Abstract
SecA ATPase is a critical member of the Sec family, which is important in the translocation of membrane and secreted polypeptides/proteins in bacteria. Small molecule inhibitors can be very useful research tools as well as leads for future antimicrobial agent development. Based on previous virtual screening work, we optimized the structures of two hit compounds and obtained SecA ATPase inhibitors with IC50 in the single digit micromolar range. These represent the first low micromolar synthetic inhibitors of bacterial SecA and will be very useful for mechanistic studies.
1. Introduction
With the rapid emergence of drug resistant bacteria, there is an urgent need for the development of new antimicrobial agents, especially those with a unique mechanism of action. With this ultimate goal in mind, we are interested in the development of inhibitors of bacterial protein translocation. Several protein transport mechanisms exist in bacteria.1 Among them, the Sec machinery (or translocase) provides a major pathway of protein translocation from the cytosol across or into the cytoplasmic membrane. The Sec machinery has seven proteins including SecA, SecD, SecE, SecF, SecG, SecY, and YajC. Assembly and complex formation are required to yield the functional translocase. Among the Sec proteins, SecA is found both in the cytoplasm and bound to the inner membrane. When SecA is bound to the SecYEG complex, acidic phospholipids and a precursor protein such as proOmpA (the precursor of outer membrane protein A), it becomes fully active as an ATPase and a protein translocase.2, 3 Recently, several seminal papers described in intricate details as to how the SecA machinery functions in transporting proteins.4–6
It has been said that in any given organism, membrane and secreted polypeptides/proteins comprise more than 30% of the proteome; and no less than 10% of proteins cross a membrane before arriving at their final locations of function.7, 8 Such actions are often mediated by protein translocases. Therefore SecA is essential for bacterial survival. We envision that inhibitors of SecA can be very useful tools for studying bacterial protein transport and potential antimicrobial agents, especially because SecA has no human counterpart. We have previously reported effort in using virtual screening against the Escherichia coli SecA crystal structure9 to search for possible structural features suitable for SecA inhibitor development.10 In this paper, we describe our effort in optimizing the structural features of the initial hits for the development of bacterial SecA inhibitors. Several low µM inhibitors have been found. Considering the fact that currently inorganic azide, which is a SecA inhibitor with an IC50 value of about 3 mM, has cross reactivities against a number of enzymes,11, 12 and is the primary research tool for probing bacterial protein translocation, the newly discovered SecA inhibitors will be very important.
2. Results and Discussions
2.1. Chemistry
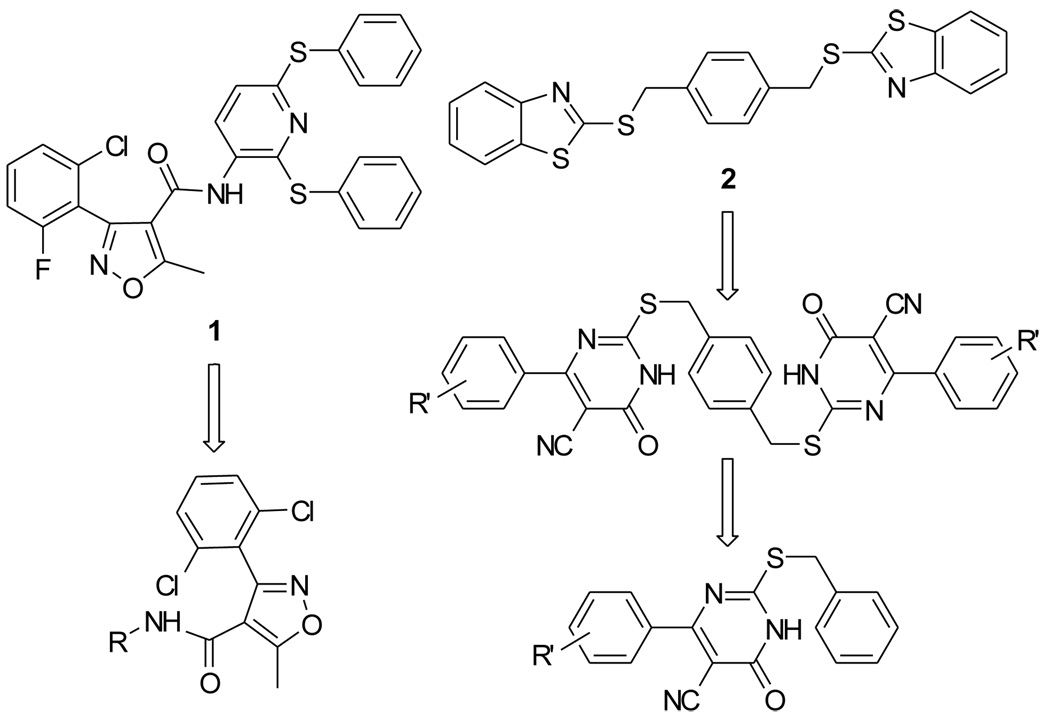
In our earlier virtual screening efforts, two hits, 1 (SEW-05929) and 2 (HTS-12302), were shown to have modest SecA inhibitory activities (IC50 values of about 100 µM).10, 13 Since there were no other known SecA inhibitors except one natural product, for which the true inhibition mechanism was not known,14 our effort to search for potent SecA inhibitors started with the optimization of these two modest inhibitors (Figure 1).
Figure 1.
Two hit compounds and their derivatives
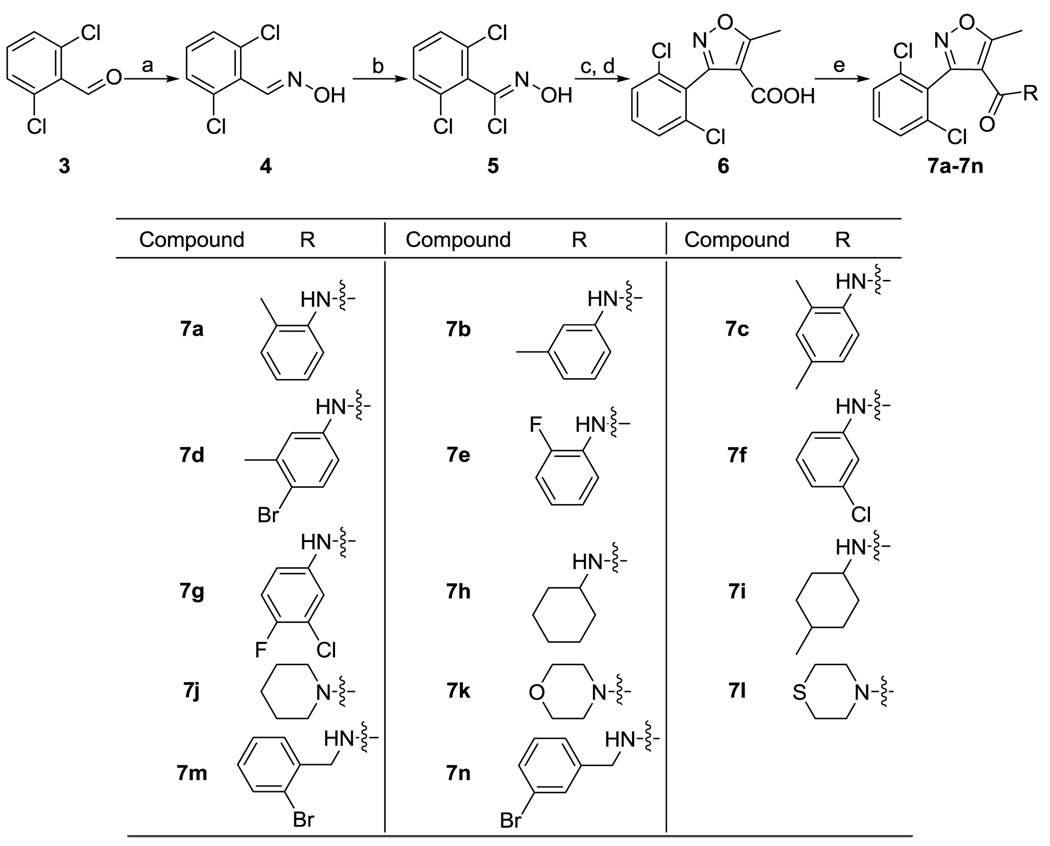
Our optimization effort first started with the isoxazole carboxamide series (1) with the focus being on optimizing the aryl group attached to the amide. In this series, 14 analogs were synthesized. The synthesis started with conversion of halogenated benzaldehyde 3 to the corresponding oxime 4 (Scheme 1). Isoxazole acid 6 was prepared by reacting 5 with ethyl acetoacetate followed by hydrolysis.15 Subsequent coupling/amidation reactions using EDCI and DMAP gave the final isoxazole carboxamide derivatives 7a–7n. In this series, there were amides of aniline compounds 7a–g, primary alkylamines 7h,i, secondary alkyamines 7j–l, and benzylamines 7m,n.
Scheme 1.
