Abstract
Semantic short-term memory (STM) deficits have been traditionally defined as an inability to maintain semantic representations over a delay (R. Martin, Shelton & Yaffee, 1994). Yet some patients with semantic STM deficits make numerous intrusions of items from previously presented lists, thus presenting an interesting paradox: Why should an inability to maintain semantic representations produce an increase in intrusions from earlier lists? In this study, we investigated the relationship between maintenance deficits and susceptibility to interference in a group of 20 aphasic patients characterized with weak semantic or weak phonological STM. Patients and matched control participants performed a modified item-recognition task designed to elicit semantic or phonological interference from list items located one, two, or three trials back (Hamilton & R. Martin, 2007). Controls demonstrated significant effects of interference in both versions of the task. Interference in patients was predicted by the type and severity of their STM deficit; that is, shorter semantic spans were associated with greater semantic interference and shorter phonological spans were associated with greater phonological interference. We interpret these results through a new perspective, the reactivation hypothesis, and we discuss their importance for accounts emphasizing the contribution of maintenance mechanisms for STM impairments in aphasia as well as susceptibility to interference.
Keywords: WORKING-MEMORY, RECENT-NEGATIVES, INHIBITION, SEMANTIC, PHONOLOGICAL
Though it is uncontroversial to state that short-term memory (STM) deficits are common in acquired aphasia (N. Martin & Ayala, 2004), a debate is currently in progress regarding the root of these patients’ limited capacity to temporarily hold linguistic information in mind. The lightning rod in the discussion is a patient called ML; following a left fronto-parietal stroke, his performance on standard delayed-probe and immediate serial recall tasks was found to be dramatically deficient compared to age- and education-matched controls (Freedman & R. Martin, 2001; R. Martin & He, 2004; R. Martin & Lesch, 1996). Notably, his STM deficit was pure in that there was minimal language impairment outside of STM, and the deficit was more pronounced for semantic information. His serial recall span was affected by phonological variables such as phonological similarity and length, and his rhyme probe span was greater than his category probe span (Freedman & R. Martin, 2001). A considerable body of work attributes the absence of semantic effects on his STM span (i.e., lexical status, concreteness) to a rapid loss of semantic representations from a specialized buffer (Freedman & R. Martin, 2001; R. Martin & Freedman, 2001; R. Martin, Lesch, & Bartha, 1999; R. Martin & Romani, 1994; R. Martin, Shelton, & Yaffee, 1994). At odds with this interpretation, however, was the nature of ML’s errors on serial recall tasks: he made numerous intrusions of items from previously presented lists (Hamilton & R. Martin, 2005; R. Martin & Lesch, 1996). This piece of data presents an intriguing paradox: why should an inability to maintain semantic representations produce an increase in intrusions from earlier words?
Hamilton and R. Martin (2005, 2007) offered an alternative to the standard account: for patients with deficient semantic STM, performance may be reduced on delayed probe and immediate serial recall tasks because of a cognitive control impairment, namely an inability to inhibit previously presented items. The notion that STM capacity can be defined in terms of the integrity of control mechanisms (e.g., inhibition) that operate upon currently activated representations is not new (Engle, Kane, & Tulhosky, 1999; Hasher & Zacks, 1988). Older adults’ STM capacity improves when proactive interference is reduced in reading span tasks (May, Hasher & Kane, 1999), consistent with the idea that inhibition operates to prevent irrelevant information from entering or remaining in STM. The presence of distracting stimuli during a delay period in short-term recognition tasks causes worse performance in older relative to younger adults (Gazzaley, Sheridan, Cooney, & D’Esposito, 2007). Even within younger participants, individuals with higher STM spans are those who have a greater ability to resist shifting their eyes towards an irrelevant stimulus (Kane, Bleckley, Conway, & Engle, 2001). In sum, there exists a positive relationship between STM capacity and the ability to manage interference (Awh & Vogel, 2008; Cowan & Morey, 2006; Hedden & Yoon, 2007; Kane, Conway, Hambrick, & Engle, 2007; Vogel, McCullough, & Machizawa, 2005).
Hamilton and R. Martin (2005) supported their inhibition hypothesis for STM deficits with evidence of exaggerated interference effects in ML’s performance on a few verbal tasks (i.e., Stroop, recent-negatives task) in which accurate responses putatively require an inhibitory mechanism. In a subsequent experiment (Hamilton & R. Martin, 2007), they examined ML’s susceptibility to proactive interference by means of an adaptation of Monsell’s (1978) item-recognition task, a variant of which was also used in the current study. This task called for ML to briefly view three words sequentially on a computer screen (called the “memory set”). A one-second delay period was followed by a test item (the “probe”), at which point ML decided (YES or NO) if the probe matched any item in the memory set. Half the trials necessitated a negative response and half a positive response, though for present purposes only the NO trials are of interest. Interference was created in the task through a manipulation of semantic or phonological similarity: some probes were semantically or phonologically related to one of the items from the same memory set (“Related-same”) or the previous trial’s memory set (“Related-previous”). On the rest of the trials, probes were not related to any item from the prior three memory sets (“Unrelated” trials). On NO trials, ML had significantly longer response latencies and more errors on Related relative to Unrelated trials. This occurred on both phonologically and semantically related trials, and in both Related-same and Related-prior conditions. Two unimpaired control groups (older adults: n = 14; younger adults: n = 24) also demonstrated semantic and phonological interference, albeit only with the latency measure and only with Related-same probes.
Hamilton and R. Martin (2007; see also Hamilton & R. Martin, 2005) proposed that these interference effects could be accounted for if ML’s deficits were defined as an inability to inhibit already activated verbal representations in STM. In particular, for tasks such as the item-recognition task described above, an inhibition mechanism allows for the ‘deletion’ of memory traces from one trial to the next. If this mechanism is intact, the contents of working memory are restricted to task-relevant information through the gating of task-irrelevant, dominant responses from previous trials (see Hamilton & R. Martin, 2005; see also, Hasher & Zacks, 1988; May, Hasher, & Kane, 1999; Miller, Lin, & Desimone, 1993). However, in patients with semantic STM deficits, an impaired inhibition mechanism may not allow for the successful deletion of task-irrelevant information on a trial-to-trial basis that would bring STM back to a baseline state; as a result, this information persists in working memory causing intrusions from prior trials and, consequently, the observed susceptibility to interference on this task from semantically and phonologically related items.
