Summary
Intestinal dietary triacylglycerol absorption is a multi-step process. Triacylglycerol exit from the endoplasmic reticulum (ER) is the rate-limiting step in the progress of the lipid from its apical absorption to its basolateral membrane export. Triacylglycerol is transported from the ER to the cis Golgi in a specialized vesicle, the pre-chylomicron transport vesicle (PCTV). The vesicle-associated membrane protein 7 (VAMP7) was found to be more concentrated on PCTVs compared with ER membranes. VAMP7 has been previously identified associated with post-Golgi sites in eukaryotes. To examine the potential role of VAMP7 in PCTV trafficking, antibodies were generated that identified a 25 kDa band consistent with VAMP7 but did not crossreact with VAMP1,2. VAMP7 was concentrated on intestinal ER by immunofluorescence microscopy. Immunoelectron microscopy showed that the ER proteins Sar1 and rBet1 were present on PCTVs and colocalized with VAMP7. Iodixanol gradient centrifugation showed VAMP7 to be isodense with ER and endosomes. Although VAMP7 localized to intestinal ER, it was not present in the ER of liver and kidney. Anti-VAMP7 antibodies reduced the transfer of triacylglycerol, but not newly synthesized proteins, from the ER to the Golgi by 85%. We conclude that VAMP7 is enriched in intestinal ER and that it plays a functional role in the delivery of triacylglycerol from the ER to the Golgi.
Keywords: VAMP7, Lipid absorption, Pre-chylomicron transport vesicle, Triacylglycerol, ER-to-Golgi transport, Vesicles
Introduction
The process of absorbing long-chain triacylglycerols (TAG), the predominant fat in mammalian diets, is a complex, efficient and rapid process. In order for absorption to occur, dietary TAG must be hydrolyzed to fatty acids (FA) and sn-2-monoacylglycerol (MAG) in the intestinal lumen and re-esterified to TAG on the endoplasmic reticulum (ER) membrane. The majority of the TAG leaves the intestine in the lipid core of the chylomicron, the unique lipoprotein produced by the intestine. Chylomicrons are the largest of the circulating lipoproteins with an average diameter of 227 nm (Zilversmit, 1967). The chylomicron, once formed in the ER lumen, goes to the Golgi and then is exocytosed from the basolateral surface of the enterocyte to enter the lymph. We have reported that the rate-limiting step in the transport of TAG across the enterocyte is the exit of chylomicrons from the ER (Mansbach and Nevin, 1998; Zilversmit, 1967). Further work has shown that this step is controlled by the generation of a specialized vesicle, the pre-chylomicron transport vesicle (PCTV), which is budded from the ER membrane (Siddiqi et al., 2003).
Vesicles carrying nascent proteins from transitional sites on the ER membrane to the cis Golgi use coat protein complex II (COPII) proteins both to select proteins for cargo and vesicle budding (Aridor et al., 2001; Miller et al., 2003; Mossessova et al., 2003). These COPII-dependent vesicles, which are 60-80 nm in diameter (Matsuoka et al., 1998), transport protein cargo vectorially toward the cis Golgi (Dascher et al., 1994). Because the size of PCTVs (∼250 nm) needs to be larger than the COPII-vesicles to accommodate its chylomicron cargo and the intermittent requirement for their formation associated with dietary fat intake compared with the constant generation of protein transporting vesicles, we speculated that a protein complex different than COPII may be used for PCTV budding (Siddiqi et al., 2003). In support of this concept, we found that PCTVs, but not vesicles carrying nascent proteins, can be generated from intestinal ER membranes in either the absence of Sar1 or in the presence of inhibitory antibody to Sec31, another COPII protein (Siddiqi et al., 2003). The vesicles formed under these conditions, like those formed in native cytosol, are membrane-bound structures (Siddiqi et al., 2003). PCTVs generated in the absence of COPII proteins, however, do not have the requisite protein(s) on their surface required for them to fuse with the cis Golgi (Siddiqi et al., 2003).
All intracellular transport vesicles studied to date have a protein on their surface, a vesicle-associated soluble N-ethylmaleimide-sensitive factor attachment protein receptor (v-SNARE) that directs the docking and subsequent fusion of the vesicle with its target membrane. The fusion event is controlled by the formation of a SNARE complex prior to fusion in which the v-SNARE is paired with specific SNARE proteins on the target membrane (t-SNAREs). With respect to mammalian systems, only two SNARE complexes have been described for ER-derived vesicles that fuse with the cis Golgi. In one, Sec22b was the v-SNARE and syntaxin5, membrin and rBet1 were the t-SNAREs in NRK cells (Xu et al., 2000). In the other, also using NRK cells, Ykt6 was the v-SNARE with syntaxin5, GOS28, and rBet1 as the t-SNAREs (Zhang and Hong, 2001). Both Ykt6 and Sec22b have been shown to have similar N-termini (Jang et al., 2002). Because of their N-terminal extensions to the coiled-coil domains of the synaptobrevin-like SNARE motif of most v-SNAREs, these SNAREs have been called longins (Filippini et al., 2001). VAMP7, an additional member of the longin family (TI-VAMP, SYBL1) (Filippini et al., 2001), has not been described as an ER-related SNARE, but has been shown to be predominately associated with endosomes (Advani et al., 1999) in mammals. VAMP7 (Advani et al., 1999) and Sec22b have both been shown to have a widespread tissue distribution whereas Ykt6 is predominantly found in brain in a lysosome-related compartment (Hasegawa et al., 2003).
Because the N-terminus directs the subcellular localization of at least some longins (Zeng et al., 2003), we questioned whether a longin, different from the ER-associated Ykt6 and Sec22b, could be associated with intestinal ER and PCTV generation. We focused our attention on Sec22b and VAMP7 because of their widespread tissue distribution. The present study demonstrates that VAMP7 is specifically associated with intestinal ER and regulates the intracellular trafficking of PCTVs.
Results
Since PCTVs are derived from the ER (Siddiqi et al., 2003), we reasoned that a potential v-SNARE would be more concentrated on PCTVs compared with ER. To detect the protein of interest, we performed 2D gels of PCTV membranes and carbonate-treated ER membranes (data not shown). One protein spot concentrated on PCTVs was VAMP7, Ykt6 or Sec22b according to their molecular mass and pI. Immunoblot analysis demonstrated that VAMP7, not Ykt6 or Sec22b (Fig. 2), was concentrated on PCTV rather than ER membranes supporting further investigation of VAMP7 distribution and functionality.
Fig. 2.
