Abstract
Myelopathy is an uncommon complication of VZV infection and may develop in the absence of rash. We report the rare recurrence of myelopathy in an immunocompetent adult who initially developed myelopathy after ophthalmic-distribution zoster. Recurrent myelopathy two years later caused by VZV was verified by the presence of new- onset clinical symptoms and signs consistent with myelopathy, new lesions in the spinal cord detected by MRI, and evidence of intrathecal synthesis of anti-VZV IgG antibody in CSF. After virological verification and antiviral therapy, myelopathy did not recur and anti-VZV IgG antibody could not be detected in CSF. In contrast to earlier cases of recurrent VZV myelopathy in immunocompetent adults that developed weeks to months after the first episode of myelopathy, this is the first instance of recurrence years later.
Keywords: VZV, recurrent myelopathy, immunocompetent
1. Introduction
Myelopathy is an uncommon complication of VZV infection and may develop in the absence of rash. Not unexpectedly, recurrent and protracted VZV myelopathy has been described in immunocompromised individuals [1,2]. In addition, there have been five reports of recurrent VZV myelopathy weeks to months after an initial episode of myelopathy in immunocompetent adults. We describe a case of virologically confirmed recurrent myelopathy caused by VZV in an immunocompetent adult that developed years after an initial episode of myelopathy following ophthalmic-distribution zoster.
2. Case report
In April 2004, a 56-year-old woman developed left ophthalmic-distribution zoster. Despite treatment with famciclovir 500 mg TID for one week, facial pain persisted for a year. In June 2004, she developed a Lhermitte’s symptom and left leg weakness. In August 2004, she noted urinary urgency, rectal paresthesias and loss of sensation during stool evacuation and when wiping herself. In September 2004, a cervical MRI revealed abnormal signal extending from the medulla to the thoracic region with enhancement at C7-T1 (Fig.1, panels a–c; Fig. 2 panels a,b). A neurological examination was not documented, and no antiviral treatment was given. In May 2005, enhancement resolved, and there was significant improvement in cord signal (Fig.1, panels d–f; Fig. 2, panels c,d). In July 2006, she developed back pain that radiated down the left leg. In December 2006, a cervical MRI revealed recurrent multifocal lesions from C2 to T1 (Fig. 1, panels g–i; Fig. 2, panels e,f). CSF examination was normal. In February 2007, neurological examination revealed a spastic paraparesis, worse on the left, hyperactive DTRs and clonus bilaterally, with a left extensor plantar response; all sensory modalities were intact. The following studies were negative or normal: ESR, CRP, RPR, serum vitamin B12, ACE levels, ANA, HIV, Lyme, NMO-IgG, anti-SSA or -SSB antibodies and visual evoked responses. In March 2007, routine CSF examination was normal. PCR of the CSF did not reveal amplifiable HSV-1, HSV-2 or VZV DNA, but did reveal anti-VZV IgG antibody, and the serum/CSF ratio of anti-VZV IgG was reduced compared to ratios for total IgG and albumin, consistent with intrathecal synthesis of anti-VZV IgG antibody. She was treated with intravenous acyclovir, 10 mg/kg for 14 days followed by oral valacyclovir 500 mg BID for 3 months. In July 2007, she was asymptomatic; a repeat CSF examination was normal, and contained neither VZV DNA or anti-VZV IgG antibody. In October 2007, she had a residual spastic paraparesis, worse on the left, hyperactive DTRs, clonus bilaterally, extensor plantar responses bilaterally and a wide-based spastic gait. All sensory modalities were intact.
Fig. 1.
