Abstract
The goal of the study is to examine the relationship between the sensor molecules, Hypoxia Inducible Factor-1 (HIF-1), AMP activated Protein Kinase (AMPK) and mammalian Target of Rapamycin (mTOR) in chondrocyte survival and autophagy. We showed that chondrocytes expressed the energy sensor AMPK-1 and that activation increased with maturation. In addition, we showed that thapsigargin treatment activated AMPK and autophagy in a HIF-1 dependent manner. Using serum-starved AMPK-silenced cells, we demonstrated that AMPK was required for the induction of the autophagic response. We also noted a change in chondrocyte sensitivity to apoptogens, due to activation of caspase-8 and cleavage and activation of the pro-apoptotic protein, BID. To test the hypothesis that AMPK signaling directly promoted autophagy, we inhibited AMPK activity in mTOR silenced cells and showed that while mTOR suppression induced autophagy, AMPK inhibition did not block this activity. Based on these findings, it is concluded that due to the micro-environmental changes experienced by the chondrocyte, autophagy is activated by AMPK in a HIF-1 dependent manner.
Keywords: AMPK, mTOR, HIF-1, autophagy, chondrocyte
INTRODUCTION
Chondrocytes embedded within the growth-plate survive in an almost avascular and hypoxic microenvironment. In this milieu, they undergo a series of maturational changes, following which the hypertrophic chondrocytes die through the induction of programmed cell death. Within the cartilage matrix, a complex macromolecular network directly influences the fate and function of resident chondrocytes. Adaptation to this avascular environment is mediated by Hypoxia Inducible Factors, HIF-1 and –2. While HIF-1 acts as a cellular metabolic sensor and stimulates chondrocyte glycolytic flux (1, 2) and autophagy (3, 4), HIF-2 is a potent negative regulator of the autophagic flux (5, 6). In addition to HIF-1, another important metabolic sensor is the AMP-activated protein kinase (AMPK). AMPK activity is responsive to many metabolic signals including hypoxia and hyperosmotic stress (7). The kinase is sensitive to the AMP/ATP ratio: binding of AMP activates AMPKand induces phosphorylation by the tumor suppressor LKB1 (8). Furthermore, when bound, AMP inhibits it’s dephosphorylation by Protein Phosphatase 2C (24). Accordingly, AMPK functions as an energy sensor that triggers catabolic pathways that produce ATP, while inhibiting energy-consuming anabolic activities.
In a low energy state, or under stress condition, cells survive for long time periods by activating autophagy, a process which results in the bulk degradation of cellular proteins through an evolutionarily conserved autophagolysosomal pathway (9, 10). The autophagic state is regulated, in part, by mTOR, a serine/threonine kinase that modulates translation and cell division (11, 12). Recent studies indicate that mTOR integrates multiple signals including those from growth factors and amino acids (13, 14). In addition to this latter function, mTOR activity is also regulated by AMPK; activation of AMPK inhibits mTOR-dependent signaling (15). This activity is consistent with AMPK’s energy sensing function: suppression of ATP utilization and activation of autophagy.
To date, little is known of mTOR and AMPK function in cartilage. However, in a recent study of the growth plate, we have shown that prior to their apoptotic cell death, maturing chondrocytes survive in an autophagic state (3, 16). In addition, we have shown that autophagy is stimulated by HIF-1 (3) and that in concert with chondrocyte maturation, there is a profound decrease in ATP and a concomitant rise in AMP, key regulators of AMPK (17). However, the detailed relationship between HIF-1, AMPK and autophagy has not been determined. The goal of the study reported herein is to examine the relationship between HIF-1 and AMPK in the regulation of autophagy in chondrocytes. We show that AMPK is activated during chondrocyte maturation and increased intracellular calcium flux, in a HIF 1-dependent manner. Furthermore, we demonstrate that the induction of autophagy is also dependent on the activities of this kinase and mTOR.
MATERIALS AND METHODS
Reagents
Cell culture reagents were purchased from Fisher Scientific (Malvern, PA). Alpha Minimal Essential Medium and transfection reagents were obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum was from Atlanta Biological (Norcross, GA). Mammalian Protein Extraction Reagent (M-PER) was obtained through Pierce (Rockford, IL). This company also supplied HRP secondary antibody. AMPKα1, AMPKα2 and mTOR antibodies were from Cell Signaling (Danvers, MA). LC-3 and Beclin-1 antibodies were obtained from Abgent (San Diego, CA) and Novus Biologicals (Golden CO), respectively. Bcl-2 and BID antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Alexafluor 594-labeled and fluorescein-labeled secondary antibodies (Southern Biotechnology, Birmingham, AL) were used in the immunohistochemical studies. Reagents for Western blotting, were from Bio-Rad, (Hercules, CA). The caspase-8 FLICA detection kit was purchased from Chemicon (Temecula, CA). Unless indicated, all other reagents were from Sigma-Aldrich (St. Louis, MO).
Chondrocyte Culture
N1511 mouse chondrocytes and derived cell lines were cultured in alpha-MEM (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, 0.2% L-glutamine, 0.2% penicillin/streptomycin, and 0.2% sodium pyruvate. All cells were incubated at 37°C, 5%, CO2, 95% air. In some cases, the cells were treated for 10 min with the AMPK inhibitor, Compound C (6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine; Calbiochem, San Diego, CA) at final concentration of 10 μM. Thapsigargin (Sigma-Aldrich, St. Lois, MO) was used at a concentration of 100nM in some experiments.
siRNA Plasmid Construction
An siRNA construction kit (Ambion) was utilized to down regulate the expression of the genes of interest. The following phosphorylated oligonucleotides were used to create the siRNAs:
AMPK-α 1 (F) gatccgatcggccactacatcctgttcaagagacaggatgtagtggccgatcttttttggaaa
AMPK-α1 (R) agcttttccaaaaaagatcggccactacatcctgtctcttgaacaggatgtagtggccgatcg
mTOR (F) gatcccgtgaggagaccagggccaaattcaagagatttggccctggtctcctcattttttggaaa
mTOR ( R) agcttttccaaaaaatgaggagaccagggccaaatctcttgaatttggccctggtctcctcacgg
Permanent cell lines were generated using 80% confluent monolayers transfected with the desired siRNA vector followed by clonal selection using 800 μg/mL of hygromycin B (Invitrogen, Carlsbad, CA). Cell-lines transfected with backbone vector and a scrambled sequence served as control.
