Abstract
The molecular mechanisms responsible for postpollination changes in floral scent emission were investigated in snapdragon cv Maryland True Pink and petunia cv Mitchell flowers using a volatile ester, methylbenzoate, one of the major scent compounds emitted by these flowers, as an example. In both species, a 70 to 75% pollination-induced decrease in methylbenzoate emission begins only after pollen tubes reach the ovary, a process that takes between 35 and 40 h in snapdragon and ∼32 h in petunia. This postpollination decrease in emission is not triggered by pollen deposition on the stigma. Petunia and snapdragon both synthesize methylbenzoate from benzoic acid and S-adenosyl-l-methionine (SAM); however, they use different mechanisms to downregulate its production after pollination. In petunia, expression of the gene responsible for methylbenzoate synthesis is suppressed by ethylene. In snapdragon, the decrease in methylbenzoate emission is the result of a decrease in both S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase (BAMT) activity and the ratio of SAM to S-adenosyl-l-homocysteine (“methylation index”) after pollination, although the BAMT gene also is sensitive to ethylene.
INTRODUCTION
To improve reproductive success, flowering plants have evolved floral scent in addition to visual and tactile cues as a means of attracting pollinators. Similar to flower colors and forms, there is a large diversity of floral scents that play a prominent role in the location and selection of flowers by insects (Dobson, 1994; Knudsen et al., 1999). Closely related plant species that rely on different types of insects for pollination produce different fragrances, reflecting the olfactory sensitivities or preferences of their pollinators (Henderson, 1986; Raguso and Pichersky, 1995). The fact that insects can distinguish between these complex mixtures makes floral scent patterns of key importance for plant–insect interactions. Such species-specific floral scent may enhance pollinator specificity, which, in turn, may reduce the chance of pollen loss and unsuccessful interspecific pollination. Although species-specific pollination appears to be advantageous to plants, many angiosperms are pollinated by several species to ensure their reproductive success (e.g., orchids are pollinated by beetles, butterflies, bees, moths, flies, wasps, ants, and birds) (Dafni and Bernhardt, 1990; Waser et al., 1996). However, even in generalized pollination systems, pollinators use the combination of specific odor and visual cues to learn and return to target flowers (Gegear and Laverty, 2001).
The composition of floral scent, as well as the total output, changes during the life span of the flower in relation to flower age (Tollsten, 1993; Pichersky et al., 1994; Wang et al., 1997; Dudareva et al., 1998, 2000), pollination status (Tollsten, 1993; Euler and Baldwin, 1996; Schiestl et al., 1997), environmental conditions (Jakobsen and Olsen, 1994), and diurnal endogenous rhythms (summarized by Dudareva et al., 1999). Plants tend to emit scent at maximal levels when the flowers are ready for pollination and concomitantly when their potential pollinators are active. The rhythmic release of flower scent during the day and night generally coincides with the foraging activities of potential pollinators (Matile and Altenburger, 1988; Loughrin et al., 1990; Nielsen et al., 1995; Dudareva et al., 2000). In addition, newly opened and young flowers, which are not ready to function as pollen donors because their anthers have not yet dehisced, usually produce less scent and therefore are less attractive to pollinators (Jones et al., 1998; Dudareva et al., 2000; summarized by Dudareva et al., 1999). In several cases, an age-related decrease in scent production also was shown in unpollinated flowers (Tollsten, 1993; Pichersky et al., 1994; Wang et al., 1997; Dudareva et al., 1998, 2000, 2003).
The scent of many flowers is reduced markedly soon after pollination. Such quantitative and/or qualitative postpollination changes in floral bouquets, shown mostly in orchids (Arditti, 1979; Tollsten and Bergstrom, 1989; Tollsten, 1993; Schiestl et al., 1997), decrease the attractiveness of these flowers and increase the overall reproductive success of the plant by directing pollinators to unpollinated flowers. This is particularly important for plants with a low visitation rate, in which reproductive success is mostly pollinator limited (Neiland and Wilcock, 1998).
Although the effects of pollination on the level of emission and the composition of floral scent have been demonstrated (summarized by Dudareva et al., 1999), there is no information about the specific molecular and biochemical mechanisms responsible for these postpollination changes of floral scent in plants. The recent isolation and characterization of several genes responsible for the formation of floral scent volatiles has allowed the investigation of the developmental regulation of scent biosynthesis. Transcriptional regulation of the expression of these genes at the site of emission and the level of supplied substrates for the reactions were found to be the major factors that control scent production and, indirectly, scent emission during snapdragon flower development (Dudareva et al., 1999, 2000, 2003). Moreover, the level of substrate (benzoic acid) also plays a major role in the regulation of the circadian emission of methylbenzoate in diurnally (snapdragon) and nocturnally (petunia cv Mitchell and Nicotiana suaveolens) emitting flowers (Kolosova et al., 2001a).
In this study, we investigate the molecular mechanisms responsible for postpollination changes in floral scent emission in snapdragon cv Maryland True Pink and petunia cv Mitchell flowers using a volatile ester, methylbenzoate, as an example. Although petunia cv Mitchell is a man-made hybrid and therefore did not evolve specific mechanisms for pollinator attraction like its wild relatives, it appears to be a useful model system for understanding physiological changes after pollination. In both species, methylbenzoate is one of the major scent compounds (Dudareva et al., 2000; Kolosova et al., 2001a; Verdonk et al., 2003). Its emission is controlled by a circadian clock and reaches a maximum level during the day in snapdragon and at night in petunia (Kolosova et al., 2001a). We found that pollinated snapdragon and petunia flowers stop producing scent only after pollen tubes reach the ovary, thereby ensuring that successful fertilization likely has occurred. We also show that genes responsible for methylbenzoate production in both plant systems can be downregulated transcriptionally by ethylene. However, only petunia uses ethylene to downregulate the expression of the gene responsible for methylbenzoate biosynthesis after pollination, whereas in snapdragon, the combination of decreases in S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase (BAMT) activity and in the ratio of S-adenosyl-l-methionine (SAM) to S-adenosyl-l-homocysteine (SAH) (“methylation index”) after pollination accounts for the decrease in methylbenzoate emission.
RESULTS
Effect of Pollination on Methylbenzoate Emission in Snapdragon Flowers
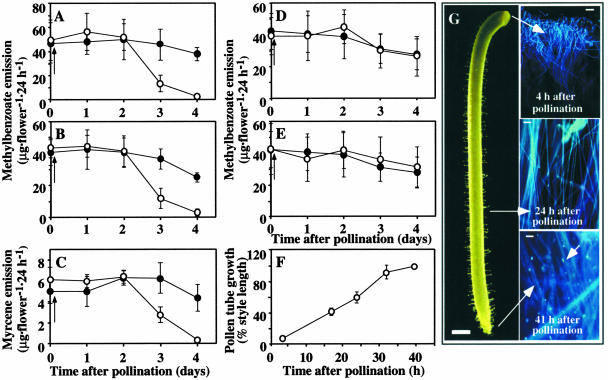
Methylbenzoate is one of the most abundant scent compounds detected in the majority of snapdragon varieties, and its emission is regulated developmentally (Dudareva et al., 2000). To determine the effect of pollination on floral scent emission, snapdragon flowers were emasculated at the bud stage, 2 or 3 days before opening, and then hand self-pollinated at different times after anthesis (4 and 5 days). Floral volatiles were collected from single, living flowers for 24 h before pollination (time 0 in Figures 1A to 1E) and for 4 days after pollination at 24-h intervals and analyzed by gas chromatography–mass spectrometry. No new volatile compounds were detected in pollinated flowers compared with unpollinated flowers (data not shown). When flowers were pollinated on the 4th or 5th day after anthesis, a time when the stigma is receptive to pollen and seed set is highest (Jones et al., 1998), the quantitative changes in methylbenzoate emission were very similar (Figures 1A and 1B). During the first 2 days after pollination, there were no differences in total amounts of methylbenzoate emitted from pollinated and unpollinated flowers. A 70% decrease in emission was found on day 3 after pollination, followed by an additional 20% decrease in the next 24-h period, leaving pollinated flowers with a small amount of emitted volatile ester (2.7 and 3.0 μg·flower−1·24 h−1 for flowers pollinated on days 4 and 5, respectively) compared with corresponding unpollinated control flowers (38 and 25 μg·flower−1·24 h−1) (Figures 1A and 1B). Pollinated flowers still were fully turgid, although often freshly abscised, by day 4 after pollination. Unpollinated emasculated control flowers also showed some decrease in methylbenzoate emission by the end of the experiment, similar to the developmental changes in normal unpollinated flowers (Dudareva et al., 2000), indicating that this decrease is the result of aging and not emasculation. Similar patterns were found for the other two major scent compounds, the monoterpenes myrcene (Figure 1C) and (E)-β-ocimene (data not shown).
Figure 1.
Effect of Pollination on Floral Scent Emission and Pollen Tube Movement in Snapdragon Flowers.
(A) and (B) Emission of methylbenzoate in snapdragon flowers pollinated with self pollen on day 4 (A) and day 5 (B) after anthesis.
(C) Emission of myrcene in snapdragon flowers pollinated with self pollen on day 5 after anthesis.
(D) and (E) Emission of methylbenzoate in snapdragon flowers pollinated on day 5 after anthesis with foreign pollen (D) and heat-inactivated self-pollen (E).
Closed circles represent emission in unpollinated flowers, and open circles represent emission in pollinated flowers. Arrows indicate the time of pollination. Standard deviations are indicated by vertical bars. Each point is the average of 7 and 10 independent scent collections for unpollinated and pollinated flowers, respectively, in (A), 8 independent scent collections for unpollinated and pollinated flowers in (B), 4 and 7 independent scent collections for unpollinated and pollinated flowers, respectively, in (C), and 10 and 6 independent scent collections for unpollinated and pollinated flowers, respectively, in (D) and (E).
(F) and (G) Pollen tube growth in snapdragon styles pollinated on day 5 after anthesis.