It is important to note that Hamilton and R. Martin’s (2007) revision of semantic STM deficits as an impaired inhibitory mechanism operating on general verbal information does not necessarily account for the domain-specific effects observed in serial recall tasks. It is not obvious how a weak inhibition mechanism would give rise to an absence of a lexicality effect but presence of a phonological similarity effect in serial recall; a pattern that typically indicates a weaker semantic relative to phonological STM. However, Hamilton and R. Martin (2007) note that patients with semantic STM deficits often demonstrate weak phonological STM as well (R. Martin, Freedman, Jackson, & Lesch, 2003). This weakness is not as dramatic as that found in patients with deficient phonological STM, but it may be enough to allow for subtle yet functionally significant differences in serial recall and also produce an inhibition impairment in tasks like item-recognition.
Moreover, based on the correspondence between ML’s lesion location and the localization of control mechanisms from neuroimaging studies with normal subjects in the inferior frontal gyrus (e.g., Jonides et al., 1998; Thompson-Schill, Jonides, Marshuetz, Smith, D’Esposito, Kan, Knight, & Swick, 2002), Hamilton and R. Martin (2005, 2007) argued that this radical recharacterization of STM deficits might apply selectively to patients with deficient semantic STM. That is, the semantic STM deficit is due to weak inhibition, which predisposes to interference. In contrast, the phonological STM deficit is due to a rapid loss of phonological information from STM. Hamilton and R. Martin (2007) offered as evidence for this distinction the case of a patient with deficient phonological STM who did not demonstrate similar exaggerated interference effects.1
Yet, attempts to replicate the results of Hamilton and R. Martin (2005, 2007) raised challenging questions. In a detailed case analysis of two aphasic patients with STM deficits, the patient with greater phonological (relative to semantic) STM deficit showed exaggerated interference effects across four tasks (Barde, Schwartz, & Thompson-Schill, 2006). Although the patient with greater semantic STM deficit also exhibited interference effects, these effects were much smaller than those shown by the phonological STM patient. Furthermore, these differences were not an effect of aphasia severity. On the Western Aphasia Battery (Kertesz, 1982) the patient with the biggest interference effects (i.e., the phonological STM patient) had an aphasia quotient of 91.5, while the patient showing less interference (i.e., the semantic STM patient) had an aphasia quotient of 82.9. Thus, in contrast to Hamilton and R. Martin’s (2007) prediction that only semantic STM patients would show susceptibility to interference, the findings of Barde et al. have shown that it is possible to observe exaggerated interference effects both in patients diagnosed with semantic and in those diagnosed with phonological STM deficits.
These contradictions between Hamilton and R. Martin (2007) and Barde et al. (2006) led us to the present study, in which we examined susceptibility to interference in a large group of aphasic patients with either semantic or phonological STM deficits. We sought to determine (1) if susceptibility to interference would be reliably associated with weak semantic STM, weak phonological STM, or both; and (2) if there was a specific relationship between type of STM deficit (semantic or phonological) and type of interference (semantic, phonological, or both). Following Hamilton and R. Martin (2007), we posited that a general inhibition (i.e., for verbal information) impairment account would predict that patients with semantic (but not phonological) STM deficits would demonstrate interference effects, whereas a fractionated inhibition account would predict both STM deficit types (semantic and phonological) to be associated with interference susceptibility, as suggested by the data from Barde et al. (2006). We further posited that a general inhibitory account would predict semantic STM deficits to be related to both semantic and phonological interference, whereas a fractionated account would predict semantic STM deficits to be associated with semantic interference and phonological STM deficits to be associated with phonological interference.
Method
Participants
We present data from 20 aphasic patients (12 male, 8 female; M = 58.6 years, SD = 9.9 years). Patients were recruited from the Philadelphia Cognitive Rehabilitation Research Registry (Schwartz, Brecher, Whyte, & Klein, 2005) with the following criteria: (1) left cerebrovascular accident etiology, (2) native speaker of English, (3) right-handed, (4) between the ages of 21 and 80 years, (5) 6 months or longer post-stroke. The patients were all judged to have mild to moderate aphasia based on aphasia quotient scores near or above the normal cut-off (Western Aphasia Battery; Kertesz et al., 1982) and performance on a custom battery of language tests used in our laboratory. Importantly, on the tests of single word semantic and phonological processing (N. Martin, Schwartz, & Kohen, 2005; N. Martin & Saffran, 1997; R. Martin, Yelton, & Shaffee, 1994), all patients scored at or near normal limits. Demographic and clinical data are presented in Table 1, which also includes classification of weaker semantic or phonological STM (based on information presented later). Language data are presented in Table 2. For lesion information, see Figure 1. All participants gave informed consent according to the guidelines of the Albert Einstein Medical Center Institutional Review Board, and were paid for their participation.
Table 1.
Demographic and clinical information for the patients in the study.
| Patient | Gender | Agea | Yrs of Educ. | Mos. Post Onset | Aphasia Quotientb | Lesion Locationc | Weaker STMd |
|---|---|---|---|---|---|---|---|
| BAC | M | 60 | 19 | 144 | 86.6 | F, T, P | P |
| CAC | F | 46 | 12 | 30 | 82.9 | T, P | S |
| CN | F | 76 | 16 | 218 | 72 | F, P | S |
| DU | F | 64 | 12 | 84 | 81.1 | F | P |
| EAC | F | 51 | 16 | 60 | 87.1 | F, T | S |
| EBC | M | 69 | 12 | 312 | 89.8 | P | S |
| EP | F | 51 | 16 | 36 | 84.7 | F, T, P | P |
| IG | M | 71 | 16 | 80 | 89.5 | F | P |
| KBE | M | 51 | 18 | 29 | 62.8 | F, T, P | S |
| KCX | M | 63 | 16 | 24 | 89 | F, T, P | S |
| KD | F | 55 | 12 | 73 | 95.2 | T, P | S |
| KL | M | 59 | 16 | 13 | 92.4 | F, T | P |
| MD | M | 55 | 12 | 104 | 92.2 | F | S |
| MH | M | 52 | 12 | 74 | 96.5 | F | P |
| NCC | F | 70 | 12 | 13 | 93.2 | F | S |
| NH | M | 67 | 12 | 120 | 84.2 | F, P | P |
| NU | M | 47 | 11 | 84 | 95.1 | F | P |
| OE | M | 63 | 12 | 72 | 93.6 | F, T | P |
| TB | F | 37 | 12 | 24 | 91.5 | F, P | S |
| XAI | M | 65 | 12 | 34 | 72.2 | F, P | S |
| Mean | -- | 58.6 | 13.8 | 83.8 | 86.7 | -- | -- |
| St. Dev. | -- | 9.9 | 2.5 | 75.2 | 9.1 | -- | -- |
| Range | -- | 37–76 | 12–19 | 13–312 | 62.8 – 96.5 | -- | -- |
Years at test.
