Abstract
We studied the effects of exercise (primarily running), calorie restriction (dieting), and a low-fat, high-carbohydrate diet on changes in lipoprotein subfractions in moderately overweight men in a randomized controlled clinical trial. After 1 year, complete data were obtained for 39 men assigned to lose weight through dieting without exercise, 37 men assigned to lose weight through dieting with exercise (primarily running), and 40 nondieting sedentary controls. We instructed both diet groups to consume no more than 30% total fat, 10% saturated fat, and 300 mg/d of cholesterol, and at least 55% carbohydrates, and the controls were instructed to maintain their usual food choices. Analytic ultracentrifugation was used to measure changes in plasma lipoprotein mass concentrations. In addition, the absorbance of protein-stained polyacrylamide gradient gels was used as an index of concentrations for five high-density lipoprotein (HDL) subclasses that have been identified by their particle sizes, ie, HDL3c, (7.2 to 7.8 nm), HDL3b (7.8 to 8.2 nm), HDL3a (8.2 to 8.8 nm), HDL2a (8.8 to 9.7 nm), and HDL2b (9.7 to 12 nm). Relative to controls, weight decreased significantly in men who dieted with exercise (net difference ± SE, −3.3 ± 0.4 kg/m2) and in men who dieted without exercise (−2.0 ± 0.4 kg/m2). Dieting with exercise significantly decreased very-low-density lipoprotein (VLDL)-mass concentrations and significantly increased plasma HDL2-mass, HDL3a, HDL2a, and HDL2b relative to both control and dieting without exercise. There were no significant changes in lipoprotein mass and HDL protein for dieters who did not run. Adjustment for weight loss by analysis of covariance eliminated the significant decrease in VLDL-mass and increases in HDL2-mass and HDL2b in men who both increased exercise and dieted. Thus, the addition of exercise to dieting appears to increase HDL2-mass and HDL2b through metabolic processes associated with weight loss, and to increase HDL2a and HDL3a through processes that are independent of weight loss. Previous exercise studies that report changes in HDL that are independent of weight loss may be measuring increases in HDL2a and HDL3a rather than HDL2b.
High levels of high-density lipoprotein (HDL)2, low levels of small, dense low-density lipoprotein (LDL), and low levels of very-low-density lipoprotein (VLDL) are all associated with a low risk of coronary heart disease (CHD) [1–4]. As compared with sedentary men, endurance runners have higher plasma concentrations of HDL2 and lower concentrations of small LDL and VLDL, suggesting that exercise may reduce CHD risk [5].
Several investigators attribute the HDL differences primarily to the skeletal muscle adaptations to endurance (aerobic) training [6–8]. We have hypothesized that metabolic processes associated with weight loss may be primarily responsible [9–14]. More recently, we have shown that exercise induced weight loss specifically affects the HDL2b component [14], raising the possibility that the differences in opinion could be due to different effects of muscle adaptations and weight loss on specific HDL components.
This report examines l-year changes in lipoprotein subfractions in moderately overweight men who were assigned to lose weight through a combined program of exercise (primarily running) and dieting while following nutritional guidelines of no more than 30% of calories as total fat, 10% of total calories as saturated fat, and 300 mg/d cholesterol, and at least 55% carbohydrates. Their changes are compared with those of men who were assigned to lose weight through dieting without exercise while following these nutritional guidelines, and with those of nondieting sedentary controls who were instructed to maintain their usual caloric intake and food choices. A combined program of exercise, calorie restriction, and a low-fat high-carbohydrate diet may involve opposing influences on HDL levels [13,15]. Changes in lipoprotein cholesterol and apolipoproteins A-I and B from this trial have been previously described by Wood et al. [16] The present report uses analytic ultracentrifugation and gradient gel electrophoresis to provide more detailed measurements of the changes in lipoprotein subfractions induced in overweight men by these interventions.
SUBJECTS AND METHODS
Subjects and Experimental Design
We recruited 132 moderately overweight men aged 25 to 49 years who were nonsmokers, nonhypertensive (blood pressure < 160/95 mm Hg), and not on medication that might affect lipid metabolism or blood pressure [16]. All were relatively sedentary, ie, exercising no more than 30 minutes twice per week. Their plasma total cholesterol concentrations were less than 260 mg/dL, and their plasma triglyceride concentrations were less than 500 mg/dL. Baseline body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters) was required to be between 28 and 34 kg/m2. After their baseline evaluation, the men were assigned at random into one of three experimental conditions, ie, dieting without exercise (calorie restriction without increasing exercise); dieting with exercise (calorie restriction and physical activity increase, primarily running); and control (no change in diet or exercise) [16].
Clinical and Laboratory Measurements
At baseline and 1 year, the men reported to our clinic in the morning after having abstained for 12 to 16 hours from all food and drink (except water) and any vigorous activity. We estimated body compositions by hydrostatic weighing, and maximal oxygen uptake (VO2max) by recording gas exchange during graded exercise treadmill tests to exhaustion [17]. Energy intakes were estimated by computer analysis of food records maintained by the participants over a 7-day diet period, during which the assessment laboratory staff interviewed them at regular intervals to ensure completeness [18]. The runners recorded exercise duration and frequency in monthly activity logs. These entries were verified by the training staff.
Blood samples were collected in EDTA (1.5 mg/mL). Plasma samples were kept at 4° to 6°C until being transported to Donner Laboratory for analysis. Lipoprotein-containing fractions were prepared and assayed by analytic ultracentrifugation as previously described [19,20]. Concentrations of total lipoprotein mass were estimated using computer techniques for 15 HDL flotation intervals between F1.20 0–9 (half-integer increments from 0 to 6 and integer increments thereafter), 11 LDL flotation intervals between Sf 0–12 (integer increments between Sf 0–10 and then Sf 10–12), four intermediate-density lipoprotein (IDL) flotation intervals between Sf 12–20 (2-unit increments), and 14 VLDL flotation intervals between Sf 20–400 (10 unit increments < Sf 100 and 50 unit increments thereafter) [19,20]. Results are also presented for plasma mass concentrations of HDL2 (F1.20 3.5–9), HDL3 (F1.20 0–3.5), small LDL (Sf 0–7), large LDL (Sf 7–12), IDL (Sf 12–20), and VLDL (Sf 20–400), and LDL peak flotation (Sf) rates (ie, the mode of the distribution of LDL particles) [19,20].
Electrophoresis of HDL in the ultracentrifuged d < 1.20 g/mL fraction and LDL in whole plasma was performed on a Pharmacia Electrophoresis Apparatus (GE 4–11) using slab gradient gels (PAA 4/30 and PAA 2/16. Pharmacia, Piscataway, NJ) [21–23]. Protein-stained HDL and lipid-stained LDL were scanned at a wavelength of 603 and 555 nm, respectively, using a model RFT densitometer [22]. HDL and LDL distributions were converted from the migration distance to the particle diameter scale by transformation of variables [24]. The absorbance of protein-stained polyacrylamide gradient gels was used as an index of mass concentrations for five HDL subclasses that have been identified by their particle sizes: HDL3c (7.2 to 7.8 nm), HDL3b (7.8 to 8.2 nm), HDL3a (8.2 to 8.8 nm), HDL2a (8.8 to 9.7 nm), and HDL2b (9.7 to 12 nm) [25]. Seven LDL subclasses have been defined by their particle sizes, as follows: LDL-IVB (22.0 to 23.2 nm), LDL-IVA (23.3 to 24.1 nm), LDL-IIIB (24.2 to 24.6 nm), LDL-IIIA (24.7 to 25.5 nm), LDL-II (25.5 to 26.4 nm), LDL-I (26.0 to 28.5 nm), and IDL (28.0 to 30.0 nm) [23,26]. The gradient gel HDL and LDL distributions are displayed with mean absorbance represented by the height of the curve at each diameter value [25].