(F) Average style length is 2.02 ± 0.02 cm (n = 30). Five styles were used at each time point.
(G) Styles were fixed and stained with aniline blue at different times after pollination. Stained pollen tubes were visualized by fluorescence microscopy (at right). Bars = 1 mm (pistil), 50 μm (4 h after pollination), and 25 μm (24 and 41 h after pollination).
To determine if this pollination-induced decrease in methylbenzoate emission is triggered by pollen deposition on the stigma or if pollen tube growth through the style and/or fertilization is necessary, snapdragon flowers were pollinated with foreign pollen from petunia and heat-inactivated (killed) self-pollen. Previously, it was shown that killed or foreign pollen, both of which fail to germinate, elicit the initial response to pollination but do not elicit postpollination responses in the corolla (Hoekstra and Weges, 1986; O'Neill, 1997; O'Neill and Nadeau, 1997). Pollination of snapdragon flowers on day 5 after anthesis with petunia pollen resulted in no decrease in methylbenzoate emission (Figure 1D). Similar results were obtained with heat-inactivated self pollen, which failed to germinate (Figure 1E). In both experiments, emission of methylbenzoate followed a developmentally regulated profile similar to that of unpollinated flowers (Figures 1D and 1E).
To associate pollen tube growth with the pollination-induced decrease in scent emission, cytological staining experiments monitoring pollen tube penetration through the stigma and style were performed. Snapdragon styles pollinated on day 5 after anthesis were stained with aniline blue at different times after pollination, and callose deposits in growing pollen tubes were visualized by fluorescence microscopy (Figure 1G). The distance between the top of the stigma and the callose deposits most distal to the pollen grains was measured (Figure 1F). Compatible self-pollination of snapdragon flowers resulted in efficient pollen germination and tube growth through the style. Four hours after pollination, pollen grains had germinated and penetrated into the top of the style (Figure 1G). By 24 h after pollination, most pollen tubes had grown through the upper 60% of the 2-cm-long style, and they had reached the style base between 35 and 40 h (Figures 1F and 1G). These results indicate that the pollination-induced decrease in methylbenzoate emission begins after pollen tubes reach the ovary and is not triggered by pollen deposition on the stigma.
Effect of Pollination on Methylbenzoate Emission in Petunia Flowers
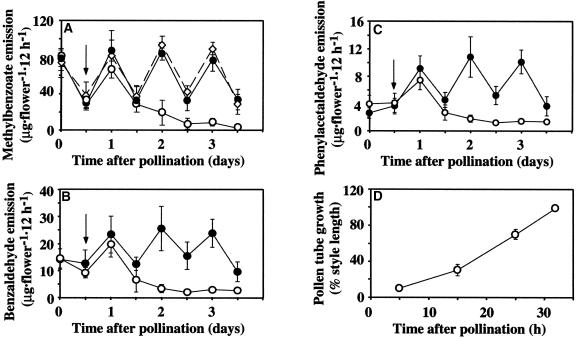
To determine whether the postpollination decrease in scent emission occurs after pollen tubes reach the ovary in another plant species, floral scent emission and pollen tube growth through the style after pollination were analyzed in petunia cv Mitchell. Petunia cv Mitchell emits methylbenzoate with maximum emission at approximately midnight (Kolosova et al., 2001a; Verdonk et al., 2003). Petunia flowers were emasculated 1 day before corolla opening and hand-pollinated with self pollen on the night of the 2nd day after anthesis, when scent production reaches its maximum (data not shown). As in snapdragon, floral volatiles were collected from single, living flowers for 1 day before pollination and for 3 days after pollination at 12-h intervals. During the 1st day after pollination, there was no decrease in methylbenzoate emission, which continued to oscillate in the same manner as in unpollinated flowers, with a maximum at midnight (Kolosova et al., 2001a) (Figure 2A). A 75% decrease in methylbenzoate emission was found at 36 h after pollination. By 60 h after pollination (the last night of scent collection), the amount of emitted methylbenzoate decreased by an additional 15%, accounting for ∼10% (8.3 μg·flower−1·12 h−1) of the amount emitted from flowers before pollination (77 μg·flower−1·12 h−1) and from corresponding unpollinated control flowers (77.5 μg·flower−1·12 h−1) (Figure 2A). The other major scent compounds, benzaldehyde (Figure 2B) and phenylacetaldehyde (Figure 2C), exhibited similar patterns of emission after pollination. Analysis of pollen tube growth through the style in petunia flowers pollinated with self pollen on the night of day 2 after anthesis revealed that pollen tubes reached the base of the 42-mm style at ∼32 h after pollination (Figure 2D). These results indicate that, as in snapdragon, the decrease in scent emission begins after fertilization.
Figure 2.
Effect of Pollination on Floral Scent Emission and Pollen Tube Movement in Petunia Flowers.
(A) Emission of methylbenzoate in wild-type and etr1-1 petunia flowers pollinated with self pollen on the night of day 2 after anthesis.
(B) and (C) Emission of benzaldehyde (B) and phenylacetaldehyde (C) in wild-type petunia flowers pollinated with self pollen on the night of day 2 after anthesis. Increases in the amount of benzaldehyde and phenylacetaldehyde at 1 day after pollination relative to the emission on the day of pollination reflect developmental changes.
Closed circles represent emission in unpollinated wild-type flowers, and open circles and open diamonds represent emission in pollinated wild-type and etr1-1 flowers, respectively. Arrows indicate the time of pollination. Standard deviations are indicated by vertical bars. Each point is the average of five and six independent scent collections for unpollinated and pollinated wild-type flowers, respectively, and four independent scent collections for etr1-1 flowers.
(D) Pollen tube growth in petunia styles pollinated on the night of day 2 after anthesis. Average style length is 4.15 ± 0.03 cm (n = 30). Three styles were used at each time point. Standard deviations are indicated by vertical bars.
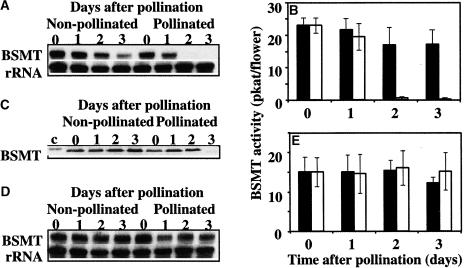
Effects of Pollination on BAMT Gene Expression, Level of BAMT Protein, BAMT Activity, the Endogenous Pool of Benzoic Acid, and Methylation Index in Snapdragon Flowers
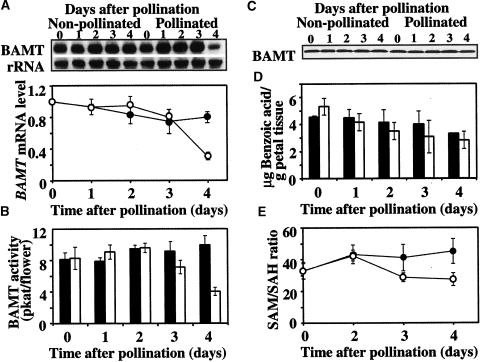
In snapdragon flowers, the volatile ester methylbenzoate is made predominantly in the conical cells of upper and lower petal lobes by the enzymatic methylation of benzoic acid in the reaction catalyzed by BAMT (Dudareva et al., 2000; Murfitt et al., 2000; Kolosova et al., 2001b). Its biosynthesis during flower development is regulated at the level of BAMT gene expression as well as by the amount of benzoic acid, which is the substrate for the reaction (Dudareva et al., 2000). To determine the molecular mechanisms responsible for postpollination changes in methylbenzoate emission, we analyzed BAMT gene expression, level of BAMT protein, BAMT activity, and the endogenous pool of benzoic acid in pollinated and unpollinated snapdragon flowers. For these experiments, emasculated snapdragon flowers were hand self-pollinated on day 5 after anthesis, and samples were prepared from upper and lower petal lobes at 24 h before pollination (time 0) and at different times (1 to 4 days) after pollination. Unpollinated emasculated flowers of the same age were used as a control. When the levels of BAMT transcripts were examined in pollinated and unpollinated flowers by RNA gel blot analysis, there were no differences in BAMT gene expression during the first 3 days after pollination, whereas a 50% decrease in BAMT expression was observed on the 4th day after pollination (Figure 3A). Similarly, pollination of emasculated snapdragon flowers led to a slight decrease in BAMT activity on day 3 after pollination and to a 50% decrease in BAMT activity on day 4 after pollination (4.03 ± 0.53 pkat/pollinated flower compared with 9.94 ± 1.17 pkat/unpollinated control flower) (Figure 3B).
Figure 3.
Effect of Pollination on BAMT Gene Expression, BAMT Activity, the Levels of BAMT Protein and Benzoic Acid, and SAM/SAH Ratio in Snapdragon Flowers Pollinated on Day 5 after Anthesis.
(A) RNA gel blot analysis of steady state BAMT mRNA levels in unpollinated and pollinated snapdragon flowers at different times after pollination. Total RNA was isolated from upper and lower petal lobes of snapdragon flowers pollinated on day 5 after anthesis at the times indicated at top, and 5 μg of total RNA was loaded in each lane. The top gel represents the results of hybridization with a BAMT probe. Autoradiography was performed overnight. The blot was rehybridized with an 18S rDNA probe (bottom gel) to standardize samples. RNA gel blots were scanned with a PhosphorImager, and values were used to generate a graph of fluctuation in relative BAMT mRNA levels after pollination. Each point on the graph is the average of eight independent experiments The transcript level on the day before pollination was taken as 1. Closed and open circles represent transcript levels in unpollinated and pollinated flowers, respectively. Standard error values are indicated by vertical bars.
(B) BAMT activity in unpollinated and pollinated snapdragon flowers at different times after pollination. For each time point, enzyme assays were run in duplicate on at least three independent crude extract preparations for unpollinated and pollinated flowers, and the standard deviations (indicated by vertical bars) were obtained. Closed bars represent BAMT activity in unpollinated flowers, and open bars represent BAMT activity in pollinated flowers.
