Abstract
A recent shortage of erythromycin ointment has resulted in the use of alternative agents for newborn ocular infection prophylaxis in the United States. We report a series of 26 newborns who developed a characteristic periocular ulcerative dermatitis following gentamicin ointment administration at two Philadelphia hospitals.
Erythromycin ophthalmic ointment is used routinely for newborn ocular infection prophylaxis in the United States and other countries. A recent manufacturing shortage of erythromycin has resulted in the use of alternative agents. In a collaborative statement, the Centers for Disease Control (CDC), Food and Drug Administration (FDA), and multiple health organizations released a joint recommendation for alternative medications during the shortage.(1) Azithromycin drops were the preferred option, considering chlamydial and gonoccocal antibiotic coverage, but are expensive and in relative short supply. Aminoglycosides were the next tier medications, including gentamicin and tobramycin ointments. Fluoroquinolones were the last option but considered less desirable due to gonococcal resistance.
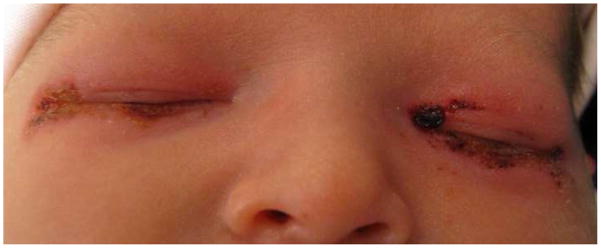
We report a series of 26 newborns who developed a characteristic periocular ulcerative dermatitis following gentamicin ointment administration for newborn ocular prophylaxis at two Philadelphia hospitals (Figure). This study was approved by the joint institutional review board of both institutions.
Figure.
A periocular ulcerative dermatitis, characterized by bilateral eyelid erythema, edema, and ulcerative lesions with serous and hemorrhagic exudate, associated with administration of gentamicin ophthalmic ointment after birth. Photograph courtesy of Stefanie L. Davidson, MD.
Case Series
Gentamicin ointment use for neonatal ocular prophylaxis at the Hospital of the University of Pennsylvania began on September 20, 2009, after erythromycin supplies were exhausted. The index case was an infant born the next day. He was a full-term infant, weighing 3200 grams, born via spontaneous vaginal delivery to a 25 year-old primagravida mother with negative prenatal labs and no history of Herpes Simplex Virus infection. The mother developed a fever prior to delivery, with maternal and fetal tachycardia, suggesting a clinical diagnosis of chorioamnionitis, and he was admitted to the intensive care nursery. He had received gentamicin sulfate ophthalmic ointment (Gentak, Akorn Pharmaceuticals) to both eyes in the delivery room. The next day, he developed bilateral eyelid erythema and edema with multi-focal ulcerative lesions. These lesions produced a sero-sanguinous exudate, and the surrounding skin had a somewhat violacious hue. There was no significant ocular surface inflammation. The baby was otherwise afebrile, feeding, and acting well.
Following ophthalmological and dermatological consultations, bacterial cultures and HSV PCR testing were obtained using specimens from the palpebral conjunctiva, eyelid lesions, serum, and cerebrospinal fluid. Systemic oxacillin, clindamycin, and acyclovir were begun. Bacitracin—polymyxin B sulfate ophthalmic ointment was applied to the ulcerative lesions. Subsequently, HSV testing and cultures returned negative, the baby continued to be otherwise asymptomatic, and acyclovir was discontinued. The periorbital eruption gradually improved, and he was discharged at one week of life on oral cefuroxime, with almost complete resolution of the dermatitis.
Within two days of the first child’s presentation, a second infant presented with identical symptoms, following gentamicin administration. A similar work-up for herpetic disease was negative, and the ulcerative dermatitis improved over one to two weeks. With the second case, the treating neonatologist performed a literature search and identified a previous report that implicated gentamicin ointment as a potential irritant causing contact dermatitis in the newborn.(2) By October 11, fourteen additional cases were identified in the Hospital of the University of Pennsylvania well baby nursery without extensive infectious diagnostic testing. After the fifth case, instructions were given to delivery room personnel to wipe excess gentamicin ointment from the eyelids after administration. The subsequent cases of ulcerative dermatitis were considerably milder in severity. All cases had onset on days two of life, had minimal to no conjunctival involvement, were managed with topical bacitracin-polymixin ophthalmic ointment, and gradually resolved without sequelae over a two-week period.