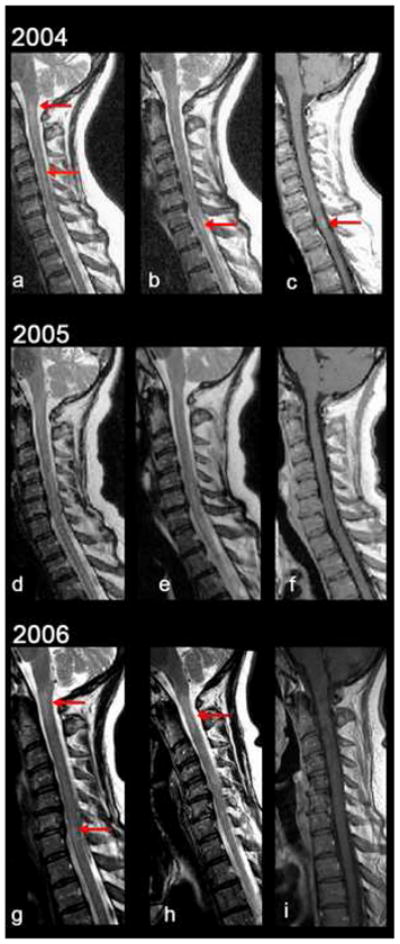
T2- and enhanced T1-weighted sagittal images of the cervical spine. In 2004, there was high T2 signal in the cord at the cervico-medullary junction and C4-5 (a, arrows) and at C7-T1 (b). The lesion at C7-T1 enhanced (c). In 2005, the T2 signal abnormalities significantly improved (d,e) and enhancement resolved (f). In 2006, there was recurrence of high T2 signal in the spinal cord especially at C2 (g,h) and at C6-7 (g), without enhancement (i).
Fig. 2.
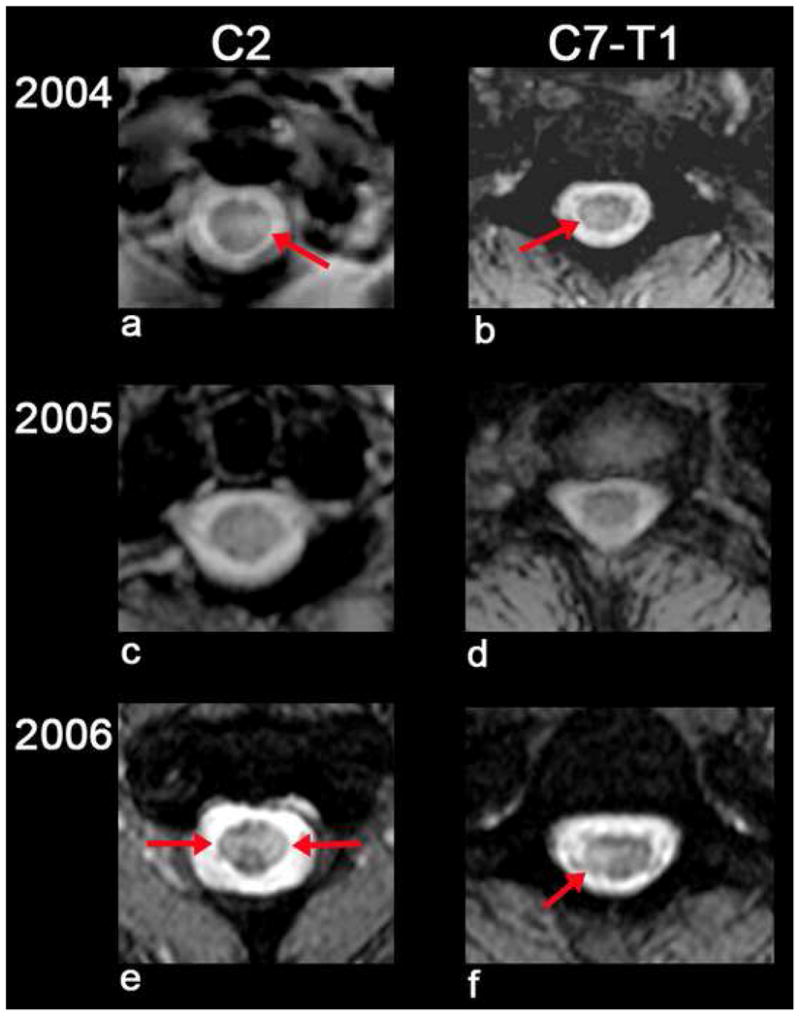
T2 axial images at the level of C2 and C7-T1 showing abnormal high signal in the spinal cord on the left at C2 (a) and right at C7-T1 (b). These areas nearly resolved in 2005 (c,d). In 2006, there was recurrent cord signal abnormality bilaterally at C2 (e) and left at C7-T1 (f).