Determination of expression of AMPK in chondrocytes by RT-PCR
Cells were washed with cold PBS and total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA). RNA yield was determined spectrophotometrically. Each reaction contained 50 ng of total RNA and 2 pM of primer. The RNA was reverse transcribed and then amplified using with Superscript One-Step RT-PCR Kit with Platinum Taq (Invitrogen, Carlsbad, CA). The following primers specific for the mouse AMPK α1 gene was used for RT-PCR analysis:
(F) gattcggagccttgacgt (R) agcaggacgttctcaggt
Detection of AMPKa1 in cartilage
Expression of AMPKα1was assessed in the growth plate of distal tibial sections of new-born mice. Paraffin-embedded sections (5 μM) were permeabilized with proteinase K (10 μg/mL), and fixed in 4.0% paraformaldehyde. Next, serial sections were treated with an AMPKα1 antibody at a dilution of 1:50 v/v. Following treatment with the primary antibody, sections were treated with Alexafluor 594 labeled secondary antibodies, counterstained with DAPI, and visualized by fluorescent microscopy.
Immunohistochemical localization of LC-3 in chondrocytes in culture
LC-3 expression was assessed by immunohistochemistry. Cells were prepared at a density of 40,000 cells/mL in a 24-well plate and maintained in hypoxia or normoxia for 16 h. After washing 3 times in Phosphate Buffered Saline (PBS), cells were fixed with 3.7% paraformaldehyde in PBS [pH 8.0] for 10 min and then washed. The cells were then permeabilized with 0.5% Triton-X 100 in PBS for 5 min. and washed three times in PBS. Antigenic sites were blocked in 10% calf serum in PBS for 1 h. After blocking, the cells were incubated with LC-3 antibody at a dilution of 1:200 overnight at 4°C. Subsequently, the treated cells were incubated with a fluorescein labeled secondary antibody, for 1 h at room temperature. The cells were then washed in PBS three times for 5 min and mounted in CrystalMount. Proteins were visualized by confocal microscopy (Olympus Fluoview, Japan).
Western blot analysis of cultured chondrocytes
Cells were lysed with MPER supplemented with Complete Protease inhibitors (Roche, Indianapolis, IN), 150 mM NaCl, 1 mM DTT, 0.4 mM Na3VO4, 10 mM NaF. Total protein concentration of the supernatent was quantified by the Bradford method. 100 μg of protein was then loaded onto 4–20% Tris gradient gels. Following transfer to PVDF membranes and blocking, blots were treated overnight with the appropriate primary antibody. Membranes were then washed and treated with HRP labeled secondary antibody. Blots were visualized with Lumigen TMA-6 (Amersham Biosciences, Piscataway, NJ).
Cell Viability Challenge Assay
Chondrocytes were plated in 24-well culture dishes at 40,000 cells/mL and grown to 80% confluence. The chondrocytes were treated with 10 μM H2O2 in serum-free DMEM. Viability was determined by the addition of 20 μl MTT to the cultures. The reaction was stopped after 3 h and the change in color quantified using a plate reader at 590 nm.
Detection of caspase-8 activation in siAMPK chondrocytes
Caspase-8 activation was measured using a commercial kit and used according to the manufacturers instructions. Briefly, cells were plated at a density of 40,000 cells/mL in 24-well plates and grown for 24 h at 37°C. Cells were then serum-starved for 4 h. Activation of caspase 8 was assayed by the addition of the FLICA reagent solution, at a 1:30 dilution, to the media for 1 h at 37°C. Cells were then washed twice with wash buffer, fixed and fluorescence, at 490 nm, evaluated by confocal microscopy.
Immunoprecipitation of Bcl-2- Beclin complex
siAMPK cells were serum-starved for 4 h and whole cell extracts were prepared as described previously (16). Cell lysates (150 μg) were pre-cleared using 20 μL of protein A/G Sepharose beads (Pierce Biotechnology, Rockford, IL) and 10 μL of rabbit antiserum for 1 h at 4°C. The supernatant was then incubated with 10 μL of Beclin-1 antibody and 20 μL of protein A/G Sepharose beads overnight at 4°C. Following incubation, the beads were then washed 4× in lysis buffer. Proteins were then resolved by SDS-PAGE followed by Western blot analysis for Bcl-2 and Beclin-1.