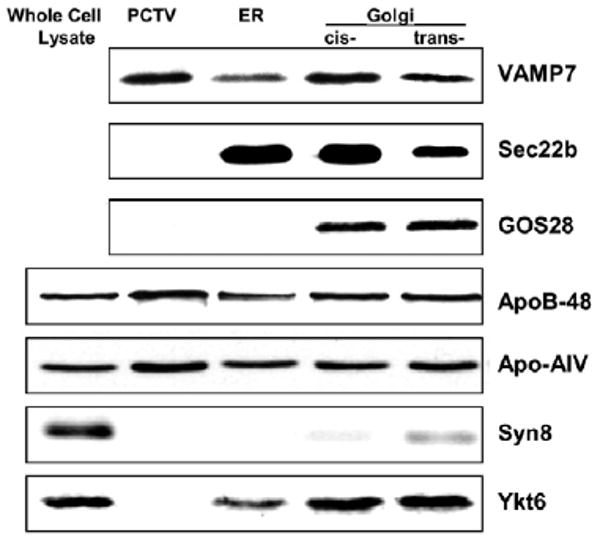
Immunoblots of VAMP7, GOS28, apoB48, apoAIV, Ykt6, syntaxin8 and Sec22b in subcellular fractions of rat intestinal cells. Whole-cell lysate, PCTV, ER and Golgi proteins (30 μg protein) were separated by 12% SDS-PAGE, transblotted onto nitrocellulose membranes and immunoblotted for VAMP7, Sec22b, GOS28, apoB48, apoAIV, Ykt6 and Syntaxin8.
Anti-VAMP7 antibodies
To investigate the potential presence of VAMP7 in intestinal ER, we first generated polyclonal antibodies to rat VAMP7. To distinguish VAMP1 or 2 from VAMP 7, antibodies were raised to a unique VAMP7 N-terminal 19-mer synthetic peptide (see Materials and Methods). These antibodies recognized 25, 47 and 150 kDa bands on an immunoblot of rat intestinal ER membranes (Fig. 1A). Each of these bands was VAMP7 as shown by MALDI-TOF with a Z score of 2.4 for each band. We assumed that the higher molecular mass bands represented multimers of the expected 25 kDa VAMP7 but reducing agents such as DTT (50 mM), 2-mercaptoethanol (0.35 M), tributyl-phosphine (5 mM), Bond-Breaker TCEP [tris (2-carboxyethyl phosphine)] (Pierce Biotechnology, Rockford, IL) (10 mM) reduced the higher molecular mass bands only slightly. To be more certain that our antibody was identifying only VAMP7, we performed an immunoblot on rat intestinal ER membranes separated on a two-dimensional (2D) gel and probed the blot with our anti-VAMP7 antibody. The 150 kDa band is no longer identified and the band at 47 kDa is greatly attenuated (Fig. 1B). A major and a minor band at 25 kDa, the expected size for VAMP7, are the most prominent of the proteins identified by the antibody. We assume that the urea used in the ER-solubilizing buffer de-aggregated the VAMP7. The data support the thesis that our antibody identifies uniquely VAMP7.
Fig. 1.
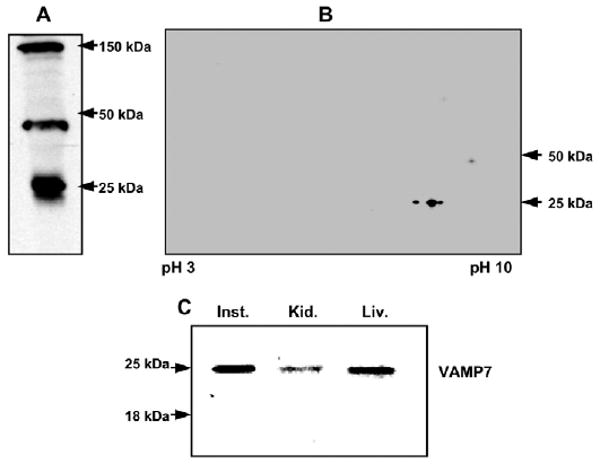
Immunoblots demonstrating that VAMP7 is found in enterocytes and concentrated on PCTVs. (A) Immunoblot of rat intestinal ER (30 μg protein) separated by SDS-PAGE, transblotted to nitrocellulose membrane, and probed with anti-VAMP7 antibody. The molecular sizes of identified bands are indicated. (B) Immunoblot of ER membrane separated by 2D SDS-PAGE (8-18%). ER membranes (200 μg) were solubilized and separated first by pI and subsequently by molecular size (Materials and Methods). The proteins were transblotted onto nitrocellulose membranes and probed with anti-VAMP7 antibodies. Detection was by ECL. (C) Immunoblots of VAMP7 in different rat organs. of Post nuclear supernatant (30 μg protein) of rat intestinal (Inst.), rat kidney (Kid.), and rat liver (Liv.) were separated by 12% SDS-PAGE, transblotted onto nitrocellulose membranes and immunoblotted for VAMP7. Bands at 25 and 18 kDa are indicated.
Because of the homology of the C-terminus of VAMP7 with VAMP1,2, we performed an immunoblot of post-nuclear supernatants (PNS) formed from rat small intestine, liver and kidney (Fig. 1C) to be certain that our antibody did not identify a protein with an size consistent with VAMP1,2 (18 kDa). Our anti-VAMP7 antibody identified only a 25 kDa band. The antibody did not identify the 47 and 150 kDa bands in the PNS of liver and kidney (data not shown).
VAMP7 is present in intestinal ER
To confirm the presence of VAMP7 on PCTVs and to investigate its presence in the intestinal ER and Golgi, we separated PCTV, ER and Golgi proteins by 12% SDS-PAGE and performed an immunoblot using our anti-VAMP7 antibodies. A band corresponding to 25 kDa was detected in each subcellular fraction, suggesting the presence of VAMP7 in each (Fig. 2). Although the PCTV concentrated VAMP7, it contained no Sec22b or Ykt6 v-SNAREs associated with the ER (Fig. 2). Confirming our previous observations (Siddiqi et al., 2003), PCTV contained apoB48 and apoAIV but did not contain the Golgi marker, GOS28, nor the endosomal marker, Syntaxin 8, nor the Golgi/endosomal v-SNARE, Ykt6, which can also have an ER localization (Liu and Barlowe, 2002) as it does in the rat intestine (Fig. 2). The ER preparation was not contaminated by Golgi as suggested by the lack of GOS28. Furthermore, ER or cis-Golgi preparations did not contain endosomes as suggested by the absence of syntaxin 8. Minimal syntaxin 8 was present in the trans Golgi (Fig. 2). In total, these data support the conclusion that the VAMP7 antibody is specific and that VAMP7 is enriched in intestinal ER.
To extend these observations, immunofluorescence localizations were performed on isolated intestinal cells (Fig. 3). To label the ER we used anti-protein disulfide isomerase (PDI) antibodies (green) and to label the location of VAMP7, we used anti-VAMP7 antibodies (red). Both the anti-VAMP7 and the anti-PDI antibodies stained linear structures typical of the ER (arrows, Fig. 3). When merged, these structures became yellow suggesting the colocalization of VAMP7 with PDI. In the presence of pre-immune IgG, only a black background with no fluorescence was found (data not shown).
Fig. 3.