3. Discussion
Herein, we describe recurrent myelopathy caused by VZV in an immunocompetent adult years after zoster. The first clinical episode of cervical myelopathy developed 2–3 months after ophthalmic-distribution zoster and was corroborated by an enhancing lesion in the cervical spinal cord that resolved within a year. A second episode of myelopathy 2-1/2 years later without rash was confirmed by recurrent multifocal lesions in the cervical spinal cord, during which time CSF analysis revealed intrathecal synthesis of anti-VZV IgG antibody. Treatment with intravenous acyclovir followed by oral valacyclovir halted progression of disease, concomitant with the disappearance of anti-VZV IgG antibody in CSF.
Recurrent myelopathy in an immunocompetent adult raises the diagnosis of multiple sclerosis. However, the patient never developed neurological symptoms or signs indicative of disease above the neck, visual evoked responses were normal and serial MRIs did not reveal demyelinating lesions in the brain or brainstem. Furthermore, repeated CSF examinations never revealed increased amounts of IgG or oligoclonal bands. Neuromyelitis optica was ruled out by the absence of visual symptoms and signs, normal visual evoked responses and the absence of serum antibody to aquaporin-4. A search for antibodies often found in vasculitic and granulomatous disease was negative, and there was no evidence of vitamin B12 deficiency.
The detection of anti-VZV IgG antibody together with reduced serum/CSF ratios compared to normal serum/CSF ratios of total IgG and albumin indicates intrathecal synthesis of anti-VZV IgG at the time of recurrent myelopathy. The presence of anti-VZV IgG antibody, but not VZV DNA, in CSF at the time of recurrence parallels virological findings in other cases of VZV myelopathy [3] and protracted vasculopathy [4], and emphasizes the diagnostic usefulness of detecting VZV antibody in CSF in the absence of VZV DNA [5], particularly since, as indicated above, VZV myelopathy occurs without rash. Our case exemplifies the importance of timely diagnosis of VZV myelopathy, since after virological verification and antiviral therapy, there was no recurrence of myelopathy and anti-VZV IgG antibody could not be detected in CSF. Improvement of myelopathy with clearing of anti-VZV IgG antibody from CSF after treatment strongly supports the notion that VZV caused the second attack of myelopathy.
This sixth recorded case of recurrent VZV myelopathy in an immunocompetent adult illustrates two notable features. First, while all earlier cases of recurrent VZV myelopathy developed weeks to months after the first episode of myelopathy [1,6–8], this is the first instance of recurrence years later. Second, recurrent myelopathy developed in the absence of rash in our patient, as in three of the five previously described cases. Awareness of the potential presentation of VZV myelopathy, VZV vasculopathy, cerebellitis and retinal necrosis in the absence of rash is likely to lead to earlier diagnosis and treatment.
Acknowledgments
This work was supported in part by Public Health Service grants AG006127 and NS32623 from the National Institutes of Health. The authors thank Marina Hoffman for editorial review and Cathy Allen for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilden DH, Beinleich BR, Rubinstien EM, Stommel E, Swenson R, Rubinstein D, Mahalingam R. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818–23. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- 2.de Silva SM, Mark AS, Gilden DH, Mahalingam R, Balish M, Sandbrink F, Houff S. Zoster meyelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–31. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- 3.Orme HT, Smith AG, Nagel MA, Bert RJ, Mickelson TS, Gilden DH. VZV spinal cord infarction identified by diffusion-weighted MRI (DWI) Neurology. 2007;69:298–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- 4.Gilden DH, Lipton HL, Wolf JS, Akenbrandt W, Smith JE, Mahalingam R, Forghani B. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Engl J Med. 2002;347:1500–3. doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- 5.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, et al. The varicella zoster vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–60. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano T, Awaki E, Araga S, Takai H, Inoue K, Takahashi K. Recurrent herpes zoster myelitis treated with human interferon alpha: a case report. Acta Neurol Sci. 1992;85:372–5. doi: 10.1111/j.1600-0404.1992.tb04064.x. [DOI] [PubMed] [Google Scholar]
- 7.Baik JS, Kim WC, Heo JH, Zhang HY. Recurrent herpes zoster myelitis. JKMS. 1997;12:360–3. doi: 10.3346/jkms.1997.12.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilhuis HJ, Visser CE, Portegies P. Recurrent varicella-zoster virus myelitis in an immunocompetent patient. Eur Neurol. 2004;52:121–2. doi: 10.1159/000080270. [DOI] [PubMed] [Google Scholar]