RESULTS
AMPKa1 is expressed by cells of the growth plate and by N1511 chondrocytes
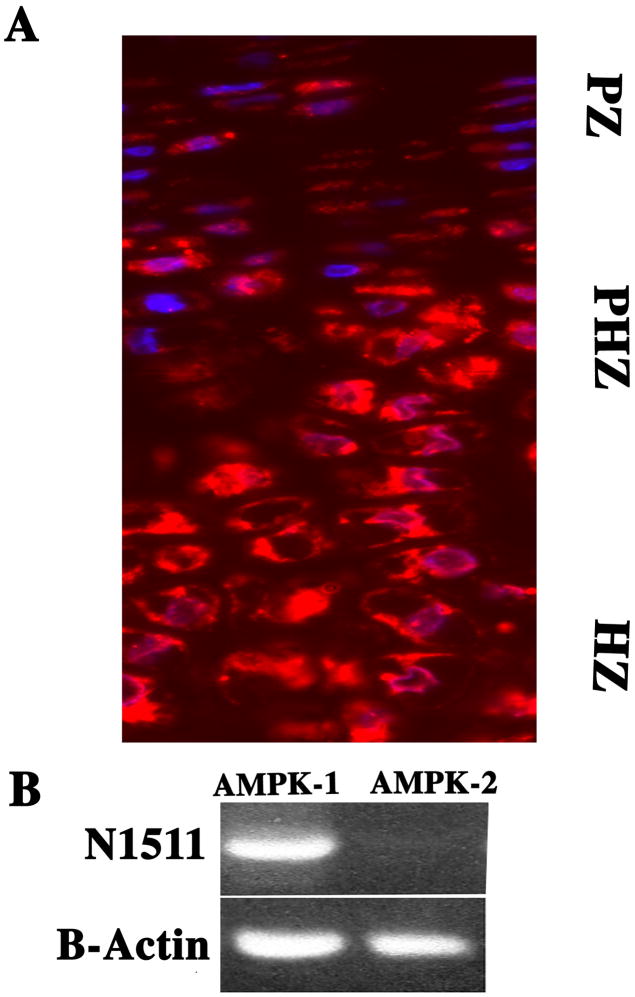
Longitudinal sections of the mouse growth plate were immunostained for both AMPKa1 and AMPKa2. Figure 1A shows that epiphyseal chondrocytes express AMPKa1; AMPKa2 was not detected (data not shown). Noteworthy, while the AMPKa1 stained cells are present throughout the growth plate, the most densely stained cells are localized in the pre-hypertrophic and hypertrophic region. In addition, we examined the expression of AMPKa1 in the N1511 chondrocytes. As shown in Fig. 1B, RT-PCR analysis indicates that AMPKa1is expressed by the cultured chondrocytes; again AMPKa2 was not detected in chondrocytes but was expressed by osteoblasts (not shown).
Figure 1. Expression of AMPKa1 in cells of the epiphyseal growth plate and in N1511 chondrocytes.
(A) Longitudinal section through the mouse growth plate stained with an antibody to AMPKa1. Paraffin-embedded section of the proximal tibial epiphysis was treated with an antibody against AMPKa1. Subsequently the section was treated with Alexafluor 594 labeled secondary antibodies and counterstained with DAPI. Rabbit IgG was used as a negative control. Note the robust expression of AMPKa1 in proliferating (PZ) and pre-hypertrophic cells (PHZ) and decreased expression in hypertrophic cells (HZ) (mag. × 200). (B) AMPKa1 expression in N1511 chondrocytes. AMPKa1 and AMPKa2 expression was assessed by RT-PCR analysis. There was robust expression of AMPKa1 and an absence of AMPKa2. 18S RNA served as a loading control.
Activation of AMPK by thapsigargin requires HIF-1
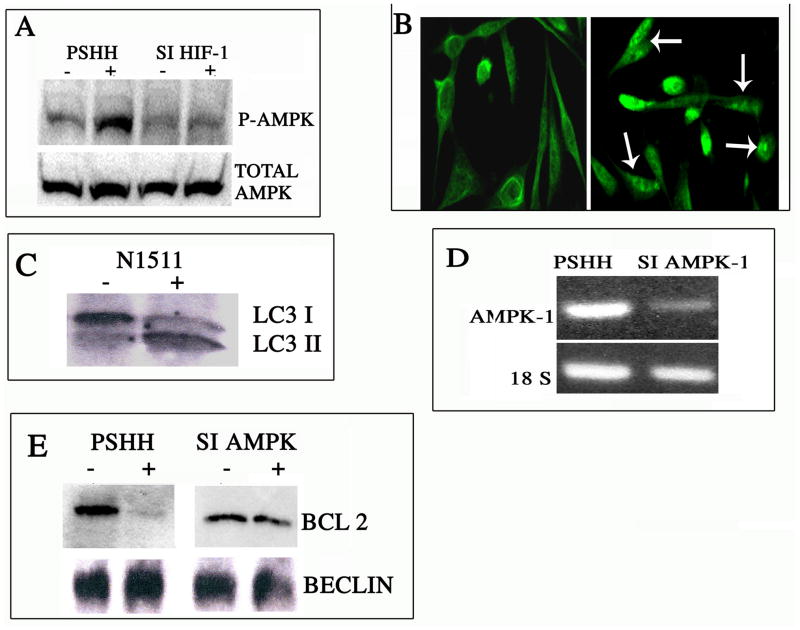
Thapsigargin is an inhibitor of sarco/endoplasmic reticulum Ca2+ ATPases (SERCA). It raises the cytosolic calcium concentration by blocking the ability of the cell to pump calcium into the sarcoplasmic and endoplasmic reticula (ER; as a result, these stores become depleted, resulting in ER stress and ultimately cell death. In a number of tissues, AMPK is activated both by a decrease in energy charge and by an increase in intracellular calcium flux. To examine calcium activation of AMPK, we treated N1511 chondrocytes with thapsigargin. Figure 2A shows that thapsigargin treatment results in activation of AMPK as evidenced by an increase in the expression of phosphorylated AMPK (p-AMPK). We next determined if HIF-1 modulated calcium-dependent AMPK activity. For this study, we measured AMPK and p-AMPK in thapsigargin-treated control and (previously described) HIF-1 silenced chondrocytes (3). Figure 2A shows that when treated with thapsigargin, AMPK is highly expressed by both control and HIF-1 silenced cells. However, since the silenced cells exhibit very low levels of activation of AMPK, it is clear that the activation of this enzyme is dependent on the expression of HIF-1.
Figure 2. Regulation of autophagy by AMPK.