Statistics
The effects of dieting without exercise and dieting with exercise are estimated by subtracting the mean changes of the control group from those of each diet group. The net change is then presented ± 1 SE. The significance of these differences is evaluated by ANOVA. Pearson correlation coefficients describe the pairwise associations between changes in lipoprotein concentrations, weight loss, distance run per week, maximum aerobic capacity (VO2max), and energy intake. Analysis of covariance was used to adjust changes in lipoproteins for changes in BMI. This procedure uses parallel regression lines to describe the relationship between the dependent variable and the covariate. Separate intercepts are fitted to the regression lines of the three groups, and the distances between the parallel lines are used to test for significant group differences. The analysis assumes that the relationship between the dependent variable and covariate is the same within each group. The equality of the regression slopes was tested before adjustment.
Conversion from absorbance to plasma concentration is not necessary for analyzing protein-stained HDL and lipid-stained LDL levels from the gradient gels. The statistical tests used in this report (ie. t tests, Pearson’s correlation coefficients, ANOVA, and analysis of covariance) are invariant to translations of scale or location. This means that the statistics and significance levels for absorbance will be identical to those based on unknown plasma concentrations when the conversion involves the addition and/or multiplication of numerical constants. In fact, different constants may be used at each diameter, so that variation in chromogenicity across the lipoprotein particle size spectrum will not affect the results.
RESULTS
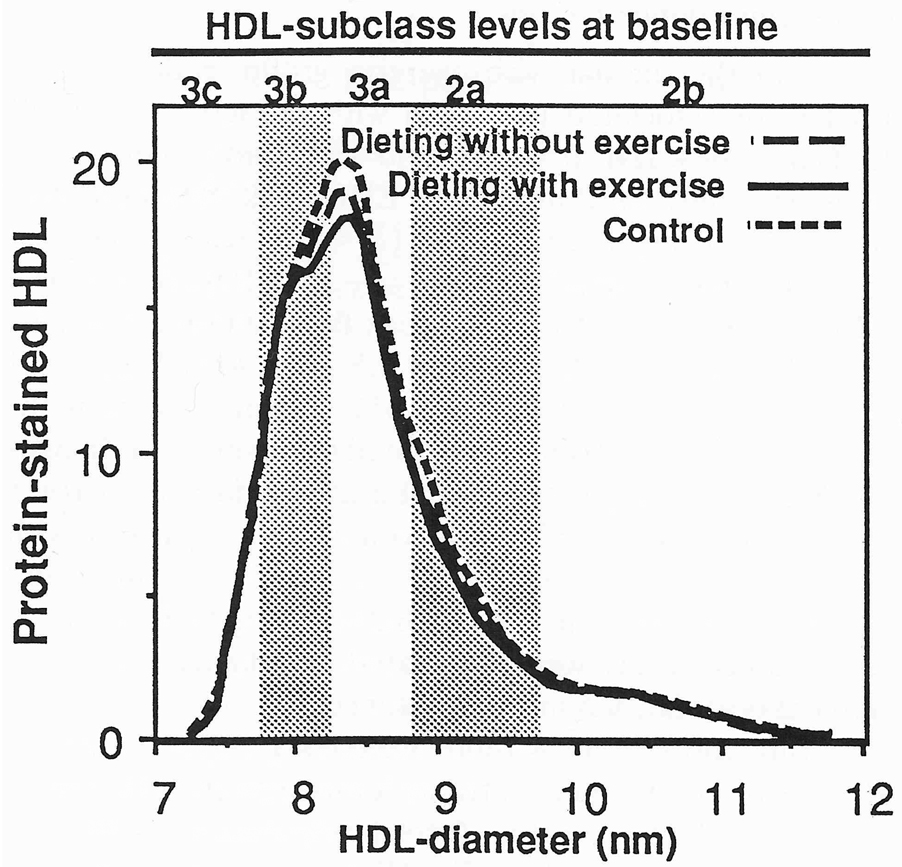
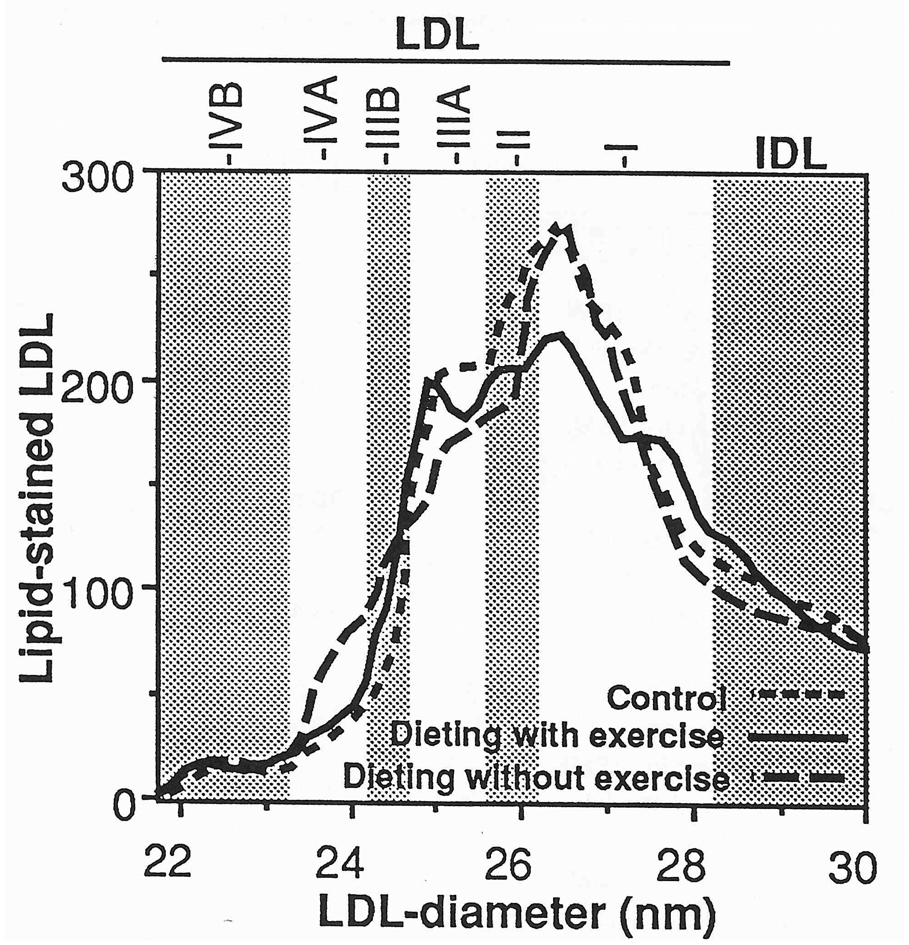
Complete data on lipoprotein mass concentrations and diet were obtained for 39 (of 45) men assigned to dieting without exercise, 37 (of 43) men assigned to dieting with exercise, and 40 (of 44) men assigned to nondieting sedentary control. Table 1 shows that the three groups were well matched for most variables. The dieting with exercise group had lower HDL3-mass concentrations and lower alcohol intake than controls. The proportion of men reporting fat intakes of 30% or less were similar in the diet with exercise (2.7%), diet without exercise (7.7%), and control (2.5%) groups. Similarly, the proportions of men reporting carbohydrate intakes of 55% or more were not different in the diet with exercise (0.0%), diet without exercise (0.0%), and control (2.5%) groups. Figure 1 and Figure 2 display the men’s protein-stained HDL and lipid-stained LDL at baseline. There were no significant group differences, except that men in the dieting with exercise group had significantly higher HDL protein between 8.20 and 8.32 nm than did controls at baseline.