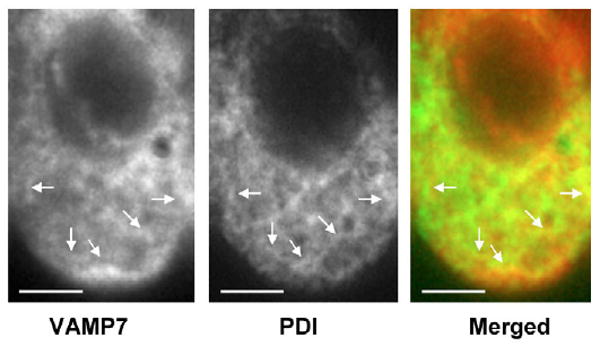
Deconvoluted microscopy of isolated rat intestinal cells showing colocalization of VAMP7 with the ER marker, PDI. Optical horizontal sections stained with anti-VAMP7 antibodies (Texas Red), anti-PDI antibody (FITC green) and the merged image. Arrows indicate structures resembling ER. Bars, 5 μm.
To provide additional morphological evidence of the localization of VAMP7 to intestinal ER, we tested whether we could colocalize VAMP7 with the COPII protein, Sar1. Sar1 is the initiating protein for the COPII-dependent budding of ER-derived vesicles (Aridor et al., 1998; Barlowe et al., 1994). We also wished to know if the ER SNARE, rBet1, would colocalize with VAMP7. Since PCTVs are derived from the ER and concentrate Sar1, rBet1 and VAMP7 (Siddiqi et al., 2003) (data not shown) we reasoned that the presence of both rBet1 and Sar1 with VAMP7 on PCTV would support the presence of VAMP7 in intestinal ER. To test this, we used immunogold antibody labeling of the PCTV and examined the results by electron microscopy using the negative-staining technique (Fig. 4). Pre-immune IgG yielded one gold particle in a typical low-power field (Fig. 4A). By contrast, when gold labeled anti-Sar1 and anti-VAMP7 antibodies were used, multiple 10 and 15 nm gold particles were associated with PCTV indicating the colocalization of Sar1 and VAMP7, respectively (Fig. 4B). Similarly, rBet1 colocalized with VAMP7 on PCTV in a low-power electron micrograph when their respective gold-labeled antibodies were tested (Fig. 4C). The co-labeling of tubular structures suggest that vesicular-tubular complexes (VTC), an intermediate compartment in ER-to-Golgi vesicle transport, may be present in this preparation (Fig. 4B,C). The data presented in Figs 3 and 4 provide strong morphological evidence that VAMP7 is concentrated on both intestinal ER and PCTVs.
Fig. 4.
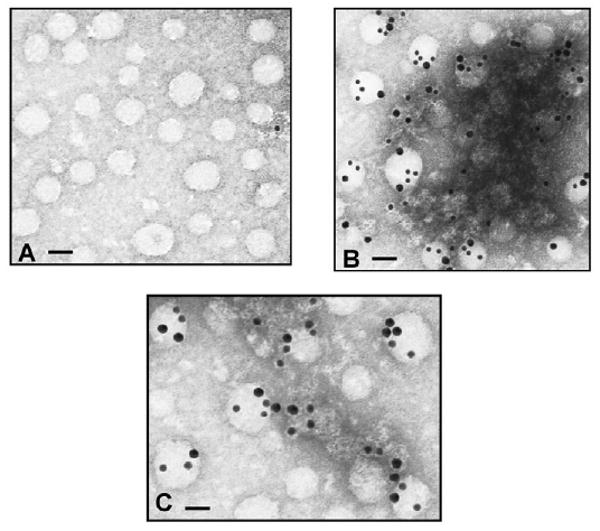
Immunogold labeling of PCTV showing VAMP7 and either Sar1 or rBet1 to be present on the same membrane as shown by electron microscopy. PCTVs were placed on formvar-coated Nickel grids and exposed to pre-immune rabbit IgG (A), anti-Sar1 and anti-VAMP7 (B) or anti-rBet1 and anti-VAMP7 antibodies (C). Rabbit anti-VAMP7 antibodies were labeled with 15 nm immunogold particles. Rabbit anti-Sar1 and mouse anti-rBet1 antibodies were labeled with 10 nm immunogold particles. Bars, 100 μm (A); 80 μm (B,C).
New cell organelle isolation techniques have enabled the separation of endosomes, the expected intracellular location of VAMP7, from the ER (Woods et al., 2002; Siddiqi et al., 2003). To develop an additional line of evidence for the localization of VAMP7, the PNS of intestinal cellular homogenates was separated using iodixanol density gradient (Woods et al., 2002). Fractions from the gradient were then probed with marker antibodies (Fig. 5). Rab11 and syntaxin 8 were used to identify the location of endosomes (Ullrich et al., 1996; Prekeris et al., 1999) and calreticulin and Sec22b the ER. In the resolved gradient, Rab11 was present in fractions 3 to 5 and Syntaxin 8 in fractions 3 to 8 as was VAMP7 (fractions 3 to 7), confirming prior studies that localized VAMP7 to endosomes (Advani et al., 1999). Calreticulin and Sec22b, as expected, localized to the denser part of the gradient occupied by the ER, fractions 14 to 17. A strong VAMP7 signal was also obtained in fractions 15 and 16 indicating the localization of VAMP7 to the intestinal ER in agreement with the morphological and immunological data.
Fig. 5.
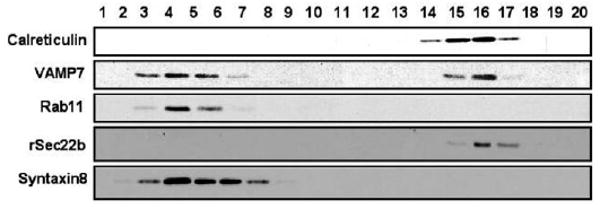
Distribution of rat intestinal subcellular organelles separated on an iodixanol gradient identifying VAMP7 in both ER and endosomes. Post nuclear supernatant protein (2 mg) was separated on a 10-40% iodixanol gradient. The gradient was centrifuged and resolved as described in the Materials and Methods and the fractions obtained indicated. The proteins in each fraction (50 μl) were separated by 12% SDS-PAGE, transblotted to a membrane and the location of calreticulin, VAMP7, Rab11, Sec22b and Syntaxin8 identified by immunoblotting with their specific antibodies.
Since an ER localization of VAMP7 has not been heretofore described, we wondered whether this was unique to the intestine or was a more generalized finding in the rat. We tested ER from the other TAG-exporting organ in mammalian systems, the liver, and a non-TAG-exporting organ, the kidney, for the presence of VAMP7 by immunoblotting (Fig. 6). Using PNS separated by the iodixanol gradient, we found that VAMP7 was present in the portion of the gradient expected for endosomes in intestine (Fig. 6A), liver (Fig. 6B), and kidney (Fig. 6C). By contrast, in the part of the gradient expected for ER, a signal for VAMP7 was present only in the intestine (Fig. 6A).