(A) Thapsigargin-mediated AMPK activation is dependent on HIF-1: Cells were treated with a low dose of thapsigargin and AMPK activation monitored by Western blot analysis. Note the robust expression of phospho-AMPKa1 upon drug treatment in control pSHH cells (the integrated plasmid contains a scrambled HIF-1a sequence), indicating AMPK activation. On the other hand, activation of AMPK is suppressed in HIF-1 silenced chondrocytes. (B & C) Induction of autophagy by thapsigargin. To examine the effect of thapsigargin treatment on the induction of autophagy, chondrocytes were treated with a low dose of this drug and autophagy monitored by immunofluorescence microscopy and Western blot analysis for LC3 expression. Figure 2B shows that drug treatment resulted in the redistribution of LC3 into puncta indicative of autophagosome formation. (mag. × 200). This was confirmed by an increase in the LC3II isoform by Western blot analysis (Fig 2C). (D) AMPK suppression by silencing technology. siRNA technology was used to silence AMPKa1 (siAMPKa1). AMPKa1 expression was assessed by RT-PCR analysis. Note, there was suppression of AMPKa1 in the silenced cells. The control cells (pSHH) displayed a robust expression of AMPKa1. (E) Beclin-Bcl2 association in AMPK suppressed chondrocytes. AMPK suppressed cells were treated with a low dose of thapsigargin, lysed, immunoprecipitated with an anti-Beclin-1 antibody and subjected to Western blot analysis. There was a minimal change in immunoprecipitated Bcl2 levels when the AMPK suppressed cells were treated with the drug. In contrast, in control cells, thapsigargin treatment caused loss of Bcl2 from Beclin-1.
Thapsigargin treatment induces chondrocyte autophagy
We next examined the relationship between ER dependent calcium flux and autophagy. Chondrocytes were treated with thapsigargin and the distribution of LC3 protein examined. When treated with thapsigargin, the protein redistributes into distinct puncta, a characteristic of cells undergoing autophagy (Fig. 2B). In contrast, in untreated cells the protein remains distributed in a diffuse manner. Western blot analysis reveals that following drug treatment there is an increase in the levels of the LC3II isoform (Fig. 2C). Since this isoform characterizes autophagosome formation, it confirms that the cells are undergoing autophagy.
Induction of autophagy is blocked by AMPK silencing
We next examined AMPK’s role in the induction of the autophagic process. First, AMPKα1 was silenced using siRNA technology. As shown in Fig. 2D, the silenced cells exhibit a profound decrease in the expression of AMPKα1. Autophagy was induced by treating the cells with thapsigargin. Whole cell extracts were subsequently immunoprecipitated with a Beclin-1 antibody. Figure 2E shows that when control cells are treated with the drug, the interaction between Beclin-1 and Bcl-2 is disrupted and minimum quantities of Bcl-2 are co-immunoprecipitated with Beclin-1. However, upon thapsigargin treatment, Bcl2 remains associated with Beclin-1 in AMPK silenced cells. This result suggests that AMPK regulates the induction of autophagy by modulating the association between Bcl-2 and Beclin-1 as has been shown previously for HIF-1 (3).
Serum-starved AMPK suppressed cells are refractory to H2O2 challenge
Building on the observation that when AMPK was silenced, autophagy was suppressed, we next evaluated the sensitivity of these cells to an apoptogen challenge. We had previously shown that serum-starvation results in the induction of autophagy in chondrocytes (3, 16). Cells were serum-starved, challenged with a low dose of H2O2 and cell viability was assessed by the MTT assay. As shown in Fig. 3A, serum-starvation sensitizes control cells to the apoptogen challenge. Thus, only about 40% of the cells remain viable after this treatment. In contrast, after serum starvation, AMPK silenced cells are refractory to the H2O2 challenge.
Figure 3. Viability and regulation of caspase-8 activity and BID cleavage and Beclin-Bcl2 association by AMPK.
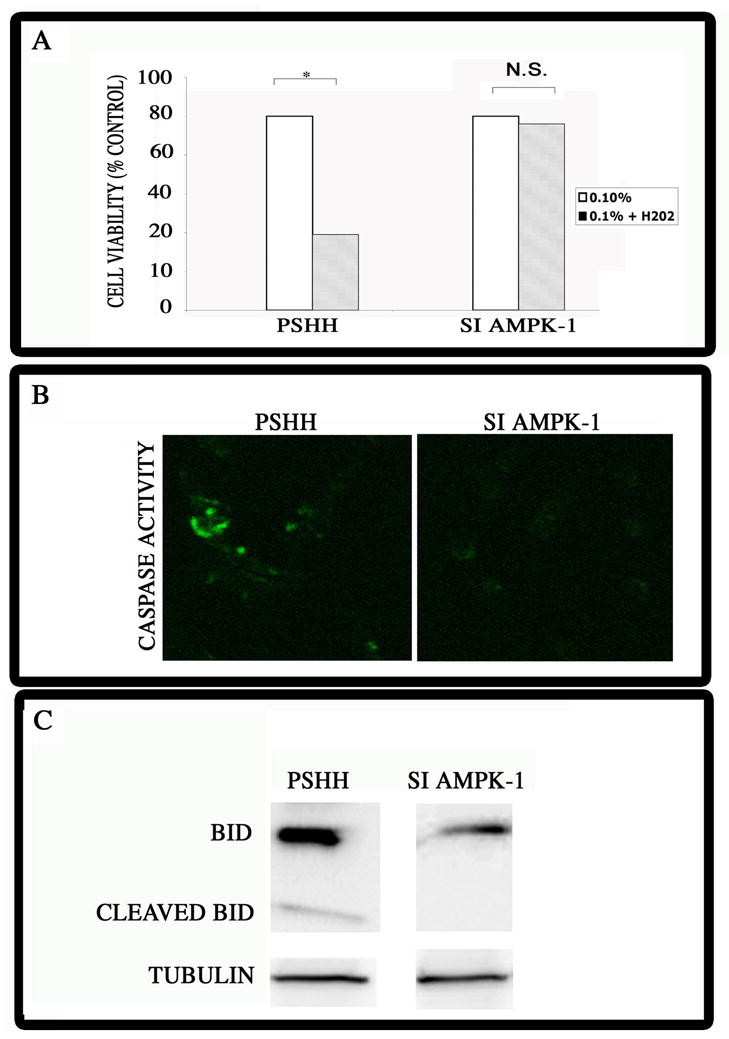
(A) Survival of AMPK silenced cells following treatment with H2O2. Serum-starved chondrocytes were treated with low dose H2O2 and death was determined by the MTT assay. Note the increased viability of the silenced (siAMPK-1) cells, compared with control chondrocytes. Values shown are mean +/− SEM; n=3; *significantly different from serum-starved cells, p<0.05; ns = not significant. (B) Caspase-8 activation in siAMPK cells. Cells were serum-starved for 4 h, stained with FLICA reagent and caspase-8 activity evaluated by confocal microscopy. Note the low level capase-8 activity in control cells; there was no discernable caspase-8 activity in the AMPK silenced cells. (mag x 200) (C) BID and cleaved BID levels in AMPK suppressed cells. AMPK silenced chondrocytes were serum-starved and BID protein levels were examined by Western blot analysis. There was a robust expression of BID and low levels of cleaved BID in control (pSHH) cells. In contrast, in the AMPK silenced cells, there was a decrease in BID expression levels, and no evidence of cleaved BID. Tubulin was used as a loading control.