Table 1.
Baseline characteristics of the dieting without exercise, dieting with exercise, and control groups.
| Dieting without exercise |
Dieting with exercise |
Control | |
|---|---|---|---|
| Sample size | 37 | 39 | 40 |
| BMI (kg/m2) | 30.7 ± 2.1 | 30.4 ± 2.2 | 30.7 ± 2.2 |
| Percent body fat | 27.5 ± 4.2 | 27.6 ± 4.5 | 28.8 ± 4.6 |
| VO2 max (mL/kg/min) | 33.8 ± 5.4 | 34.7 ± 5.3 | 33.6 ± 3.8 |
| Resting heart rate (bpm) | 69.4 ± 7.0 | 67.8 ± 7.7 | 68.2 ± 7.6 |
| Total calorie intake (kcal/day) | 2,628 ± 582 | 2,650 ± 627 | 2,613 ± 507 |
| Alcohol (% of total calories) | 3.5 ± 3.2* | 4.6 ± 4.2 | 6.0 ± 5.9 |
| Fat (% of total calories) | 38.8 ± 4.5 | 37.8 ± 5.6 | 37.7 ± 4.2 |
| Carbohydrates (% of total calories) | 42.4 ± 6.4 | 42.1 ± 6.3 | 41.4 ± 7.5 |
| Starch (% of total calories) | 19.6 ± 3.9 | 20.0 ± 4.3 | 20.0 ± 3.7 |
| HDL2-mass (mg/dL) | 21.1 ± 25.7 | 20.8 ± 23.2 | 27.3 ± 22.9 |
| HDL3-mass (mg/dL) | 181.8 ± 31.3* | 190.2 ± 36.5 | 203.5 ± 37.0 |
| Small LDL-mass (mg/dL) | 196.2 ± 70.0 | 189.0 ± 65.6 | 208.8 ± 69.3 |
| Large LDL-mass (mg/dL) | 107.1 ± 41.8 | 106.7 ± 40.0 | 121.2 + 39.0 |
| IDL-mass (mg/dL) | 31.0 ± 18.1 | 28.0 ± 15.5 | 32.8 ± 20.1 |
| VLDL-mass (mg/dL) | 114.5 ± 83.6 | 105.3 ± 77.2 | 99.1 ± 62.5 |
| LDL-peak flotation rate (Sf) | 5.76 ± 1.21 | 5.88 ± 1.30 | 5.94 ± 1.31 |
Difference between exercisers and controls was significant at P <0.0l.
Fig 1.
Baseline levels of protein-stained HDL from nondenaturing polyacrylamide gradient gel electrophoresis in men. The levels of the curves were determined by averaging individual values at each diameter value for the 37 men of the dieting with exercise group, 39 men of the dieting without exercise running group, and 40 controls. Men who both ran and dieted had significantly higher HDL between 8.2 and 8.32 nm than controls. The subfraction intervals defined by Blanche et al [21] are provided for reference.
Fig 2.
Baseline levels of lipid-stained LDL from nondenaturing polyacrylamide gradient gel electrophoresis in men. The levels of the curves were determined by averaging individual values at each diameter value for the 37 men of the dieting with exercise group, 39 men of the dieting without exercise group, and 40 controls. The subfraction intervals defined by Krauss and Burke [23] are provided for reference.
Table 2 shows that as compared with controls, both intervention groups significantly increased intakes of total carbohydrates (specifically starch) and significantly reduced BMI, percent body fat, and reported calorie intake. Men who dieted with exercise ran an average of (mean ± SE) 13.4 ± 1.5 km/wk and showed greater reductions in BMI and percent body fat and greater increases in VO2max than men who dieted without exercise. The greater fat loss among exercisers compared with nonexercising dieters presumably occurred because of the additional calories expended during exercise. The average increase in VO2max was significant in men who dieted with exercise compared with controls. After 1 year, there were more men reporting fat intakes of 30% or less and carbohydrate intakes of 55% or more in the dieting with exercise (54.1% and 21.6%, respectively) and dieting without exercise groups (41.0% and 18.0%) than in the control group (10% and 0.0%). Alcohol intake was not significantly changed during the trial [16].
Table 2.
Mean ± SD changes in weight, body composition, fitness, resting heart rate and calorie intake
| Dieting With Exercise |
Dieting Without Exercise |
Control | |
|---|---|---|---|
| Sample size | 37 | 39 | 40 |
| ΔTotal calorie intake (kcal/day) | −582 ± 690* | −665 ± 588* | 38 ± 630 |
| ΔAlcohol (% of total calories) | 0.9 ± 3.7 | 0.3 ± 3.8 | −0.7 ± 3.0 |
| ΔFat (% of total calories) | −9.6 ± 7.O*† | −6.1 ± 6.9* | 0.8 ± 5.2 |
| ΔCarbohydrates (% of total calories) | 7.9 ± 8.0*† | 4.5 ± 6.7* | −0.9 ± 5.4 |
| ΔStarch (% of total calories) | 5.9 ± 5.1* | 3.7 ± 6.3* | −0.8 ± 3.8 |
| ΔBMI (kg/m2) | −2.8 ± 1.8*† | −1.5 ± 1.7 | 0.5 ± 1.5 |
| ΔPercent body fat | −6.6 ± 3.8*† | −3.1 ± 4.0* | 0.6 ± 2.7 |
| ΔVO2max (mL/kg/min) | 8.6 ± 5.8*† | 1.5 ± 5.0 | −0.2 ± 4.1 |
| ΔResting heart rate (bpm) | −10.4 ± 7.7*† | −2.3 ± 8.9 | −0.1 ± 6.7 |
Significant difference between dieting with exercise vs. control or dieting without exercise vs. control at P < 0.0002.
significant difference between dieting with exercise vs. dieting without exercise at P ≤ 0.05.
Group Differences
Table 3 displays the mean changes in lipoprotein mass concentrations and LDL-peak flotation rate. As compared with controls, dieting with exercise significantly increased HDL2-mass and HDL3-mass and significantly decreased total VLDL-mass. More specifically, mass concentrations increased significantly for individual flotation intervals between F1.20 2–9 and decreased for intervals between Sf18–350 (data not presented). Adjustment for change in BMI eliminated the significant increases in HDL2-mass and the significant decrease in VLDL-mass. Changes in HDL2-mass and VLDL-mass were also not different from controls when simultaneously adjusted for changes in lean and fat body mass (analyses not displayed). These adjustments did not eliminate the significant increase in HDL3-mass in men who both ran and dieted.
Table 3.