Fig. 6.
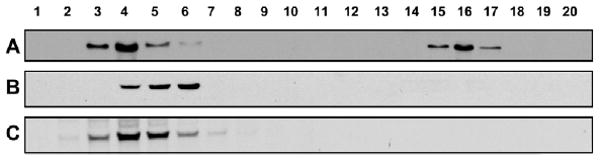
Distribution of VAMP7 in the postnuclear supernatants of rat intestine, liver and kidney. Post-nuclear supernatant (2 mg protein) of intestine (A), liver (B) and kidney (C) were separated by 10-40% iodixanol gradient (Materials and Methods) and the gradient resolved by aspiration. Fractions (50 μl) were separated by 12% SDS-PAGE, transblotted to nitrocellulose membranes, and VAMP7 was identified by immunoblotting with anti-VAMP7 antibodies.
VAMP7 functions as an ER derived v-SNARE for PCTV To test the functionality of VAMP7 as the potential PCTV v-SNARE, the effects of anti-VAMP7 antibodies on the delivery of 3H-TAG from the ER to intestinal cis Golgi was examined (Fig. 7A). In this assay 3H-TAG-loaded ER was exposed to pre-immune IgG (bar A) or anti-VAMP7 antibodies (bar B) then excess antibodies were removed and the resulting ER incubated with cis Golgi (Siddiqi et al., 2003). Post incubation, the cis Golgi was isolated on a sucrose gradient. The ER is in the 1.22 M sucrose fraction and pellet under these conditions whereas the cis Golgi is at the 0.86/1.15 M sucrose interface. Twelve percent of the ER 3H-TAG migrated to the cis Golgi in the presence of pre-immune IgG. Pre-incubation of the ER with anti-VAMP7 antibodies (Fig. 7A, bar B) suppressed the delivery of PCTV-3H-TAG to the cis Golgi compared with pre-immune IgG incubated controls (bar A) by nearly 90% suggesting a role for VAMP7 in targeting PCTV to the cis Golgi and fusing with it. Further, the data presented in Fig. 2 showing the absence of Syntaxin8 in the cis-Golgi preparations suggest that PCTVs are not fusing with endosomes in this assay. To further clarify these findings, we also pre-incubated intestinal cis Golgi with anti-VAMP7 antibodies under similar conditions but this had no effect on PCTV transport to the cis Golgi (Fig. 7A, bar C) compared with ER pre-incubated with IgG (bar A). These data suggest that it is the VAMP7 in the ER that is required for PCTV export to the Golgi, not the VAMP7 known to be present in the cis-Golgi fraction (Fig. 2). Intestinal ER was also incubated with antibodies to the endosomal SNARE, Syntaxin 8 (Fig. 7A, bar D) and the ER v-SNARE Sec22b (bar E). In both cases there was no effect on the transport of TAG in PCTVs to the cis Golgi compared with incubation of the ER with pre-immune IgG, suggesting that steric hindrance or other non-specific antibody effects by the anti-VAMP7 antibody were not the cause of the reduced PCTV transport to the cis Golgi.
Fig. 7.
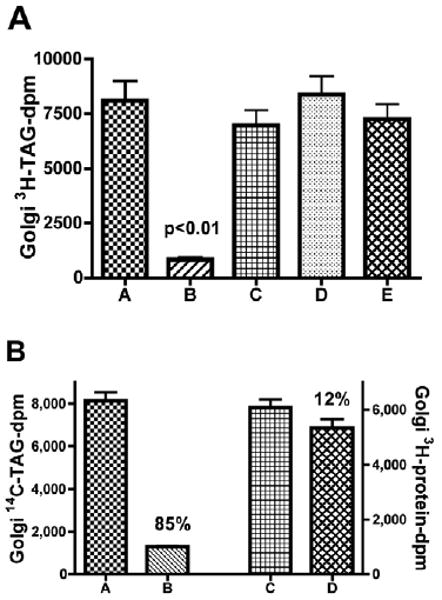
Anti-VAMP7 antibodies inhibit the transfer of TAG but only modestly reduce the transfer of newly synthesized proteins from the ER to the cis Golgi. 3H-TAG-loaded rat intestinal ER (300 μg protein) were incubated with 10 μl pre-immune IgG (A), ER treated with 10 μl of anti-VAMP7 antibody (B), cis Golgi treated with 10 μl anti-VAMP7 antibodies (C), ER treated with anti-syntaxin8 antibodies (D) or ER treated with anti-Sec22b antibodies (E). Unbound antibody was removed by washing and the ER was incubated with 1 mg native rat cytosol, 500 μg rat cis Golgi, an ATP-generating system and buffer B for 30 minutes at 35°C. The cis Golgi was isolated on a sucrose step gradient (see Materials and Methods) and obtained by aspiration. TAG was extracted and the dpm determined. The data are the mean ± s.e.m., n=4. There was a significant difference in 3H-TAG transfer (P<0.01) between VAMP7 antibody-treated ER and the pre-immune control. (B) ER (500 μg protein) loaded with 14C-TAG and 3H-protein was treated with pre-immune IgG (10 μl) (A and C), or anti-VAMP7 antibody (10 μl) (B and D), excess antibody was removed by washing, and incubated with native rat intestinal cytosol (1 mg protein), an ATP-generating system and cis Golgi (500 μg protein). The Golgi fraction was isolated on a sucrose gradient and the amount of TAG (left y axis) or protein (right y axis) dpm determined (Materials and Methods). The percentage reduction in transport upon VAMP7 antibody treatment is shown above the bars. A compared with B, P<0.001; C compared with D, P>0.05. The data are the mean ± s.e.m. (n=4).
Since VAMP7 appears to play a role in the movement of a specialized vesicle from the ER to the Golgi in the intestine, we wondered if it also played a role in the transport of vesicles carrying newly synthesized proteins. To address this issue, intestinal ER was loaded with 14C-TAG and 3H-protein. The ER was pre-incubated with either pre-immune IgG or anti-VAMP7 antibodies, the excess antibodies removed, and the treated ER incubated with cytosol, cis Golgi and an ATP-generating system. After incubation, the cis Golgi was isolated and the content of 3H-protein and 14C-TAG was determined to assay the transport of newly synthesized proteins or TAG from the ER to the cis Golgi (Fig. 7B). In agreement with Fig. 7A, incubation of the ER with anti-VAMP7 antibody reduced transport of TAG to the Golgi by 85% compared with incubation with pre-immune IgG (Fig. 7B, compare bars A and B). However, the transport of newly synthesized proteins was reduced by only 41% after ER treatment with anti-VAMP7 antibodies compared with pre-immune IgG. We questioned whether a portion of the observed reduction in protein transport to the Golgi could be accounted for by the lack of transport of 3H-proteins in PCTVs under these conditions. To test this, the same double-labeled ER preparation incubated under the same conditions as used in Fig. 7A was used but without acceptor cis Golgi. PCTVs and protein vesicles were collected (Siddiqi et al., 2003) and 3H-protein measured in each. In these experiments, the protein vesicles contained 4520±502 3H-dpm and the PCTVs 2280±296 3H-dpm. The data suggest that 29% ([2280/4520+2280] ×0.85) of the 3H-protein-dpm available to be transported to the cis Golgi are inhibited by the effect of VAMP7 antibodies on PCTV transport, 85% of which were blocked. Taking these data into account, the effective reduction in newly synthesized protein transport to the Golgi by anti-VAMP7 antibody treatment was only 12% (41-29%) (Fig. 7B, compare bars C and D) suggesting that the anti-VAMP7 antibodies had little effect on the anterograde movement of newly synthesized proteins. In sum, the data are consistent with the thesis that ER-localized VAMP7 participates in the anterograde movement of TAG via PCTV but that newly synthesized protein movement is VAMP7 independent.