Synthesis of isoxazole carboxamides 7a–n. Reagents and conditions: (a) HONH2·HCl, NaOH, EtOH, H2O, reflux; (b) NCS, DMF; (c) Ethyl acetoacetate, MeONa, THF; (d) NaOH, EtOH, H2O; (e) EDCI, HOBt, DMAP, DMF
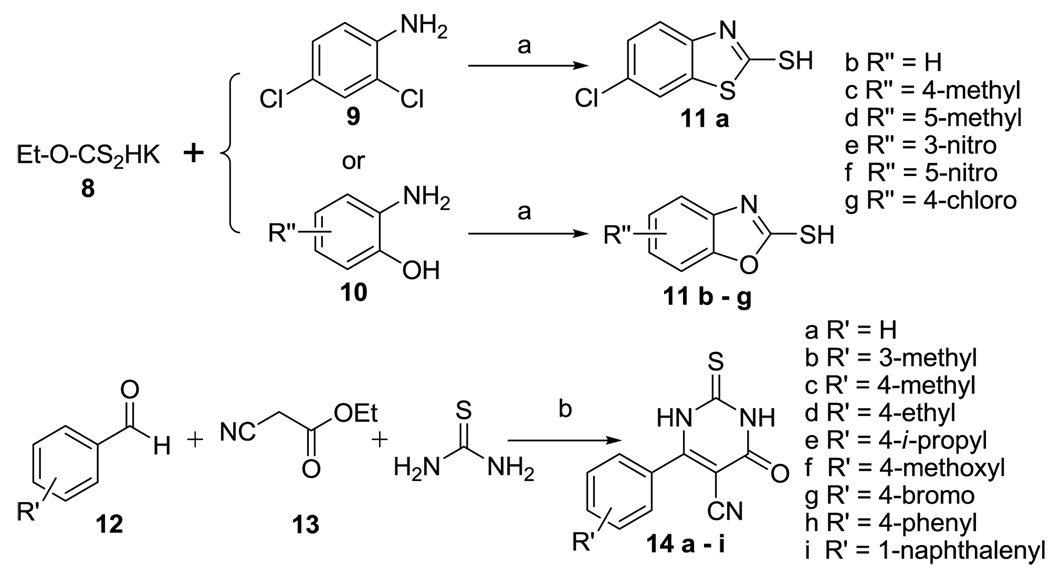
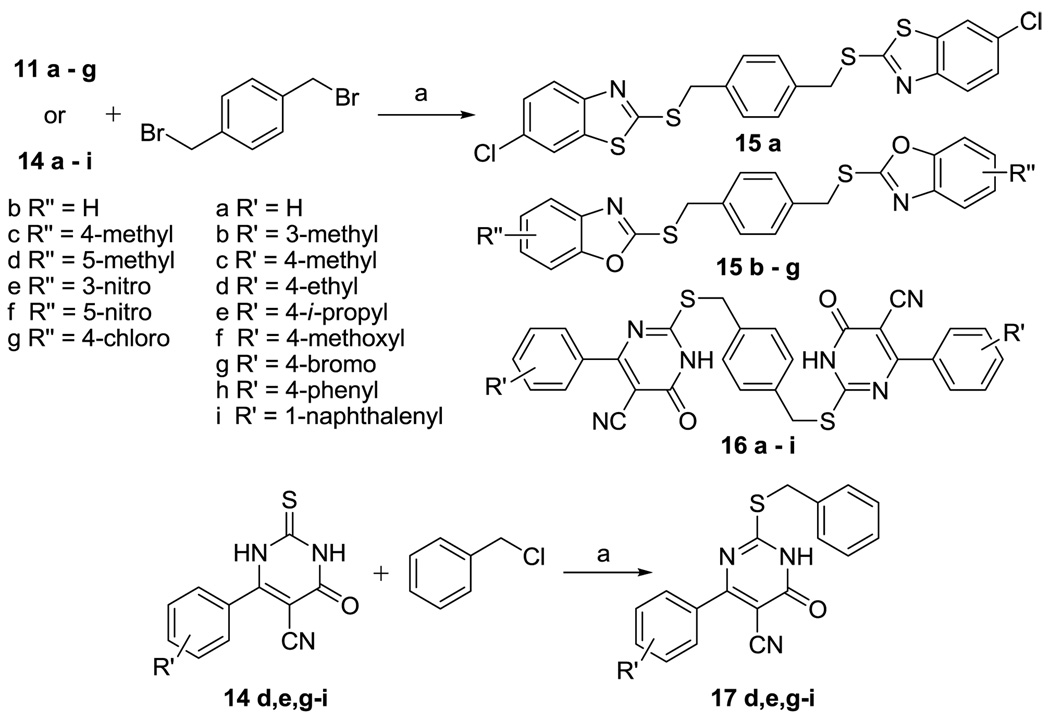
In optimizing the second series (2, Figure 1), we first started by testing different aryl structures flanking the central ring. In our initial effort, 6-chloro-2-mercaptobenzothiazole and 2-mercaptobenzoxazole derivatives were prepared by reacting potassium ethylxanthate 8 with 2,4-dichloroaniline 9 or substituted 2-aminophenol 10 (Scheme 2). Further, 5-cyano-6-aryl-2-thiouracils were prepared “by condensation of an aldehyde with ethyl cyanoacetate and thiourea in the presence of piperidine”.16 The symmetrical compounds 15a–g or 16a–i were obtained by reacting two equivalents of compounds 11a–g or 14a–i with p-xylylene dibromide in acetonitrile in the presence of K2CO3 (Scheme 3). One successful series of analogs was the 2,2'-(α,α’-xylene)bis(sulfanediyl)bis-(6-aryl-5-cyano-4-oxopyrimidine) 16a–i (see below for biological results). For this series, we were interested in further simplifying the structure to understand the core structural need. Therefore, "monomer" series 17d,e,g–i was prepared by benzylation of compounds 14d,e,g–i with benzyl chloride, and the difference in activities between the "dimer" and "monomer" series was also studied.
Scheme 2.
Synthesis of compounds 11a–g and 14a–i. Reagents and conditions: (a) EtOH, reflux; (b) piperidine, EtOH, reflux
Scheme 3.
Synthesis of compounds 15a–g, 16a–i and 17d,e,g–i. Reagents and conditions: (a) K2CO3, CH3CN, reflux
2.2. Biological evaluation
2.2.1. In vitro study
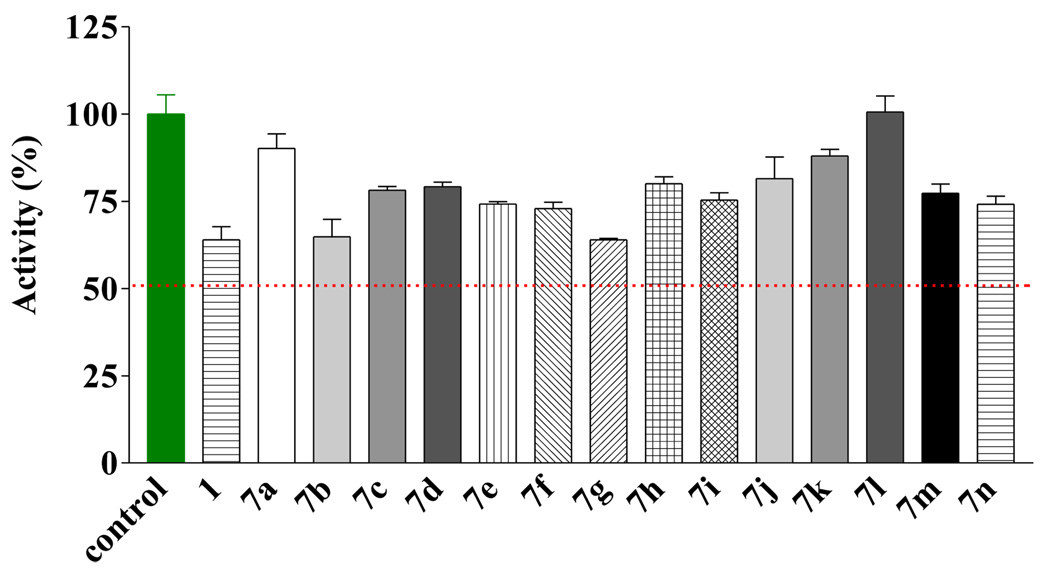
The synthesized compounds were first evaluated using EcN68 SecA, which is a truncated version without the C-terminal regulatory/inhibitory domain, by following procedures published earlier.10 Briefly, ATPase activities were determined by the release of phosphate (Pi), which can be detected spectrophotometrically using malachite green.14 For compounds 7a–n, none of them showed improved activities over the original hit (1) or significant inhibition at 100 µM (Figure 2). Such results coupled with the weak activities of the original hit compound led to the decision of not pursuing this class of compounds any further.
Figure 2.
Inhibitory effect of compounds 1 and 7a–n at 100 µM against EcN68 Sec A.
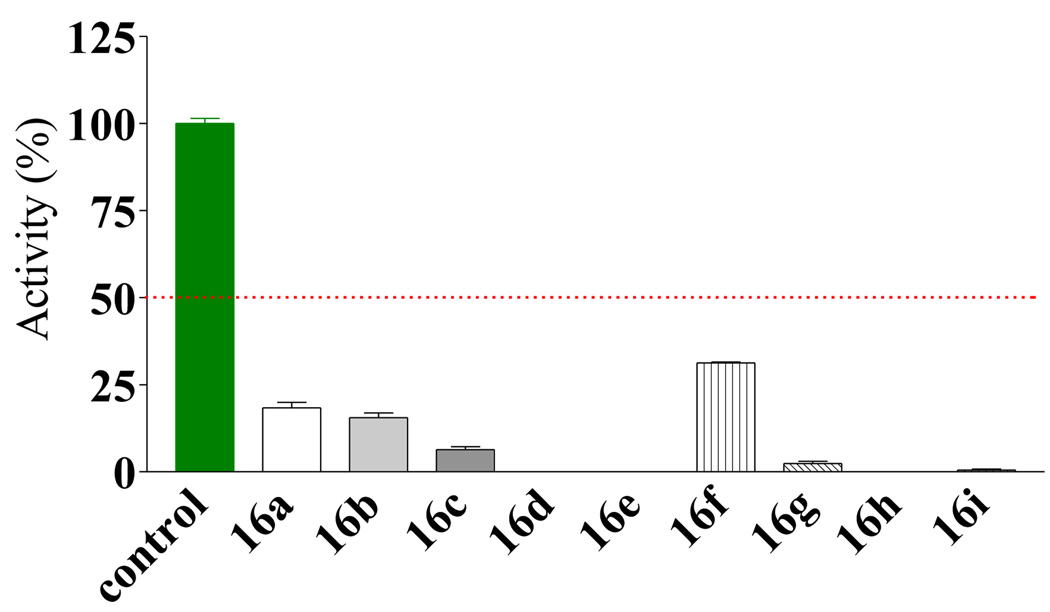
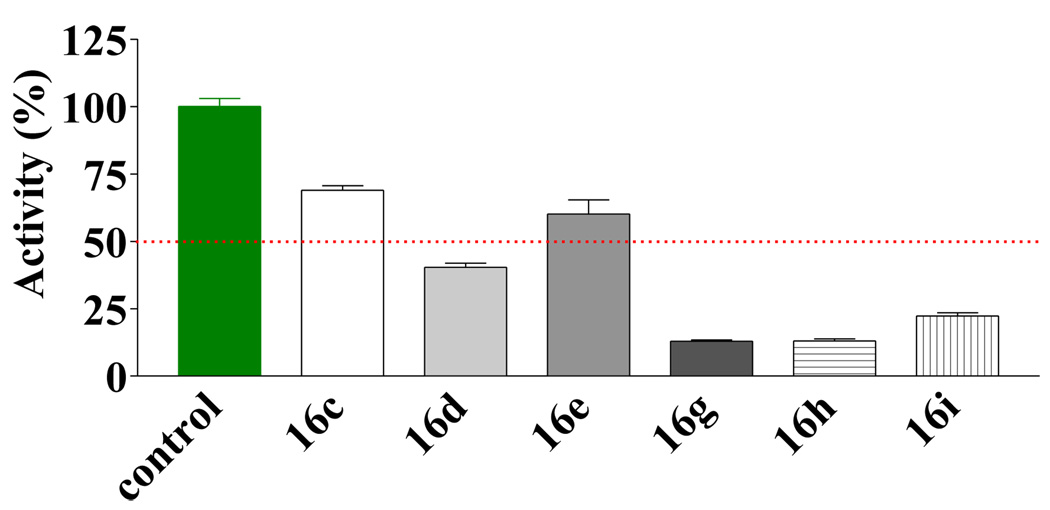
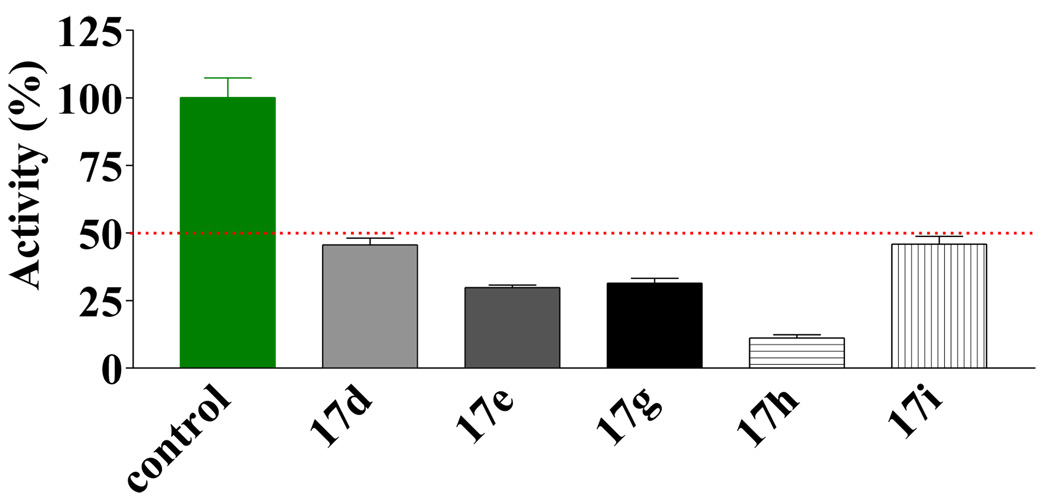
For analogs of 2, compounds 15a–g did not show significant improvement over the initial hit (data not shown). However, the substituted thiouracils (16) showed very significant activities when screened at 100 (data not shown) and 30 µM (Figure 3). Those compounds that showed potent inhibition at 30 µM were further screened at 5 µM (Figure 4). Within the symmetrical compound series 16a–i, there were two substitution patterns: one with a phenyl ring substituted at