Changes in lipoprotein mass concentrations and LDL-peak flotation rate in the dieting with exercise, dieting without exercise, and control group
| Controls (mean ± SD) |
Group differences for l-year change scores (mean differences ± SE)§ | |||
|---|---|---|---|---|
| Dieting with exercise- controls |
Dieting without exercise- controls |
Dieting with exercise-Dieting without exercise |
||
| HDL2-mass (mg/dL) | ||||
| Unadjusted | −0.2 ± 19.5 | 16.5 ± 5.0‡ | 6.8 ± 4.9 | 9.7 ± 5.0* |
| Adjusted for ΔBMI | 5.6 ± 6.2 | 0.0 ± 5.4 | 5.6 ± 5.1 | |
| HDL3-mass (mg/dL) | ||||
| Unadjusted | −1.0 ± 23.8 | 15.1 ± 6.9* | 3.9 ± 6.8 | 11.2 ± 7.0 |
| Adjusted for ΔBMI | 20.4 ± 8.9* | 7.2 ± 7.7 | 13.2 ± 7.3 | |
| Small LDL-mass (mg/dL) | ||||
| Unadjusted | −10.6 ± 54.3 | −15.2 ± 13.1 | −6.1 ± 12.9 | −9.1 ± 13.2 |
| Adjusted for ΔBMI | 23.0 ± 15.9 | 17.7 ± 13.7 | 5.3 ± 13.0 | |
| Large LDL-mass (mg/dL) | ||||
| Unadjusted | −3.4 ± 34.9 | 10.1 ± 8.0 | −0.5 ± 7.9 | 10.6 ± 8.0 |
| Adjusted for ΔBMI | 13.2 ± 10.3 | 1.4 ± 8.9 | 11.8 ± 8.4 | |
| IDL-mass (mg/dL) | ||||
| Unadjusted | 0.1 ± 15.7 | −3.6 ± 3.7 | 0.3 ± 3.6 | −3.9 ± 3.7 |
| Adjusted for ΔBMI | 8.1 ± 4.4 | 7.5 ± 3.8* | 0.5 ± 3.6 | |
| VLDL-mass (mg/dL) | ||||
| Unadjusted | 11.5 ± 60.7 | −52.5 ± 15.1‡ | −13.2 ± 14.9 | −39.2 ± 15.2† |
| Adjusted for ΔBMI | −9.8 ± 18.4 | 13.3 ± 15.9 | −23.1 ± 15.1 | |
| LDL-peak flotation rate (Sf) | ||||
| Unadjusted | −0.08 ± 0.92 | −0.46 ± 0.25 | 0.41 ± 0.25 | 0.05 ± 0.25 |
| Adjusted for ΔBMI | 0.08 ± 0.32 | 0.17 ± 0.27 | −0.10 ± 0.26 | |
Significant differences between groups: * P≤ 0.05,
P≤ 0.01,
P≤ 0.001;
The column entries represent the net effect of 1 year’s intervention relative to the changes in the control group or relative to the changes in the dieting without exercise group.
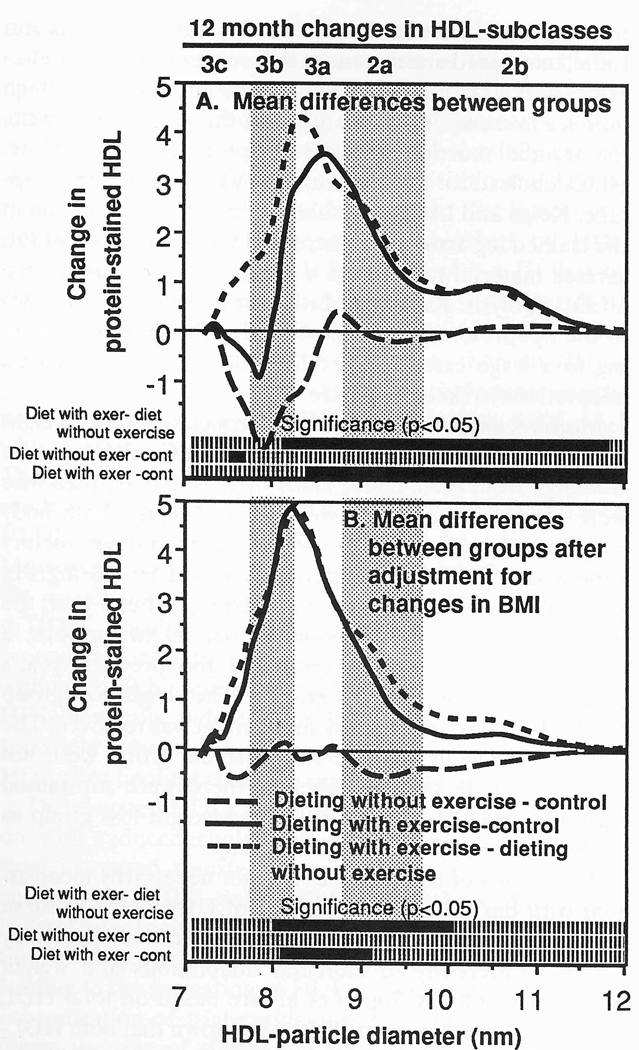
Plasma concentrations of VLDL-mass decreased significantly more for dieting with exercise than for dieting without exercise (P < 0.05 for individual intervals between Sf 20–40 and between Sf 60–200). Adjustment for change in BMI eliminated this difference. Figure 3 displays the group differences for changes in protein-stained HDL by particle size. Significant differences (ie, P≤ 05) from two-sample t tests are shown at the bottom. Dieting with exercise significantly increased HDL3a, HDL2a, and HDL2b as compared with both control (specifically for protein between 8.37 and 11.69 nm) and dieting without exercise (specifically between 8.10 and 11.80 nm). Adjustment for change in BMI eliminated the significant increases in HDL2b; however, differences within HDL3a and HDL2a (ie. between 8.09 and 8.56 nm) remained significant as compared with controls. Differences in HDL3a and HDL2a (7.99 to 9.93 nm) between the runners and nonrunners also persisted when adjusted.
Fig 3.
Mean differences for changes in protein-stained HDL by particle diameter between the dieting with exercise, dieting without exercise, and control groups. Results are displayed (A) before and (B) after adjustment for change in BMI. Bars at the bottom of the figures designate significance at P < .05 by two-sample t tests (A) and analysis of covariance (B).
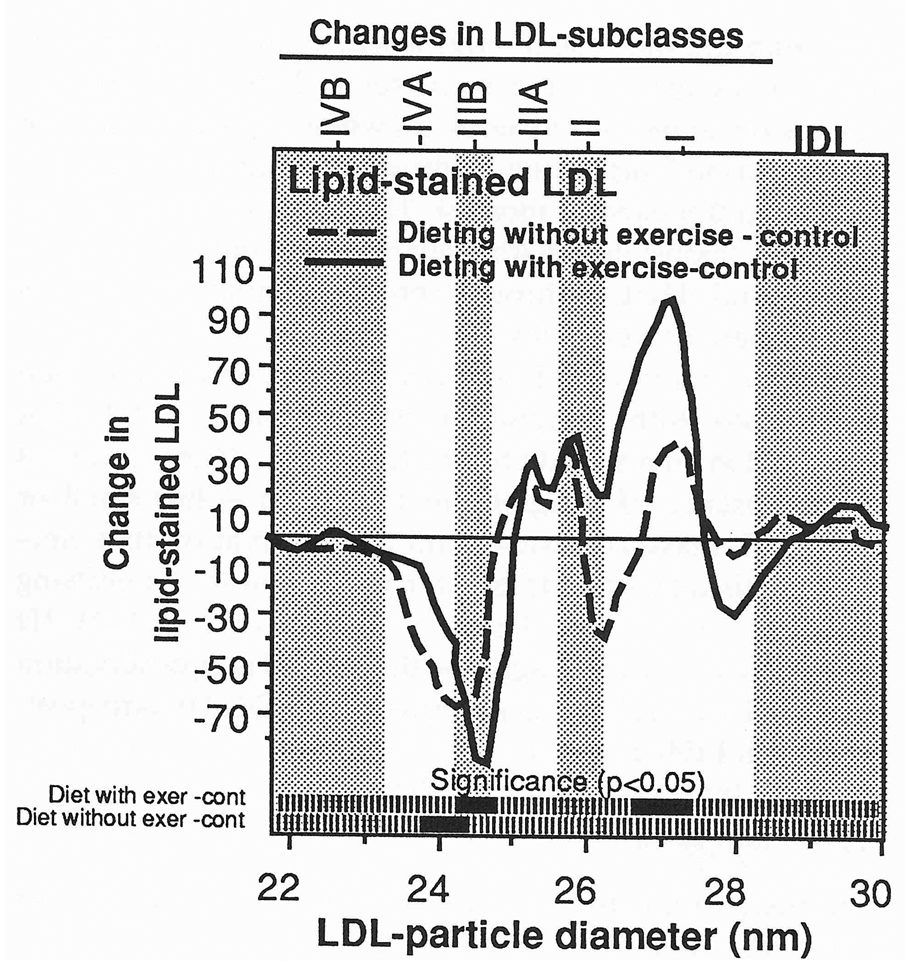
Figure 4 displays the group differences for changes in lipid-stained LDL by particle size. As compared with controls, dieting with exercise significantly reduced lipid-stained LDL within the LDL-IIIB subclass (specifically between 24.3 and 24.8 nm) and increased lipid-stained LDL within the LDL-I subclass (specifically between 26.7 and 27.4 nm). Dieting without exercise decreased LDL within LDL-III (specifically between 23.8 and 24.4 nm) as compared with controls, but there were no significant differences between dieting with exercise and dieting without exercise. These differences were largely unaffected by adjustment for change in BMI (analyses not displayed).