(C) BAMT protein levels in unpollinated and pollinated snapdragon flowers at different times after pollination. The representative protein gel blot shows the 49-kD protein recognized by anti-BAMT antibodies. Proteins were extracted from upper and lower petal lobes at the times after pollination indicated at top, and 11 μg of protein was loaded in each lane.
(D) Amount of benzoic acid in unpollinated and pollinated snapdragon flowers at different times after pollination. Benzoic acid was extracted from upper and lower petal lobes by supercritical carbon dioxide extraction and analyzed by HPLC. Closed bars represent benzoic acid levels in unpollinated flowers, and open bars represent benzoic acid levels in pollinated flowers. Standard error values are indicated by vertical bars. Each point is the average of three independent experiments.
(E) Levels of the SAM/SAH ratio in unpollinated and pollinated snapdragon flowers at different times after pollination. Closed and open circles represent the SAM/SAH ratio in unpollinated flowers and pollinated flowers, respectively. Standard error values are indicated by vertical bars. Each point is the average of three independent experiments. SAM and SAH levels on the day before pollination were 38.99 ± 4.01 and 1.21 ± 0.14 nmol/g fresh weight, respectively, where ± corresponds to a 95% confidence interval of the mean.
BAMT protein levels in upper and lower petal lobes of pollinated and unpollinated snapdragon flowers were determined by the chemiluminescence protein gel blot technique using polyclonal anti-BAMT antibodies (Dudareva et al., 2000). The antibodies selectively recognized one protein with an apparent molecular mass of 49 kD in crude petal extracts separated by SDS-PAGE. The level of BAMT protein remained stable after pollination (Figure 3C), indicating that post-translational regulatory mechanisms are responsible for the decrease in BAMT activity on day 4 after pollination.
The level of benzoic acid in petal tissue was shown to be involved in the regulation of developmental and rhythmic methylbenzoate emission in snapdragon (Dudareva et al., 2000; Kolosova et al., 2001a). To investigate its possible involvement in the regulation of methylbenzoate emission after pollination, we measured the endogenous pools of benzoic acid in petal tissue of pollinated and unpollinated control flowers. Pollination did not affect the levels of benzoic acid (2.84 ± 1.39 μg/g petal tissue for pollinated flowers on day 4 after pollination and 3.3 ± 0.01 μg/g petal tissue for corresponding unpollinated control flowers; Figure 3D), suggesting that it does not contribute to the postpollination decrease in methylbenzoate emission, in contrast to its involvement in the regulation of developmental and rhythmic emission (Dudareva et al., 2000; Kolosova et al., 2001a).
Because the decrease in BAMT activity on the 3rd day after pollination could not account completely for the 70% decrease in methylbenzoate emission (Figure 1B), we analyzed the intracellular levels of SAM and SAH, the methyl donor and product, respectively, of the BAMT-catalyzed reaction (Murfitt et al., 2000). The SAM/SAH ratio (methylation index) is used frequently to reflect the cellular methylation potential and to indicate whether methylation reactions in tissues are likely to be inhibited (Cantoni et al., 1979). Pollination of snapdragon flowers led to an increase in SAH level and a decrease in SAM level, resulting in a 30% decrease in the methylation index from 41.12 ± 8.3 to 28.73 ± 2.73 at 3 days after pollination, with an additional 10% decrease the next day (Figure 3E). These results show that the intracellular methylation capacity changed, contributing to the downregulation of methylbenzoate biosynthesis after pollination (Figure 1B).
Isolation and Characterization of Carboxyl Methyltransferase cDNAs That Encode Enzymes Responsible for Methylbenzoate Biosynthesis in Petunia
To isolate the gene(s) responsible for methylbenzoate formation in petunia, we searched ESTs generated by random sequencing of several petunia flower cDNA libraries constructed at the University of Florida for potential carboxyl methyltransferases. This search initially revealed one EST clone of 390 nucleotides (ID 63582) with high amino acid identity to known S-adenosyl-l-methionine:salicylic acid carboxyl methyltransferases (SAMT). Two methods, cDNA library screening and 5′ rapid amplification of cDNA ends, were used to recover the corresponding full-length clone for this EST. A second full-length cDNA clone was revealed by further sequencing of random clones. These two full-length cDNAs, 1473 and 1425 bp in size and designated BSMT1 and BSMT2, respectively (for S-adenosyl-l-methionine:benzoic acid/salicylic acid carboxyl methyltransferase; see below), are 99% identical to each other in their coding regions and 65% identical in their 3′ untranslated regions. They encode proteins of 357 amino acids that are 99% identical, differing in three amino acids located within the last 23 amino acids of their 3′ ends. Two of these differences represent substitutions by similar amino acids. The proteins encoded by these cDNAs are 84% identical to SAMT from Atropa belladonna and 57 to 62% identical to SAMTs from Stephanotis floribunda (Pott et al., 2002), Antirrhinum majus (Negre et al., 2002), and Clarkia breweri (Ross et al., 1999). They also share 44% identity with snapdragon BAMT (Dudareva et al., 2000; Murfitt et al., 2000). To determine the enzymatic activity of these almost identical proteins, their coding regions were subcloned into the expression vector pET-28a and expressed in Escherichia coli, and recombinant proteins were purified to near homogeneity using nickel-based affinity chromatography. The purified recombinant proteins as well as petal cell-free extracts were used to determine substrate specificity (Table 1). General catalytic properties and kinetic parameters were determined for recombinant proteins (Table 2).
Table 1.
Relative Activity of Crude Extracts from Petunia Petals and of Purified BSMTs with a Variety of Substrates
| Substrate | Crude Extracts |
BSMT1 | BSMT2 |
|---|---|---|---|
| Salicylic acid | 100 | 100 | 100 |
| Benzoic acid | 22.45 ± 0.25 | 16.34 ± 3.71 | 27.27 ± 5.49 |
| 3-Hydroxybenzoic acid | 1.14 ± 0.09 | 0.65 ± 0.14 | 1.32 ± 0.20 |
| trans-Cinnamic acid | 1.00 ± 0.02 | 0.41 ± 0.05 | 0.69 ± 0.11 |
| p-Coumaric acid | 0.47 ± 0.01 | 0.07 ± 0.04 | 0.29 ± 0.12 |
| m-Coumaric acid | 0.51 ± 0.02 | 0.24 ± 0.05 | 0.50 ± 0.14 |
| o-Coumaric acid | 0.48 ± 0.02 | 0.14 ± 0.07 | 0.25 ± 0.05 |
| Benzyl alcohol | 0.92 ± 0.03 | 0.07 ± 0.02 | 0.33 ± 0.14 |
Values are averages of three independent measurements. All substrates were tested at a concentration of 2 mM. The BSMT activity with salicylic acid, set as 100%, was 32.15 ± 0.79 pkat/mg protein for crude extracts, 284.9 ± 4.1 pkat/mg protein for BSMT1, and 243.3 ± 20.4 pkat/mg protein for BSMT2.
Table 2.
Properties of Petunia BSMTs
| Enzyme | Holoenzyme Mass by Gel Filtration (D) |
Subunit Mass by SDS-PAGE (D) |
Calculated Subunit Mass (D) |
Substrate | pH Optimum |
Apparent Km for Substrate (μM) |
kcat × 10−2 (s−1) | kcat/Km (mM−1·s−1) |
|---|---|---|---|---|---|---|---|---|
| BSMT1 | 78,430 | ∼45,000 | 40,659 | SAM SA | 1.93 ± 0.18 | 1.45 ± 0.024 | 7.65 ± 0.618 | |
| SAM BA | 15.78 ± 1.34 | 0.75 ± 0.023 | 0.48 ± 0.047 | |||||
| Salicylic acid | 7.0 to 8.0 | 51.55 ± 2.73 | 1.2 ± 0.16 | 0.23 ± 0.038 | ||||
| Benzoic acid | 7.5 | 1273.2 ± 37 | 0.39 ± 0.04 | 0.003 ± 0.0003 | ||||
| BSMT2 | 77,100 | ∼45,000 | 40,673 | SAM SA | 2.76 ± 0.04 | 0.57 ± 0.099 | 2.07 ± 0.015 | |
| SAM BA | 30.78 + 0.78 | 0.79 ± 0.06 | 0.26 ± 0.015 | |||||
| Salicylic acid | 7.0 to 7.5 | 69.6 ± 1.31 | 0.4 ± 0.028 | 0.057 ± 0.005 | ||||
| Benzoic acid | 6.5 to 7.5 | 2355.9 ± 88 | 0.32 ± 0.0096 | 0.0014 ± 0.0084 |
The highest activity was found with salicylic acid as a substrate, and approximately fivefold lower activity was detected with benzoic acid for both cell-free extracts and recombinant proteins (Table 1). Activities with the other tested substrates, including cinnamic acid, salicylic acid and benzoic acid derivatives, did not exceed 1.5% of the highest activity. Similar to other carboxyl methyltransferases (Ross et al., 1999; Murfitt et al., 2000; Negre et al., 2002), the active enzymes exist as homodimers, as was determined by comparison of the molecular masses obtained by gel filtration chromatography and SDS-PAGE (Table 2). Both recombinant enzymes possessed a pH optimum of ∼7 when benzoic or salicylic acid was used as a substrate. BSMT1 and BSMT2 had similar kinetic parameters with regard to SAM, with apparent Km values 8 to 11 times higher with benzoic acid than with salicylic acid (Table 2). Both enzymes also had similar apparent Km values for salicylic acid, which were 24 to 33 times lower than the apparent Km values for benzoic acid. Similar Km values for salicylic and benzoic acids (65.65 ± 1.36 and 2280.3 ± 243 μM, respectively) were obtained for partially purified plant proteins.