Expression of caspase-8 and BID in AMPK silenced chondrocytes
To explore the cause of the refractory response of the AMPK silenced cells, we evaluated the relationship between AMPK and apoptosis. First, we examined caspase-8 activity in the AMPK silenced cells. Cells were serum-starved for 4 h, stained with the FLICA reagent and caspase-8 activity evaluated by confocal microscopy. Predictably, there is a low level capase-8 activity in the control cells (Fig. 3B). However, there is no discernable caspase-8 activity in the AMPK-silenced cells. Next, we examined BID and BID cleavage in the silenced cells. Using siAMPK chondrocytes that were serum-starved, we measured BID protein levels by Western blot analysis. As shown in Fig. 3C, in the AMPK silenced cells, a decrease in BID expression levels is apparent, and there is no evidence of cleaved BID. In contrast, in control (pSHH) chondrocytes, a robust expression of BID is seen, together with low levels of cleaved BID.
AMPK silencing stimulates Akt activation
One likely explanation for the decrease in apoptogen sensitization is the increase in survival activity of the silenced cells. To examine this possibility, we examined the expression and activation of Akt-1, a major transducer of survival signaling. Figure 4A shows that AMPK suppression leads to a significant activation of Akt-1. Thus, there is a robust increase in the expression of phosphorylated Akt-1 in the silenced cells, relative to the control cells. Noteworthy, while AMPK activity regulates the activity of the Akt-1 pathway, it has minimal effect on total Akt-1 expression.
Figure 4. Activation of Akt-1, mTOR and S6K in AMPKa1 suppressed chondrocytes.
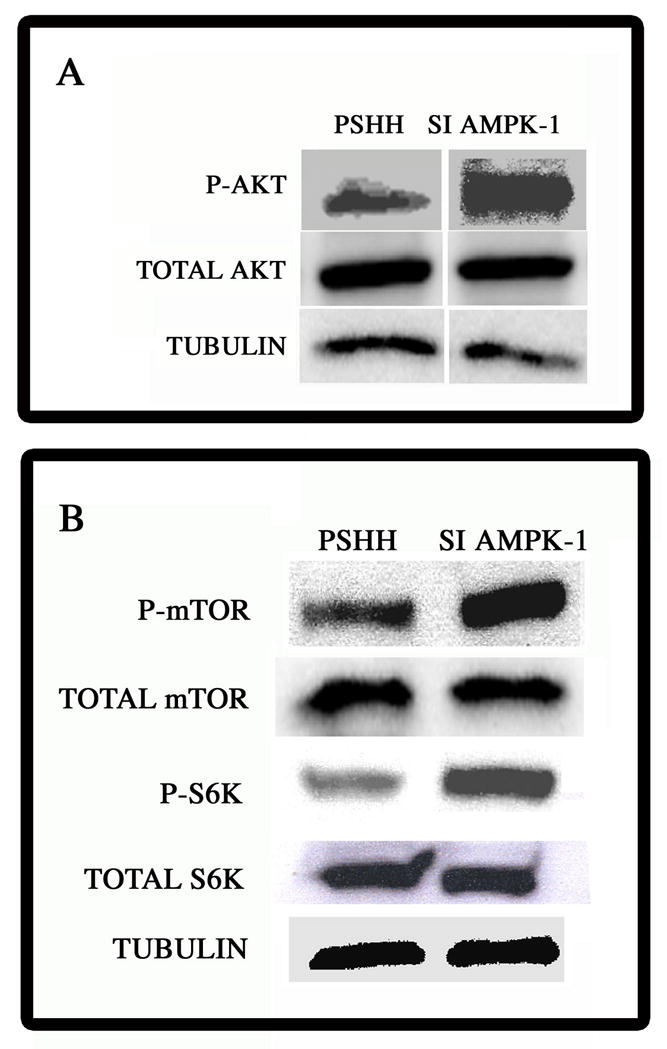
(A) pAkt expression in AMPK suppressed cells. Following silencing, Akt activation (p-Akt) was assessed by Western blot analysis. There was robust activation of Akt (p-Akt) in AMPK suppressed cells. Thre vwas no difference in the expression of total Akt-1 between control and silenced cells. Tubulin was used as a loading control. (B) Expression of mTOR and S6K in chondrocytes. Western blot analysis of expression of mTOR, phospho-mTOR (p-TOR) and phospho-S6K (p-S6K) in AMPK-suppressed cells There was an increase in p-mTOR and p-S6K in AMPK silenced cells. Expression of total mTOR was unaffected by AMPK suppression. Control (pSHH) cells contained the scrambled sequence. Tubulin was used as a loading control.