the 4-position and the other with a phenyl ring substituted at the 3-position. The results showed that the 4-substituted analogs were more potent than the 3-substituted class, which was in turn slightly more potent than the ones without phenyl substituent. For example the activities of the 4-methyl substituted (16c, Figure 3) was higher than the 3-methyl analog (16b, Figure 3), which was in turn higher than the unsubstituted one (16a, Figure 3). With the initial indication that derivatives with a phenyl ring bearing a 4-substituent were more active, the subsequent effort was focused on optimizing this series of compounds. One approach adopted was to use relatively bulky alkyl groups at the 4-position. It turned out that these compounds were more potent than the corresponding 4-methyl substituted compounds. Among these compounds, those with an electron donating (e.g., methoxy) substituent seemed to be less active than the unsubstituted ones (e.g., 16f < 16a, Figure 3). At 5 µM, analogs with a halogen or aryl group substitution at the 4-position were more potent than the analogues with an alkyl substitution (e.g., 16g,h,i > 16c,d,e Figure 4). For the examination of the difference between the “dimers” and “monomers”, S-benzyl-2-thiouracils analogues 17d, e, g–i were also tested (Figure 5). First of all, both thiouracil-based “dimer” and “monomer” compounds showed more potent inhibition than the benzothiazole or benzoxazole compounds 15a–g. However, the "dimer" series 16d,e,g–i were more potent than the "monomer" series 17d,e,g–i, respectively. This higher potency for the “dimer” series seems to come from better fitting of the binding pocket of these compounds (see below). In the “monomer” series, it was observed that a large sized R’ group seemed to confer high potency (e.g., 17h > 17g ≈ 17e > 17d). However, when the substituented phenyl ring was replaced by a larger 1-naphthyl group, the activity seemed to decrease slightly.
Figure 3.
Inhibitory effect of compounds 16a–i at 30 µM against EcN68 Sec A.
Figure 4.
Inhibitory effect of compounds 16c–e, g–i at 5 µM against EcN68 Sec A.
Figure 5.
Inhibitory effect of compounds 17d, e, g–i at 30 µM against EcN68 Sec A.
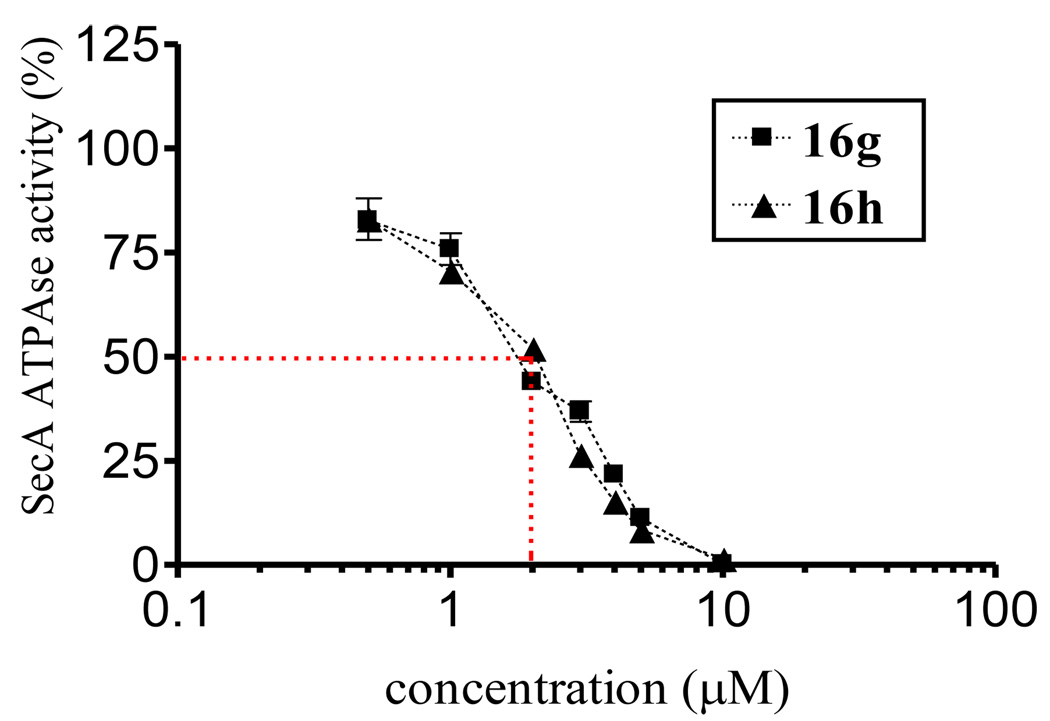
We determined the IC50 values of compound 16 g and 16 h since they showed the most potent activities of all the compounds screened at 5 µM. The result showed they had low micro molar inhibition (IC50: 2 µM, Figure 6), which is 50-fold more potent than the hit compound 2 (IC50: 100 µM).10
Figure 6.
The inhibitory curves of the two most potent compounds, 16g and 16h, against EcN68 Sec A.
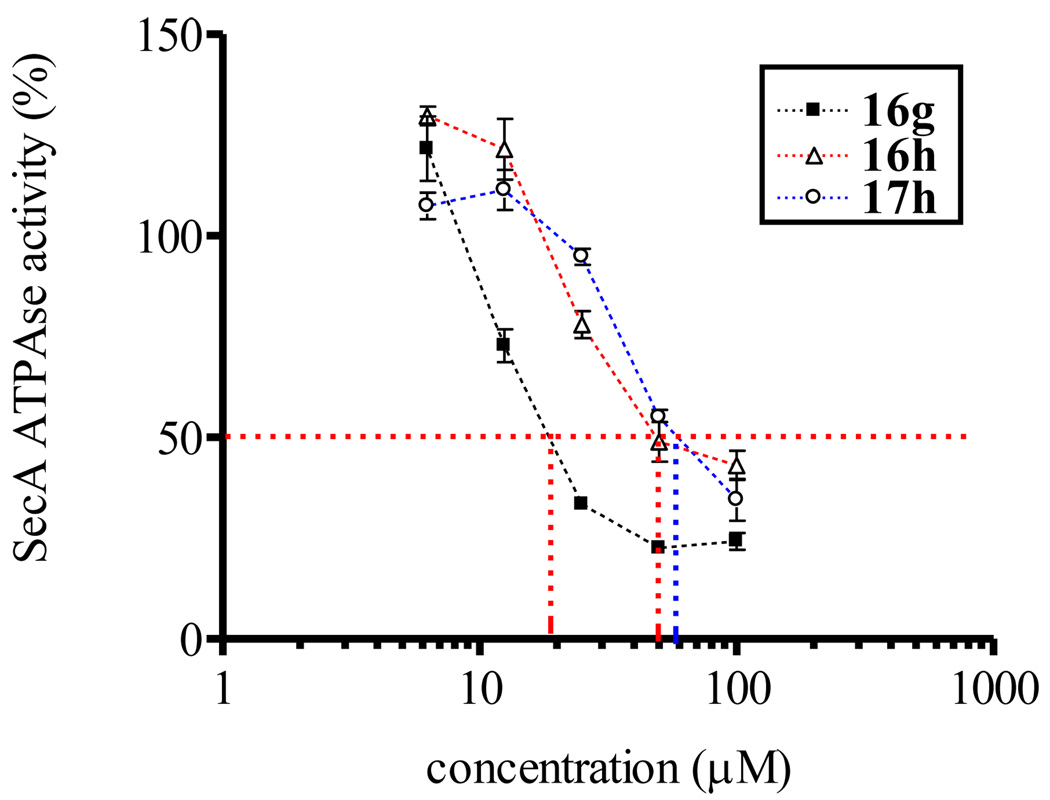
Inhibition tests using whole EcSecA gave similar results (16g IC50: 20 µM, 16h IC50: 50 µM and 17h IC50: 60 µM, Figure 7), which suggested that the EcN68 inhibition assay was more sensitive than the whole SecA inhibition assay. This is understandable since EcSecA contains a regulatory domain, which is essentially an inhibitor.
Figure 7.
The inhibitory curves of 16g,h and 17h against EcSecA.
