or enteropathogenic Escherichia coli. Folia Microbiol. (Praha) 2004;49:751–756. doi: 10.1007/BF02931560. [DOI] [PubMed] [Google Scholar]
- Splichalova A., Trebichavsky I., Muneta Y., Mori Y., Splichal I. Effect of bacterial virulence on IL-18 expression in the amnion infected with Escherichia coli. Am. J. Reprod. Immunol. 2005;53:255–260. doi: 10.1111/j.1600-0897.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Sterzl J., Riha I. vols. I and II. Academia Publishing House of the Czechoslovak Academy of Sciences; Prague: 1970. (Developmental Aspects of Antibody Formation and Structure). 1054 pp. [Google Scholar]
- Sternberger L.A., Hardy P.H., Jr., Cuculis J.J., Meyer H.G. The unlabelled antibody enzyme method of immunohistochemistry. Preparation and properties of soluble anagen-antibody comples (horseradish peroxidase-anti-horseradish peroxidase) and its use in identification of spirochetes. J. Histochem. Cytochem. 1970;18:315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Sun J., Butler J.E. Molecular characteristics of VDJ transcripts from a newborn piglet. Immunology (British) 1996;88:331–339. doi: 10.1046/j.1365-2567.1996.d01-676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Butler J.E. Sequence analysis of swine switch μ, Cμ and Cμm. Immunogenetics. 1997;46:452–460. doi: 10.1007/s002510050305. [DOI] [PubMed] [Google Scholar]
- Sun J., Kacskovics I., Brown W.R., Butler J.E. Expressed swine VH genes belong to a small VH gene family homologous to human VH III. J. Immunol. 1994;153:5618–5627. [PubMed] [Google Scholar]
- Sun J., Hayward C., Shinde R., Christenson R., Ford S.P., Butler J.E. Antibody repertoire development in fetal and neonatal piglets. I. Four VH genes account for 80% of VH usage during 84 days of fetal life. J. Immunol. 1998;161:5070–5078. [PubMed] [Google Scholar]
- Sun J., Sinkora J., Wertz N., Moravkova A., Butler J.E. Characterization of porcine CD19 and anti-CD19 monoclonal antibodies. Mol. Immunol. 2004;41:929–938. doi: 10.1016/j.molimm.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Thacker E.L. Lung inflammatory responses. Vet. Res. 2006;37:469–486. doi: 10.1051/vetres:2006011. [DOI] [PubMed] [Google Scholar]
- Thorbecke G.J. Some histological and functional aspects of lymphoid tissue in germfree animals. I. Morphological studies. Ann. NY Acad. Sci. 1959;78:237–246. doi: 10.1111/j.1749-6632.1959.tb53106.x. [DOI] [PubMed] [Google Scholar]
- Travnicek J., Mandel L., Lanc A., Ruzicka R. The breeding of microbe-free piglets. Cesk. Fysiol. 1966;15:240–244. [PubMed] [Google Scholar]
- Trebichavsky I., Kovaru F., Rehakova Z., Mandel L., Pospisil R. Skin response to tuberculin in pig foetuses and germ-free piglets. Immunol. Lett. 1992;33:271–276. doi: 10.1016/0165-2478(92)90072-v. [DOI] [PubMed] [Google Scholar]
- Trebichavsky I., Splichal I., Zahradnickova M., Splichalova A., Mori Y. Lipopolysaccharide induces inflammatory cytokines in the pig amnion. Vet. Immunol. Immunopathol. 2002;87:11–18. doi: 10.1016/s0165-2427(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Trebichavsky I., Splichalova A., Rychlik I., Hojna H., Muneta Y., Mori Y., Splichal I. Attenuated aroA Salmonella enterica serovar Typhimurium does not induce inflammatory response and early protection of gnotobiotic pigs against parental virulent LT2 strain. Vaccine. 2006;24:4285–4289. doi: 10.1016/j.vaccine.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Trexler P.C., Reynolds L.I. Flexible film apparatus for rearing and use of germfree animals. Appl. Microbiol. 1957;5:406–412. doi: 10.1128/am.5.6.406-412.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerman, J.-P., 1970. Studies on IgA immunoglobulins in man and animals. PhD Thesis, Universite Catholique de Louvain, 292 pp.
- Vaerman J.P., Arbuckle J.B., Hereman J.F. Immunoglobulin A in the pig. II. Sow's colostral and milk IgA: quantitative studies and molecular size estimates. Arch. Allergy Appl. Immunol. 1970;39:323–333. [PubMed] [Google Scholar]
- Van Reeth K., Labarque G., Nauwynck H., Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Nauwynck H., Pensaert M. Clinical effects of experimental dual infections with porcine reproductive and respiratory syndrome virus followed by swine influenza virus in conventional and colostrum-deprived pigs. J. Vet. Med. B: Infect. Dis. Vet. Public Health. 2001;48:283–292. doi: 10.1046/j.1439-0450.2001.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Hulst M.M. Monoclonal antibodies against porcine immunoglobulin isotypes. Vet. Immunol. Immunopathol. 1987;16:23–26. doi: 10.1016/0165-2427(87)90171-1. [DOI] [PubMed] [Google Scholar]
- Vernersson M., Pejler G., Kristersson T., Alving K., Hellman L. Cloning, structural analysis and expression of the pig IgE ɛ-chain. Immunogenetics. 1997;46:461–468. doi: 10.1007/s002510050306. [DOI] [PubMed] [Google Scholar]
- Wardmann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Werhahn E., Klobasa F., Butler J.E. Investigation of some factors which influence the absorption of IgG by the neonatal piglet. Vet. Immunol. Immunopathol. 1981;2:35–51. doi: 10.1016/0165-2427(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Wikler M., Deneur C., Sweasme G., Urban J. Immunoregulatory role of maternal idiotypes—ontogeny of immune networks. J. Exp. Med. 1980;152:1024–1035. doi: 10.1084/jem.152.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostmann B.S. Serum proteins in germfree vertebrates. Ann. NY Acad. Sci. 1959;78:254–260. [PubMed] [Google Scholar]
- Zhang W., Berberov E.M., Freeling J., He D., Moxley R.A., Francis D.H. Significance of heat-stable and heat-labile enterotoxin in porcine colibacillosis in an additive model for pathogenicity studies. Infect. Immun. 2006;74:3107–3114. doi: 10.1128/IAI.01338-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Kacskovics I., Pan Q., Liberies D.A., Geli J., Davis K., Rabbani H., Hammarström L. Artiodactyl IgD: the missing link. J. Immunol. 2002;169:4408–4416. doi: 10.4049/jimmunol.169.8.4408. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Pan-Hammarstrom Q., Kacskovics I., Hammarstrom L. The porcine Igδ gene: Unique chimeric splicing of the first constant region domain in its heavy chain transcripts. J. Immunol. 2003;171:1312–1318. doi: 10.4049/jimmunol.171.3.1312. [DOI] [PubMed] [Google Scholar]