The apparent catalytic efficiency (kcat/Km ratio) of BSMT1 and BSMT2 was 40- to 75-fold higher with salicylic acid than with benzoic acid, indicating that salicylic acid is the preferred substrate. Although we detected high carboxyl methyltransferase activity toward salicylic acid in petals (24.6 pkat/flower in 2-day-old flowers), petunia flowers do not emit methylsalicylate (Verdonk et al., 2003). This finding could be explained by a very small internal pool of free salicylic acid that was ∼10 times lower than the apparent Km values of these enzymes for salicylic acid (J. Boatright and A. Enyedi, unpublished data) and that might not be available to the enzymes as a result of compartmentalization. This indicates that the catalytic activities of BSMT enzymes toward salicylic acid do not contribute to floral scent.
The apparent Km values of BSMT1 and BSMT2 toward benzoic acid are very similar to that of the snapdragon BAMT (1.1 and 1.5 mM for plant and recombinant proteins, respectively [Murfitt et al., 2000]). Moreover, the level of benzoic acid at midnight in 1-day-old petunia petals is 70.5 ± 15.5 μg/g fresh weight (Kolosova et al., 2001a), which corresponds to a concentration of ∼7 mM and is in the range of Km values for benzoic acid for these enzymes, suggesting that both proteins could be involved in methylbenzoate emission, which was confirmed genetically by RNA interference knockout. Using this approach, the resulting transformants showed a significant decrease in BSMT expression as well as a decrease in methylbenzoate emission (B. Underwood and D.G. Clark, unpublished data).
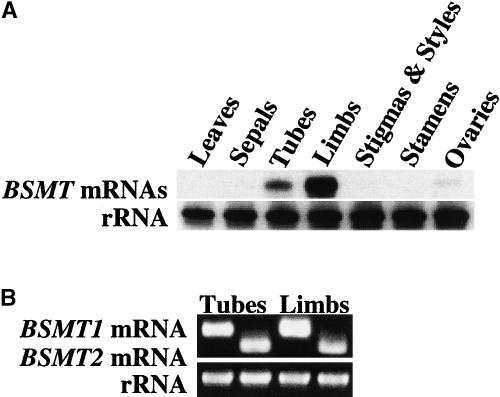
Examination of BSMT gene expression in leaves and different flower organs of 2-day-old petunia flowers by RNA gel blot analysis using the BSMT coding region as a probe revealed BSMT mRNA transcripts predominantly in the limbs and tubes of petunia corollas (Figure 4A). This expression pattern is very similar to that of other known genes involved in scent production, with petals being the principal emitters of volatiles (Dobson, 1994; Dudareva et al., 1999). BSMT1 had a 1.8 times lower Km value for benzoic acid and was catalytically more efficient (kcat/Km ratio) than BSMT2 (Table 2), suggesting that BSMT1 might contribute to methylbenzoate biosynthesis to a greater extent. Reverse transcriptase (RT)–PCR with 3′ untranslated region gene-specific primers was performed to determine the contribution of each gene to the total expression level, because the signals detected in the RNA gel blot experiments represent the sum of transcripts for both BSMT genes. When total RNA isolated from the limbs and tubes of 2-day-old petunia corollas was used in RT-PCR, similar expression patterns were found for each BSMT (Figure 4B), revealing that both genes contribute to methylbenzoate emission. Although the biochemical properties of the isolated enzymes are very similar to those of SAMTs described previously (Ross et al., 1999; Negre et al., 2002; D'Auria et al., 2003), they are involved in methylbenzoate formation in petunia flowers and therefore are designated BSMTs.
Figure 4.
Tissue Specificity of Petunia BSMT Gene Expression.
(A) RNA gel blot of total RNA (5 μg per lane) isolated from young leaves, sepals, tubes and limbs of corollas, pistils, stamens, and ovaries of 2-day-old petunia flowers. The top gel represents the results of hybridization with a coding region of the BSMT genes as a probe. The length of the BSMT mRNA was estimated as 1.5 kb using RNA molecular markers in an adjacent lane. Autoradiography was performed overnight. The blot was rehybridized with an 18S rDNA probe (bottom) to standardize samples.
(B) Contribution of each BSMT gene to total BSMT expression. RT-PCR with gene-specific primers was performed on RNA isolated from the limbs and tubes of 2-day-old petunia corollas. The amplified products were run on a 1.2% agarose gel and stained with ethidium bromide. The RT-PCR products for rRNA are shown at bottom.
Effect of Pollination on BSMT Gene Expression, BSMT Activity, and Protein Level in Petunia Flowers
In contrast to its effects in snapdragon, BSMT gene expression (Figure 5A) and corresponding enzyme activity toward benzoic acid (Figure 5B) in pollinated petunia flowers were tightly correlated with methylbenzoate emission (Figure 2A). Two days after pollination, BSMT mRNA transcripts and BSMT activity were barely detectable (Figures 5A and 5B) in petal tissue of petunia flowers, resulting in a significant decrease in methylbenzoate emission (Figure 2A). To determine if, as in snapdragon, post-translational regulation is involved in the decrease of BSMT activity, we used polyclonal anti-SAMT antibodies raised against the denatured snapdragon SAMT protein overexpressed in E. coli, which were able to recognize both denatured pure petunia BSMT proteins, as determined by immunoblot analysis (Figure 5C), and the native proteins, as determined by immunoprecipitation of enzymatic activity in crude extracts, partially purified plant proteins, and pure recombinant proteins (data not shown). In crude extracts from petunia petals separated by SDS-PAGE, these antibodies predominantly recognized a protein with an apparent molecular mass of 45 kD. Immunoblot analysis of two-dimensional gels of crude extracts as well as a mixture of BSMT1 and BSMT2 confirmed the specificity of the antibodies. The BSMT proteins still were detected in petal crude extracts on day 2 after pollination by immunoblot analysis of both one-dimensional (Figure 5C) and two-dimensional (data not shown) gels, whereas BSMT activity already had decreased significantly (Figure 5B), indicating the involvement of post-translational regulatory mechanisms. The presence of the BSMT proteins on the 2nd day after pollination, when the level of BSMT transcripts was reduced significantly, could be the result of a lag between transcription and translation processes and the stability of the BSMT proteins.
Figure 5.
Effect of Pollination on BSMT Gene Expression, BSMT Activity toward Benzoic Acid, and Protein Level in Wild-Type and etr1-1 Petunia Flowers.
(A) and (D) BSMT gene expression in unpollinated and pollinated wild-type (A) and etr1-1 (D) petunia flowers at different times after pollination. Total RNA was isolated from the limbs and tubes of corollas of petunia flowers pollinated on day 2 after anthesis at the times indicated at top, and 5 μg of total RNA was loaded in each lane. The top gels represent the results of hybridization with a BSMT probe. Autoradiography was performed overnight. The blots were rehybridized with an 18S rDNA probe (bottom) to standardize samples. Each blot represents a typical result of three independent experiments.
(B) and (E) BSMT activity toward benzoic acid in unpollinated and pollinated wild-type (B) and etr1-1 (E) petunia flowers at different times after pollination. For each time point, enzyme assays were run in duplicate on at least four (B) and three (E) independent crude extract preparations for unpollinated and pollinated flowers. Closed and open bars represent BSMT activity toward benzoic acid in unpollinated and pollinated flowers, respectively. Standard error values are indicated by vertical bars.
(C) BSMT protein levels in unpollinated and pollinated petunia flowers at different times after pollination. The representative protein gel blot shows the 45-kD protein recognized by anti-SAMT antibodies. Proteins were extracted from petunia petals at the times after pollination indicated at top, and 12 μg of protein was loaded in each lane. A total of 0.76 μg of pure BSMT2 protein was loaded in the first (left) lane and used as a control. The blot shown here represents a typical result of three independent experiments.
Does Ethylene Play a Role in the Downregulation of Methylbenzoate Emission after Pollination?
There is considerable evidence that many postpollination processes in flowers, such as corolla abscission and/or senescence, pigmentation changes in the perianth, and ovary maturation, are mediated by endogenous ethylene that is produced as a consequence of pollination (Clark et al., 1997; Wilkinson et al., 1997; Ketsa et al., 2001; Kato et al., 2002). In petunia, pollination leads to two distinct phases of ethylene production: a sharp increase in ethylene synthesis in the stigma at ∼3 h after pollination, followed by a burst of synthesis in the corolla at ∼36 h after pollination (Jones et al., 2003). To determine whether ethylene plays a regulatory role in the downregulation of methylbenzoate emission in petunia, ethylene-insensitive transgenic petunia cv Mitchell plants (etr1-1) (Wilkinson et al., 1997) were used in experiments. These transgenic plants contain the dominant mutant ethylene receptor etr1-1 gene from Arabidopsis driven by the 35S promoter of Cauliflower mosaic virus and display an ethylene-insensitive delayed-petal-senescence phenotype (Wilkinson et al., 1997). Pollination of emasculated etr1-1 transgenic plants on the night of day 2 after anthesis did not affect the emission of methylbenzoate. Indeed, 3 days after pollination, methylbenzoate emission remained unchanged in etr1-1, whereas in the wild type, there was no methylbenzoate emission (Figure 2A). Analysis of BSMT gene expression and corresponding enzyme activity toward benzoic acid in etr1-1 transgenic plants after pollination revealed no changes in BSMT mRNA (Figure 5D) and BSMT activity (Figure 5E) at 3 days after pollination, whereas in the wild type, BSMT mRNA (Figure 5A), protein (Figure 5C), and activity (Figure 5B) were undetectable.