Discussion
These studies show a previously undescribed functional association of VAMP7 with ER and a distinct subset of ER-derived transport vesicles. These conclusions are based on morphological data showing that VAMP7 colocalizes with the ER marker PDI using immunofluorescence microscopy and is localized to the PCTV, an ER-derived vesicle, with the ER COPII protein, Sar1 and the ER SNARE, rBet1 upon immunoelectron microscopy. Additional support for these conclusions is provided by cell fractionation studies that show VAMP7 is present in the same density fractions as the ER markers, calreticulin and Sec22b. Further, antibody inhibition studies demonstrate that ER-localized VAMP7 is required for the transport of ER-TAG to the cis Golgi but not that of newly synthesized proteins. In sum, the combination of morphological as well as physiological studies provide compelling evidence that VAMP7 is present in rat intestinal ER and PCTV and that it participates in the transfer of ER-TAG to the cis Golgi.
VAMP7 or TI-VAMP is an integral membrane protein (type 2) that has an N-terminal extension differentiating it from VAMP1 and VAMP2 (Galli, 1998). Since the transmembrane region of VAMP7 is at the C-terminal end, the N-terminal extension would be expected to provide an elongated cytoplasmic sequence compared with that of VAMP1 (Filippini et al., 2001). Crystallographic evidence suggests the N-terminal extension has a protein-binding sequence (Gonzalez et al., 2001). Because the shorter VAMPs were called brevins, Filippini et al. proposed the term, longin, to describe the VAMP7 family (Filippini et al., 2001). The N-terminal end of VAMP7 shows a considerable structural homology with the VAMP family member, Sec22b, a v-SNARE (Gonzalez et al., 2001) involved in ER-to-Golgi protein transport (Hay et al., 1996). Since both proteins are present in intestinal ER, these homologous N-terminal regions may contain information required for the ER targeting of these proteins. Support for this idea has been obtained in Arabidopsis in which Uemura et al. propose that the longin domain determines in part the organelle targeting of VAMP7 (Uemura et al., 2005), which in this plant includes the ER (Uemura et al., 2004).
There is evidence that VAMP7 is required in intestinal ER for successful export of chylomicrons from intestinal cells. Intestine-like CaCo2 cells synthesize TAG rapidly (Trotter and Storch, 1991) and synthesize the requisite lipoproteins associated with chylomicrons (Traber et al., 1987) but are unable to deliver TAG in an efficient manner to the basal medium. VAMP7 is expressed in CaCo2 cells but it is not found in their ER (Galli, 1998) suggesting that its absence reduces chylomicron export. Consistent with these results is our finding that anti-VAMP7 antibodies block TAG movement to the cis Golgi. However, the causal relationship between ER-localized VAMP7 and successful export of chylomicrons remains to be investigated further.
Identifying VAMP7 as a potential v-SNARE for PCTV is a surprising result because VAMP7 is usually considered as a post-Golgi SNARE protein. It was originally found to be associated with lysosomes in a rat kidney cell line (Advani et al., 1998) and with the apical plasma membranes of both intestine-like CaCo2 epithelial cells (Galli, 1998) and Madin-Darby canine kidney (MDCK) cells (Bogdanovic et al., 2002). Thus far in eukaryotes, VAMP7 has been described to mediate endosomal function including protein transport to lysosomes (Advani et al., 1998; Bogdanovic et al., 2002; Wade et al., 2001), the post-Golgi transport of raft-associated proteins and lipids (Lafont et al., 1999), and peripheral microtubules and vesicles in neuronal cells (Coco et al., 1999) associated with neurite growth (Martinez-Arca et al., 2000).
VAMP7 is concentrated on intestinal ER, but not the kidney or liver ER, the latter being a TAG-exporting tissue. The mechanism of the distinct VAMP7 targeting in the intestine is unknown. VAMP7 does not contain a FFAT domain, which in combination with VAMP-associated protein-A (VAP-A), could direct it to the ER as occurs with oxysterol binding protein (OSBP) (Wyles et al., 2002). VAMP7 can be ectopically located to the ER under the unusual circumstances of transfecting nSec1 in HeLa cells, which keeps syntaxin1 in its closed conformation. Under these conditions, syntaxin1 becomes localized to the ER (Martinez-Arca et al., 2003), as does its cognate SNARE partner, SNAP23. These two t-SNARES bind the v-SNARE, VAMP7, which completes a VAMP7-containing SNARE complex (Martinez-Arca et al., 2003). However, we did not detect syntaxin1 in intestinal ER (data not shown). Interestingly, SNAP23 was detected in intestinal ER, but not in liver or kidney ER (data not shown). It is unclear what SNARE pairing, if any, binds VAMP7 to the ER but SNAP23 may play a role because PCTV concentrates not only VAMP7 but also SNAP23 from intestinal ER membranes.
Although VAMP7 plays a role in the anterograde movement of TAG, it does not appear to be involved with the transport of newly synthesized proteins carried in COPII-dependent protein vesicles to the cis Golgi. These data are consistent with prior information that would suggest a major role for the COPII proteins in protein export from the ER (Aridor et al., 1998). The unique presence of VAMP7 in intestinal ER may be linked to the intermittent requirement for cargo transport associated with lipid meals when compared with the more constant demands of newly synthesized proteins for distribution throughout the cell or for secretion. In this paradigm, VAMP7 would play a central role in the intermittent formation of a vesicle whose specialized cargo segregates it from the anterograde movement of proteins in COPII-dependent vesicles that continuously form from the ER membrane. In conclusion, we show that VAMP7 is concentrated in intestinal ER and on PCTVs where it plays a functional role in the export of chylomicrons from the ER to the cis Golgi.