2.2.2. In vivo study
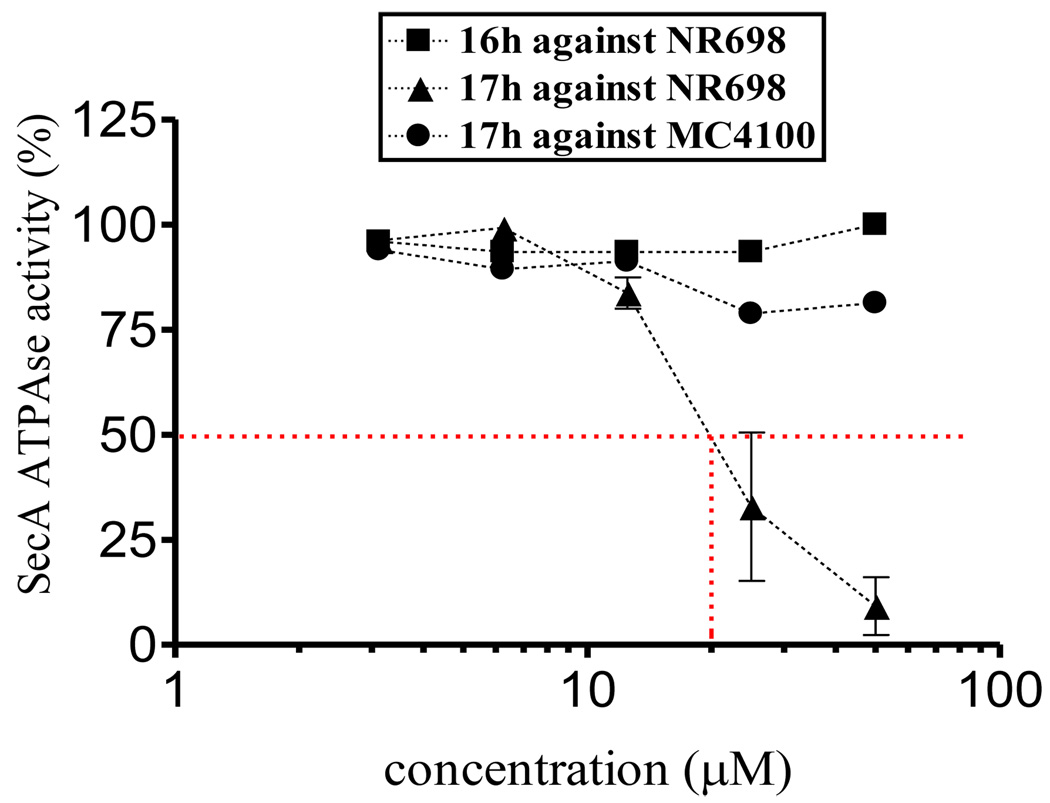
The biological activities of “dimer” and “monomer” compounds 16h and 17h were assessed against leaky mutant NR698 and wild type E. coli strain MC4100 by determining the minimum inhibition concentration (MIC) (Figure 8). “Monomer” compound 17h exhibited the most potent inhibition effects against NR698, whereas “dimer” compounds 16h did not exhibit significantly antimicrobial activities. However, neither 17h nor 16h exhibited inhibition effects against wild type E. coli strain MC4100. Such results suggested that the permeability of 16h against NR698 and 17h against MC4100 might be a key factor and for in vivo applications future studies should focus on low molecular weight compounds such as 17h for structural optimization.
Figure 8.
The inhibitory curves of 16g,h and 17h against EcSecA.
2.3. Computational modeling
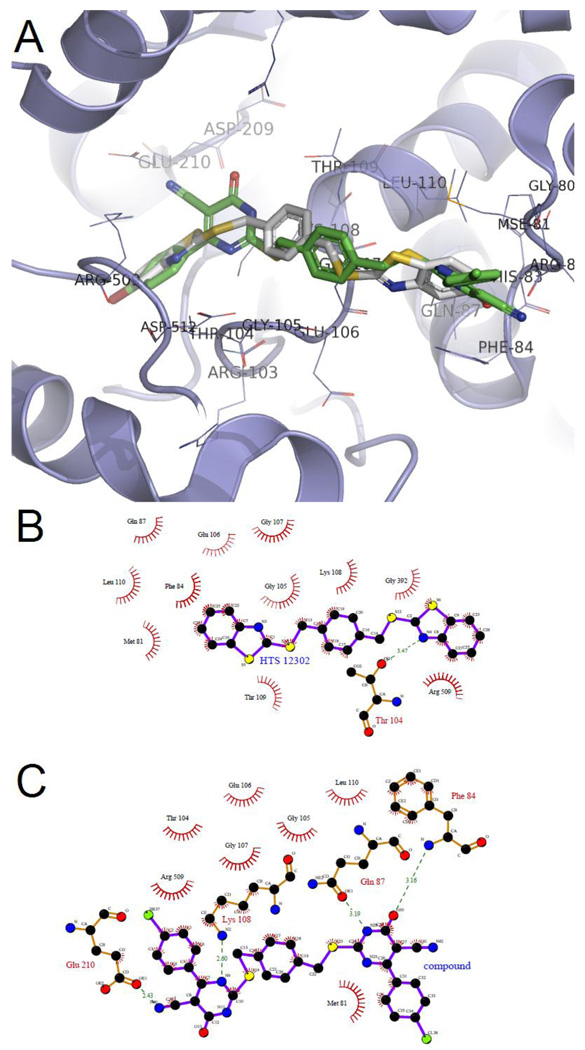
In order to achieve a detailed understanding the binding mode between SecA and our compounds, in silico modeling was conducted by using molecular simulation.17–20 Herein, the parent compound, HTS-12302 and the most active compound, 16g, were docked into the ATP site of SecA using DOCK 5.4. The docked complexes were then optimized by molecular mechanics and molecular dynamics simulation implemented in AMBER 8. Finally, the possible ligand-protein interactions were examined by following similar procedures we used in previous studies.10 After molecular simulation, compound HTS-12302 seems to bind SecA through interactions with Thr 104 by forming hydrogen bond and with Met 81, Phe 84, Gln 87, Gly 105, Glu 106, Gly 107, Lys 108, Thr 109, Leu 110, Gly 392 and Arg 509 through hydrophobic interactions (Figure 9). Compound 16g has a similar binding conformation and orientation, in which it seems to engage in more hydrogen bond interactions with Phe 84, Gln 87, Lys 108 and Glu 210. Moreover, compound 16g still bears hydrophobic interactions with Met 81, Thr 104, Gly 105, Glu 106, Gly 107, Leu 110 and Arg 509. Upon analysis of the structural features of these two compounds, it seems that the inclusion of the thiouracil moiety may contribute to the inhibitory activity because of more hydrophobic interaction and hydrogen bonds when compared with lead compound HTS-12302. Such structural insights will play a very critical role in future design of potent SecA inhibitors and in further structural optimizations.
Figure 9.
(A) The proposed docking conformation of HTS-12302 (white sticks) and compound 16g (green sticks) around SecA ATP-site; (B) The proposed schematic interactions of HTS-12302 with SecA; (C) The proposed schematic interactions of compound 16g with SecA
2.4. Conclusion
Through optimization of two hit compounds 1 (SEW-05929) and 2 (HTS-12302) identified from virtual screening, we have found a series of thiouracil derivatives that are much more potent than the primary hits. The two most potent compounds, 16g and 16h, are 50-fold more active than the hit compounds. Results of antimicrobial tests suggest that future work should focus on low molecular weight analogs of 17h for in vivo applications. These compounds are the first in its class and should be very useful as research tools in studying bacterial protein transport. The new inhibitory structural features identified should also be very useful for further structural optimization in search of even more potent inhibitors as potential antimicrobial agents.
3. Experimental
3.1. Chemistry
General Chemical Methods
All reagents and solvents were reagent grade or were purified by standard methods before use. Column chromatography was carried out on flash silica gel (Sorbent 230–400 mesh). TLC analysis was conducted on silica gel plates (Sorbent Silica G UV254). NMR spectra were recorded at 1H (400 MHz) and 13C (100 MHz) with a Bruker instrument. Chemical shifts (δ values) and coupling constants (J values) are given in ppm and Hz, respectively, using TMS (1H NMR) and solvents (13C NMR) as internal standards.
General procedure for the preparation of isoxazole carboxamide derivatives (7a–7n)
Under N2 atmosphere, a solution of an isoxazole carboxylic acid (6, 0.1 mmol), amine (0.12 mmol), EDCI (23 mg, 0.12 mmol), DMAP (14.7 mg, 0.12 mmol) and HOBt (27 mg, 0.2 mmol) in DMF (2.5 mL) was stirred at room temperature overnight. Then most of the solvent was removed under reduced pressure. To the residue was added 10 mL H2O and 10 mL EtOAc. Then the aqueous solution was extract by EtOAc (20 mL × 2). The organic layer was subsequently washed with brine (20 mL). The crude compound was purified by flash chromatography on silica gel using hexane and EtOAc (9:1) as the mobile phase to give 7a–7n.
3-(2,6-Dichlorophenyl)-5-methyl-N-m-tolylisoxazole-4-carboxamide (7a)
Yield 76%; 1H NMR (CDCl3) δ 1.70 (s, 3H), 2.86 (s, 3H), 6.74 (bs, 1H), 7.00–7.07 (m, 2H), 7.18 (td, 1H, J = 1.6 Hz, 7.2 Hz), 7.44 (dd, 1H, J = 6.4 Hz, 9.6 Hz), 7.50 (m, 2H), 7.90 (d, 1H, J = 8.0 Hz); 13C NMR (CDCl3) δ 13.7, 16.9, 112.0, 123.0, 125.4, 127.0, 127.3, 128.3, 129.1, 130.6, 132.7, 135.3, 136.6, 155.7, 159.0, 176.4. HRMS-ESI (+): Calc. for C18H15N2O2Cl2: 361.0511. Found: 361.0527 [M+H]+.
N-(3-Bromobenzyl)-3-(2,6-dichlorophenyl)isoxazole-4-carboxamide (7b)
Yield 71%; 1H NMR (CDCl3) δ 2.27 (s, 3H), 2.84 (s, 3H), 6.85 (m, 3H), 7.11 (m, 2H), 7.48 (dd, 1H, J = 6.4 Hz, 9.6 Hz), 7.54 (m, 2H); 13C NMR (CDCl3) δ 13.5, 21.7, 112.1, 117.1, 120.8, 125.8, 127.3, 129.0, 129.0, 132.7, 136.4, 137.2, 139.3, 155.9, 158.9, 175.7. HRMS-ESI (+): Calc. for C18H15N2O2Cl2: 361.0511. Found: 361.0516 [M+H]+.
(3-(2,6-Dichlorophenyl)-5-methylisoxazol-4-yl)(morpholino)methanone (7c)
Yield 81%; 1H NMR (CDCl3) δ 1.63 (s, 3H), 2.18 (s, 3H), 2.80 (s, 3H), 6.62 (bs, 1H), 6.82 (s, 1H), 6.92 (d, 1H, J = 8.4 Hz), 7.37 (dd, 1H, J = 6.4 Hz, 9.6 Hz), 7.44 (m, 2H), 7.66 (d, 1H, J = 8.4 Hz); 13C NMR (CDCl3) δ 13.7, 16.9, 21.0, 112.0, 123.2, 127.4, 127.5, 128.6, 129.0, 131.2, 132.7, 135.3, 136.6, 155.7, 159.0, 176.2. HRMS-ESI (+): Calc. for C19H17N2O2Cl2: 375.0667. Found: 375.0679 [M+H]+.
(3-(2,6-Dichlorophenyl)-5-methylisoxazol-4-yl)(piperidin-1-yl)methanone (7d)
Yield 67%; 1H NMR (CDCl3) δ 2.24 (s, 3H), 2.78 (s, 3H), 6.70 (dd, 1H, J = 2.4 Hz, 8.4 Hz), 6.75 (bs, 1H), 7.12 (d, 1H, J = 2.4 Hz), 7.29 (d, 1H, 8.8 Hz), 7.43 (dd, 1H, J = 6.4 Hz, 9.6 Hz), 7.49 (m, 2H), 7.62 (m, 5H); 13C NMR (CDCl3) δ 13.6, 23.3, 111.9, 118.8, 120.1, 122.2, 127.2, 129.0, 132.8, 132.8, 136.3, 136.5, 139.0, 155.8, 158.8, 175.9. HRMS-ESI (+): Calc. for C18H14N2O2Cl2Br: 438.9616. Found: 438.9633 [M+H]+.