Aphasia quotient, from the Western Aphasia Battery (WAB; Kertesz, 1982).
Damage to left hemisphere, based on MRI or CT-scan reports: F = frontal, P = parietal, T = temporal.
Designation of weaker phonological or semantic STM is taken from Table 8 (see text).
Table 2.
Patients’ performance on tests of single-word semantic and phonological processing.
| Semantic Processing |
Phonological Processing |
|||||
|---|---|---|---|---|---|---|
| Patient | PNVT-Sa | PPVTb | CP-LL1c | PNVT-Pd | ADNDe | RP-LL1f |
| BAC | 95 | 100 | 93 | 98 | 98 | 98 |
| CAC | 96 | 83 | 95 | 96 | 95 | 95 |
| CN | 93 | 83 | 80 | 98 | 90 | 98 |
| DU | 94 | 74 | 85 | 97 | 95 | 93 |
| EAC | 95 | 79 | 93 | 94 | 75 | 98 |
| EBC | 96 | 89 | 93 | 99 | 98 | 100 |
| EP | 99 | 88 | 95 | 100 | 95 | 100 |
| IG | nt | 99 | 90 | nt | 80 | 95 |
| KBE | 92 | 83 | 90 | 99 | 98 | 95 |
| KCX | 98 | 91 | 90 | 98 | 100 | 100 |
| KD | 97 | 86 | 95 | 93 | 93 | 100 |
| KL | 98 | 99 | 95 | 98 | 100 | 98 |
| MD | 96 | 86 | 95 | 96 | 93 | 98 |
| MH | 98 | 90 | 98 | 97 | 88 | 98 |
| NCC | 96 | 79 | 93 | 97 | 98 | 98 |
| NH | 99 | 88 | 85 | 97 | 97 | 95 |
| NU | 99 | 92 | 98 | 99 | 93 | 90 |
| OE | 100 | 86 | 95 | 98 | 80 | 95 |
| TB | 99 | 75 | 90 | 99 | 98 | 100 |
| XAI | nt | 92 | 85 | nt | 93 | 95 |
Philadelphia Noun Verification Task, Semantic Foils Condition (N. Martin, Schwartz, & Kohen, 2005). Score is expressed in terms of percent correct rejections of the semantic foil.
Peabody Picture Vocabulary Test, Form III-A (Dunn & Dunn, 1997). Standard score is reported, where 100 is the (normal) mean and 15 points delineate a standard deviation.
Category Probe Task (Freedman & R. Martin, 2001), list length = 1 item. Score is expressed in terms of percent correct of 40 trials (100% is maximum).
Philadelphia Noun Verification Task, Phonological Foils Condition (N. Martin, Schwartz, & Kohen, 2005). Score is expressed in terms of percent correct rejections of the phonological foil.
Auditory Discrimination (N. Martin & Saffran, 1997), no delay. Score is expressed in terms of percent correct (100% is maximum).
Rhyme Probe Task (Freedman & R. Martin, 2001), list length = 1 item. Score is expressed in terms of percent correct of 40 trials (100% is maximum).
Figure 1.
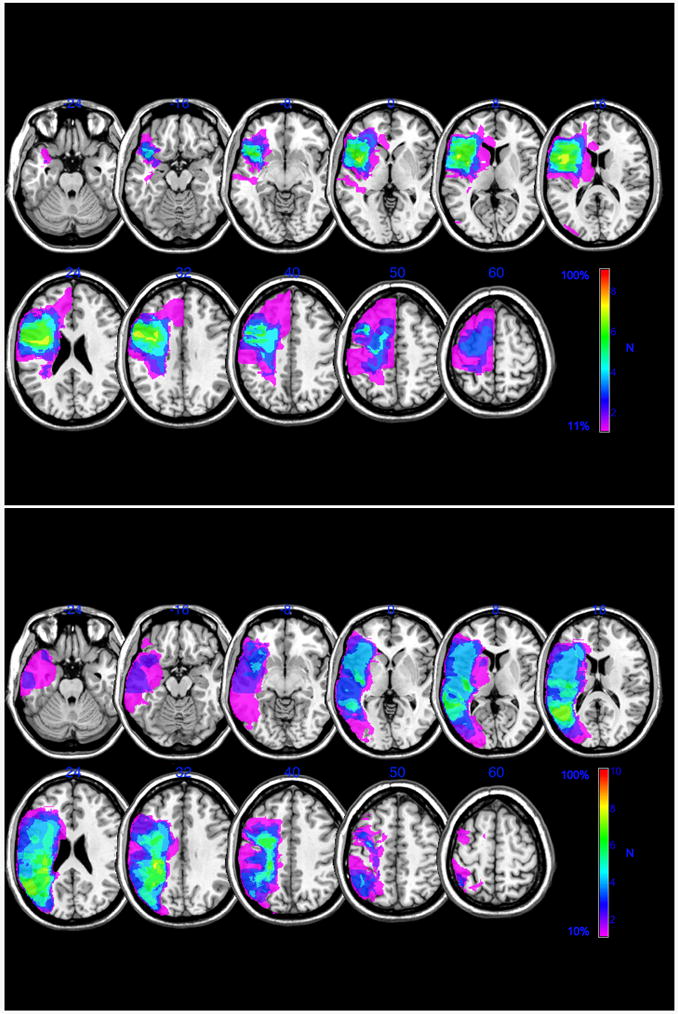
Lesion overlays of patients identified from composite short-term memory scores as predominantly suffering from phonological short-term memory deficits (top panel, n = 9, patients: BAC, DU, EP, KL, MH, NH, NU, OE, TB) or semantic short-term memory deficits (bottom panel, n = 10, patients: CAC, CN, EAC, EBC, KBE, KCX, KD, MD, NCC, XAI). Color bars indicate degree of lesion overlap across patients in each group. Slices correspond to standard Talaraich slices −24, −16, −8, 0, 8, 16, 24, 32, 40, 50, 60 mm. One patient with weaker phonological short-term memory (patient IG) did not wish to have a diagnostic imaging scan; for this reason there were no lesion data available for that patient.