Fig 4.
Mean differences for changes in lipid-stained LDL by particle diameter between the dieting with exercise, dieting without exercise, and control groups. Bars at the bottom of the figures designate significance at P < .05 by two-sample t tests. There were no significant differences between the dieting with exercise and dieting without exercise groups. Significance levels were largely unchanged when adjusted for changes in body mass (analyses not displayed).
Correlations Within Groups
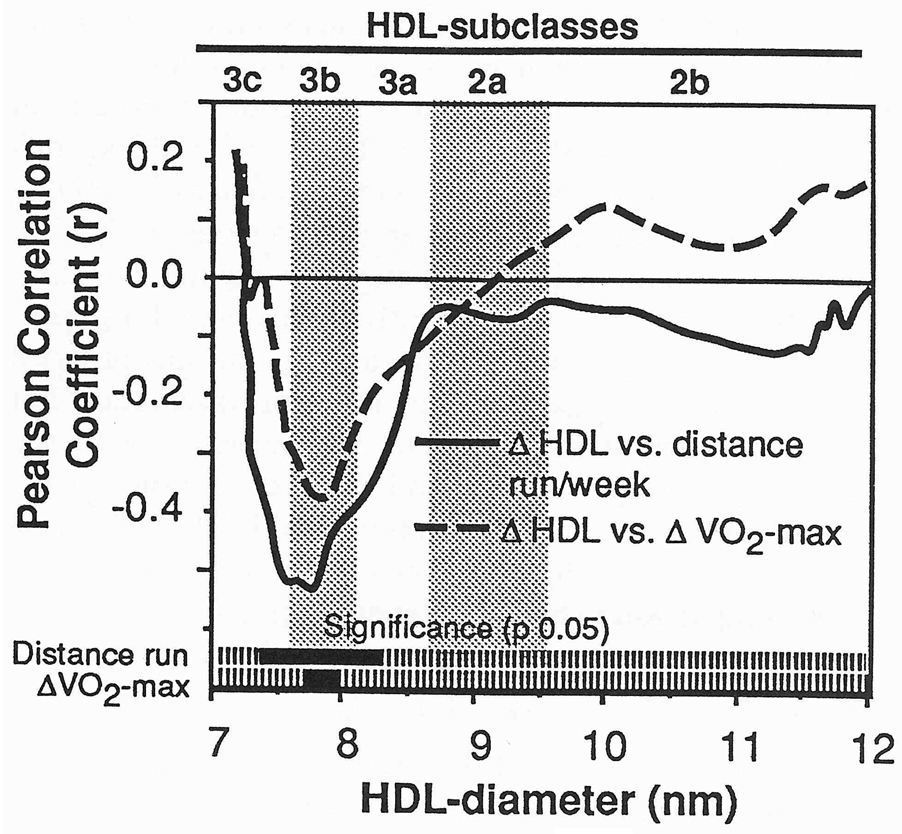
Within the dieting with exercise group, reductions in BMI were associated (P < 0.05) with reductions in small LDL (r = 0.36), IDL (r = 0.39), and VLDL-mass concentrations (r = 0.40) and increased LDL-peak flotation rate (r = −0.33). Changes in VO2max were associated (P < 0.05) with changes in small LDL (r = −0.35), IDL (r = −0.34), VLDL-mass (r = −0.53), LDL-peak flotation rate (r = 0.35), and HDL-mass within F1.20 3–3.5 (r = 0.41), F1.203.5–4 (r = 0.37), and F1.204–4.5 (r = 0.35). Changes in protein-stained HDL3c and HDL3b correlated positively with changes in BMI (specifically between 7.51 and 8.05 nm) and percent body fat (between 7.53 and 7.86 nm) and negatively with changes in VO2max (between 7.71 and 7.97 nm) and distance run (between 7.37 and 8.27 nm, Fig 5). Changes in lipid-stained LDL were unrelated to changes in BMI, percent body fat, VO2max, or distance run.
Fig 5.
Plot of the correlation of change in protein-stained HDL by distance run and ΔVO2max in men who dieted with exercise. Bars at the bottom of the figures designate correlations that are significantly different from zero at P≤0.05.
Within the dieting without exercise group, changes in BMI correlated significantly with changes in HDL2-mass (r = −0.39), HDL2b protein (specifically 9.83 to 11.89 nm), and IDL-mass (r = 0.33). The dieter’s decrease in total calories was correlated with their increases in HDL2-mass (r = −0.34) and their decreases in small LDL-mass (r = 0.53).
DISCUSSION
This report is the second of two studies of lipoprotein subfractions in moderately overweight men who lost weight through exercise and dieting. In the previous (first) study [4,14,17], the men either ran or dieted (not both) while maintaining their usual food choices. In the current study, the men dieted, with or without exercise, while reducing fat and cholesterol intake and increasing carbohydrates. The two studies suggest that running-induced weight loss, with or without dieting, increases HDL3-mass, HDL2-mass, and HDL2b protein and decreases VLDL-mass. Both studies show that adjustment for the change in BMI eliminates the significant increases in HDL2-mass and HDL2b and the significant decreases in VLDL-mass in the runners, suggesting that metabolic processes associated with weight loss may be responsible. The results of these two studies are consistent with analyses of cross-sectional studies suggesting that reduced adiposity explains most of the HDL cholesterol differences between runners and sedentary men [12]. They are also consistent with meta-analyses suggesting that weight loss largely determines whether HDL cholesterol is increased during training [27].
Our study (Fig 3) suggests that HDL3a and HDL2a are also increased when exercise is added to dieting. Whereas the significant increase in HDL2b was eliminated by adjustment for weight loss, the increases in HDL2a and HDL3a remained significant in exercisers when adjusted. Thus, on the prescribed diets, the addition of running to caloric restriction appears to increase HDL2b through metabolic processes associated with weight loss, and to increase HDL2a and HDL3a through processes that are largely independent of weight loss.
Modest decreases in LDL-III subclasses occurred in men who dieted with exercise or dieted alone, and LDL-I increased in men who dieted with exercise. These occurred in the absence of a significant change in either small or large LDL-mass, consistent with the observation that electrophoresis may provide greater resolution for identifying effects for specific subclasses [28]. The decrease in LDL-III and increase in LDL-I agree with our previous observation that HDL2 correlates negatively with LDL-III and positively with LDL-I [28].