3-(2,6-Dichlorophenyl)-N-(2,4-dimethylphenyl)-5-methylisoxazole-4-carboxamide (7e)
Yield 55%; 1H NMR (CDCl3) δ 2.87 (s, 3H), 6.92–6.99 (m, 2H), 7.08 (t, 1H, J = 7.6 Hz), 7.20 (bs, 1H), 7.47 (dd, 1H, J = 6.4 Hz, 9.6Hz), 7.52 (m, 2H), 8.33 (td, 1H, J = 1.6 Hz, 8.0 Hz); 13C NMR (CDCl3) δ 13.7, 111.8, 114.7, 114.9, 121.6, 124.7, 124.8, 124.8, 124.8, 126.3, 129.1, 132.8, 136.3, 153.4, 155.9, 158.8, 176.4. HRMS-ESI (+): Calc. for C17H12N2O2Cl2F: 365.0260. Found: 365.0269 [M+H]+.
N-(3-Chlorophenyl)-3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carboxamide (7f)
Yield 42%; 1H NMR (CDCl3) δ 2.79 (s, 3H), 6.81 (bs, 1H), 6.84 (m, 1H), 6.98 (m, 1H), 7.09 (t, 1H, J = 8.0 Hz), 7.30 (t, 1H, J = 1.6 Hz), 7.45(dd, 1H, J = 6.4 Hz, 9.6 Hz), 7.50 (m, 2H); 13C NMR (CDCl3) δ 90.2, 113.8, 128.2, 128.6, 132.0, 158.2, 160.2, 176.0. HRMS-ESI (+): Calc. for C17H12N2O2Cl3: 380.9964. Found: 380.9962 [M+H]+.
N-Cyclohexyl-3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carboxamide (7g)
Yield 59%; 1H NMR (CDCl3) δ 2.83 (s, 3H), 6.83 (bs, 1H), 6.87 (m, 1H), 6.98 (t, 1H, J = 8.8 Hz), 7.42 (dd, 1H, J = 2.8 Hz, 6.8 Hz), 7.50 (dd, 1H, J = 6.4 Hz, 9.6 Hz), 7.55 (m, 2H); 13C NMR (CDCl3) δ 13.6, 111.7, 116.8, 117.0, 119.6, 119.7, 121.4, 121.6, 122.4, 127.1, 129.1, 132.9, 133.8, 136.3, 154.0, 155.7, 156.4, 158.9, 176.1. HRMS-ESI (+): Calc. for C17H11N2O2FCl3: 398.9870. Found: 398.9885 [M+H]+.
3-(2,6-Dichlorophenyl)-5-methyl-N-o-tolylisoxazole-4-carboxamide (7h)
Yield 79%; 1H NMR (CDCl3) δ 0.83 (m, 2H), 1.04 (m, 1H), 1.19–1.32 (m, 4H), 1.39 (m, 1H), 2.02 (m, 2H), 2.73 (s, 3H), 3.74 (m, 1H), 5.02 (m, 1H), 7.39 (dd, 1H, J = 6.4 Hz, 9.6 Hz), 7.45 (m, 2H); 13C NMR (CDCl3) δ 13.3, 24.1, 25.5, 32.5, 47.4, 112.0, 127.8, 128.7, 132.4, 136.3, 156.1, 159.9, 174.7. HRMS-ESI (+): Calc. for C17H19N2O2Cl2: 353.0824. Found: 353.0838 [M+H]+.
N-(4-Bromo-3-methylphenyl)-3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carboxamide (7i)
Yield 82%; 1H NMR (CDCl3) δ 0.28 (m, 2H), 0.65–0.70 (m, 4H), 0.81 (d, 2H, J = 6.8 Hz), 0.94 (m, 2H), 1.16–1.25 (m, 2H), 1.31–1.44 (m, 4H), 1.49–1.58 (m, 4H), 1.75 (m, 2H), 3.63 (m, 1H), 4.10 (m, 1H), 7.39–7.51 (m, 5H); 13C NMR (CDCl3) δ13.2, 13.4, 22.2, 22.3, 29.6, 29.8, 31.5, 31.9, 32.9, 33.6, 44.3, 48.3, 111.8, 112.0, 127.7, 127.9, 128.7, 128.9, 132.3, 132.6, 136.2, 136.4, 156.0, 156.2, 159.8, 160.0, 174.5, 175.3. HRMS-ESI (+): Calc. for C18H21N2O2Cl2: 367.0980. Found: 367.0991 [M+H]+.
(3-(2,6-Dichlorophenyl)-5-methylisoxazol-4-yl)(thiomorpholino)methanone (7j)
Yield 85%; 1H NMR (CDCl3) δ 1.34 (bs, 4H), 1.52 (m, 2H), 2.56 (s, 3H), 3.38 (bs, 4H), 7.32 (m, 1H), 7.40 (m, 2H); 13C NMR (CDCl3) δ 12.4, 24.4, 25.9, 113.6, 127.7, 128.4, 131.5, 135.9, 157.5, 161.6, 169.6. HRMS-ESI (+): Calc. for C16H17N2O2Cl2: 339.0667. Found: 339.0668[M+H]+.
3-(2,6-Dichlorophenyl)-N-(2-fluorophenyl)-5-methylisoxazole-4-carboxamide (7k)
Yield 84%; 1H NMR (CDCl3) δ 2.52 (s, 3H), 3.36 (bs, 8H), 7.30 (dd, 1H, J = 6.4 Hz, 9.6Hz), 7.37 (m, 2H); 13C NMR (CDCl3) δ 12.6, 66.7, 112.8, 127.4, 128.6, 131.8, 135.9, 157.3, 161.9, 170.4. HRMS-ESI (+): Calc. for C15H15N2O3Cl2: 341.0460. Found: 341.0466 [M+H]+.
N-(3-Chloro-4-fluorophenyl)-3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carboxamide (7l)
Yield 72%; 1H NMR (CDCl3) δ 2.30 (bs, 4H), 2.52 (s, 3H), 3.62 (bs, 4H), 7.30 (dd, 1H, J = 6.4 Hz, 9.6Hz), 7.37 (m, 2H); 13C NMR (CDCl3) δ 12.6, 27.8, 113.0, 127.4, 128.6, 131.9, 135.9, 157.2, 162.2, 170.4. HRMS-ESI (+): Calc. for C15H15N2O2SCl2: 357.0231. Found: 357.0237 [M+H]+.
N-(2-Bromobenzyl)-3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carboxamide (7m)
Yield 73%; 1H NMR (CDCl3) δ 2.73 (s, 3H), 4.37 (d, 2H, J = 6.0 Hz), 5.62 (bs, 1H), 7.04 (m, 1H), 7.16 (m, 2H), 7.26–7.33 (m, 3H), 7.38 (d, 1H, J = 8.0 Hz); 13C NMR (CDCl3) δ 13.4, 43.9, 111.6, 123.9, 127.3, 127.9, 128.8, 129.5, 130.6, 132.3, 132.8, 136.2, 136.9, 156.1, 160.6, 175.2. HRMS-ESI (+): Calc. for C18H14N2O2Cl2Br: 438.9616. Found: 438.9627 [M+H]+.
3-(2,6-Dichlorophenyl)-5-methyl-N-(4-methylcyclohexyl)isoxazole-4-carboxamide (mixture of cis & trans) (7n)
Yield 93%; 1H NMR (CDCl3) δ 2.74 (s, 3H), 4.28 (d, 2H, J = 5.6 Hz), 5.27 (bs, 1H), 6.91 (d, 1H, J = 7.2 Hz), 7.04 (m, 2H), 7.29 (d, 2H, J = 7.6 Hz), 7.34 (d, 2H, J = 7.6 Hz); 13C NMR (CDCl3) δ 13.4, 42.9, 111.5, 122.9, 126.2, 127.3, 128.8, 130.2, 130.4, 132.5, 136.0, 139.8, 156.1, 160.7, 175.2. HRMS-ESI (+): Calc. for C18H14N2O2Cl2Br: 438.9616. Found: 438.9635 [M+H]+.
General procedures for the preparation of and 2-mercaptobenzoxazole (11b – 11g)
To a solution of a substituted 2-aminophenol (3 mmol) was added potassium ethylxanthate (484 mg, 3 mmol) in absolute ethanol (10 mL). The resulting mixture was heated under reflux overnight and then cooled to room temperature. The precipitate was dissolved in H2O (10 mL) and washed with ethyl acetate (10 mL × 3) and the aqueous solution was then neutralized to pH = 5 by slow addition of glacial acetic acid. Then the product precipitated (crystallized) out to give 11b – 11g.
2-Mercaptobenzoxazole (11b)
Yield 52%; 1H NMR (DMSO-d6) δ 7.29 (m, 3H), 7.53 (d, 1H, J = 7.6 Hz), 13.90 (bs, 1H); 13C NMR (DMSO-d6) δ 110.0, 110.5, 123.7, 125.1, 131.2, 148.1, 180.1. HRMS-ESI (+): Calc. for C7H6NOS: 152.0170. Found: 152.0170 [M+H]+.
2-Mercapto-5-methylbenzoxazole (11c)
Yield 45%; 1H NMR (DMSO-d6) δ 2.37 (s, 3H), 7.05 (m, 2H), 7.34 (m, 1H), 13.73 (bs, 1H); 13C NMR (DMSO-d6) δ 20.8, 109.4, 110.4, 124.2, 131.2, 134.7, 146.3, 180.2. HRMS-ESI (+): Calc. for C8H8NOS: 166.0327. Found: 166.0333 [M+H]+.
2-Mercapto-6-methylbenzoxazole (11d)
Yield 64%; 1H NMR (DMSO-d6) δ 2.39 (s, 3H), 7.10 (d, 1H, J = 7.6 Hz), 7.16 (t, 1H, J = 7.6 Hz), 7.31 (d, 1H, J = 7.6 Hz), 13.95 (bs, 1H); 13C NMR (DMSO-d6) δ 16.1, 107.2, 121.1, 123.6, 126.0, 130.4, 147.9, 180.1. HRMS-ESI (+): Calc. for C8H8NOS: 166.0327. Found: 166.0333 [M+H]+.
2-Mercapto-3-nitrobenzoxazole (11e)
Yield 14%; 1H NMR (DMSO-d6) δ 7.44 (t, 1H, J = 8.0Hz), 7.91 (d, 1H, J = 8.0 Hz), 8.06 (d, 1H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ 115.8, 119.8, 123.4, 128.0, 131.4, 149.8, 181.7. HRMS-ESI (+): Calc. for C7H5N2O3S: 197.0021. Found: 197.0013 [M+H]+.