Thirty unimpaired older adults (7 male, 23 female; M = 62.8 years, SD = 9.9 years) were also recruited for participation in this study from the Control Subject Research Registry at the Moss Rehabilitation Research Institute. All participants were native speakers of English without history of neurological injury. All were right-hand dominant, their score on the Mini-Mental Status Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) was ≥ 28, and the group was matched for age (p = .20) and education (p = .45) to the aphasic patients. They gave informed consent according to the guidelines of the Albert Einstein Medical Center Institutional Review Board and were paid for their participation.
Materials and Procedure
Short-Term Memory
We utilized a multi-measurement approach in the evaluation of patients’ phonological and semantic STM2 (Freedman & R. Martin, 2001; N. Martin, Schwartz, & Kohen, 2005; N. Martin & Saffran, 1997; R. Martin, Shelton, & Yaffee, 1994). The auditory-verbal STM assessment battery consisted of four immediate serial recall (ISR) and two delayed-probe (DP) tests; written STM span was not assessed. In each ISR test, lists of items (words or nonwords) were presented in increasing list lengths, beginning with two items. A given list was read aloud at a rate of 1 item per second. Participants repeated the list aloud and in the correct serial order. For each list length, 10 lists were presented until the participant fell at or below 50% performance. ISR span was calculated following R. Martin and colleagues (R. Martin, Shelton, & Yaffee, 1994), that is, last list length plus [proportion lists correctly recalled / .5]. For example, if a patient correctly recalled 3 of 10 lists at list length 3, span was calculated as 2 + [.3 / .5] = 2.6.
For the two DP tests, each participant listened to a list of 1–7 items and responded “yes” or “no” (verbally) to a probe item. In the Rhyme Probe test, participants decided if the probe item rhymed with any of the items in the list. In the Category Probe test, the participants decided if the probe matched any item in the list by semantic category. Testing was discontinued when participants performed at or below 75% correct at any list length. DP span (Rhyme and Category Probe tasks) was calculated as “last list length plus (% correct at last list length – 75)/(% correct at last list length – % correct at current list length).” For example, if a patient scored 84% correct at length 2 and 68% correct at length 3, span was determined to be 2 + [(84 − 75)/(84 − 68)] = 2.56.3
Three tests (resulting in two measures) were used to index the strength of semantic STM: (1) the difference between the immediate serial recall of Words versus NonWords (R. Martin, Shelton, & Yaffee, 1994; Freedman & R. Martin, 2001), and (2) the Category Probe span (R. Martin, Shelton, & Yaffee, 1994; Freedman & R. Martin, 2001). Three tests (resulting in two measures) were used to assess the strength of phonological STM: (1) the difference between the immediate serial recall (ISR) of Non-Rhyming words and Rhyming words (R. Martin, Shelton, & Yaffee, 1994; Freedman & R. Martin, 2001), and (2) the Rhyme Probe span (R. Martin, Shelton, & Yaffee, 1994; Freedman & R. Martin, 2001).
Semantic and Phonological Interference
Similar to Hamilton and R. Martin (2007), we used a modified item recognition task designed to elicit interference from semantic or phonological features shared between a probe and a list item. However, our task differed from theirs in that Related probes shared features with list items appearing either one, two, or three trials back, and not in the current trial. For example, on Related trials in the phonological condition, the probe rhymed with an item in one of three prior memory sets (e.g., sign-wine, see Figure 2, Panel A). We made special effort to reduce the occurrence of orthographically similar rhymes (e.g., line-wine) across the experiment; however, when it did occur we attempted to balance these kinds of pairs across conditions. On Related trials in the semantic condition, the probe shared semantic category membership with an item in one of the three prior memory sets (e.g., shoe-sock, “items of clothing”). Nine categories consisting of 24 category exemplars were taken from a category probe span task (Freedman & R. Martin, 2001). Probe items on Unrelated trials were not related to any list item in the same trial, nor were they related to a list item in the three preceding trials. Different from Hamilton and R. Martin (2007), we did not include the Related-Same condition. We chose not to include the Related-Same condition in the present study to avoid the possibility of influencing the performance of our control subjects by revealing the purpose of the experiment. We will return to this difference in procedure relative to Hamilton and R. Martin (2007) in the discussion.
Figure 2.
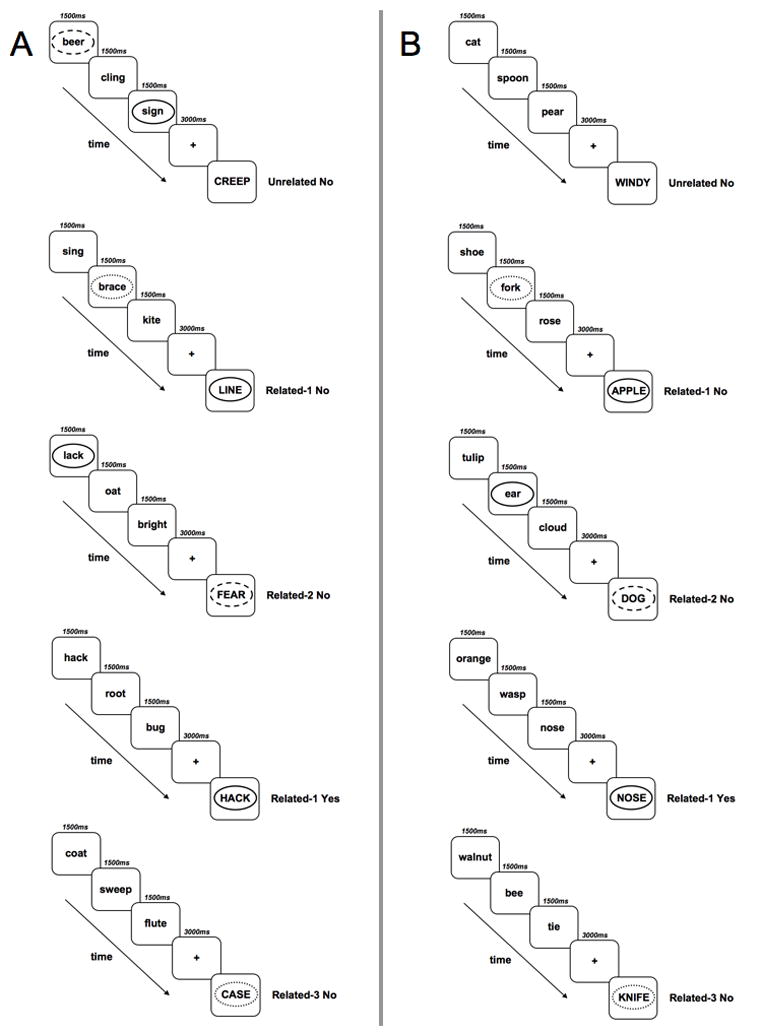
Example trial conditions from our item-recognition task are presented. Circles are for the reader’s ease in understanding the experimental design, and do not appear in the actual task. Panel A: The phonological version. Panel B: The semantic version. Actual trials are presented in pseudorandom order. See text for additional details.