Dietary Influences
In the current study, the low-fat, high-carbohydrate diet may have attenuated the lipoprotein responses to weight loss. Low-fat, high-carbohydrate diets have been found to increase the relative proportion of small, dense LDL and to reduce HDL [16,29]. Diets that are high in complex carbohydrates decrease lipoprotein lipase activity of both skeletal muscle and adipose tissue of runners [30]. As compared with our previous study [9,17], there was a substantially greater proportion of runners eating 30% fat or less (current vs. previous: 54% vs. 4%) and 55% or more carbohydrates (22% vs. 0%) at the end of 1 year. The present study also finished with a significantly greater proportion of nonexercising dieters eating 30% or less fat (41% vs. 4%) and 55% or more carbohydrates (18% vs 0%). Three quarters of the carbohydrate increase in the runners was due to increased starch consumption. These dietary differences may explain the smaller lipoprotein changes in the current study vis-a-vis the previous study. The current study required more than twice the change in BMI (−3.3 ± 1.8 vs. −1.4 ± 0.3 kg/m2) to produce approximately the same increases in HDL2-mass and HDL2b in runners as in the previous study. The low-fat, high-carbohydrate diet may also explain why dieting without exercising significantly increased HDL2b protein, HDL2-mass, and HDL3-mass and significantly reduced small LDL-mass and VLDL-mass in the previous study [9] but not in the current study (Table 3). Consistent with the findings by Thompson et al [15] and Keins et al [31], our results suggest that exercise increases HDL2 and HDL-cholesterol even on a high-carbohydrate, low-fat diet, albeit somewhat less in comparison to higher-fat diets.
Prior Studies of Exercise and Weight Loss
In a recent editorial, Thompson discussed two studies that appear to show that aerobic conditioning increases HDL cholesterol independently of weight loss [8]. The study by Sopko et al [6] showed that HDL cholesterol was significantly increased in sedentary men who participated in a 3-month running program when weight remained constant through increased caloric intake [6]. The study by Keins and Lithell measured arteriovenous differences in VLDL triglycerides and HDL cholesterol in trained and untrained thigh muscles in men [7]. As compared with the untrained muscle, the trained muscle increased lipoprotein lipase activity, HDL2 cholesterol production, and VLDL triglyceride uptake. Keins and Lithell postulated that HDL2 formation in the trained leg arose from increased transference of VLDL surface material to HDL as a consequence of heightened VLDL lipolysis. Keins and Lithell surmised that “changes in the lipoprotein profile associated with endurance training to a large extent are explainable by training-induced adaptations to skeletal muscle.”
More recently, Thompson et al concluded that “weight loss is not required to increase HDL-C with exercise training” from their l-year study of 17 sedentary men who were trained 4 hours per week while keeping both body weight and percent body fat constant through dietary supplement [32]. HDL cholesterol increased by 3.8 mg/dL, primarily due to a 33% increase in HDL2. After 1 year, the men were assigned at random to one of two groups, a weight-stable group (ie, continuing the previous year’s protocol) and a weight loss group [33]. The weight loss group lost 9.4 kg when their dietary supplement was removed. The lipoprotein changes in the weight-stable group were not sustained by 18 months, whereas there were substantial increases in HDL2-cholesterol in the weight loss group as compared with the weight-stable group [33].
The results of these studies are not necessarily inconsistent with our own. Measurements of HDL levels in these studies include multiple components that we believe may respond differently to muscular adaptations and weight loss. The results by Sopko et al [6] are based on total HDL cholesterol measurements. We have shown that both HDL2 and HDL3 are increased in men who exercise [9,31] (Table 3), and whereas the HDL3 increase is independent of weight loss, the HDL2 increase is not. The findings of Sopko et al [6] that “exercise and weight loss contribute separately and independently increase HDL-cholesterol, and their effects are additive” may represent, in part, separate and additive contributions of HDL3a and HDL2a (independent of weight loss) and HDL2b cholesterol (dependent on weight loss).
Two studies to date have examined arteriovenous HDL cholesterol production across exercising muscle, Ruys et al [34] and Keins and Lithell [7]. The increase was ascribed to HDL3 in the former study and to HDL2 in the latter study. The former report is consistent with the runners’ increase in HDL3 in Table 3 and Fig 3. The increase in HDL2 cholesterol reported by Keins and Lithell is made up of two components, ie, HDL2a, which contains predominantly both apo A-I and apo A-II, and HDL2b, which contains predominantly apo A-I only [35,36]. We believe that the increase in HDL2 cholesterol may have been due to increased HDL2a rather than HDL2b. Our conjecture is based on numerous published reports on the effects of transference of apo A-I, phospholipids, and cholesterol to HDL during lipolysis in vivo after a fat meal [37–39], and after infusion of heparin or artificial fat emulsions [40,41], and on in vitro incubations of plasma lipoproteins [42–45] or model complexes [46]. In these studies, the alterations in HDL are characterized as general shifts in the buoyancy, density, or size of the total HDL distribution [39,45,47,48] or as the formation of light HDL3 that are isolated within the HDL2 range [44,49]. Increases within the HDL2a range during lipolysis are substantially greater and occur sooner than any increases within the HDL2b range [37,43]. James and Pometta have argued that lipoprotein surface materials are more readily absorbed by HDL particles that contain apo A-II, i.e., HDL(A-I with A-II), than by particles that contain no apo A-II, i.e., HDL(A-I without A-11), because the HDL(A-I with A-II) particles have lower surface-to-core partial volumes [50]. If true, then an HDL2a product is expected in Kein’s study since cholesterol enrichment of HDL3(A-I with A-II) should yield an HDL2(A-I with A-II) product [36,51–53], ie, HDL2a rather than HDL2b. This agrees with the observation that apo A-I and apo A-II are both increased in the HDL2 range during lipolysis of VLDL, chylomicrons, or an artificial fat emulsion [40,41]. Moreover, the short exposure of HDL to VLDL lipolysis in the muscle may be inadequate for LCAT to convert HDL3 to HDL2b. Incubation studies show that the LCAT reaction is essential for the formation of larger HDL from HDL3(A-I without A-II) but not HDL3(A-I with A-II) [36,43]. Changes in HDL generally fade 6 to 8 hours after fat feeding or Intralipid infusion [40], suggesting that the shift toward larger HDL after lipolysis is not necessarily sustained.
The elevated HDL2 levels of runners may have more to do with reduced cholesteryl ester triglyceride exchange than increased transfer of apo A-I, phospholipids, and cholesterol to HDL during VLDL lipolysis. Reduced cholesteryl ester triglyceride exchange may cause the accumulation of cholesteryl ester within HDL(A-I without A-II), leading to the formation of HDL2b. In normal subjects, the concentration of triglyceride-enriched lipoproteins determines the rate of cholesteryl ester transfer from HDL [54]. Accelerated lipolysis of VLDL or chylomicrons due to increased lipoprotein lipase activity may have a greater and more lasting effect on the size of the triglyceride-rich lipoprotein pool than the amount of surface material transferred to HDL. It is significant therefore that in the studies by Sopko et al [6] and Thompson et al [32] triglycerides were at best marginally reduced when weight loss was prevented, but decreased precipitously when natural weight loss was allowed to occur [6,33]. Thus, the overfeeding in the studies by Sopko et al [6] and Thompson et al [32] may have prevented the reduction of plasma triglyceride that usually accompanies exercise, thereby eliminating a principal cause for cholesteryl ester accumulation in HDL. Overfeeding may fundamentally alter lipoprotein metabolism in runners, rather than simply eliminating the confounding effects of weight loss.