AMPKα1 silencing activates mTOR and stimulates the activity of its target gene, S6K
A major regulator of autophagy is the protein kinase mTOR. While active mTOR represses autophagy, it also activates Akt. In addition to its regulation by extracellular nutrients, mTOR is suppressed by AMPK. Accordingly, we determined if mTOR is activated in AMPK silenced cells. Figure 4B indicates that suppression of AMPK increases the levels of phosphorylated mTOR while total mTOR expression is unchanged. To determine if mTOR is functional, we evaluated the expression of the mTOR target gene S6K. AMPKα1 suppressed cells display robust S6K activation. Total S6K levels are not significantly altered by AMPK silencing.
mTOR suppression promotes chondrocyte autophagy
To further explore the relationship between AMPK, mTOR and autophagy, we next suppressed mTOR expression using siRNA technology. Western blot analysis indicates that there is significant suppression of mTOR expression in the siRNA-transfected cells (Fig. 5A). To examine the importance of mTOR in autophagy, cells were stained with LC3. While both control (pSHH) and mTOR silenced chondrocytes express LC3, the puncate organization of the protein was evident only in the silenced cells (Fig. 5B). As LC3 puncta are observed under serum replete conditions, it confirms that mTOR suppresses autophagy. In a parallel experiment, we evaluated the association of Bcl-2 with Beclin-1 in the mTOR silenced cells. Figure 5C shows that significantly less Bcl-2 is associated with Beclin-1 in the mTOR silenced cells than the control cells even under serum-rich conditions. We next examined the autophagic flux in control and mTOR silenced cells. For this study, to block the maturation of the autophagolysosome, cells were treated with bafilomycin, and expression of LC3II examined by Western blot analysis. Figure 5D shows low levels of the LC3II isoform in control cells; in the mTOR silenced chondrocytes there is an increase in this isoform. Upon bafilomycin treatment, the increase in LC3II in both cell types is indicative of an active autophagic flux. These results suggest that the interaction between Beclin-1 and Bcl-2, during the induction of the autophagic flux, is controlled by mTOR.
Figure 5. Effect of mTOR silencing on the induction of autophagy.
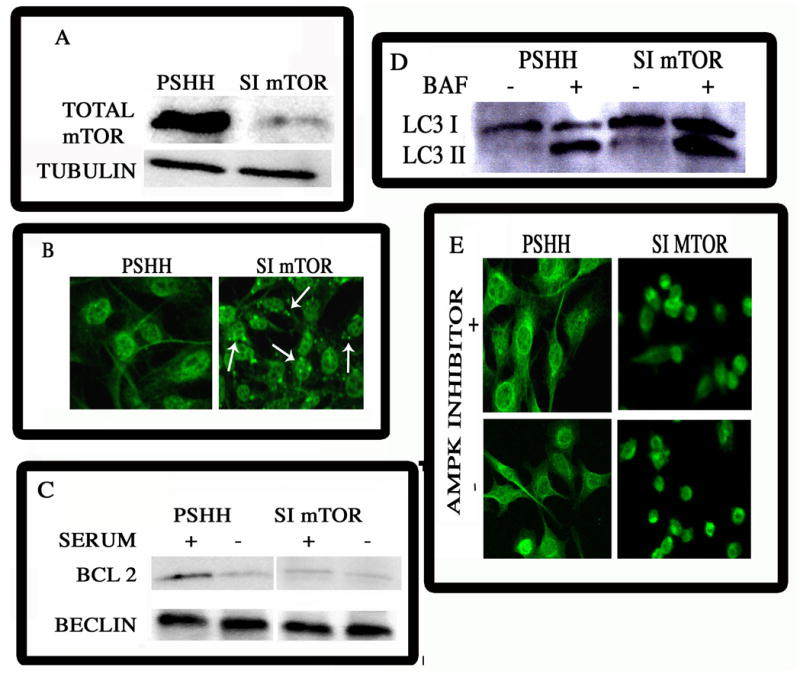
(A) mTOR suppression by silencing technology. siRNA technology was used to silence mTOR in N1511 chondrocytes. mTOR expression was assessed by Western blot analysis. There was suppression of mTOR in the silenced cells. The control cells (pSHH) displayed a robust expression of mTOR. (B) LC-3 expression in mTOR suppressed cells. N1511 chondrocytes were maintained in culture and LC3 expression was examined by immunofluorescence analysis. Note the diffuse pattern of expression of LC3 in control cells. In contrast, LC3 was reorganized into speckled structures, indicative of autophagy in mTOR silenced chondrocytes (mag. × 200). (C) Beclin-Bcl2 association in mTOR suppressed cells. Silenced and control N1511 chondrocytes were maintained under serum-replete conditions. Whole cell extracts were immunoprecipitated with an anti-Beclin-1 antibody. Western blot analysis of lysed control cells indicated that Bcl-2 was associated with Beclin-1. In contrast, when mTOR was silenced, the association between Bcl-2 and Beclin was disrupted. (D) Effect of mTOR silencing on the autophagic flux. LC3 expression was detected by Western blot analysis. The autophagic flux was assayed by treating the cells with Bafilomycin A1 and monitoring the accumulation of the LC3II isoform by Western blot analysis. Note the greater accumulation of this isoform in mTOR silenced cells than control cells, indicating an increase in the autophagic flux upon mTOR suppression (E) LC-3 organization in mTOR silenced chondrocytes treated with the AMPK inhibitor, Compound C. mTOR silenced chondrocytes (simTOR) were maintained in culture and LC3 expression was examined by immunofluorescence analysis. The diffuse pattern of expression of LC3 in control cells, contrasts with the reorganized speckled LC3 structures in mTOR silenced chondrocytes. Treatment with the AMPK inhibitor did not influence LC3 organization in the silenced cells. Note the diffuse pattern of expression of LC3 in control cells, with or without, the inhibitor. (mag. × 200).
AMPK regulation of the autophagic flux is transduced by mTOR
Finally, to explore the possibility that suppression of AMPK alone is sufficient to inhibit autophagy, we treated mTOR silenced chondrocytes with the AMPK inhibitor, Compound C. As shown in Fig. 5E, when mTOR is silenced, autophagy is induced; noteworthy, inhibition of AMPK does not block this activity. Furthermore, Compound C treatment blocked both serum-starvation and thapsigargin-mediated autophagy in wildtype cells (not shown). Thus, the effect of AMPK on the induction of autophagy is mediated through its regulatory affects on mTOR.