2-Mercapto-5-nitrobenzoxazole (11f)
Yield 25%; 1H NMR (DMSO-d6) δ 7.17 (d, 1H, J = 8.8 Hz), 7.95 (d, 1H, J = 2.4 Hz), 7.99 (dd, 1H, J = 2.4 Hz, 8.8 Hz); 13C NMR (DMSO-d6) δ 101.8, 112.3, 119.8, 139.7, 150.3, 153.2, 188.4. HRMS-ESI (+): Calc. for C7H5N2O3S: 197.0021. Found: 197.0018 [M+H]+.
2-Mercapto-5-chlorobenzoxazole (11g)
Yield 36%; 1H NMR (DMSO-d6) δ 7.30 (d, 2H, J = 7.6 Hz), 7.53 (d, 1H, J = 8.0 Hz), 14.04 (bs, 1H); 13C NMR (DMSO-d6) δ 110.3, 111.2, 123.5, 129.3, 132.6, 147.0, 180.8. HRMS-ESI (+): Calc. for C7H5NOSCl: 185.9780. Found: 185.9789 [M+H]+.
General procedures for the preparation of 2-thiouracils 14a – 14i
To a solution of an aldehyde (RCHO, 10 mmol), ethyl cyanoacetate (1.0 mL, 10 mmol), and thiourea (0.76 g, 10 mmol) in absolute ethanol (50 mL) was added piperidine (2.0 mL, 20 mmol); the mixture was heated under reflux overnight and then cooled to room temperature. The precipitate was dissolved in 0.5M NaOH (20 mL) and washed with ethyl acetate (10 mL × 3). The aqueous solution was then neutralized to pH = 2 by slow addition of 1M HCl. Then the product precipitated (crystallized) out to give 14a – 14i.
5-Cyano-6-phenyl-2-thiouracil (14a)
Yield 67%; 1H NMR (DMSO-d6) δ 7.62 (m, 5H), 13.19 (s, 1H), 13.32 (bs, 1H); 13C NMR (DMSO-d6) δ 90.2, 113.8, 128.2, 128.6, 132.0, 158.2, 160.2, 176.0. MS-ESI (+): 252.0 [M+Na]+.
5-Cyano-6-(3-tolyl)-2-thiouracil (14b)
Yield 31%; 1H NMR (DMSO-d6) δ 2.39 (s, 3H), 7.46 (m, 4H), 13.17 (s, 1H), 13.26 (bs, 1H); 13C NMR (DMSO-d6) δ 20.9, 90.6, 114.7, 125.9, 128.4, 129.1, 129.2, 132.8, 137.9, 158.5, 160.9, 176.2. HRMS-ESI (+): Calc. for C12H10N3OS: 244.0545. Found: 244.0555 [M+H]+.
5-Cyano-6-(4-tolyl)-2-thiouracil (14c)
Yield 43%; 1H NMR (DMSO-d6) δ 2.45 (s, 3H), 7.41 (d, 2H, J = 7.6 Hz), 7.61 (d, 2H, J = 8.4 Hz); 13C NMR (DMSO-d6) δ 12.2, 81.7, 105.9, 118.4, 120.1, 121.2, 135.3, 150.9, 153.2, 168.2. HRMS-ESI (+): Calc. for C13H12N3OS: 258.0701. Found: 258.0702 [M+H]+.
5-Cyano-6-(4-ethylphenyl)-2-thiouracil (14d)
Yield 25%; 1H NMR (DMSO-d6) δ 1.22 (t, 3H, J = 7.6 Hz), 2.71 (q, 2H, J = 7.6 Hz), 7.42 (d, 2H, J = 8.0 Hz), 7.61 (d, 2H, J = 8.0 Hz), 13.15 (bs, 1H); 13C NMR (DMSO-d6) δ 15.2, 28.1, 90.4, 114.8, 126.6, 127.9, 128.9, 148.6, 158.5, 160.9, 176.2. HRMS-ESI (+): Calc. for C14H14N3OS: 272.0858. Found: 272.0867 [M+H]+.
5-Cyano-6-(4-isopropylphenyl)-2-thiouracil (14e)
Yield 37%; 1H NMR (DMSO-d6) δ 1.24 (d, 6H, J = 6.8 Hz), 3.00 (septet, 1H, J = 6.8 Hz), 7.46 (d, 2H, J = 8.0 Hz), 7.62 (d, 2H, J = 8.0 Hz), 13.15 (bs, 2H); 13C NMR (DMSO-d6) δ 23.6, 33.5, 90.3, 114.9, 126.5, 126.7, 129.0, 153.1, 158.6, 160.8, 176.2. HRMS-ESI (+): Calc. for C14H14N3OS: 272.0858. Found: 272.0867 [M+H]+.
5-Cyano-6-(4-methoxyphenyl)-2-thiouracil (14f)
Yield 21%; 1H NMR (DMSO-d6) δ 3.86 (s, 3H), 7.12 (d, 2H, J = 8.8 Hz), 7.68 (d, 2H, J = 8.8 Hz), 13.13 (bs, 2H); 13C NMR (DMSO-d6) δ 55.7, 89.9, 114.0, 115.2, 121.1, 131.0, 158.8, 160.6, 162.5, 176.3. HRMS-ESI (+): Calc. for C12H10N3O2S: 260.0494. Found: 260.0496 [M+H]+.
5-Cyano-6-(4-bromophenyl)-2-thiouracil (14g)
Yield 39%; 1H NMR (DMSO-d6) δ 7.63 (d, 2H, J = 8.4 Hz), 7.80 (d, 2H, J = 8.4 Hz), 13.21 (s, 1H), 13.37 (bs, 1H); 13C NMR (DMSO-d6) δ 91.1, 114.6, 125.9, 128.5, 130.9, 131.6, 158.4, 160.0, 176.2. HRMS-ESI (+): Calc. for C11H7N3OSBr: 307.9493. Found: 307.9504 [M+H]+.
5-Cyano-6-(biphenyl-4-yl)-2-thiouracil (14h)
Yield 75%; 1H NMR (DMSO-d6) δ 7.45 (t, 1H, J = 7.2 Hz), 7.53 (t, 2H, J = 7.2 Hz), 7.78 (d, 4H, J = 8.4 Hz), 7.89 (d, 2H, J = 8.4Hz) 13.19 (s, 1H), 13.35 (bs, 1H); 13C NMR (DMSO-d6) δ 90.6, 114.8, 126.6, 127.0, 128.1, 128.4, 129.2, 129.6, 138.7, 143.7, 158.5, 160.5, 176.2. HRMS-ESI (+): Calc. for C17H12N3OS: 306.0701. Found: 306.0714 [M+H]+.
5-Cyano-6-(1-naphthyl)-2-thiouracil (14i)
Yield 22%; 1H NMR (DMSO-d6) δ 7.64 (m, 3H), 7.74 (d, 1H, J = 6.8 Hz), 7.99 (dd, 1H, J = 6.0 Hz, 6.4 Hz), 8.06 (dd, 1H, J = 6.0 Hz, 6.4 Hz, ), 8.15 (d, 1H, J = 8.4 Hz), 13.11 (s, 1H), 13.46 (bs, 1H); 13C NMR (DMSO-d6) δ 93.1, 114.5, 124.7, 125.2, 126.8, 127.2, 127.5, 128.3, 128.5, 129.5, 131.2, 132.8, 158.7, 161.1, 176.8. HRMS-ESI (+): Calc. for C15H10N3OS: 280.0545. Found: 280.0554 [M+H]+.
General procedure for the preparation of 2,2'-(α,α’-Xylene)bis(sulfanediyl)bisbenzothiazole (15a), 2,2'-(α,α’-Xylene)bis(sulfanediyl)bisbenzoxazole (15b–g), and 2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-4-oxopyrimidine (16a–i)
To a solution of the 2-mercaptobenzothiazole, 2-mercaptobenzoxazole, or 2-thiouracil derivatives (11a–g or 10a–h, 0.1 mmol) and α, α’-xylenedibromide (12 mg, 0.045 mmol) in acetonitrile (2.5 mL) was added K2CO3 (42 mg, 0.3 mmol). The mixture was heated under reflux overnight and then cooled to room temperature. The liquid was removed on a rotavapor and the residue was washed with 0.5M NaOH (20 mL). Then the white solid residue was dried in vacuum oven at 40 °C overnight to give 15a–15g or 16a–16h.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-chlorobenzothiazole) (15a)
Yield 84%; 1H NMR (DMSO-d6) δ 4.62 (s, 4H), 7.45 (s, 4H), 7.47 (dd, 2H, J = 2.0 Hz, 8.4 Hz), 7.84 (d, 2H, J = 8.4 Hz), 8.11 (d, 2H, J = 2.0 Hz); 13C NMR (DMSO-d6) δ 36.3, 120.8, 121.7, 126.2, 128.7, 135.4, 135.9, 151.0, 166.6. HRMS-ESI (+): Calc. for C22H15N2S4Cl2: 504.9495. Found: 504.9499 [M+H]+.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis(benzoxazole) (15b)
Yield 35%; 1H NMR (DMSO-d6) δ 4.60 (s, 4H), 7.34 (m, 4H), 7.49 (m, 4H), 7.65 (m, 4H); 13C NMR (DMSO-d6) δ 35.1, 110.2, 118.3, 124.3, 124.6, 129.2, 136.1, 141.2, 151.3, 163.8. HRMS-ESI (+): Calc. for C22H17N2O2S2: 405.0731. Found: 405.0732[M+H]+.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(5-methylbenzoxazole) (15c)
Yield 57%; 1H NMR (DMSO-d6) δ 2.40 (s, 6H), 4.57 (s, 4H), 7.12 (d, 2H, J = 8.0 Hz), 7.46 (m, 8H); 13C NMR (DMSO-d6) δ 20.9, 35.1, 109.6, 118.3, 125.1, 129.2, 134.0, 136.2, 141.4, 149.5, 163.6. HRMS-ESI (+): Calc. for C24H21N2O2S2: 433.1044. Found: 433.1041 [M+H]+.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-methylbenzoxazole) (15d)
Yield 86%; 1H NMR (DMSO-d6) δ 2.49 (s, 6H), 4.59 (s, 4H), 7.18 (m, 4H), 7.43 (d, 2H, J = 7.6 Hz), 7.49 (s, 4H); 13C NMR (DMSO-d6) δ 16.0, 35.2, 107.5, 124.0, 125.1, 128.3, 129.3, 136.2, 140.4, 151.0, 162.6. HRMS-ESI (+): Calc. for C24H21N2O2S2: 433.1044. Found: 433.1050 [M+H]+.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(4-nitrobenzoxazole) (15e)