Each participant completed one semantic and one phonological version of the item-recognition task, counterbalanced for order. Thirty-six trials each of Related No, Unrelated No, Related Yes, and Unrelated Yes conditions were pseudorandomly arranged in three equal blocks. Two filler trials began each block, for a total of 150 trials per version. To reduce the likelihood that repetition might cue ‘yes’ responses, a category (semantic or phonological) repeated across trials (Monsell, 1978; Thompson-Schill, et al., 2002). Word stimuli were not repeated across the two versions of the task. Lastly, we used the same number of semantic and phonological categories and approximately the same number of exemplars from each category so that any difference in the magnitude of interference between conditions was less likely to be attributable to stimulus properties.
Participants were not informed of the semantic or phonological relationship between Related probes and prior memory set items. Phonological and semantic versions of the task were blocked such that these trial types were performed separately. All participants completed one version (phonological, semantic) per session, and there were at least two days between sessions. Semantic and phonological interference sessions were given following the STM battery. In a post-experiment debriefing interview, participants were asked if they had noticed anything special about the words used in the task. No participant claimed knowledge or awareness of semantic or phonological relationships between words.
Results
Short Term Memory
Tables 3 and 4 present patients’ spans on ISR and DP tests of phonological and semantic STM, respectively. Investigators sometimes combine ISR difference scores and DP spans into composite scores indexing phonological and semantic STM (Freedman & R. Martin, 2001; N. Martin, Schwartz, & Kohen, 2005; N. Martin & Saffran, 1997; R. Martin, Shelton, & Yaffee, 1994). To assess whether the data justified such a move, we first examined the correlation matrix of phonological and semantic ISR and DP.
Table 3.
Patients’ STM span as measured by ISR and DP tests designed to reflect the integrity of phonological STM. The difference between NonRhyme and Rhyme ISR spans is called the Phonological Similarity Effect: smaller differences indicate weaker phonological STM.
| Immediate Serial Recall Span |
Delayed Probe Span | |||
|---|---|---|---|---|
| Phonological Similarity Effect | ||||
| Subject | NonRhyme | Rhyme | Diff | Rhyme |
| BAC | 3.8 | 2.2 | 1.4 | 1.50 |
| CAC | 4.2 | 2.6 | 1.6 | 5.19 |
| CN | 2.0 | 1.8 | 0.2 | 3.75 |
| DU | 3.8 | 2.8 | 1.0 | 2.29 |
| EAC | 3.6 | 1.6 | 2.0 | 6.00 |
| EBC | 5.8 | 4.0 | 1.8 | 5.00 |
| EP | 3.6 | 3.0 | 0.6 | 2.30 |
| IG | 3.0 | 2.4 | 0.6 | 1.66 |
| KBE | nt | nt | nt | 3.40 |
| KCX | 3.8 | 2.6 | 1.2 | 6.12 |
| KD | 3.8 | 2.6 | 1.2 | 3.81 |
| KL | 4.0 | 2.4 | 1.6 | 3.24 |
| MD | 3.4 | 2.2 | 1.2 | 3.68 |
| MH | 3.8 | 2.0 | 1.8 | 2.00 |
| NCC | nt | nt | nt | 6.80 |
| NH | 2.4 | 2.0 | 0.4 | 1.50 |
| NU | 4.2 | 3.8 | 0.4 | 3.33 |
| OE | 4.2 | 2.8 | 1.4 | 3.50 |
| TB | 4.4 | 3.0 | 1.4 | 4.62 |
| XAI | 4.2 | 2.8 | 1.4 | 3.50 |
| Pt. Mean | 3.8 | 2.6 | 1.2 | 3.73 |
| St. Dev. | 0.8 | 0.6 | 0.5 | 1.6 |
Table 4.
Patients’ STM span as measured by ISR and DP tests designed to reflect the integrity of semantic STM. The difference between Word and NonWord ISR spans is called the Lexicality Effect: smaller differences indicate weaker semantic STM.
| Immediate Serial Recall Span |
Delayed Probe Span | |||
|---|---|---|---|---|
| Lexicality Effect | ||||
| Subject | Word | NonWord | Diff | Category |
| BAC | 3.0 | 2.2 | 0.8 | 3.00 |
| CAC | 3.0 | 2.8 | 0.2 | 3.00 |
| CN | 2.0 | 2.2 | −0.2 | 1.50 |
| DU | 3.0 | 2.4 | 0.6 | 2.79 |
| EAC | 2.4 | 1.8 | 0.6 | 2.45 |
| EBC | 4.4 | 3.8 | 0.6 | 4.62 |
| EP | 2.0 | 1.0 | 1.0 | 5.00 |
| IG | 2.6 | 2.4 | 0.2 | 2.00 |
| KBE | nt | nt | nt | 1.75 |
| KCX | 3.4 | 2.4 | 1.0 | 2.71 |
| KD | 3.2 | 2.4 | 0.8 | 2.71 |
| KL | 3.0 | 2.4 | 0.6 | 4.00 |
| MD | 2.6 | 2.4 | 0.2 | 2.33 |
| MH | 2.8 | 1.4 | 1.4 | 3.70 |
| NCC | nt | nt | nt | 1.80 |
| NH | 2.4 | 1.6 | 0.8 | 2.55 |
| NU | 3.8 | 2.4 | 1.4 | 4.62 |
| OE | 3.4 | 1.8 | 1.6 | 3.00 |
| TB | 4.2 | 3.0 | 1.2 | 3.32 |
| XAI | 4.0 | 3.0 | 1.0 | 1.66 |
| Pt. Mean | 3.1 | 2.4 | 0.8 | 2.82 |
| St. Dev. | 0.7 | 0.6 | 0.5 | 0.9 |
Correlations are shown Table 5. Phonological ISR marginally correlated with rhyme probe span (r = .45, p = .06) and not with category probe span (r = .13, p = .61; these measures were statistically different from each other (t(15) = 2.03, p < .05), suggesting that the phonological ISR tests specifically measured phonological STM strength. Turning to the semantic tests, we found semantic ISR score correlated marginally with category probe span (r = .46, p = .05), but not with rhyme probe span (r = −.09, p = .73); again, these correlations were statistically different from one another (t(15) = 1.82, p < .05). Finally, rhyme and category probe spans did not correlate (r = −.15, p = .54), nor did phonological and semantic ISR measures correlate (r = .19, p = .44).