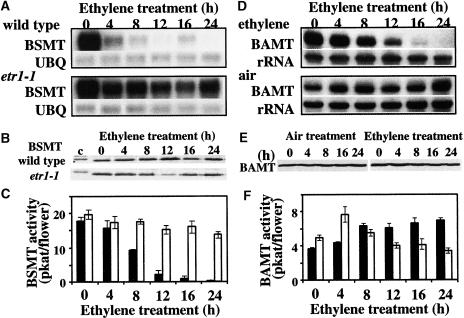
To confirm that the decrease in methylbenzoate emission in wild-type petunia after pollination is the result of ethylene action, both wild-type and etr1-1 petunia flowers were treated with 2 μL/L ethylene for 4, 8, 12, 16, and 24 h, and BSMT gene expression, protein level, and activity were analyzed after these different treatment periods (Figures 6A to 6C). Ethylene treatments had a drastic effect on BSMT gene expression and corresponding enzyme activity toward benzoic acid in wild-type petunia plants. Four hours of ethylene treatment resulted in a significant reduction of BSMT expression, which continued to decrease to an undetectable level with an extension of the duration of ethylene treatment (Figure 6A). In etr1-1 petunia flowers, the expression of BSMT and corresponding enzyme activity remained unaffected by ethylene treatment (Figures 6A and 6C), indicating that the BSMT gene is ethylene sensitive. However, the transcriptional downregulation of BSMT expression by ethylene in wild-type flowers did not result in a decrease of BSMT protein level after a 24-h ethylene treatment (Figure 6B). The 50% decrease in BSMT activity toward benzoic acid detected after an 8-h ethylene treatment (9.16 ± 0.29 pkat/flower) compared with BSMT activity in corresponding ethylene-treated etr1-1 flowers (17.6 ± 0.73 pkat/flower) (Figure 6C) probably is the result of post-translational regulation.
Figure 6.
Effect of Ethylene Treatment on BSMT and BAMT Gene Expression, Protein Levels, and Corresponding Enzyme Activities in Petunia and Snapdragon Flowers.
(A) BSMT gene expression in wild-type and etr1-1 petunia petals after different periods of ethylene treatment. Poly(A) mRNA was isolated from whole flowers at anthesis after the periods of ethylene treatment indicated at top, and 2 μg of mRNA was loaded in each lane. The top gels for the wild type and etr1-1 represent the results of hybridization with a BSMT probe. Autoradiography was performed overnight. The blots were rehybridized with a ubiquitin probe (UBQ) to standardize samples. Each blot represents a typical result of three independent experiments.
(B) BSMT protein levels in wild-type and etr1-1 petunia flowers after different periods of ethylene treatment. Representative protein gel blots show the 45-kD protein recognized by anti-SAMT antibodies. Proteins were extracted from petunia petals after the periods of ethylene treatment indicated at top, and 12 μg of protein was loaded in each lane. A total of 0.76 μg of pure BSMT2 protein was loaded in the first (left) lane and used as a control (c). Each blot represents a typical result of three independent experiments.
(C) BSMT activity toward benzoic acid in wild-type and etr1-1 petunia flowers after different periods of ethylene treatment. Closed and open bars represent BSMT activity toward benzoic acid in wild-type and etr1-1 flowers, respectively. For each time point, enzyme assays were run in duplicate on at least four independent crude extract preparations for both wild-type and etr1-1 flowers, and the standard deviations indicated by vertical bars were obtained.
(D) BAMT gene expression in upper and lower petal lobes of snapdragon flowers after different periods of ethylene treatment. Flowers treated with air were used as a control. Total RNA was isolated from upper and lower petal lobes of 5-day-old snapdragon flowers after the periods of ethylene or air treatment indicated at top, and 5 μg of total RNA was loaded in each lane. The top gel for each experiment represents the results of hybridization with a BAMT probe. Autoradiography was performed overnight. The blots were rehybridized with an 18S rDNA probe (bottom gels) to standardize samples. Each blot represents a typical result of three independent experiments.
(E) BAMT protein levels in snapdragon flowers treated with ethylene and air (control) after different periods of treatment. The representative protein gel blot shows the 49-kD protein recognized by anti-BAMT antibodies. Proteins were extracted from upper and lower petal lobes, and 11 μg of protein was loaded in each lane. The blot shown represents a typical result of three independent experiments.
(F) BAMT activity in snapdragon flowers treated with ethylene and air (control) after different periods of treatment. Enzyme assays were run in duplicate for each time point on at least five independent crude extract preparations for both ethylene- and air-treated flowers, and the standard deviations indicated by vertical bars were obtained. Closed and open bars represent BAMT activity in air- and ethylene-treated snapdragon flowers.
To determine if the snapdragon BAMT gene also is sensitive to ethylene, snapdragon flowers were treated with air or 2 μL/L ethylene for the same time used in petunia treatments, and BAMT gene expression, protein level, and activity were analyzed. A decrease in BAMT gene expression was found after 12 h of ethylene exposure, resulting in no detectable transcripts after 24 h of treatment, whereas BAMT expression in control flowers was stable (Figure 6D). These results indicate that, similar to the petunia BSMT gene, the snapdragon BAMT is sensitive to ethylene. An ∼35% decrease in BAMT activity also was found after 12 h of ethylene treatment (3.95 ± 1.04 pkat/ethylene-treated flower versus 6.07 ± 0.58 pkat/air-treated flower) (Figure 6F) and remained level after 16- and 24-h ethylene treatments. However, no changes in the BAMT protein level were detected during the experiments (Figure 6E), indicating the stability of the BAMT protein and suggesting the involvement of post-translational mechanisms in the regulation of BAMT activity after ethylene treatment.
DISCUSSION
Pollination-Induced Changes in Methylbenzoate Emission in Snapdragon and Petunia Flowers
In nature, floral structure, color, and scent are critical factors in attracting pollinators to flowers. Although floral structure and color provide important information for pollinators at a close range, floral scent can function as both a long- and short-distance attractant and as a nectar guide to a variety of insect pollinators (Dobson, 1994). Pollination of flowers by insects leads to changes in scent emission, which, to our knowledge, has been shown previously only in orchids (Tollsten and Bergstrom, 1989; Tollsten, 1993; Schiestl et al., 1997). These changes in scent emission after pollination include a general decrease in all scent compounds, as found in Platanthera bifolia (Orchidaceae) (Tollsten and Bergstrom, 1989; Tollsten, 1993), or a decrease in some compounds while others increase or remain unchanged, as was discovered in Ophrys orchids (Schiestl et al., 1997; Schiestl and Ayasse, 2001). Although alterations in scent emission in response to pollination have been shown, the actual mechanisms responsible for postpollination changes of floral scent have not been investigated because of the lack of knowledge about the enzymes responsible for the biosynthesis of scent compounds.
Here, we used snapdragon and petunia as model systems to identify the molecular mechanisms responsible for changes in scent emission after pollination. We found that in both species, all major scent compounds decreased after pollination similar to P. bifolia (Tollsten and Bergstrom, 1989; Tollsten, 1993) (Figures 1A to 1C and 2A to 2C). Floral scent of snapdragon and petunia flowers is dominated by the volatile ester methylbenzoate (Dudareva et al., 2000; Kolosova et al., 2001a; Verdonk et al., 2003), which is synthesized from benzoic acid and SAM in the reaction catalyzed by BAMT in snapdragon (Dudareva et al., 2000; Murfitt et al., 2000) and, as shown in the present study, by BSMT in petunia. We observed that a significant pollination-induced decrease in methylbenzoate emission (∼70 to 75%; Figures 1B and 2A) begins after pollen tubes reach the ovary, a process that takes between 35 and 40 h in snapdragon (Figures 1F and 1G) and ∼32 h in petunia (Figure 2D). This postpollination decrease in scent emission is not triggered by pollen deposition on the stigma. When snapdragon flowers were pollinated with foreign pollen from petunia and heat-inactivated self pollen, no changes in scent emission were found compared with that in unpollinated flowers (Figures 1D and 1E), suggesting that fertilization is a prerequisite for the reduction of floral scent after pollination. By decreasing scent emission only after pollen tubes reach the embryo sac, plants protect their reproductive process, thereby ensuring that successful fertilization will occur.
Pollination did not completely eliminate floral scent, leaving snapdragon flowers on day 4 after pollination with low scent emission (2.7 and 3.0 μg·flower−1·24 h−1 when flowers were pollinated on days 4 and 5 after anthesis, respectively; Figures 1A and 1B). In petunia, flowers on day 3 after pollination also produced a low amount of methylbenzoate (8.3 μg·flower−1·12 h−1), which accounts for 10% of the amount emitted by corresponding unpollinated control flowers (Figure 2A). This retained floral scent continues to contribute to the overall fragrance of the whole inflorescence or plant, likely enhancing the orientation cue for pollinator attraction.
Different Molecular Mechanisms Are Responsible for the Postpollination Downregulation of Methylbenzoate Emission in Snapdragon and Petunia Flowers
Analysis of BAMT activity in snapdragon flowers pollinated on day 5 after anthesis revealed only a slight decrease in BAMT activity on day 3 after pollination (Figure 3A), which could not account completely for the 70% decrease in methylbenzoate emission (Figure 1B). This raises at least two questions. (1) Does pollination affect the release of methylbenzoate, leading to its accumulation in floral tissue? (2) Does pollination affect the level of benzoic acid and/or the level of SAM, which is the methyl group donor for the BAMT-catalyzed reaction (Dudareva et al., 2000; Murfitt et al., 2000), thereby decreasing methylbenzoate emission after pollination?
We found no accumulation of methylbenzoate within snapdragon petal tissue at 3 and 4 days after pollination (data not shown). When the endogenous pool of benzoic acid was measured in pollinated flowers, no differences were found between pollinated and unpollinated flowers (Figure 3D). These data indicate that the benzoic acid level does not contribute to the regulation of methylbenzoate emission after pollination in snapdragon flowers, unlike its involvement in the regulation of developmental and rhythmic emission (Dudareva et al., 2000; Kolosova et al., 2001a). However, pollination affected the methylation index (i.e., the SAM/SAH ratio), leading to a 40% total decrease by day 4 after pollination (Figure 3E). These results suggest that the postpollination decrease in methylbenzoate emission in snapdragon flowers is the result of a decrease in both the methylation index and BAMT activity.