Yield 61%; 1H NMR (DMSO-d6) δ 4.66 (s, 4H), 7.50 (t, 4H, J = 8.0 Hz), 7.52 (s, 4H), 8.01 (d, 2H, J = 8.0 Hz), 8.08 (d, 2H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ 35.3, 115.6, 119.8, 123.5, 128.8, 135.4, 136.9, 152.6, 168.1. HRMS-ESI (+): Calc. for C22H15N4O6S2: 495.0433. Found: 495.0438 [M+H]+.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-nitrobenzoxazole) (15f)
Yield 81%; 1H NMR (DMSO-d6) δ 4.67 (s, 4H), 7.53 (s, 4H), 7.85 (d, 2H, J = 8.4 Hz), 8.27 (dd, 2H, J = 2.0 Hz, 8.4 Hz), 8.62 (d, 2H, J = 2.0 Hz); 13C NMR (DMSO-d6) δ 35.4, 106.9, 118.2, 121.0, 129.4, 135.9, 143.9, 146.6, 150.6, 170.0. HRMS-ESI (+): Calc. for C22H15N4O6S2: 495.0433. Found: 495.0431 [M+H]+.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(5-chlorobenzoxazole) (15g)
Yield 49%; 1H NMR (DMSO-d6) δ4.60 (s, 4H), 7.36 (dd, 2H, J = 2.0 Hz, 8.8 Hz), 7.48 (s, 4H), 7.67 (d, 2H, J = 8.8 Hz), 7.76 (d, 2H, J = 2.0 Hz); 13C NMR (DMSO-d6) δ 35.2, 111.5, 118.1, 124.3, 129.0, 129.3, 136.0, 142.5, 150.1, 165.9. HRMS-ESI (+): Calc. for C22H15N2O2S2Cl2: 472.9952. Found: 472.9974 [M+H]+.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-phenyl-5-cyano-4-oxopyrimidine) (16a)
Yield 80%; 1H NMR (DMSO-d6) δ 4.26 (s, 4H), 7.31 (s, 4H), 7.44 (m, 6H), 7.77 (m, 4H); 13C NMR (DMSO-d6) δ 33.6, 88.8, 119.9, 127.8, 127.9, 128.6, 129.3, 137.2, 137.7, 166.8, 170.3, 171.5. HRMS-ESI (−): Calc. for C30H19N6O2S2: 559.1011. Found: 559.0989 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(3-tolyl)-5-cyano-4-oxopyrimidine) (16b)
Yield 62%; 1H NMR (DMSO-d6) δ 2.35 (s, 6H), 4.24 (s, 4H), 7.31 (m, 8H), 7.54 (s, 4H); 13C NMR (DMSO-d6) δ 21.0, 33.6, 88.9, 120.1, 125.3, 127.9, 128.6, 128.8, 130.2, 137.2, 137.4, 137.8, 167.2, 170.5, 171.6. HRMS-ESI (−): Calc. for C32H23N6O2S2: 587.1324. Found: 587.1348 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(4-tolyl)-5-cyano-4-oxopyrimidine) (16c)
Yield 80%; 1H NMR (DMSO-d6) δ 2.36 (s, 6H), 4.25 (s, 4H), 7.26 (d, 4H, J = 8.0 Hz), 7.32 (s, 4H), 7.68 (d, 4H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ 21.0, 33.7, 88.6, 120.2, 128.1, 128.6, 128.9, 134.9, 137.4, 139.4, 166.9, 170.7, 171.7. HRMS-ESI (−): Calc. for C32H23N6O2S2: 587.1324. Found: 587.1306 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(4-ethylphenyl)-5-cyano-4-oxopyrimidine) (16d)
Yield 96%; 1H NMR (DMSO-d6) δ 1.21 (t, 6H, J = 7.6 Hz), 2.65 (q, 4H, J = 7.6 Hz), 4.26 (s, 4H), 7.29 (d, 4H, J = 8.0 Hz), 7.32 (s, 4H), 7.70 (d, 4H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ 15.5, 28.1, 33.7, 88.6, 120.3, 127.5, 128.2, 128.9, 135.2, 137.4, 145.6, 166.9, 170.7, 171.7. HRMS-ESI (−): Calc. for C34H27N6O2S2: 615.1637. Found: 615.1613 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(4-isopropylphenyl)-5-cyano-4-oxopyrimidine) (16e)
Yield 87%; 1H NMR (DMSO-d6) δ 1.25 (d, 12H, J = 7.2 Hz), 2.98 (septet, 2H, J = 7.2 Hz), 4.52 (s, 4H), 7.36 (s, 4H), 7.38 (d, 4H, J = 8.4 Hz), 7.87 (d, 4H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ 22.7, 32.7, 33.7, 91.8, 114.9, 125.8, 128.2, 128.4, 132.2, 135.3, 152.1, 161.1, 165.3, 166.4. HRMS-ESI (−): Calc. for C36H31N6O2S2: 643.1950. Found: 643.1943 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(4-methoxyphenyl)-5-cyano-4-oxopyrimidine) (16f)
Yield 46%; 1H NMR (DMSO-d6) δ 3.81(s, 6H), 4.28 (s, 4H), 6.97 (d, 4H, J = 8.8 Hz), 7.30 (s, 4H), 7.82 (d, 4H, J = 8.8 Hz); 13C NMR (DMSO-d6) δ 33.7, 55.3, 88.2, 113.5, 120.4, 128.9, 129.8, 130.0, 137.4, 160.6, 166.3, 170.6, 171.4. HRMS-ESI (−): Calc. for C32H23N6O4S2: 619.1222. Found: 619.1230 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(4-bromophenyl)-5-cyano-4-oxopyrimidine) (16g)
Yield 95%; 1H NMR (DMSO-d6) δ 4.25 (s, 4H), 7.31 (s, 4H), 7.67 (d, 4H, J = 8.4 Hz), 7.72 (d, 4H, J = 8.8 Hz); 13C NMR (DMSO-d6) δ 33.7, 88.9, 119.9, 123.3, 128.9, 130.2, 131.1, 136.8, 137.3, 165.9, 170.2, 171.8. HRMS-ESI (−): Calc. for C30H18Br2N6O2S2: 714.9221. Found: 714.9213 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(biphenyl-4-yl)-5-cyano-4-oxopyrimidine) (16h)
Yield 68%; 1H NMR (DMSO-d6) δ 4.28 (s, 4H), 7.35 (s, 4H), 7.40 (t, 2H, J = 7.2 Hz), 7.49 (t, 4H, J = 7.6 Hz), 7.75 (m, 8H), 7.88 (d, 4H, J = 8.4 Hz); 13C NMR (DMSO-d6) δ 33.7, 88.8, 120.3, 126.3, 126.8, 127.8, 128.7, 128.9, 129.0, 136.7, 137.4, 139.5, 141.3, 166.4, 170.4, 171.8. HRMS-ESI (−): Calc. for C42H27N6O2S2: 711.1637. Found: 711.1661 [M−H]−.
2,2'-(α,α’-Xylene)bis(sulfanediyl)bis-(6-(1-naphthyl)-5-cyano-4-oxopyrimidine) (16i)
Yield 92%; 1H NMR (CD3OD) δ 4.37 (s, 4H), 7.32 (s, 4H), 7.44–7.57 (m, 8H), 7.74 (d, 2H, J = 8.0 Hz), 7.91 (d, 2H, J = 8.2 Hz), 7.96 (dd, 2H, J = 2.0 Hz, 7.0 Hz); 13C NMR (CD3OD) δ 35.7, 94.1, 119.1, 126.2, 126.5, 127.4, 127.8, 127.9, 129.5, 130.3, 130.9, 132.0, 135.2, 136.6, 138.5, 171.6, 174.0, 175.0. HRMS-ESI (−): Calc. for C38H23N6O2S2: 659.1324. Found: 659.1343 [M−H]−.
General procedure for the preparation of S-benzyl-2-thiouracils (17d, e, g–i)
To a solution of the 2-thiouracil derivatives (10d, e, g–i, 2 mmol) and benzylchloride (253 mg, 2 mmol) in acetonitrile (10 mL) was added K2CO3 (829 mg, 6 mmol). The mixture was heated under reflux for 8 h and then cooled to room temperature. The liquid was removed on a rotavapor, and the residue was washed by H2O (20 mL). Then the solid was dried in a vacuum oven at 40 °C overnight to give 17d, e, g–i.
S-Benzyl-5-cyano-6-(4-ethylphenyl)-2-thiouracil (17d)
Yield 28%; 1H NMR (DMSO-d6) δ 1.27 (t, 3H, J = 7.6 Hz), 2.71 (q, 2H, J = 7.6 Hz), 4.40 (s, 2H), 7.21 (m, 1H), 7.28 (m, 4H), 7.41 (d, 2H, J = 7.2 Hz), 7.74(d, 2H, J = 8.4 Hz); 13C NMR (DMSO-d6) δ 16.0, 29.8, 36.0, 90.6, 120.1, 128, 128.8, 129.2, 129.4, 129.8, 130.1, 136.0, 139.8, 148.2, 170.2, 174.9. HRMS-ESI (+): Calc. for C20H18N3OS: 348.1171. Found: 348.1185 [M+H]+.
S-Benzyl-5-cyano-6-(4-isopropylphenyl)-2-thiouracil (17e)
Yield 40%; 1H NMR (DMSO-d6) δ 1.23 (d, 6H, J = 6.8 Hz), 2.94 (septet, 1H, J = 6.8 Hz), 4.30 (s, 2H), 7.22 (t, 1H, J = 6.8 Hz), 7.31 (m, 4H), 7.40 (d, 2H, J = 7.2 Hz), 7.73 (d, 2H, J = 7.6 Hz); 13C NMR (DMSO-d6) δ 23.7, 33.3, 33.8, 88.7, 120.3, 126.0, 126.7, 128.2, 128.3, 128.8, 135.3, 139.0, 150.2, 166.8, 170.5, 171.5. HRMS-ESI (+): Calc. for C21H20N3OS: 362.1327. Found: 362.1335 [M+H]+.
S-Benzyl-5-cyano-6-(4-bromophenyl)-2-thiouracil (17g)
Yield 33%; 1H NMR (DMSO-d6) δ 4.27 (s, 2H), 7.22 (t, 1H, J = 7.2 Hz), 7.29 (t, 2H, J = 7.2 Hz), 7.39 (d, 2H, J = 7.2 Hz), 7.67 (d, 2H, J = 8.4 Hz), 7.71 (d, 2H, J = 8.4 Hz); 13C NMR (DMSO-d6) δ 33.8, 88.9,120.0, 123.2, 126.7, 128.3, 128.9, 130.2, 131.1, 136.9, 139.0 165.8, 170.1, 171.8. HRMS-ESI (+): Calc. for C18H13N3OSBr: 397.9963. Found: 397.9950 [M+H]+.