Table 5.
Correlation matrix of STM tasks.
| Category Probe | Rhyme Probe | Semantic ISR | Phonological ISR | ||
|---|---|---|---|---|---|
| Category | r | −.15 | .46 | .13 | |
| Probe | p | .54 | .05 | .61 | |
| Rhyme | r | −.09 | .45 | ||
| Probe | p | .73 | .06 | ||
| Semantic | r | .19 | |||
| ISR | p | .44 | |||
| Phonological | r | ||||
| ISR | p | ||||
Taken together, the above results argue in favor of creating composite scores as previously described in the literature (Freedman & R. Martin, 2001). Briefly, we calculated four z-scores (Semantic ISR, Phonological ISR, Category Probe, and Rhyme Probe) from the patients’ mean and standard deviation for each STM task. Averaging relevant z-scores together created the two composite scores of interest: (1) Semantic ISR and Category Probe z-scores were averaged to create the Semantic Composite score and (2) Phonological ISR and Rhyme Probe z-scores were averaged to created the Phonological Composite score. Composite STM scores are shown in Table 6. Phonological and Semantic Composite scores did not correlate (r = −.08, p = .75).
Table 6.
Composite STM scores. Phonological composite is the average of Phonological ISR and Rhyme Probe z-scores. Semantic composite is the average of Semantic ISR and Category Probe z-scores.
| Patient | Phon ISR | Rhyme DP | PComp | Sem ISR | Categ DP | SComp |
|---|---|---|---|---|---|---|
| BAC | 0.42 | −1.38 | −0.48 | 0.07 | 0.07 | 0.07 |
| CAC | 0.79 | 0.98 | 0.88 | −1.19 | 0.07 | −0.56 |
| CN | −1.83 | 0.06 | −0.89 | −2.03 | −1.39 | −1.71 |
| DU | −0.33 | −0.88 | −0.60 | −0.35 | −0.13 | −0.24 |
| EAC | 1.54 | 1.49 | 1.52 | −0.35 | −0.46 | −0.41 |
| EBC | 1.16 | 0.86 | 1.01 | −0.35 | 1.65 | 0.65 |
| EP | −1.08 | −0.84 | −0.96 | 0.49 | 2.03 | 1.26 |
| IG | −1.08 | −1.28 | −1.18 | −1.19 | −0.90 | −1.05 |
| KBE | n.t. | −0.17 | −0.17 | n.t. | −1.15 | −1.15 |
| KCX | 0.04 | 1.57 | 0.81 | 0.49 | −0.21 | 0.14 |
| KD | 0.04 | 0.10 | 0.07 | 0.07 | −0.21 | −0.07 |
| KL | 0.79 | −0.27 | 0.26 | −0.35 | 1.05 | 0.35 |
| MD | 0.04 | −.01 | 0.03 | −1.19 | −0.58 | −0.89 |
| MH | 1.16 | −1.06 | 0.05 | 1.33 | 0.76 | 1.04 |
| NCC | n.t. | 2.01 | 2.01 | n.t. | −1.10 | −1.10 |
| NH | −1.45 | −1.38 | −1.42 | 0.07 | −0.37 | −0.15 |
| NU | −1.45 | −0.21 | −0.83 | 1.33 | 1.65 | 1.49 |
| OE | 0.42 | −0.10 | 0.16 | 1.75 | 0.07 | 0.91 |
| TB | 0.42 | 0.61 | 0.51 | 0.91 | 0.39 | 0.65 |
| XAI | 0.42 | −0.10 | 0.16 | 0.49 | −1.24 | −0.37 |
Semantic and Phonological Interference
To enable comparison between the unimpaired control group and our patients, we transformed the response times on the item-recognition task to correct for differences in baseline performance. YES and NO trials were analyzed separately. For each participant, task version (phonological, semantic), and condition (Related-1, Related-2, Related-3) we calculated the effect of relatedness as a percent difference score relative to the Unrelated condition on correct trials only, following the deletion of outliers (latencies greater than 2.5 standard deviations above the participant’s mean for that condition). Negative trials were expected to produce an interference effect, where it takes longer to respond to a Related probe.4
Older adult controls
Controls’ mean difference scores and mean accuracies for each version and condition, NO trials only, are presented in Table 7. The phonological Related-1 condition yielded a significant interference effect of 77.6 ms, or 6.3% (paired-sample t-test: t(29) 5.22, p < .001, see Figure 3). Similarly, the semantic Related-1 condition yielded a significant interference effect of 39.4 ms, or 4%, (paired-sample t-test: t(29) = 2.98, p = .006). With accuracy as the dependent variable, there were no significant interference effects in either phonological or semantic Related-1 conditions.
Table 7.
Mean interference scores (% difference) and mean accuracy (% correct) for the control group, by version (phonological, semantic) and relatedness condition (Related-1, Related-2, Related-3), for NO trials only. See text for detail regarding calculation of difference scores.
| Mean Difference Scores | |||||
|---|---|---|---|---|---|
| Phonological | Semantic | ||||
| Related-1 | Related-2 | Related-3 | Related-1 | Related-2 | Related-3 |
| 6.3 | 4.2 | 1.7 | 4.0 | 3.3 | 0.5 |
| Mean Accuracy | |||||
|---|---|---|---|---|---|
| Phonological | Semantic | ||||
| Related-1 | Related-2 | Related-3 | Related-1 | Related-2 | Related-3 |
| 98.4 | 99.5 | 99.8 | 98.4 | 99.5 | 100 |
Figure 3.
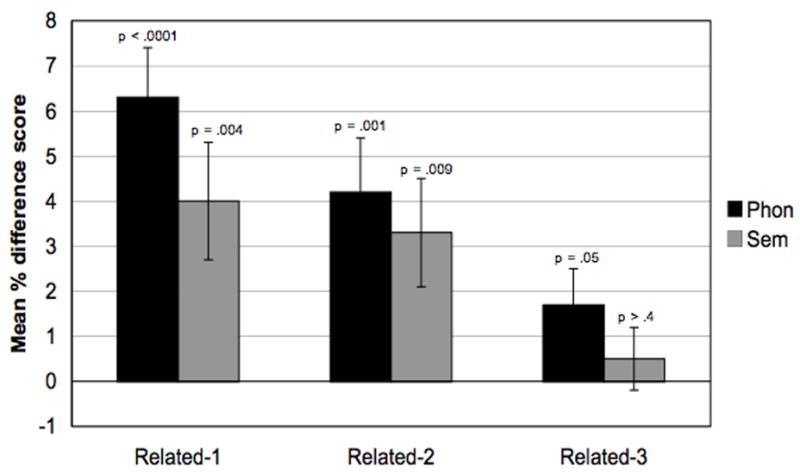
Mean percent difference scores for the control group are denoted by phonological (black columns) and semantic (gray columns) versions of the item-recognition task, NO trials only. Error bars denote one standard error of the condition mean. The p-values denote where one-sample t-tests revealed the magnitude of interference to be significantly greater than zero.