Materials and Methods
Materials
[3H]oleic acid (9.2 Ci/mM), [14C]oleic acid (56 mCi/mM), and [3H]leucine (180 Ci/mM) were obtained from New England Nuclear (Boston, MA). Iodixanol (Optiprep) was purchased from Axis-Shield PoC (Greiner-Bio-One, Longwood, FL). Immunoblot reagents were purchased from Bio-Rad (Hercules, CA). Enhanced chemiluminescence (ECL) reagents were procured from Amersham International (Piscataway, NJ). Protease inhibitor cocktail tablets were obtained from Boehringer Mannheim (Indianapolis, IN). Other biochemicals used were of analytical grade and purchased from local companies. Rats, 150-200 g, were purchased from Harlan (Indianapolis, IN).
Antibodies
Polyclonal antibodies against rat VAMP7 were raised in rabbits commercially (Genemed Synthesis, San Francisco, CA) using a synthetic 19-mer peptide corresponding to amino acids 105-123 of rat VAMP7. The antibody was purified against its immunogenic peptide. The antibody recognized VAMP7 but did not cross-react with VAMP1,2 by immunoblot (Fig. 1C). Rabbit anti-Sar1 antibodies were raised commercially (ProteinTech Group, Chicago, IL) using recombinant Sar1 protein (Siddiqi et al., 2003). Rabbit anti-rat apolipoproteinB48 (apoB48) antibodies were made from amino acid sequence 2055-2067 by ProteinTech Group; Rabbit anti-apolipoprotein AIV was a generous gift from Patrick Tso (University of Cincinnati, Cincinnati, OH). Mouse monoclonal antibodies to rBet1, GOS-28 and membrin were procured from Stressgen (Victoria, Canada). Mouse anti-protein disulfide isomerase (PDI) monoclonal antibodies were purchased from Affinity Bioreagents (Golden, CO). Rabbit anti-Rab11, rabbit anti-calreticulin polyclonal antibodies, rabbit anti-Ykt6 antibodies, FITC-conjugated goat anti-mouse, Texas-Red-conjugated goat anti-rabbit IgGs, goat anti-rabbit IgG, goat anti-mouse IgG, and goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-syntaxin8 antibodies were from BD Biosciences (San Jose, CA). Anti-rabbit IgG and anti-mouse IgG conjugated with gold particles were obtained from Electron Microscopy Sciences (Fort Washington, PA).
Isolation of ER, cytosol, Golgi, and metabolic labeling of enterocytes
Enterocytes from the proximal half of rat small intestine were isolated and radiolabeled with 3H-TAG essentially as described (Kumar and Mansbach, 1997). In brief, enterocytes were released from intestinal villi, collected, incubated with albumin bound [3H]oleate, and washed with 2% BSA in PBS to remove excess [3H]oleate. The cells were homogenized and the ER and Golgi isolated using a sucrose step gradient which was repeated to purify the ER. Double labeling of intestinal ER was achieved by incubation of similarly isolated enterocytes with [14C]oleate and [3H]leucine (Siddiqi et al., 2003). To determine if the ER preparation was contaminated by cis Golgi, it was probed with antibodies to the cis Golgi marker, GOS28 (Hay et al., 1997; Nagahama et al., 1996). The immunoblot of both ER and cis Golgi (Fig. 2) shows no detectible GOS28 in the ER fraction whereas the cis Golgi shows a strong GOS28 signal. Cytosol was isolated from rat enterocytes (Siddiqi et al., 2003).
SDS-PAGE, 2D electrophoresis and immunoblotting
Proteins separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) were transblotted onto nitrocellulose membranes (Bio-Rad) (Siddiqi et al., 2003). Proteins were detected by their specific antibodies and peroxidase-conjugated secondary antibodies using ECL and Biomax film (Eastman Kodak, Rochester, NY). For 2D gels, ER membranes (200 μg protein) were solubilized in 500 μl sample buffer [7 M urea, 2 M thiourea, 4% CHAPS, 2 mM tri-butyl phosphine (TBP), 0.5% carrier ampholyte (pH 3-10), 40 mM Tris] for 1 hour at 37°C. The solubilized ER protein sample was loaded onto immobilized pI gradient gel (IPG) strips (pH 3-10) (Amersham-Pharmacia) by incubation at room temperature overnight. Isoelectric focusing (IEF) was performed using a Multiphor II apparatus (Amersham-Pharmacia). A total of 50,500 volt-hours were applied to each IPG strip. After IEF, the IPG strip was first equilibrated in buffer I [0.5 M Tris-HCl pH 6.8, 6 M urea, 30% glycerol (v/v), 2% SDS, 25 mM DTT] for 10 minutes and then in buffer II [0.5 M Tris-HCl pH 6.8, 6 M urea, 30% glycerol (v/v), 2% SDS, 12 mM iodoacetamide] for 10 minutes. Proteins were separated on an 8-18% SDS polyacrylamide gel, transblotted onto nitrocellulose membranes, and probed with anti-VAMP7 antibodies.
Isolation of subcellular organelles using an Iodixanol gradient
Enterocytes from the proximal half of rat small intestine, freshly isolated rat hepatocytes, or kidney cells were re-suspended in buffer A (20 mM Tris-HCl, pH 8.0, 0.25 M sucrose, 140 mM NaCl, 1 mM EDTA, protease inhibitor cocktail). The cellular membranes were disrupted by 15 passes of a Potter-Elvehjem tissue homogenizer with a PTFE pestle and the cell lysate was centrifuged at 700 g for 10 minutes at 4°C to obtain a post-nuclear supernatant (PNS). The PNS (2 mg protein) was overlaid onto the top of a linear gradient of 10-40% iodixanol in buffer A and centrifuged at 48,000 g for 18 hours at 4°C using a Beckman SW-41 rotor as described (Woods et al., 2002). The gradient was fractionated into 0.5 ml fractions. The proteins from each fraction were precipitated with trichloroacetic acid, resolved by 12% SDS-PAGE and probed for Rab11, calreticulin, Sec22b, syntaxin8, and VAMP7 by immunoblotting as indicated.
In vitro PCTV formation
PCTVs containing 3H-TAG, were formed from 3H-TAG loaded ER (Siddiqi et al., 2003). In brief, ER (500 μg protein) was incubated with cytosol (1 mg protein) and an ATP-generating system at 37°C for 30 minutes in the absence of Golgi acceptor (Siddiqi et al., 2003). The incubation mixture was resolved on a continuous sucrose gradient and PCTV isolated from the light portions of the gradient. PCTVs thus formed were concentrated to 5.0 mg/ml protein using a Centricon-10 filter (Millipore, Bedford, MA).