Separating HDL subclasses by gradient gel electrophoresis provides a possible explanation for the discrepancy between studies that attribute the high HDL levels of runners to reduced adiposity [9,11–14], and those that attribute the high levels to muscular adaptations [6,7]. Previous exercise studies that report changes in HDL that are independent of weight loss may be measuring increases in HDL2a and HDL3a rather than HDL2b [6,7]. Our study suggests that the addition of running to dieting appears to increase HDL2-mass and HDL2b through metabolic processes associated with weight loss, and appears to increase HDL2a and HDL3a through processes that are largely independent of weight loss.
ACKNOWLEDGMENT
We wish to thank Laura Hall, Charlotte Brown, and Bahareh Sahami for laboratory analysis of gradient gel electrophoresis. Analytic ultracentrifuge measurements were made by Joseph Orr. We wish to thank Dr Darlene M. Dreon, Barbara Frey-Hewitt, Sharon Bortz, Nancy Ellsworth. Steven Hake. and Walter Bortz for their help in completing the study.
Supported in part by Grants No. HL-24462. HL-02183, HL-49828, and HL-18574 from the National Heart, Lung, and Blood Institute of the National Institutes of Health, and a grant funded by the National Dairy Promotion and Research Board, administered in cooperation with the National Dairy Council and conducted under Department of Energy Grant No. DE-ACO3-SF00098 to the University of California.
REFERENCES
- 1.Krauss RM, Lindgren FT, Williams PT, et al. Intermediate density lipoproteins and progression of coronary artery disease in hypercholesterolaemic men. Lancet. 1987;2:62–66. doi: 10.1016/s0140-6736(87)92734-6. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne FC, Clark RS, Simpson HS, et al. High density and low density lipoprotein subfractions in survivors of myocardial infarction and in control subjects. Metabolism. 1982;31:433–437. doi: 10.1016/0026-0495(82)90230-x. [DOI] [PubMed] [Google Scholar]
- 3.Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966;34:679–697. doi: 10.1161/01.cir.34.4.679. [DOI] [PubMed] [Google Scholar]
- 4.Austin MA, Breslow JL, Hennekens CH, et al. Low density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–1921. [PubMed] [Google Scholar]
- 5.Williams PT, Krauss RM, Wood PD, et al. Lipoprotein subfractions of runners and sedentary men. Metabolism. 1986;35:45–52. doi: 10.1016/0026-0495(86)90094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sopko G, Leon AS, Jacobs DR. The effects of exercise and weight loss on plasma lipids in young obese men. Metabolism. 1985;34:227–236. doi: 10.1016/0026-0495(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 7.Keins B, Lithell H. Lipoprotein metabolism influenced by training-induced changes in human skeletal muscle. J Clin Invest. 1989;83:558–564. doi: 10.1172/JCI113918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson PD. What do muscles have to do with lipoproteins? Circulation. 1990;81:1428–1431. doi: 10.1161/01.cir.81.4.1428. [DOI] [PubMed] [Google Scholar]
- 9.Williams PT, Krauss RM, Vranizan KM, et al. Changes in lipoprotein subfractions during diet-induced and exercise-induced weight loss in moderately overweight men. Circulation. 1990;81:1293–1304. doi: 10.1161/01.cir.81.4.1293. [DOI] [PubMed] [Google Scholar]
- 10.Wood PD, Haskell WL, Klein H, et al. The distribution of plasma lipoproteins in middle-aged male runners. Metabolism. 1976;25:1249–1257. doi: 10.1016/s0026-0495(76)80008-x. [DOI] [PubMed] [Google Scholar]
- 11.Williams PT, Wood PD, Krauss RM, et al. Does weight loss cause the exercise induced increase in plasma high-density lipoproteins? Atherosclerosis. 1983;47:173–185. doi: 10.1016/0021-9150(83)90153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams PT. Weight setpoint and the high-density lipoprotein concentrations of long-distance runners. Metabolism. 1990;39:460–467. doi: 10.1016/0026-0495(90)90003-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams PT, Krauss RM, Vranizan KM, et al. The effects of long-distance running and weight loss on plasma low-density lipoprotein subfraction concentrations in men. Arteriosclerosis. 1989;9:623–632. doi: 10.1161/01.atv.9.5.623. [DOI] [PubMed] [Google Scholar]
- 14.Williams PT, Krauss RM, Vranizan KM, et al. Effects of weight loss by exercise and by diet on apolipoprotein A-I and A-II and the particle size distribution of high-density lipoproteins in men. Metabolism. 1992;41:441–449. doi: 10.1016/0026-0495(92)90082-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson PD, Cullinane EM, Eshleman R, et al. The effects of high carbohydrate and high fat diets on the serum lipid and lipoprotein concentrations of endurance athletes. Metabolism. 1984;33:1003–1010. doi: 10.1016/0026-0495(84)90228-2. [DOI] [PubMed] [Google Scholar]
- 16.Wood PD, Stefanick ML, Williams PT, et al. The effects on plasma lipoproteins of a weight-reducing prudent diet, with and without exercise, in overweight men and women. N Engl J Med. 1991;325:461–466. doi: 10.1056/NEJM199108153250703. [DOI] [PubMed] [Google Scholar]
- 17.Wood PD, Stefanick ML, Dreon D, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 18.Nutrition Coordinating Center: Reference Food Table, Version 10. Minneapolis, MN: University of Minnesota; 1984. [Google Scholar]
- 19.Lindgren FT, Jensen LC, Hatch FT. The isolation and quantitative analysis of lipoproteins. In: Nelson GJ, editor. Blood Lipids and Lipoproteins: Quantitation, Composition and Metabolism. New York: Wiley-Interscience; 1972. pp. 181–274. [Google Scholar]
- 20.Lindgren FT, Jensen LC, Wills RD, et al. Flotation rates, molecular weights and hydrated densities of the low-density lipoproteins. Lipids. 1969;4:337–344. doi: 10.1007/BF02531003. [DOI] [PubMed] [Google Scholar]
- 21.Blanche PJ, Gong EL, Forte TM, et al. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta. 1981;665:408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- 22.Nichols AV, Krauss RM, Musliner TA. Nondenaturing polyacrylamide gradient gel electrophoresis. In: Segest JP, Albers JJ, editors. Methods in Enzymology. vol 128: Plasma Lipoproteins. A. Preparation and Molecular Biology. Orlando, FL: Academic; 1986. pp. 417–431. [DOI] [PubMed] [Google Scholar]
- 23.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]
- 24.Williams PT, Krauss RM, Nichols A, et al. Identifying the predominant peak diameter of high-density (HDL) and low density (LDL) lipoproteins by electrophoresis. J Lipid Res. 1990;31:1131–1139. [PubMed] [Google Scholar]
- 25.Williams PT, Krauss RM, Vranizan KM, et al. Associations of lipoproteins and apolipoproteins with gradient gel electrophoresis estimates of high density lipoprotein subfractions in men and women. Arterioscler Thromb. 1992;12:332–340. doi: 10.1161/01.atv.12.3.332. [DOI] [PubMed] [Google Scholar]