The phonological Related-2 condition produced a significant interference effect of 48.3 ms (6.7%), t(29) = 3.18, p = .004, as did the semantic Related-2 condition (34.1ms, 3.3%), t(29) = 2.50, p = .018. For Related-3, there was a marginal interference effect in the phonological version (18.4 ms, 1.7%), t(29) = 1.81, p = .08 but no effects were observed in the semantic version (4 ms, 0.5%), p = .59. Again, accuracy measures did not reveal any interference effects in either Related-2 or Related-3 conditions.
Patients
Table 8 presents individual difference scores for the Related-1, Related-2, and Related-3 conditions in each version, NO trials only; shading identifies scores ≥ 2 SD of the control mean for that condition. Notably, it is not only the patients with weak semantic STM that demonstrate exaggerated interference on this measure, but patients with weak phonological STM do so as well. Additionally, of the 13 patients who demonstrated at least one exaggerated interference score, 11 patients showed specificity, that is, they showed either semantic or phonological interference.
Table 8.
Interference scores for each patient, grouped by test version (phonological, semantic) and condition (Related-1, Related-2, Related-3), for NO trials only. Boxes denote scores that were ≥ 2SD (shaded) relative to the control mean. Weaker STM denotes the lower of the two composite scores (see text).
| Weaker | Phonological Interference | Semantic Interference | |||||
|---|---|---|---|---|---|---|---|
| Subject | STM | Related-1 | Related-2 | Related-3 | Related-1 | Related-2 | Related-3 |
| BAC | P | 20.0 | 25.3 | 10.6 | −2.5 | 6.8 | −1.3 |
| CAC | S | −4.0 | −7.0 | −3.8 | 7.6 | 0.4 | 1.8 |
| CN | S | 16.2 | 8.5 | 2.4 | 33.6 | 14.5 | 7.6 |
| DU | P | 7.7 | −3.8 | −8.6 | 21.7 | −2.2 | −0.7 |
| EAC | S | −6.1 | −4.3 | 7.4 | 9.4 | 0.2 | −5.0 |
| EBC | S | 0.2 | 1.6 | 1.0 | −0.1 | 1.1 | 0.3 |
| EP | P | 11.6 | 31.6 | −1.7 | 10.6 | 3.7 | 2.2 |
| IG | P | 14.6 | 1.0 | 1.8 | 31.8 | 10.9 | −15.2 |
| KBE | S | −7.9 | 17.1 | −4.5 | 17.8 | 0.4 | 1.9 |
| KCX | S | 4.8 | 8.4 | 0.4 | 0.6 | −1.0 | −1.9 |
| KD | S | 21.3 | −9.7 | 2.2 | 5.6 | 15.4 | 6.0 |
| KL | P | 0.8 | −3.5 | 3.7 | 2.7 | 6.9 | 9.6 |
| MD | S | 0.6 | −8.5 | −8.6 | −0.8 | 8.7 | 9.5 |
| MH | P | 4.9 | −5.2 | 4.0 | −0.1 | 5.6 | −4.0 |
| NCC | S | −4.0 | 11.2 | 8.6 | 4.3 | −0.1 | −7.0 |
| NH | P | 13.8 | 42.1 | −13.0 | −0.6 | −3.7 | 11.1 |
| NU | P | 4.5 | 1.1 | 1.5 | 1.5 | 0.4 | −2.9 |
| OE | P | 24.3 | 23.7 | 7.7 | 1.0 | 14.0 | −8.8 |
| TB | S | 5.8 | 1.1 | 6.2 | 15.9 | 4.4 | 13.9 |
| XAI | S | 1.3 | 4.2 | −3.6 | 20.1 | 7.6 | −0.2 |
| Ctrl | Mn | 6.3 | 4.2 | 1.7 | 4.0 | 3.3 | 0.5 |
| Ctrl | SD | 6.1 | 6.4 | 4.4 | 6.9 | 6.4 | 3.8 |
We used stepwise multiple regression analyses to test for a specific and inverse relationship between STM span and susceptibility to interference. To test this prediction in relation to phonological interference, we entered the phonological composite measure in the first step, and the semantic composite measure in the second step. The prediction would be supported if the beta coefficient corresponding to the main predictor variable (phonological composite) was negative (indicating inverse relationship), and adding the semantic composite at step 2 did not improve the fit of the model (indicating specificity of STM span as a predictor variable). Similarly, to test the prediction in relation to semantic interference, we entered the semantic composite measure in the first step and the phonological composite measure in the second step. These semantic and phonological stepwise regressions were performed separately for Related-1, Related-2, and Related-3 conditions. We will report results for each condition in turn.
Related-1
As shown in Table 9, the Phonological composite explained 33% of the variance in phonological interference at the Related-1 position, and the model was significant (F(1,18) = 8.78, p = .008). As predicted, addition of the Semantic composite at step 2 did not add predictive power (FΔ(1,17) = .61, p = .45). The standardized beta coefficient for the phonological term was negative (β = −0.57).
Table 9.
Coefficients resulting from the stepwise multiple regression of STM composite measures onto phonological (top) and semantic (bottom) interference. In both models, interference difference score is derived from the Related-1 condition.
| Regression of STM onto Phonological Interference | |||
|---|---|---|---|
| Step 1 | B | SE of B | β |
| Phonological Comp | −5.98 | 2.02 | −.57* |
| R2 = .33, F(1,18) = 8.78, p = .008 | |||
| Step 2 | B | SE of B | β |
| Phonological Comp | −5.85 | 2.05 | −.56* |
| Semantic Comp | 1.64 | 2.10 | .15 |
| Adjusted R2 = .27, FΔ(1,17) = .61, p = .45 | |||
| Regression of STM onto Semantic Interference | |||
|---|---|---|---|
| Step 1 | B | SE of B | β |
| Semantic Comp | −6.72 | 2.48 | −.54* |
| R2 = .29, F(1,18) = 7.33, p = .01 | |||
| Step 2 | B | SE of B | β |
| Semantic Comp | −7.06 | 2.33 | −.57* |
| Phonological Comp | −4.30 | 2.26 | −.35 |
| Adjusted R2 = .33, FΔ(1,17) = 3.61, p = .08 | |||
p ≤ .05.