Comparison of BAMT activity and BAMT gene expression in snapdragon flowers after pollination revealed that they followed the same pattern and showed a strong positive correlation (Figures 3A and 3B), both decreasing by 50% on day 4 after pollination. However, the decrease in BAMT gene expression did not affect the level of BAMT protein on this day (Figure 3C), likely because of a lag between the transcription and translation processes. At least a 24-h shift between the mRNA and its corresponding protein level often has been observed for other genes involved in scent production (Wang et al., 1997; Dudareva et al., 1999, 2000). Thus, the 50% decrease in BAMT activity on the 4th day after pollination (Figure 3B) likely is attributable to regulation at the post-translational level.
Such post-translational regulation is not unique to BAMT. The involvement of post-translational modifications in the regulation of enzyme activities has been shown for several other plant enzymes (Khayat et al., 1993; Chang and Gallie, 1997; Rudrabhatla and Rajasekharan, 2002; Smith et al., 2002). Protein modifications, such as phosphorylation, methylation, carboxylation, glucosylation, acetylation, and prenylation, often contribute to the regulation of enzyme activities (Lillo et al., 1997; Siddiqui et al., 1998; Tanase et al., 2002). Future experiments involving sequencing of the post-translationally modified protein will show the nature of the modification that occurs for BAMT.
In pollinated snapdragon just before abscission (day 4 after pollination), BAMT transcripts and BAMT activity were 50% lower than those of corresponding unpollinated flowers (Figures 3A and 3B). By contrast, pollination in petunia resulted in undetectable BSMT mRNA transcripts, enzyme activity, and BSMT protein level on the last day (day 3) after pollination before flowers start to wilt (Figures 5A to 5C). There was a lag period of ∼24 h between the decrease in the BSMT transcript level at 2 days after pollination (Figure 5A) and the disappearance of the corresponding protein at 3 days after pollination (Figure 5C), indicating that, as in snapdragon, post-translational regulation also contributes to a decrease in BSMT activity soon after fertilization (day 2 after pollination). However, unlike in snapdragon, the postpollination decrease in methylbenzoate emission in petunia was the result of a decrease in BSMT activity toward benzoic acid, which is regulated at the transcriptional and post-translational levels.
Involvement of Ethylene in the Regulation of Methylbenzoate Emission after Pollination in Petunia and Snapdragon
In flowering plants, pollination initiates a cascade of developmental events, including perianth senescence, pigmentation changes, and ovule differentiation, in which ethylene plays an important role in coordinating postpollination development (O'Neill, 1997; O'Neill and Nadeau, 1997; Ketsa et al., 2001; Kato et al., 2002). Pollination also affects floral scent emission, as was shown in this study for snapdragon and petunia and previously for orchids (Tollsten and Bergstrom, 1989; Tollsten, 1993; Schiestl et al., 1997). Because all of these species are considered ethylene sensitive, these findings raise the question of ethylene's involvement in the regulation of floral scent emission after pollination. There is growing evidence that in many cases, plant responses to ethylene are associated with changes in gene expression (Llop-Tous et al., 2000; Weterings et al., 2002). Our data show that in petunia, pollination downregulates BSMT gene expression, which is correlated tightly with BSMT activity toward benzoic acid and methylbenzoate emission (Figures 2A, 5A, and 5B), revealing the possibility of ethylene involvement in this process. Indeed, a comparative analysis of methylbenzoate emission, BSMT activity toward benzoic acid, and corresponding gene expression after pollination in ethylene-insensitive transgenic etr1-1 and wild-type petunia flowers (Figures 2A and 5) revealed the ethylene-dependent nature of the pollination-induced downregulation in scent emission. Three days after pollination in etr1-1 flowers, methylbenzoate emission (Figure 2A), BSMT gene expression (Figure 5D), and BSMT activity toward benzoic acid (Figure 5E) remained level, whereas significant decreases to almost undetectable levels were found in wild-type petunia flowers (Figures 2A, 5A, and 5B). Moreover, treatment of petunia flowers with exogenous ethylene led to the downregulation of BSMT gene expression and BSMT activity toward benzoic acid in wild-type plants but not in etr1-1 plants (Figures 6A and 6C), indicating that the BSMT gene is ethylene sensitive.
In snapdragon, the BAMT gene also is sensitive to ethylene, although the decrease in BAMT expression was found only after 12 h of ethylene exposure, in contrast to 4 h in petunia (Figures 6A and 6D), indicating that snapdragon is less sensitive to ethylene than petunia or that it responds more slowly to ethylene. In both wild-type petunia and snapdragon, ethylene treatment affected benzoic acid carboxyl methyltransferase activities, resulting in no activity in petunia and 50% lower activity than in the control in snapdragon after 24 h of ethylene treatment (Figures 6C and 6F). However, in both snapdragon and petunia, BAMT and BSMT protein levels remained unchanged after ethylene treatment (Figures 6B and 6E), suggesting that post-translational regulatory mechanisms are responsible for the decrease in corresponding enzyme activities.
In summary, our results show that scent genes are sensitive to ethylene in both model systems, with snapdragon responding more slowly than petunia (Figures 6A and 6D). In petunia, ethylene downregulates the expression of scent biosynthetic genes and the subsequent emission after pollination. Although we detected ethylene production in snapdragon flowers at 3 days after pollination (21 nL·g−1 fresh weight·h−1 compared with an undetectable level in control flowers), a 70% decrease in methylbenzoate emission on day 3 after pollination occurred without a concomitant decrease in the BAMT transcript level (Figures 1B and 3A), indicating that this decrease in emission is not the result of an ethylene-induced downregulation of BAMT expression. The 50% decrease in BAMT mRNA levels on day 4 after pollination (Figure 3A) likely is the result of ethylene action, and we would expect eventually to see a complete disappearance of BAMT transcripts if snapdragon flowers do not abscise. Together, our data indicate that in snapdragon, the decrease in methylbenzoate emission after pollination is not the result of a transcriptional downregulation of the BAMT gene but rather of decreases in methylation index and post-translationally regulated BAMT activity. Because snapdragon flowers produce ethylene after pollination, its biosynthesis contributes to the decrease in the methylation index via the use of SAM. Overall, although the effects of ethylene on petal pigmentation, abscission, and/or senescence in ethylene-sensitive flowers after pollination have been characterized thoroughly, our results demonstrate a new role of ethylene as a signal in the downregulation of floral scent emission after pollination in petunia flowers.
METHODS
Plant Material and Headspace Analysis of Floral Volatiles
Snapdragon (Antirrhinum majus cv Maryland True Pink; Ball Seed, West Chicago, IL) and petunia (Petunia hybrida cv Mitchell; Ball Seed) were grown under standard greenhouse conditions as described previously (Dudareva et al., 2000; Kolosova et al., 2001a). Headspace collections of petunia volatiles were performed in a greenhouse, and snapdragon flower volatiles were collected in growth chambers (model E8; Conviron, Asheville, NC) under previously described conditions (Dudareva et al., 2003). Flowers were emasculated at 2 to 3 days before opening for snapdragon and 1 to 2 days before opening for petunia and hand pollinated on the 2nd day after anthesis (petunia) and on days 4 and 5 after anthesis (snapdragon). The compatibility of pollen was confirmed by seed production. Emitted volatiles were determined by headspace analysis as described previously (Raguso and Pichersky, 1995; Raguso and Pellmyr, 1998) at 24-h intervals for snapdragon and 12-h intervals (beginning at 10 pm) for petunia. Trapped floral scent compounds were analyzed by gas chromatography–mass spectrometry with a FinniganMAT GCQ instrument (Thermoquest, San Jose, CA) and by gas chromatography with an Agilent 6890-series gas chromatograph (Agilent Technologies, Palo Alto, CA) as described previously (Dudareva et al., 2000; Kolosova et al., 2001a).
BAMT Enzyme Assays
Crude protein extracts were prepared from upper and lower petal lobes of snapdragon flowers and from tubes and corollas of petunia flowers as described previously (Dudareva et al., 2000). For each collection time, at least three and five flowers from different plants were combined for petunia and snapdragon, respectively. Enzyme assays were performed at the saturation level of substrates (100 μM SAM and 2 mM benzoic acid) as described previously (Dudareva et al., 2000), eliminating the effect on enzyme activity of the increased amount of SAH in the crude extracts after pollination.
RNA Isolation and RNA Gel Blot Analysis
Total RNA samples from floral tissues (upper and lower petal lobes of snapdragon flowers, and limbs and tubes of petunia corollas) were isolated as described previously (Dudareva et al., 1996, 1998, 2000; Wang et al., 1997; Kolosova et al., 2001a). A 1.3-kb EcoRI fragment containing the coding region of the BAMT gene was used as a probe for RNA gel blot analysis in snapdragon, and a 1-kb EcoRI fragment containing the coding region of the BSMT genes was used as a probe for RNA gel blot analysis in petunia. Hybridization signals were quantified with a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, CA), and mRNA transcript levels were normalized to rRNA levels to overcome errors in RNA quantitation by spectrophotometry.
Immunoblot Analysis
Crude extracts were prepared from the upper and lower petal lobes of snapdragon flowers and petunia petals as described previously (Dudareva et al., 2000). For two-dimensional electrophoresis, an immobilized linear (3 to 10) pH gradient (Immobiline DryStrip pH 3-10; Amersham Bioscience, Uppsala, Sweden) was used in an IPGphor isoelectric focusing unit (Amersham Bioscience, Piscataway, NJ). Immunodetection was performed using rabbit anti-BAMT purified polyclonal antibodies or rabbit anti-SAMT polyclonal antibodies (1:2,500 dilution), with goat anti-rabbit IgG horseradish peroxidase conjugate (1:30,000 dilution) as a secondary antibody. Antigen bands were visualized using chemiluminescence reagents (DuPont–New England Nuclear Life Science Products, Boston, MA) for protein gel blots, according to the manufacturer's protocols, and exposed on Eastman Kodak X-Omat AR film.