S-Benzyl-5-cyano-6-(biphenyl-4-yl)-2-thiouracil (17h)
Yield 37%; 1H NMR (DMSO-d6) δ 4.32 (s, 2H), 7.23 (t, 1H, J = 7.6 Hz), 7.31 (t, 2H, J = 7.6 Hz), 7.40 (m, 3H), 7.50 (t, 2H, J = 7.6 Hz), 7.74 (d, 2H, J = 8.0 Hz), 7.77 (d, 2H, J = 8.4 Hz), 7.90 (d, 2H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ 33.8, 89.1, 126.4, 126.8, 126.8, 127.9, 128.3, 128.8, 128.9, 129.0, 136.6, 139.0, 139.4, 141.4, 166.4, 169.7, 171.3. HRMS-ESI (+): Calc. for C24H18N3OS: 396.1171. Found: 396.1187 [M+H]+.
S-Benzyl-5-cyano-6-(1-naphthyl)-2-thiouracil (17i)
Yield 43%; 1H NMR (DMSO-d6) δ 4.26 (s, 2H), 7.23 (t, 1H, J = 7.2 Hz), 7.29 (t, 2H, J = 7.2 Hz), 7.39 (d, 2H, J = 7.2 Hz), 7.55 (m, 5H), 7.78 (d, 1H, J = 8.0 Hz), 7.99 (t, 2H, J = 6.8 Hz); 13C NMR (DMSO-d6) δ 33.8, 92.4, 119.3, 125.2, 125.4, 126.0, 126.2, 126.4, 126.8, 128.2, 128.3, 128.9, 130.1, 133.1, 135.9, 138.9, 168.7, 167.6, 171.4. HRMS-ESI (+): Calc. for C22H16N3OS: 370.1014. Found: 370.1015 [M+H]+.
3.2. Biological evaluation
General in vitro biological methods
EcN68, the N-terminal fragment of SecA from E. coli without the C-terminal regulatory domain, and EcSecA, the full length SecA from E. coli, were over-expressed from pIMBB-821 and pT7-SecA,22 respectively, and purified as described.23, 24 EcN68 was used for screening because it has higher intrinsic activity and is more sensitive to inhibitors.
All potential inhibitors were dissolved in 100% DMSO. The ATPase activity was determined by the release of phosphate (Pi) detected by malachite green as described3 in a modified procedure15 and in the presence of 10% DMSO. Inhibitory effect was determined by the percentage of the remaining ATPase activity as compare to the controls without test compounds. Briefly, 50 µL reaction mixture was prepared so that it contained 2.25 µg N68 or 5 µg SecA, 2 mM ATP, 50 mM Tris-HCl (pH7.6), 20 mM KCl, 20 mM NH4Cl, 1 mM DTT, and 2 mM Mg(OAc)2. Reactions took place at 40 °C for 20 min (for N68) or 40 min (for SecA) then were stopped by adding 800 µL of malachite green and then 100 µL of 34% citric acid within 1 min. The mixtures were incubated at room temperature for 40 min and then the absorption at 660 nm was measured. All assays were done at least in triplicate, and the results were presented as bar graphs with standard error of the mean.
General in vivo biological methods
Log-phase growing cells (O.D. 600nm~ 0.5 to 1.0) were diluted to an absorbance of 0.05 at O.D. 600 nm, added with indicated compounds, and followed by culturing in an Eppendorf Thermomixer R (Brinkmann instruments, Inc.) at 37 °C,1050 rpm for 10 to 12 hours. All cultures contain 5% DMSO with a final volume of 100 µl. All tested compounds were dissolved in 100% DMSO (Sigma).
Bacterial strain
NR698 (MC4100 imp4213)25 with increased outer membrane permeability. MC4100, an Ecoli K-12 wild-type strain.26
3.3. Computational method
Molecular simulation of ligand-SecA complexes
The 3D structures for these compounds were refined using the PM3 method in the MOPAC 7 program27 and assigned with AM1-BCC partial charges28–30 by the QuACPAC program. All partial charges on the atoms of the homology model were derived from AMBER 8 parameters. Docking of the ligands into SecA around the active site (included residues Gly80, Mse81, Arg82, His83, Phe84, Gln87, Arg103, Thr104, Gly105, Glu106, Gly107, Lys108, Thr109, Leu110, Arg138, Asp209, Glu210, Arg509 and Gln578) was performed by using DOCK 5.4..31 After docking, MD simulations were conducted with the ligand-receptor complexes following similar procedures we reported before. 17–20 In brief, the docked complexes were solvated by using the TIP3P water model,32 subjected to 500-steps of molecular mechanics minimization and molecular dynamics simulations at 300 K for 1.0 ns using the SANDER module in the AMBER 8 program.33 The resulting structures were then analyzed using PyMOL 1.0,34 HBPLUS 3.0635 and Ligplot 4.2236 to identify specific contacts between ligands and SecA. During the computation, molecular docking (DOCK 5.4), binding analysis (HBPLUS 3.06 and Ligplot 4.22) and visualization (PyMOL 1.0) were carried out on a Xeon-based Linux workstation. Molecular mechanics calculations and molecular dynamics simulations (AMBER 8) were performed on URSA, a 160-processor computer based on the Power5+ processor and IBM’s P series architecture.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support by the Georgia Research Alliance, Georgia Cancer Coalition, and the Molecular Basis of Disease Program at GSU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Saier MH. J. Membr. Biol. 2006;214:75. doi: 10.1007/s00232-006-0049-7. [DOI] [PubMed] [Google Scholar]
- 2.van Klompenburg W, Ridder AN, van Raalte AL, Killian AJ, von Heijne G, de Kruijff B. FEBS Lett. 1997;413:109. doi: 10.1016/s0014-5793(97)00888-0. [DOI] [PubMed] [Google Scholar]
- 3.Lill R, Dowhan W, Wickner W. Cell. 1990;60:271. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 4.Erlandson KJ, Miller SB, Nam Y, Osborne AR, Zimmer J, Rapoport TA. Nature. 2008;455:984. doi: 10.1038/nature07439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukazaki T, Mori H, Fukai S, Ishitani R, Mori T, Dohmae N, Perederina A, Sugita Y, Vassylyev DG, Ito K, Nureki O. Nature. 2008;455:988. doi: 10.1038/nature07421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmer J, Nam Y, Rapoport TA. Nature. 2008;455:936. doi: 10.1038/nature07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori H, Ito K. Trends Microbiol. 2001;9:494. doi: 10.1016/s0966-842x(01)02174-6. [DOI] [PubMed] [Google Scholar]
- 8.Vrontou E, Economou A. Biochim. Biophys. Acta. 2004;1694:67. doi: 10.1016/j.bbamcr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Papanikolau Y, Papadovasilaki M, Ravelli RB, McCarthy AA, Cusack S, Economou A, Petratos K. J. Mol. Biol. 2007;366:1545. doi: 10.1016/j.jmb.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Huang YJ, Tai PC, Wang B. Biochem. Biophys. Res. Commun. 2008;368:839. doi: 10.1016/j.bbrc.2008.01.135. [DOI] [PubMed] [Google Scholar]
- 11.Bowler MW, Montgomery MG, Leslie AG, Walker JE. Proc. Natl. Acad. Sci. U S A. 2006;103:8646. doi: 10.1073/pnas.0602915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoddard BL, Ringe D, Petsko GA. Protein Eng. 1990;4:113. doi: 10.1093/protein/4.2.113. [DOI] [PubMed] [Google Scholar]
- 13.In our earlier screening, these two compounds were found to have IC50 values of about 30 µM. However, upon more rigorous studies, 2 was found to have IC50 of 100 µM. 1 showed similar inhibition activities as 2, but started having solubility problems when approaching 100 µM.
- 14.Sugie Y, Inagaki S, Kato Y, Nishida H, Pang CH, Saito T, Sakemi S, Dib-Hajj F, Mueller JP, Sutcliffe J, Kojima Y. J Antibiot (Tokyo) 2002;55:25. doi: 10.7164/antibiotics.55.25. [DOI] [PubMed] [Google Scholar]
- 15.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, Jones SA, Willson TM. J. Med. Chem. 2000;43:2971. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 16.Abdou IM, Strekowski L. Tetrahedron. 2000;56:8631. [Google Scholar]
- 17.Li M, Wang B. Biochem. Biophys. Res. Commun. 2006;347:662. doi: 10.1016/j.bbrc.2006.06.179. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Wang B. J. Mol. Model. 2007;13:1237. doi: 10.1007/s00894-007-0245-0. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Ni N, Chou H-T, Lu C-D, Tai Phang C, Wang B. ChemMedChem. 2008;3:1242. doi: 10.1002/cmdc.200800076. [DOI] [PubMed] [Google Scholar]
- 20.Zheng S, Kaur G, Wang H, Li M, Macnaughtan M, Yang X, Reid S, Prestegard J, Wang B, Ke H. J. Med. Chem. 2008;51:7673. doi: 10.1021/jm701635j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karamanou S, Vrontou E, Sianidis G, Baud C, Roos T, Kuhn A, Politou AS. Mol. Microbiol. 1999;34:1133. doi: 10.1046/j.1365-2958.1999.01686.x. [DOI] [PubMed] [Google Scholar]
- 22.Cabelli RJ, Chen L, Tai PC, Oliver DB. Cell. 1988;55:683. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Brown T, Tai PC. J. Bacteriol. 1998;180:527. doi: 10.1128/jb.180.3.527-537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Xu H, Tai PC. J. Biol. Chem. 1996;271:29698. doi: 10.1074/jbc.271.47.29698. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz N, Falcone B, Kahne D, Silhavy TJ. Cell. 2005;121:307. doi: 10.1016/j.cell.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Casadaban MJ. J. Mol. Biol. 1976;104:541. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 27.Stewart JJ. J Comput Aided Mol Des. 1990;4:1. doi: 10.1007/BF00128336. [DOI] [PubMed] [Google Scholar]
- 28.Jakalian A, Bush BL, Jack DB, Bayly CI. J. Comput. Chem. 2000;21:132. doi: 10.1002/jcc.10128. [DOI] [PubMed] [Google Scholar]
- 29.Jakalian A, Jack DB, Bayly CI. J. Comput. Chem. 2002;23:1623. doi: 10.1002/jcc.10128. [DOI] [PubMed] [Google Scholar]
- 30.Tsai K-C, Wang S-H, Hsiao N-W, Li M, Wang B. Bioorg. Med. Chem. Lett. 2008;18:3509. doi: 10.1016/j.bmcl.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Moustakas DT, Lang PT, Pegg S, Pettersen E, Kuntz ID, Brooijmans N, Rizzo RC. J. Comput. Aided. Mol. Des. 2006;20:601. doi: 10.1007/s10822-006-9060-4. [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. J. Chem. Phys. 1983;79:926. [Google Scholar]
- 33.Case DA, Cheatham TE, 3rd, Darden T, Gohlke H, Luo R, Merz KM, Jr, Onufriev A, Simmerling C, Wang B, Woods RJ. J. Comput. Chem. 2005;26:1668. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLano WL. DeLano Scientific. San Carlos, CA, USA: 2006. http://www.pymol.org. [Google Scholar]
- 35.McDonald IK, Thornton JM. J. Mol. Biol. 1994;238:777. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- 36.Wallace AC, Laskowski RA, Thornton JM. Protein Eng. 1995;8:127. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.