DISCUSSION
The overall goal of the investigation was to examine the role of AMPK in regulating chondrocyte autophagy. We showed for the first time that chondrocytes expressed the AMPKa1 isoform and that activation of the kinase promoted autophagy. This new observation is in line with a very recent study of autophagy where it was shown that AMPK is a potent activator of the autophagic process (18, 19). Furthermore, activation of AMPK was dependent on the transcription factor HIF-1, a stimulator of autophagic flux. The study also indicated that induction of autophagy was regulated by mTOR, a second sensor of cellular metabolism (13, 20). Accordingly, an increase in AMPK, probably related to a change in the chondrocyte energy charge, would inhibit mTOR and promote autophagy; this finding is consistent with the observation that when mTOR activity is blocked, autophagy is induced (20). A second observation was that when AMPK was silenced and autophagy was suppressed, there was a marked decrease in apoptogen sensitivity, probably mediated by increased Akt-1 signaling. Based on these findings, we suggest that activation of AMPK promotes autophagy as a mechanism of survival. Related to the increase in the rate of the autophagic flux there is enhanced sensitization to local apoptogens, an event that would serve to promote apoptosis and the subsequent deletion of chondrocytes from articular cartilage.
The expression profile of AMPK in the growth plate was examined by immunohistochemistry. We showed that epiphyseal chondrocytes express AMPKa1; AMPKa2 was not detected. We also determined the expression of AMPK in N1511 chondrocytes, a cell line that undergoes rapid maturational changes in culture (23). The cultured chondrocytes expressed AMPKa1; AMPKa2 was not evident in maturing cells. While AMPKa1 positive cells were seen throughout the growth plate, the most densely stained cells were localized to the pre-hypertrophic and hypertrophic regions of the epiphysis. Remarkably, the location of the maximally positive chondrocytes was similar to that described for both Beclin-1 and LC-3, two major indicators of autophagic vacuole formation (3). We have also shown that the high HIF-1a immunoreactivity in the maturing cell zone was linked indirectly to the expression of Beclin-1 and correlated well with other determinants of chondrocyte energy status (the adenylate charge ratio, the ATP/ADP ratio and the redox ratio (1). Since these latter measures indicate that the two regions were under metabolic stress (1), it would not be unreasonable to conclude that regional HIF-dependent changes in energy metabolism are related to the rise in AMPK activity and the induction of autophagy. AMPK-dependent induction of autophagy would serve to sustain the viability of the cells during the final stages of the terminal differentiation pathway.
We investigated the relationship between AMPK and autophagy using gene-silencing technology and by activating the calcium/ER stress response. Based on earlier observations that thapsigargin-mediated release of intracellular calcium stores triggers autophagy (21, 22), we treated gene-silenced chondrocytes with low levels of the drug. Our results demonstrated that thapsigargin-mediated activation of AMPK was dependent on HIF-1, a previously described activator of autophagic flux (3, 4). Thus, when HIF-1 was silenced, AMPK activation was blocked; in contrast, there robust AMPK activation when control chondrocytes were treated with thapsigargin. We next examined this relationship from an autophagic perspective: the distribution of the microtubule-associated protein, LC3, was evaluated after thapsigargin treatment. Drug treatment caused redistribution of LC3 into distinct puncta, indicative of an induction of chondrocyte autophagy. The observation that thapsigargin treatment promoted the expression of LC3II confirmed the finding that the cells were autophagic. These results suggest that AMPK activation by thapsigargin is dependent on HIF-1, and, even more importantly, lends considerable strength to the notion that these two factors regulate the induction of thapsigargin-mediated autophagy.
To test the hypothesis that AMPK was directly linked to the onset of autophagy, we assessed the expression of two key molecules, the autophagy associated BH3 domain only protein, Beclin-1 and the pro-survival protein, Bcl-2. In this study, AMPK silenced cells were treated with thapsigargin. Immunoprecipitation followed by Western blot analysis showed that drug treatment resulted in dissociation between Beclin-1 and Bcl-2; however, when AMPK was silenced, following immunoprecipitation, the proteins remained associated even after thapsigargin treatment. Based upon these findings it is concluded that thapsigargin-mediated induction of autophagy is dependent on the activity of AMPK.
Since autophagy can serve to delay the onset of apoptosis, it raised the following question: is there a direct relationship between the induction of autophagy and apoptosis? To test this notion, we treated the AMPK silenced chondrocytes with a powerful apoptogen, H2O2. We noted that the silenced cells were almost completely refractory to the apoptogen challenge. This result was surprising, as AMPK is an important sensor of the cellular energy status and a loss of activity would be expected to compromise the viability of cells, especially when challenged with peroxide. One explanation for the observed resistance is that AMPK regulates the activity of mTOR, a protein that is responsive to the Akt survival pathway. Thus, the possibility exists that by activating a signaling pathway that promotes cell survival, AMPK suppression promotes apoptogen resistance. Indeed, we found that AMPK suppression led to a significant increase in pAkt expression. When mTOR was silenced, there was extensive reorganization of LC3, indicating that the kinase was regulating development of the autophagic state; AMPK inhibition did not block this activity. Based on this finding, it is concluded that the mechanism by which autophagy is controlled in chondrocytes is through mTOR, which is sensitive to, and regulated by, AMPK. Furthermore, caspase-8 activity and BID cleavage were reduced in the AMPK silenced cells. These findings suggested that while AMPK was required for the induction of autophagy, its suppression results in the stimulation of survival signaling and suppression of apoptosis. Accordingly, in a low energy state while AMPK is activated, inhibition of mTOR leads to decreased Akt survival signaling. Low survival pathway activity and promotion of caspase-8 and BID activities would lead to loss of viability and increased sensitivity to local apoptogens.