Extraction and Quantification of Endogenous Substrate Pools
Benzoic acid was extracted from snapdragon petal tissue (upper and lower lobes of at least 10 flowers per collection time) by supercritical carbon dioxide extraction at 414 bars and 40°C with an SFX-210 Extractor outfitted with a model-2600 pump and a temperature-controlled variable restrictor (ISCO, Lincoln, NE) and quantified by HPLC as described previously (McHugh and Krukonis, 1994; Dudareva et al., 2000; Kolosova et al., 2001a).
For the quantification of endogenous SAM and SAH, petal tissue from upper and lower lobes of at least 10 snapdragon flowers per collection time were frozen in liquid nitrogen, ground to a powder, and resuspended in 10% (w/v) trichloroacetic acid (1 μL/mg fresh weight). After centrifugation, SAM and SAH were measured as their fluorescent isoindoles as described previously (Capdevila and Wagner, 1998).
Analysis of Pollen Tube Growth
Five-day-old emasculated snapdragon flowers and 2-day-old emasculated petunia flowers were hand-pollinated with self pollen and pistils (five for snapdragon and three for petunia) and harvested at different times after pollination (3, 17, 24, 32, and 40 h for snapdragon, and 5, 15, 25, and 32 h for petunia). Callose staining of pollen tubes was performed as described (Tang and Woodson, 1996), and pollen tubes were visualized with a fluorescence microscope (E800; Nikon, Tokyo, Japan) using a 360- ± 20-nm exciter filter and a 420-nm long-pass emitter filter.
Expression of BSMT in Escherichia coli, and Purification and Characterization of Recombinant Protein
To isolate a full-length cDNA, cDNA library screening and 5′ rapid amplification of cDNA ends were performed as described previously (Dudareva et al., 2003). The coding region of BSMT was subcloned into the NdeI-EcoRI site of the expression vector pET-28a (Novagen, Madison, WI) by PCR using the appropriate oligonucleotide primers as described previously (Murfitt et al., 2000; Negre et al., 2002). E. coli BL21(DE3) cells expressing BSMT were grown in Luria-Bertani medium with 50 μg/mL kanamycin at 37°C, and induction, harvesting, and protein purification by nickel-based affinity chromatography were performed as described previously (Murfitt et al., 2000; Negre et al., 2002). For partial purification of plant BSMT proteins, crude protein extracts (up to 100 mL) obtained from 2-day-old petunia petals were loaded onto a DEAE-cellulose column (10 mL of DE53; Whatman) preequilibrated in buffer A (50 mM Bis-Tris-HCl, pH 6.9, 10% glycerol, and 10 mM β-mercaptoethanol). After washing to remove unbound proteins, the BSMT enzymes were eluted with a linear salt gradient (60 mL) from 0 to 400 mM KCl in buffer A. Fractions with the highest BSMT activity were pooled and dialyzed against 2 L of buffer A overnight at 4°C. Enzyme assays, assessment of kinetic properties, and native molecular mass determination were performed as described previously (Murfitt et al., 2000; Negre et al., 2002). In assays for pH optimum, cofactor requirements, and Km measurements, proper concentrations of purified BSMT were chosen so that the reaction velocity was proportional to the enzyme concentration and was linear with respect to time for at least 30 min. The protein content of samples was determined by the Bradford method using the Bio-Rad protein reagent (Hercules, CA) and BSA as a standard.
Reverse Transcriptase–PCR
The contribution of each BSMT gene in the expression pattern was analyzed by reverse transcriptase (RT)–PCR as described previously (Dudareva et al., 2003). One microgram of total RNA isolated from the limbs and tubes of petunia corollas was used to make cDNA using random hexamer primers with the Advantage RT-for-PCR kit according to the manufacturer's instructions (Clontech, Palo Alto, CA). Two pairs of gene-specific primers from the 3′ untranslated regions were used for PCR. For BSMT1, the primers were designed to amplify a fragment of ∼270 bp and were as follows: 5′-TCTATTTTCGGTCGAAATCCGG-3′ (forward) and 5′-CTGTGGTACAGAACCTTTAGTG-3′ (reverse). For BSMT2, the forward (5′-TGTCTGAGGAGGATTAAGAACG-3′) and reverse (5′-AGAGAGATCTGAAAGGACCCC-3′) primers amplified products of 200 bp. Reactions were performed in duplicate for 30 cycles at an annealing temperature of 54°C. Analysis of the products obtained with different amounts of cDNA in PCR (5, 10, 15, and 20 μL) showed that product increased linearly. Fifteen microliters of cDNA was chosen as optimal for further PCR steps. To ensure that an equal amount of RNA was used for all samples and that RT reactions were equally effective, PCR with the 18S rRNA gene-specific primers (5′-GATAAAAGGTCGACACGGGCTCTGC-3′ [forward] and 5′-AACGGAATTAACCAGACAAATCGCTCC-3′ [reverse]) was performed. For rDNA, PCR was performed for 15 cycles at an annealing temperature of 60°C. The amplified products were run on 1.2% agarose gels and stained with ethidium bromide.
Ethylene Measurements and Treatments of Snapdragon and Petunia Flowers
To determine ethylene production, flowers were harvested at 12-h intervals for petunia and 24-h intervals for snapdragon beginning at time 0 after pollination and placed into gas-tight 20-mL vials. After 12 h for petunia and 24 h for snapdragon, 1 mL of gas was analyzed with a gas chromatograph equipped with an activated alumina column and flame ionization detector (GC-8A; Shimadzu, Kyoto, Japan). Ten flowers were used for each time point. The concentration of ethylene was determined by comparing the sample with an authentic ethylene standard.
For ethylene treatments, petunia plants were placed in two air-proof Plexiglas chambers, and snapdragon flowers were treated individually on the inflorescences. Ethylene gas was injected to a final concentration of 2 μL/L in one of the chambers (for petunia) or in individual 0.5-L air-tight flasks (for snapdragon). For snapdragon, flowers treated with air were used as a control. Flowers of the same age were harvested at various times after ethylene injection (0, 4, 8, 12, 16, and 24 h) and frozen in liquid nitrogen. Crude extracts were prepared from petal tissue for enzyme assays and protein blot analysis, and RNA was extracted for RNA gel blot analysis. For petunia, total RNA was extracted from whole flowers as described previously (Ciardi et al., 2000), and poly(A) mRNA was isolated using the Oligotex mRNA purification system (Qiagen, Valencia, CA). To standardize samples, filters containing petunia poly(A) mRNA after hybridization with the BSMT probe were rehybridized with petunia ubiquitin cDNA (clone PhUBQ1 from the petunia EST database), which shares 80 to 90% nucleotide identity with Arabidopsis ubiquitin genes.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact N. Dudareva, dudareva@hort.purdue.edu.
Accession Numbers
The GenBank accession numbers for BSMT1 and BSMT2 are AY233465 and AY233466, respectively. The accession number for SAMT from Atropa belladonna is AB049752.
Acknowledgments
We thank Nina Gorenstein for assistance at the earlier stages of research, Stanislav Zakharov for help with gel filtration chromatography, and Mike Poling for assistance with two-dimensional gel electrophoresis. This work was supported by National Science Foundation Grant IBN-9904910 to N.D., by grants from the Fred Gloeckner Foundation and the American Floral Endowment to D.G.C. and N.D., and by U.S. Department of Agriculture Floriculture and Nursery Research Initiative and Florida Agricultural Experiment Station grants to D.G.C. This article is contribution 17060 from the Purdue University Agricultural Experimental Station.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.016766.
References
- Arditti, J. (1979). Aspects of the physiology of orchids. In Advances in Botanical Research, Vol. 7, H.W. Woolhouse, ed (London: Academic Press), pp. 421–655.
- Cantoni, G.L., Richards, H.H., and Chiang, P.K. (1979). Inhibitors of S-adenosylhomocysteine hydrolase and their role in the regulation of biological methylation. In Transmethylation, E. Usdin, R.T. Borchardt, and C.R. Creveling, eds (Amsterdam: Elsevier-North Holland), pp. 155–164.
- Capdevila, A., and Wagner, C. (1998). Measurement of plasma S-adenosylmethionine and S-adenosylhomocysteine as their fluorescent isoindoles. Anal. Biochem. 264, 180–184. [DOI] [PubMed] [Google Scholar]
- Chang, S.C., and Gallie, D.R. (1997). RNase activity decreases following a heat shock in wheat leaves and correlates with its posttranslational modification. Plant Physiol. 113, 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi, J.A., Tieman, D.M., Lund, S.T., Jones, J.B., Stall, R.E., and Klee, H.J. (2000). Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol. 123, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, D.G., Richards, C., Hilioti, Z., LindIversen, S., and Brown, K. (1997). Effect of pollination on accumulation of ACC synthase and ACC oxidase transcripts, ethylene production and flower petal abscission in geranium (Pelargonium × hortorum L.H. Bailey). Plant Mol. Biol. 34, 855–865. [DOI] [PubMed] [Google Scholar]
- Dafni, A., and Bernhardt, P. (1990). Pollination of terrestrial orchids of Southern Australia and the Mediterranean region: Systematic, ecological and evolutionary implications. Evol. Biol. 24, 193–252. [Google Scholar]
- D'Auria, J.C., Chen, F., and Pichersky, E. (2003). The SABATH family of methyltransferases in Arabidopsis thaliana and other plant species. In Recent Advances in Phytochemistry, Vol. 37, J.T. Romeo, ed (Oxford, UK: Elsevier Science), pp. 253–283.
- Dobson, H.E.M. (1994). Floral volatiles in insect biology. In Insect-Plant Interactions, Vol. 5, E. Bernays, ed (Boca Raton, FL: CRC Press), pp. 47–81.