Gentamicin ointment use began at Pennsylvania Hospital on September 21, 2009. A baby girl born the next day developed a similar periocular rash without conjunctivitis on day one of life. She too underwent systemic and ocular testing for chlamydial, gonococcal, and herpetic infection, with negative results. Subsequently, four additional cases were identified in the well-baby or intensive care nurseries, and five cases were reported back to the hospital by primary care pediatricians who had evaluated the newborns as outpatients. For these nine newborns, the association with gentamicin ointment had already been noted and no further work-up had been pursued. Again, gradual resolution occurred over one to two weeks.
Based upon the number of infants receiving gentamicin ointment, the incidence of dermatitis was 5.6 per 100 newborns, though under-reporting by outpatient physicians likely results in an underestimation. The CDC, FDA, internal hospital boards, and manufacturer were notified of the adverse reactions. It was not possible to implicate a single lot number in all of the reported cases.
DISCUSSION
The ulcerative periocular dermatitis in these newborns likely resulted from the application of gentamicin sulfate ophthalmic ointment in the delivery room. In all cases the rash was preceded by gentamicin application and was identified the same or next day. In at least one case, the father reported that he witnessed the eyelids become progressively erythematous and edematous in the hours following application. The onset of cases was also coincident with the beginning of gentamicin use at the two institutions. Finally, there was a dose-response relation noted, as the severity of the rash decreased significantly once excess ointment began to be wiped away by delivery room staff.
We identified a single previous report of infants with a similar reaction following gentamicin administration.(2) Due to a shortage of the standard prophylactic agent in Israel in 1991, gentamicin ointment was given to seventy-one newborns, five of which developed an ulcerative dermatitis with gradual resolution over two to three weeks.
We hypothesize that the underlying mechanism is a direct effect of gentamicin itself. Intraocular gentamicin injection in both humans and animals causes severe retinal ischemia with findings of an occlusive vasculopathy.(3–5) Perhaps the ulcerative dermatitis results from a direct vaso-occlusive effect of gentamicin on the blood vessels of the thin skin of the newborn’s eyelid. The clinical appearance of hemorrhagic, violacious, ulcerative lesions appears consistent with this theory. However, one cannot exclude a hypersensitivity reaction to a preservative in the ointment (e.g., benzalkonium chloride) or a synergistic action of the gentamicin and preservative.
Health care providers taking care of infants in the newborn period should be aware of this potential ulcerative eyelid skin reaction to gentamicin. Instruction to remove excess ointment from the eyelids after application to the eyes should be given to delivery room staff, as this can significantly reduce the severity of the rash. When the characteristic findings are identified in an otherwise well-appearing infant, ophthalmological consultation to exclude ocular surface involvement should be considered, but an extensive infectious work-up may not be necessary. Providers in the U.S. can resume erythromycin use once supplies have been replenished.
Acknowledgments
Supported by NIH K12-EY-01539 (G.B.). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control [homepage on the internet] Atlanta: Sep, 2009. CDC Guidance on Shortage of Erythromycin (0.5%) Ophthalmic Ointment. c2009 [updated 2009 Oct 7, cited 2009 Oct 16] [about 3 screens]. Available from: http://www.cdc.gov/std/treatment/2006/erythromycinOintmentShortage.htm. [Google Scholar]
- 2.Merlob P, Metzker A. Neonatal orbital irritant contact dermatitis caused by gentamicin ointment. Cutis. 1996;57:429–30. [PubMed] [Google Scholar]
- 3.McDonald HR, Schatz H, Allen AW, Chenoweth RG, Cohen HB, Crawford JB, et al. Retinal toxicity secondary to intraocular gentamicin injection. Ophthalmology. 1986;93:871–7. doi: 10.1016/s0161-6420(86)33648-0. [DOI] [PubMed] [Google Scholar]
- 4.Conway BP, Tabatabay CA, Campochiaro PA, D’Amico DJ, Hanninen LA, Kenyon KR. Gentamicin toxicity in the primate retina. Arch Ophthalmol. 1989;107:107–112. doi: 10.1001/archopht.1989.01070010109037. [DOI] [PubMed] [Google Scholar]
- 5.Brown GC, Eagle RC, Shakin EP, Gruber M, Arbizio VV. Retinal toxicity of intravitreal gentamicin. Arch Ophthalmol. 1990;108:1740–1744. doi: 10.1001/archopht.1990.01070140094037. [DOI] [PubMed] [Google Scholar]