Transport of newly synthesized TAG from the ER to the Golgi
To determine the transport of newly synthesized TAG to the Golgi, ER containing 3H-TAG was prepared (Kumar, 1997). Where the transport of both TAG and protein was required, ER containing 3H-protein and 14C-TAG was formed (Siddiqi et al., 2003). The ER (300 μg protein) was incubated with Golgi membranes (500 μg protein) (Siddiqi et al., 2003) in the presence of cytosol (1 mg protein), an ATP-regenerating system, and buffer B (25 mM HEPES, pH 7.2, 0.25 M sucrose, 30 mM KCl, 2.5 mM CaCl2, 2.5 mM MgCl2, 1 mM DTT, and protease inhibitor cocktail) at 35°C for 30 minutes. The reaction was stopped by adding cold buffer B and placing the tubes on ice. The density of the reaction mix was adjusted to 1.22 M sucrose (total volume 3 ml), overlaid with 2.6 ml each of 1.15 M, 0.86 M and 0.25 M sucrose, and the gradient centrifuged (Kumar and Mansbach, 1997). The cis Golgi fraction (0.86/1.15 sucrose interface) was isolated, TAG extracted, and the TAG radioactivity measured (Kumar and Mansbach, 1997). In cases where antibody treatment of ER or cis Golgi membranes was required, the indicated antibody (10 μl) was incubated with either ER or Golgi membranes for 1 hour at 4°C. Excess antibody was removed by washing twice.
Immunocytochemistry
Enterocytes were isolated (Siddiqi et al., 2003) and washed twice with cold phosphate-buffered saline (PBS). A few drops of cell suspension were placed on a poly-L-lysine-coated slide for 5 minutes and the excess removed. Cells that adhered to the slide were kept at −20°C for 2 hours and then fixed with 4% paraformaldehyde. Fixed cells were permeabilized with 0.1% Triton X-100 followed by blocking with 3% BSA in PBS for 30 minutes. The cells were incubated with rabbit anti-VAMP7 polyclonal (1:100 dilution) and mouse anti-PDI monoclonal (1:200 dilution) antibodies or pre-immune anti-rabbit and anti-mouse IgGs at 1:100 dilution for 90 minutes. The cells were washed with PBS containing 0.1% BSA and incubated with Texas-Red-conjugated goat anti-rabbit and FITC-conjugated goat anti-mouse IgGs at 1:100 dilution for 1 hour. The cells were washed with PBS five times and coverslips placed on the slides using Vectashield mounting medium. Microscopy was performed using a Nikon Diaphot inverted tissue culture microscope equipped for epi-fluorescence using a mercury lamp with UniBlitz shutter control, and a Photometrics CH250 cooled CCD camera controlled with a Macintosh PowerPC using Scanalytics IP Lab Spectrum software with a VayTech microtome and HazeBuster Macintosh plug-ins to remove contaminating light from the fluorescent micrographs. The microscope was equipped with a Nikon B-1A filter cube for FITC visualization and a Nikon G-1A filter cube for Rhodamine visualization. The immunofluorescent images were captured using a Nikon Diaphot inverted microscope with appropriate filters and images produced by deconvolution.
Immunoelectron microscopy
To examine PCTV by electron microscopy, we used the negative-staining technique (Siddiqi et al., 2003). Briefly, PCTV were collected from several experiments and concentrated using a Centricon filter with a YM 10 membrane. A formvar carbon-coated nickel grid was placed on a drop of concentrated PCTV for 1 minute, stained with 0.5% aqueous uranyl acetate, air-dried and examined using a JEOL 1200 EX electron microscope (JEOL, Peabody, MA) at 12,000× magnification.
Colocalization of VAMP7 and rBet1 on PCTV was determined by immunogold labeling as described (Mukherjee et al., 2000; Siddiqi et al., 2003). For Sar1 colocalization with VAMP7 on PCTV, the method was slightly modified (Siddiqi et al., 2003). PCTV on formvar carbon-coated grids were washed with PBS and incubated with 10% skimmed milk for 30 minutes. The samples were incubated with either anti-VAMP7 antibodies or pre-immune IgG at 1:50 dilution for 2 hours and washed with PBS followed by incubation with excess secondary antibodies (anti-rabbit IgG) conjugated to 15 nm gold particles, diluted 1:50. The samples were washed to remove unbound antibodies and then incubated either with anti-Sar1 antibodies or pre-immune IgG at 1:50 dilution for 2 hours. After removing the unbound antibodies by washing, anti-rabbit IgG conjugated to 10 nm gold particles (1:50 dilution) was added as the secondary antibody. The grids were washed five times with PBS and fixed in 1% glutaraldehyde (in 0.25 M sucrose in 10 mM HEPES). Finally, the samples were sequentially washed with PBS and distilled water, and stained with 0.5% aqueous uranyl acetate for 2 minutes. The grids were air-dried and examined under the electron microscope (Siddiqi et al., 2003) at 12,000× magnification. Because antibodies against VAMP7 and Sar1 were both generated in rabbits, we performed an additional control experiment to demonstrate that we had completely occupied all the anti-VAMP7 primary antibody with 15 nm gold-conjugated secondary antibody by reprobing the washed grid with secondary antibody containing 10 nm gold particles. No 10 nm particles were found bound to PCTV under these conditions.
Maldi-Tof
PNS from intestine, liver and kidney were separated by 10% SDS-PAGE, stained with SimplyBlue SafeStain (Invitrogen, Carlsbad, California), the bands cut from the gel, and destained with 50% acetonitrile and 100 mM ammonium bicarbonate, pH 8.0. The gel pieces were dried in a vacuum centrifuge and digested overnight with trypsin (0.5 μg) at 37°C. The digested peptides were extracted with 60% acetonitrile and 5% trifluoroacetic acid (TFA) with sonication and dried in a vacuum centrifuge. The peptides were suspended in 0.1% TFA, desalted with a ZipTip and applied to an α cyano-4-hydroxycinnamic acid (CHCA) matrix. MALDI-TOF was performed on a Voyager-DERP mass spectrometer (Applied Biosystems, Framingham, MA).
Measurement of TAG and protein radioactivity
TAG radioactivity was quantified as described (Kumar and Mansbach, 1997). Protein radioactivity was measured after precipitation with trichloroacetic acid (TCA). When double-labeling experiments were performed, the dual-isotope mode was used on the scintillation analyzer (Packard Instrument, Downer's Grove, IL).
Statistical analysis
Comparisons between means were carried out using a statistical package supplied by GraphPad Software (Instat, GraphPad Software, San Diego, CA) using a two-tailed t-test.
Acknowledgments
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK38760 (C.M.M.) and DK54201 (F.S.G.), The Stout Neuroscience Laboratory supported by NIH Grant RR1052 and a National Science Foundation Grant DB1960633, a Veterans Administration Senior Career Development Award (F.S.G.), and by the Office of R&D Medical Research Service, Department of Veteran Affairs research funds (C.M.M. and F.S.G.). The authors wish to thank Larry Tague (University of Tennessee) for help with the deconvoluting microscopy and Jesse Hay (University of Montana) for helpful discussions.