- Dudareva, N., Cseke, L., Blanc, V.M., and Pichersky, E. (1996). Evolution of floral scent in Clarkia: Novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell 8, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva, N., Martin, D., Kish, C.M., Kolosova, N., Gorenstein, N., Faldt, J., Miller, B., and Bohlman, J. (2003). (E)-β-Ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: Function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15, 1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva, N., Murfitt, L.M., Mann, C.J., Gorenstein, N., Kolosova, N., Kish, C.M., Bonham, C., and Wood, K. (2000). Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 12, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva, N., Piechulla, B., and Pichersky, E. (1999). Biogenesis of floral scent. Hortic. Rev. 24, 31–54. [Google Scholar]
- Dudareva, N., Raguso, R.A., Wang, J., Ross, J.R., and Pichersky, E. (1998). Floral scent production in Clarkia breweri. III. Enzymatic synthesis and emission of benzenoid esters. Plant Physiol. 116, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler, M., and Baldwin, I.T. (1996). The chemistry of defense and apparency in the corollas of Nicotiana attenuata. Oecologia 107, 102–112. [DOI] [PubMed] [Google Scholar]
- Gegear, R.J., and Laverty, T.M. (2001). The effect of variation among floral traits on the flower constancy of pollinators. In Cognitive Ecology of Pollination: Animal Behavior and Floral Evolution, L. Chittka and J.D. Thomson, eds (Cambridge, UK: Cambridge University Press), pp. 1–20.
- Henderson, A. (1986). A review of pollination studies in the Palmae. Bot. Rev. 52, 221–259. [Google Scholar]
- Hoekstra, F.A., and Weges, R. (1986). Lack of control by early pistillate ethylene of the accelerated wilting of Petunia hybrida flowers. Plant Physiol. 80, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen, H.B., and Olsen, C.E. (1994). Influence of climatic factors on rhythmic emission of volatiles from Trifolium repens L. flowers in situ. Planta 192, 365–371. [Google Scholar]
- Jones, K.N., Reithel, J.S., and Irwin, R.E. (1998). A trade-off between the frequency and duration of bumblebee visits to flowers. Oecologia 117, 161–168. [DOI] [PubMed] [Google Scholar]
- Jones, M.L., Langston, B.J., and Johnson, F. (2003). Pollination-induced senescence of ethylene sensitive and insensitive petunias. In Biology and Biotechnology of the Plant Hormone Ethylene II, M. Vendrell and F. Romojaro, eds (Amsterdam, The Netherlands: IOS Press), pp. 324–327.
- Kato, M., Shimizu, H., Onozaki, T., Tanikawa, N., Ikeda, H., Hisamatsu, T., and Ichimura, K. (2002). Role of ethylene in senescence of pollinated and unpollinated Campanula medium flowers. J. Jpn. Soc. Hortic. Sci. 71, 385–387. [Google Scholar]
- Ketsa, S., Bunya-atichart, K., and van Doorn, W.G. (2001). Ethylene production and post-pollination development in Dendrobium flowers treated with foreign pollen. Aust. J. Plant Physiol. 28, 409–415. [Google Scholar]
- Khayat, E., Harn, C., and Daie, J. (1993). Purification and light-dependent molecular modulation of the cytosolic fructose-1,6-bisphosphatase in sugarbeet leaves. Plant Physiol. 101, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, J.T., Andersson, S., and Bergman, P. (1999). Floral scent attraction in Geonoma macrostachys, an understorey palm of the Amazonian rain forest. Oikos 85, 409–418. [Google Scholar]
- Kolosova, N., Gorenstein, N., Kish, C.M., and Dudareva, N. (2001. a). Regulation of circadian methylbenzoate emission in diurnally and nocturnally emitting plants. Plant Cell 13, 2333–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova, N., Sherman, D., Karlson, D., and Dudareva, N. (2001. b). Cellular and subcellular localization of S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiol. 126, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo, C., Kazazaic, S., Ruoff, P., and Meyer, C. (1997). Characterization of nitrate reductase from light- and dark-exposed leaves: Comparison of different species and effects of 14-3-3 inhibitor proteins. Plant Physiol. 114, 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop-Tous, I., Barry, C.S., and Grierson, D. (2000). Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol. 123, 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin, J.H., Hamilton-Kemp, T.R., Andersen, R.A., and Hildebrand, D.F. (1990). Volatiles from flowers of Nicotiana sylvestris, N. otophora and Malus × domestica: Headspace components and day/night changes in their relative concentrations. Phytochemistry 29, 2473–2477. [Google Scholar]
- Matile, P., and Altenburger, R. (1988). Rhythms of fragrance emission in flowers. Planta 174, 242–247. [DOI] [PubMed] [Google Scholar]
- McHugh, M.A., and Krukonis, V.J. (1994). Supercritical Fluid Extraction. (Boston, MA: Butterworth-Heinemann).
- Murfitt, L.M., Kolosova, N., Mann, C.J., and Dudareva, N. (2000). Purification and characterization of S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methyl benzoate in flowers of Antirrhinum majus. Arch. Biochem. Biophys. 382, 145–151. [DOI] [PubMed] [Google Scholar]
- Negre, F., Kolosova, N., Knoll, J., Kish, C.M., and Dudareva, N. (2002). Novel S-adenosyl-l-methionine:salicylic acid carboxyl methyltransferase, an enzyme responsible for biosynthesis of methyl salicylate and methyl benzoate, is not involved in floral scent production in snapdragon flowers. Arch. Biochem. Biophys. 406, 261–270. [DOI] [PubMed] [Google Scholar]
- Neiland, M.R.M., and Wilcock, C.C. (1998). Fruit set, nectar reward, and rarity in the Orchidaceae. Am. J. Bot. 85, 1657–1671. [PubMed] [Google Scholar]
- Nielsen, J.K., Jakobsen, H.B., Hansen, P.F.K., Moller, J., and Olsen, C.E. (1995). Asynchronous rhythms in the emission of volatiles from Hesperis matronalis flowers. Phytochemistry 38, 847–851. [Google Scholar]
- O'Neill, S.D. (1997). Pollination regulation of flower development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 547–574. [DOI] [PubMed] [Google Scholar]
- O'Neill, S.D., and Nadeau, J.A. (1997). Post-pollination flower development. Hortic. Rev. 19, 1–58. [Google Scholar]
- Pichersky, E., Raguso, R.A., Lewinsohn, E., and Croteau, R. (1994). Floral scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol. 106, 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott, M.B., Pichersky, E., and Piechulla, B. (2002). Evening specific oscillations of scent emission, SAMT enzyme activity, and SAMT mRNA in flowers of Stephanotis floribunda. J. Plant Physiol. 159, 925–934. [Google Scholar]
- Raguso, R.A., and Pellmyr, O. (1998). Dynamic headspace analysis of floral volatiles: A comparison of methods. Oikos 81, 238–254. [Google Scholar]
- Raguso, R.A., and Pichersky, E. (1995). Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): Recent evolution of floral scent and moth pollination. Plant Syst. Evol. 194, 55–67. [Google Scholar]
- Ross, J.R., Nam, K.H., D'Auria, J.C., and Pichersky, E. (1999). S-Adenosyl-l-methionine:salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch. Biochem. Biophys. 367, 9–16. [DOI] [PubMed] [Google Scholar]
- Rudrabhatla, P., and Rajasekharan, R. (2002). Developmentally regulated dual-specificity kinase from peanut that is induced by abiotic stresses. Plant Physiol. 130, 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, F.P., and Ayasse, M. (2001). Post-pollination emission of a repellent compound in a sexually deceptive orchid: A new mechanism for maximising reproductive success? Oecologia 126, 531–534. [DOI] [PubMed] [Google Scholar]
- Schiestl, F.P., Ayasse, M., Paulus, H.F., Erdmann, D., and Francke, W. (1997). Variation of floral scent emission and post pollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes. J. Chem. Ecol. 23, 2881–2895. [Google Scholar]
- Siddiqui, A.A., Garland, J.R., Dalton, M.B., and Sinensky, M. (1998). Evidence for a high affinity, saturable, prenylation-dependent p21Ha-ras binding site in plasma membranes. J. Biol. Chem. 273, 3712–3717. [DOI] [PubMed] [Google Scholar]
- Smith, P.M.C., Winter, H., Storer, P.J., Bussell, J.D., Schuller, K.A., and Atkins, C.A. (2002). Effect of short-term N2 deficiency on expression of the ureide pathway in cowpea root nodules. Plant Physiol. 129, 1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase, K., Shiratake, K., Mori, H., and Yamaki, S. (2002). Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit. Physiol. Plant. 114, 21–26. [DOI] [PubMed] [Google Scholar]
- Tang, X.Y., and Woodson, W.R. (1996). Temporal and spatial expression of 1-aminocyclopropane-1-carboxylate oxidase mRNA following pollination of immature and mature petunia flowers. Plant Physiol. 12, 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollsten, L. (1993). A multivariate approach to post-pollination changes in the floral scent of Platanthera bifolia (Orchidaceae). Nord. J. Bot. 13, 495–499. [Google Scholar]
- Tollsten, L., and Bergstrom, G. (1989). Headspace volatiles of whole plants and macerated plant-parts of Brassica and Sinapis. Phytochemistry 27, 4013–4018. [Google Scholar]
- Verdonk, J.C., Ric de Vos, C.H., Verhoeven, H.A., Haring, M.A., van Tunen, A.J., and Schuurink, R.C. (2003). Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62, 997–1008. [DOI] [PubMed] [Google Scholar]
- Wang, J., Dudareva, N., Bhakta, S., Raguso, R.A., and Pichersky, E. (1997). Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the enzyme S-adenosyl-l-methionine:(iso)eugenol O-methyltransferase and phenylpropanoid emission. Plant Physiol. 114, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser, N.M., Chittla, L., Price, M.V., Williams, N.M., and Ollerton, J. (1996). Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060. [Google Scholar]
- Weterings, K., Pezzotti, M., Cornelissen, M., and Mariani, C. (2002). Dynamic 1-aminocyclopropane-1-carboxylate-synthase and -oxidase transcript accumulation patterns during pollen tube growth in tobacco styles. Plant Physiol. 130, 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, J.Q., Lanahan, M.B., Clark, D.G., Bleecker, A.B., Chang, C., Meyerowitz, E.M., and Klee, H.J. (1997). A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat. Biotechnol. 15, 444–447. [DOI] [PubMed] [Google Scholar]