In summary, we show that in chondrocytes, AMPK, an enzyme that monitors the energy status of the cell, is activated in a HIF-1 dependent manner. This enzyme responds to the energy status and intracellular calcium fluxes; inhibition of mTOR promotes development of the autophagic state. We also provide evidence that suppression of AMPK activates mTOR and survival signaling mediated by the Akt pathway. The relationship between HIF-1, AMPK, mTOR and autophagy is shown schematically in Fig. 6. The importance of these, key regulators of cell function have hitherto escaped serious study and their detailed role in chondrocyte biology has yet to be determined. However, modulation in the autophagic flux has recently been correlated with the hypertrophic and osteoarthritic phenotype (5, 6). Furthermore, the autophagic flux in the growth plate has not been studied from the perspective of growth retardation as a result of chronic renal disease. Detailed studies of the relationship among HIF-1, chondrocyte hypertrophy, chronic renal disease and autophagy, and their regulation at the AMPK-mTOR axis is currently under intense investigation.
Figure 6. Schematic of the interrelationships among AMPK, HIF-1, mTOR and autophagy.
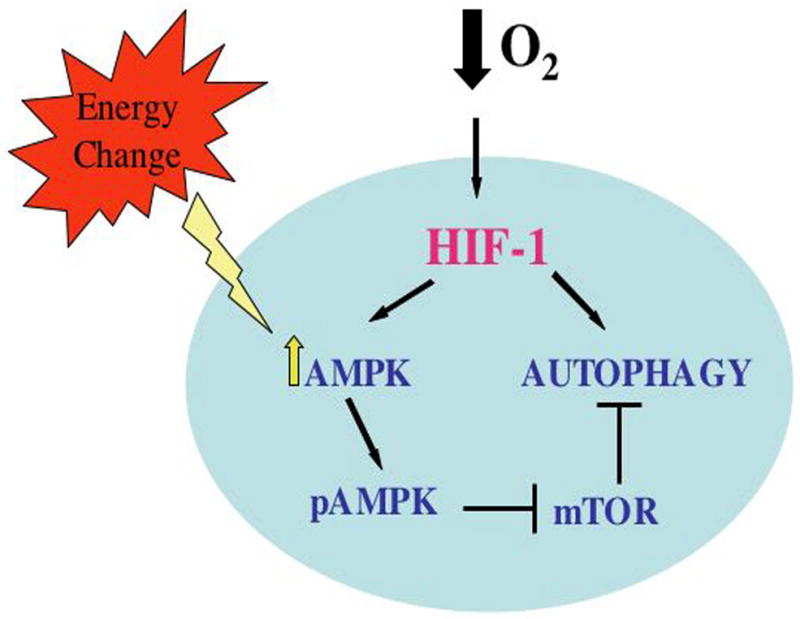
A lowering of the energy charge during tissue hypoxia activates HIF-1 which then results in the phosphorylation and activation of AMPK. Once activated, AMPK suppresses mTOR, thereby activating autophagic flux. In addition, the flux is also directly activated by HIF-1.
Acknowledgments
Contract grant sponsor: National Institutes of Health: DE 015694 and DE 016383 (to VS) and DE 010875 and DE 013319 (to IMS)
This work was supported by NIH grants DE 015694 and DE 016383 (to VS) And DE 010875 and DE 013319 (to IMS).
References
- 1.Rajpurohit R, Koch CJ, Tao Z, Teixeira CM, Shapiro IM. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. Journal of Cellular Physiology. 1996;168(2):424–432. doi: 10.1002/(SICI)1097-4652(199608)168:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15(21):2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohensky J, Shapiro IM, Leshinsky S, Terkhorn SP, Adams CS, Srinivas V. HIF-1 Regulation of Chondrocyte Apoptosis: Induction of the Autophagic Pathway. Autophagy. 2007;3(3):207–214. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Srinivas V, Bohensky J, Zahm AM, Shapiro IM. Autophagy in mineralizing tissues: Microenvironmental perspectives. Cell Cycle. 2009;8:391–393. doi: 10.4161/cc.8.3.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, Srinivas V. Regulation of Autophagy in Cartilage: HIF-2 Suppresses Chondrocyte Autophagy. Arthritis & Rheumatism. 2009;60:1406–1415. doi: 10.1002/art.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–42. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- 8.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27 (kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 9.Levine B. Unraveling the role of autophagy in cancer. Autophagy. 2006;2:65–66. doi: 10.4161/auto.2.2.2457. [DOI] [PubMed] [Google Scholar]
- 10.Klionsky DJ. Autophagy Curr Biol. 2005;15:R282–R283. doi: 10.1016/j.cub.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 12.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobukuni T, Kozma SC, Thomas G. hvps34, an ancient player, enters a growing game: mTOR Complex1/S6K1 signaling. Curr Opin Cell Biol. 2007;19:135–41. doi: 10.1016/j.ceb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 15.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 16.Bohensky J, Shapiro IM, Leshinsky S, Watanabe H, Srinivas V. PIM-2 is an independent regulator of chondrocyte survival and autophagy in the epiphyseal growth plate. J Cell Physiol. 2007;213:246–251. doi: 10.1002/jcp.21117. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto H, Debolt K, Shapiro IM. Adenine, guanine, and inosine nucleotides of chick growth cartilage: relationship between energy status and the mineralization process. Journal of Bone & Mineral Research. 1988;3(3):347–352. doi: 10.1002/jbmr.5650030315. [DOI] [PubMed] [Google Scholar]
- 18.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281(46):34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 19.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 20.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Gordon PB, Holen I, Fosse M, Røtnes JS, Seglen PO. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem. 1993;268:26107–12. [PubMed] [Google Scholar]
- 22.Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jäättelä M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Terkhorn SP, Bohensky J, Shapiro IM, Koyama E, Srinivas V. Expression of HIF prolyl hydroxylase isozymes in growth plate chondrocytes: Relationship between maturation and apoptotic sensitivity. J Cell Physiol . 2007 Jan;210(1):257–65. doi: 10.1002/jcp.20873. [DOI] [PubMed] [Google Scholar]
- 24.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–5. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]