References
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- Advani R, Prekeris R, Lee K, Klumperman J, Scheller R. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J Cell Biol. 1999;146:765–775. doi: 10.1083/jcb.146.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE. Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol. 1998;141:61–70. doi: 10.1083/jcb.141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Fish KN, Bannykh S, Weisman J, Roberts TH, Lippincott-Schwartz J, Balch WE. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152:213–229. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bogdanovic A, Kieffer S, Louwagie M, Garin J, Satre M, Bruckert F. Syntaxin7, syntaxin8, Vti1 and VAMP7 (vesicle-associated membrane protein 7) form an active SNARE complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem J. 2002;368:29–39. doi: 10.1042/BJ20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S, Raposo G, Martinez S, Fontaine JJ, Takamori S, Zahraoui A, Jahn R, Matteoli M, Louvard D, Galli T. Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J Neurosci. 1999;19:9803–9812. doi: 10.1523/JNEUROSCI.19-22-09803.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C, Matteson J, Balch WE. Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J Biol Chem. 1994;269:29363–29366. [PubMed] [Google Scholar]
- Filippini F, Rossi V, Galli T, Budillon A, D'Urso M, D'Esposito M. Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem Sci. 2001;26:407–409. doi: 10.1016/s0968-0004(01)01861-8. [DOI] [PubMed] [Google Scholar]
- Galli T. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1438. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LC, Weiss WI, Scheller RH. A novel SNARE N-terminal domain revealed by the crystal structure of Sec22b. J Biol Chem. 2001;276:24203–24211. doi: 10.1074/jbc.M101584200. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Zinsser S, Rhee Y, Vik-Mo EO, Davanger S, Hay JC. Mammalian Ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain. Mol Biol Cell. 2003;14:698–720. doi: 10.1091/mbc.E02-09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC, Hirling H, Scheller RH. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J Biol Chem. 1996;271:5671–5679. doi: 10.1074/jbc.271.10.5671. [DOI] [PubMed] [Google Scholar]
- Hay JC, Chao DS, Kuo CS, Scheller RH. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell. 1997;89:149–158. doi: 10.1016/s0092-8674(00)80191-9. [DOI] [PubMed] [Google Scholar]
- Jang SB, Kim YG, Cho YS, Suh PG, Kim KH, Oh BH. Crystal structure of SEDL and its implications for a genetic disease spondyloepiphyseal dysplasia tarda. J Biol Chem. 2002;277:49863–49869. doi: 10.1074/jbc.M207436200. [DOI] [PubMed] [Google Scholar]
- Kumar NS, Mansbach CM. Determinants of triacylglycerol transport from the endoplasmic reticulum to the Golgi in intestine. Am J Physiol. 1997;273:G18–G30. doi: 10.1152/ajpgi.1997.273.1.G18. [DOI] [PubMed] [Google Scholar]
- Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Barlowe C. Analysis of Sec22p in endoplasmic reticulum/Golgi transport reveals cellular redundancy in SNARE protein function. Mol Biol Cell. 2002;13:3314–3324. doi: 10.1091/mbc.E02-04-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach CM, II, Nevin P. Intracellular movement of triacylglycerols in the intestine. J Lipid Res. 1998;39:963–968. [PubMed] [Google Scholar]
- Martinez-Arca S, Zahraouni AP, Louvard D, Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol. 2000;149:889–900. doi: 10.1083/jcb.149.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Proux-Gillardeaux V, Alberts P, Louvard D, Galli T. Ectopic expression of syntaxin1 in the ER redirects TI-VAMP- and cellubrevin-containing vesicles. J Cell Sci. 2003;116:2805–2816. doi: 10.1242/jcs.00467. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, Orci L, Schekman R. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Siddiqi SA, Hashim S, Raje M, Basu SK, Mukhopadhyay A. Live Salmonella recruits N-ethylmaleimide-sensitive fusion protein on phagosomal membrane and promotes fusion with early endosome. J Cell Biol. 2000;148:741–753. doi: 10.1083/jcb.148.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M, Orci L, Ravazzola M, Amherdt M, Lacomis L, Tempst P, Rothman JE, Sollner TH. A v-SNARE implicated in intra-Golgi transport. J Cell Biol. 1996;133:507–516. doi: 10.1083/jcb.133.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Tang B, Oorschot V, Klumperman J, Scheller RH. Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol Biol Cell. 1999;10:3891–3898. doi: 10.1091/mbc.10.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi SA, Gorelick FS, Mahan JT, Mansbach CM., II In vitro budding of pre-chylomicron transport vesicles from the endoplasmic reticulum is COPII protein independent. J Cell Sci. 2003;116:415–427. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- Traber MG, Kayden HJ, Rindler MJ. Polarized secretion of newly synthesized lipoproteins by the Caco-2 human intestinal cell line. J Lipid Res. 1987;28:1350–1363. [PubMed] [Google Scholar]
- Trotter PJ, Storch J. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J Lipid Res. 1991;32:293–304. [PubMed] [Google Scholar]
- Uemura T, Ueda T, Ohniwa RL, Nakano A, Takeyasu K, Sato MH. Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct Funct. 2004;29:49–65. doi: 10.1247/csf.29.49. [DOI] [PubMed] [Google Scholar]
- Uemura T, Sato MH, Takeyasu K. The longin domain regulates subcellular targeting of VAMP7 in Arabidopsis thaliana. FEBS Lett. 2005;539:2842–2846. doi: 10.1016/j.febslet.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Ullrich S, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade N, Bryant NJ, Connolly LM, Simpson RJ, Luzio JP, Piper RC, James DE. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in b16 melanoma cells. J Biol Chem. 2001;276:19820–19827. doi: 10.1074/jbc.M010838200. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Roberts MS, Choudhary J, Barry ST, Mazaki Y, Sabe H, Morley SJ, Critchley DR, Norman JC. Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells. J Biol Chem. 2002;277:6428–6437. doi: 10.1074/jbc.M109446200. [DOI] [PubMed] [Google Scholar]
- Wyles JP, McMaster CR, Ridgway ND. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J Biol Chem. 2002;277:29908–29918. doi: 10.1074/jbc.M201191200. [DOI] [PubMed] [Google Scholar]
- Xu D, Joglekar A, Williams A, Hay J. Subunit structure of a mammalian ER/Golgi SNARE complex. J Biol Chem. 2000;275:39631–39639. doi: 10.1074/jbc.M007684200. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Tran TT, Tan HX, Hong W. The cytoplasmic domain of Vamp4 and Vamp5 is responsible for their correct subcellular targeting: the N-terminal extension of VAMP4 contains a dominant autonomous targeting signal for the trans-Golgi network. J Biol Chem. 2003;278:23046–23054. doi: 10.1074/jbc.M303214200. [DOI] [PubMed] [Google Scholar]
- Zhang T, Hong W. Ykt6 forms a SNARE complex with syntaxin5, GS28, and Bet1 and participates in a late state in endoplasmic reticulum-Golgi transport. J Biol Chem. 2001;276:27480–27487. doi: 10.1074/jbc.M102786200. [DOI] [PubMed] [Google Scholar]
- Zilversmit DB. Formation and transport of chylomicrons. Fed Proc. 1967;26:1599–1605. [PubMed] [Google Scholar]