- 26.Nichols AV, Krauss RM, Musliner TA. Nondenaturing polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1986;128:417–431. doi: 10.1016/0076-6879(86)28084-2. [DOI] [PubMed] [Google Scholar]
- 27.Tran ZV, Weltman A. Differential effects of exercise on serum lipid and lipoprotein levels seen in changes in body weight: A meta analysis. JAMA. 1985;254:919–924. [PubMed] [Google Scholar]
- 28.Williams PT, Vranizan KM, Krauss RM. Correlations of plasma lipoproteins with LDL subfractions by particle size in men and women. J Lipid Res. 1992;33:765–774. [PubMed] [Google Scholar]
- 29.Dreon DM, Krauss RM. Low density lipoprotein subclass phenotypes are associated with differing lipoprotein responses to reduced fat diets. Circulation. 1991;84 suppl 2:11–681. [Google Scholar]
- 30.Roberts KM, Noble EG, Hayden DB, et al. Lipoprotein lipase activity in skeletal muscle and adipose tissue of marathon runners after simple and complex carbohydrate-rich diets. Eur J Appl Physiol. 1988;57:75–80. doi: 10.1007/BF00691242. [DOI] [PubMed] [Google Scholar]
- 31.Keins B, Essen-Gustavsson B, Gad P, et al. Lipoprotein lipase activity and intramuscular triglyceride stores after long-term high-fat and high-carbohydrate diets in physically trained men. Clin Physiol. 1987;7:1–9. doi: 10.1111/j.1475-097x.1987.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PD, Yurgalevitch S, Flynn MF, et al. Weight loss is not required to increase HDL-C with exercise training. Circulation. 1992;86 suppl 1:1–590. [Google Scholar]
- 33.Terry RB, Flynn MF, Yurgalevitch S, et al. Weight loss further increases HDL2-C and apo A-I survival in trained overweight men compared to continued exercise. Circulation. 1992;86 suppl 1 I-590. [Google Scholar]
- 34.Ruys T, Sturgess I, Shaikh M, et al. Effects of exercise and fat ingestion on high density lipoprotein production by perpheral tissues. Lancet. 1989;2:1119–1122. doi: 10.1016/s0140-6736(89)91488-8. [DOI] [PubMed] [Google Scholar]
- 35.Cheung MC, Albers JJ. Characterization of lipoprotein particles isolated by immunoaffinity chromatography: Particles containing A-I and A-II and particles containing A-I but no A-II. J Biol Chem. 1984;259:12201–12209. [PubMed] [Google Scholar]
- 36.Nichols AV, Blanche PJ, Shore VG, et al. Conversion of apolipoprotein-specific high-density lipoprotein populations during incubation of human plasma. Biochim Biophys Acta. 1989;1001:325–337. doi: 10.1016/0005-2760(89)90117-3. [DOI] [PubMed] [Google Scholar]
- 37.Tall AR, Blum CB, Forester GP, et al. Changes in the distribution and composition of plasma high density lipoprotein after ingestion of fat. J Biol Chem. 1982;257:198–207. [PubMed] [Google Scholar]
- 38.Taskinen MR, Kuusi T. High density lipoprotein in postprandial lipemia. Relation to sex and lipoprotein lipase activity. Atherosclerosis. 1986;59:121–130. doi: 10.1016/0021-9150(86)90040-7. [DOI] [PubMed] [Google Scholar]
- 39.Franceshini G, Moreno Y, Apebe P, et al. Alterations in high density lipoprotein subfractions during postprandial lipidaemia induced by fat with and without ethanol. Clin Sci. 1988;75:135–142. doi: 10.1042/cs0750135. [DOI] [PubMed] [Google Scholar]
- 40.Sakuma N, Lin C, Matsumoto Y et al. Changes in HDL subfraction concentration and particle size by Intralipid in vivo. Atherosclerosis. 1988;74:91–98. doi: 10.1016/0021-9150(88)90195-5. [DOI] [PubMed] [Google Scholar]
- 41.Hailer S, Wolfram G. Influence of artificial fat emulsions on the composition of serum lipoproteins in humans. Am J Clin Nutr. 1986;43:225–233. doi: 10.1093/ajcn/43.2.225. [DOI] [PubMed] [Google Scholar]
- 42.Nichols AV, Gong EL, Blanche PJ. Interconversion of high density lipoproteins during incubation of human plasma. Biochem Biophys Res Commun. 1981;100:391–399. doi: 10.1016/s0006-291x(81)80109-x. [DOI] [PubMed] [Google Scholar]
- 43.Dieplinger H, Zechner R, Kostner GM. The in vitro formation of HDL2 during the action of L:CAT: The role of triglyceride rich lipoproteins. J Lipid Res. 1985;26:273–281. [PubMed] [Google Scholar]
- 44.Simard G, Perret B, Durand S, et al. Phosphatidylcholine and triglyceride hydrolysis in HDL as induced by hepatic lipase: Modulation of the phospholipase activity by changes in the particle surface or lipid core. Biochim Biophy Acta. 1989;1001:225–233. doi: 10.1016/0005-2760(89)90152-5. [DOI] [PubMed] [Google Scholar]
- 45.Musliner TA, Michenfelder HJ, Krauss RM. Interactions of high density lipoproteins with very low and low density lipoproteins during lipolysis. J Lipid Res. 1988;29:349–361. [PubMed] [Google Scholar]
- 46.Nichols AV, Blanche PJ, Gong EL, et al. Molecular pathways in the transformation of model discoidal lipoprotein complexes induced by 1ecithin:cholesterol acyltransferase. Biochim Biophys Acta. 1985;834:285–300. doi: 10.1016/0005-2760(85)90001-3. [DOI] [PubMed] [Google Scholar]
- 47.Manzato E, Zambon S, Marin R, et al. Modification of plasma lipoproteins after lipase activation in patients with chylomicronemia. J Lipid Res. 1986;27:1248–1258. [PubMed] [Google Scholar]
- 48.Groot PH, Scheek LM. Effects of fat injestion on high density lipoprotein profiles in human sera. J Lipid Res. 1984;25:684–692. [PubMed] [Google Scholar]
- 49.Taskinen MR, Nikkila EA, Kuusi T, et al. Changes in high density lipoprotein subfraction concentration and composition by Intralipid in vivo and by lipolysis of Intralipid in vitro. Arteriosclerosis. 1983;3:607–615. doi: 10.1161/01.atv.3.6.607. [DOI] [PubMed] [Google Scholar]
- 50.James RW, Pometta D. Immunofractionation of high density lipoprotein subclasses 2 and 3. Similarities and differences of fractions isolated from male and female populations. Atherosclerosis. 1990;83:35–45. doi: 10.1016/0021-9150(90)90128-6. [DOI] [PubMed] [Google Scholar]
- 51.Atmeh RF, Shephard J, Packard CJ. Subpopulations of apolipoprotein A-I in human high-density lipoproteins, and their metabolic properties and response to drug therapy. Biochim Biophys Acta. 1983;751:175–188. doi: 10.1016/0005-2760(83)90172-8. [DOI] [PubMed] [Google Scholar]
- 52.Rader DJ, Castro GR, Kindt MR, et al. Differential in vitro metabolism of HDL subclasses Lp A-I and LpAI,AII in man. Clin Res. 1990;38:240A. (abstr) [Google Scholar]
- 53.Rajaram OV, Barter PJ. Increases in the particle size of high density lipoproteins by purified lecithin:cholesteroI acyltransferase: Effects of low density lipoproteins. Biochim Biophys Acta. 1986;877:406–414. doi: 10.1016/0005-2760(86)90206-7. [DOI] [PubMed] [Google Scholar]
- 54.Mann CJ, Yen FT, Grant AM, et al. Mechanism of plasma cholesteryl ester transfer in hypertriglyceridemia. J Clin Invest. 1991;88:2059–2066. doi: 10.1172/JCI115535. [DOI] [PMC free article] [PubMed] [Google Scholar]