In the model predicting semantic interference, the Semantic composite explained 29% of the variance in semantic interference at the Related-1 position, and the model was significant (F(1,18) = 7.33, p = .01, see Table 9). Adding the Phonological composite did not significantly improve predictive power (FΔ (1,17) = 3.61, p = .08). The standardized beta coefficient for the semantic term was negative (β = −0.54).
Related-2
As Table 10 shows, the Phonological composite explained 16% of the variance in phonological interference from two trials back, and the model was marginally significant (F(1,18) = 3.32, p = .09). As expected, addition of the Semantic composite at step 2 did not add predictive power (FΔ (1,17) = .15, p = .70). The standardized beta coefficient for the phonological term was negative (β = −0.40).
Table 10.
Coefficients resulting from the stepwise multiple regression of STM span (as measured by DP task) onto phonological (top) and semantic (bottom) interference. In both models, interference difference score is derived from the Related-2 condition.
| Regression of STM onto Phonological Interference | |||
|---|---|---|---|
| Step 1 | B | SE of B | β |
| Phonological Comp | −6.28 | 3.45 | −.40 |
| R2 = .16, F(1,18) = 3.32, p = .09 | |||
| Step 2 | B | SE of B | β |
| Phonological Comp | −6.18 | 3.54 | −.39 |
| Semantic Comp | 1.42 | 3.65 | .09 |
| Adjusted R2 = .07, FΔ(1,17) = 0.15, p = .70 | |||
| Regression of STM onto Semantic Interference | |||
|---|---|---|---|
| Step 1 | B | SE of B | β |
| Semantic Comp | −.81 | 1.54 | −.12 |
| R2 = .02, F(1,18) = .28, p = .60 | |||
| Step 2 | B | SE of B | β |
| Semantic Comp | −.92 | 1.55 | −.14 |
| Phonological Comp | −1.41 | 1.50 | −.22 |
| Adjusted R2 = −.05, FΔ(1,17) = 0.88, p = .36 | |||
p ≤ .05.
In the model for semantic interference, the Semantic composite only explained 2% of the variance in semantic interference at the Related-2 position, and the model was nonsignificant (p = .60). The standardized beta coefficient for the semantic term was negative (β = −0.14).
Related-3
As Table 11 shows, the Phonological composite explained 19% of the variance in phonological interference from two trials back, and the model was marginally significant (F(1,18) = 4.07, p = .06). As expected, addition of the Semantic composite at step 2 did not add predictive power (FΔ (1,17) = 1.18, p = .30). The standardized beta coefficient for the phonological term was positive (β = 0.43).
Table 11.
Coefficients resulting from the stepwise multiple regression of STM span (as measured by DP task) onto phonological (top) and semantic (bottom) interference. In both models, interference difference score is derived from the Related-3 condition.
| Regression of STM onto Phonological Interference | |||
|---|---|---|---|
| Step 1 | B | SE of B | β |
| Phonological Comp | 2.97 | 1.47 | .43 |
| R2 = .19, F(1,18) = 4.07, p = .07 | |||
| Step 2 | B | SE of B | β |
| Phonological Comp | 3.10 | 1.47 | .45 |
| Semantic Comp | 1.65 | 1.51 | .23 |
| Adjusted R2 = .15, FΔ(1,17) = 1.18, p = .29 | |||
| Regression of STM onto Semantic Interference | |||
|---|---|---|---|
| Step 1 | B | SE of B | β |
| Semantic Comp | −0.09 | 1.95 | −.01 |
| R2 = .00, F(1,18) = .02, p = .99 | |||
| Step 2 | B | SE of B | β |
| Semantic Comp | −0.17 | 1.99 | −.02 |
| Phonological Comp | −1.09 | 1.94 | −.14 |
| Adjusted R2 = −0.10, FΔ(1,17) = 0.32, p = .58 | |||
p ≤ .05.
In the model for semantic interference, the Semantic composite failed to explain any variance in semantic interference at the Related-3 position, and the model was nonsignificant (p = .94). The standardized beta coefficient for the semantic term was negative (β = −0.01).
General Discussion
Our modified item recognition task elicited significant interference effects in both older control participants and patients with STM deficits. Specifically, for older adult controls strong phonological and semantic interference effects were observed for the Related-1 and Related-2 conditions, with non-reliable findings for the Related-3 condition. Patients classified with either phonological or semantic STM deficits also exhibited interference effects, often significantly more pronounced relative to control participants. Importantly, and particularly for the Related-1 condition, the magnitude of phonological interference effects was predicted by the extent of phonological STM deficit alone, while the magnitude of semantic interference effects was predicted by the extent of the semantic STM deficit alone.
These results differ from the study by Hamilton and R. Martin that inspired the present experiment (Hamilton & R. Martin, 2007; Experiment 2). In that study, the control groups (young and older) did not show significant interference effects from semantically- or phonologically-related items when these items appeared one trial back (i.e., Related-1). This difference from present findings may hinge on their inclusion of a Related-Same condition, where items related to the probe appeared in the current trial. This may have inadvertently called attention to the relatedness manipulation across the experiment and encouraged controls to adopt strategies that minimized its influence.
ML, the semantic STM patient featured in Hamilton and R. Martin (2007), did demonstrate significant interference in the Related-1 condition, and while the effects were numerically greater in the semantic version (4.5 SD above the older controls’ mean) than in the phonological version (2.2 SD above controls), they were significant in both. Hamilton and R. Martin took this as evidence that ML suffered from excessive interference due to an inability to inhibit prior-list information, both semantic and phonological. Furthermore, based on other evidence, they argued that this inhibitory deficit occurred in patients with the semantic-STM deficit profile, and not in those with the phonological-STM deficit profile.
By testing a larger group of STM patients (N = 20) than any previous study on this topic, we obtained a more representative assessment of how deficits in phonological and semantic STM correlate with interference vulnerability. We found that both semantic and phonological STM patients exhibited pronounced interference effects on the modified item recognition task (see Table 8); we further showed for the first time that semantic STM span predicted semantic and not phonological interference, whereas phonological STM span predicted phonological and not semantic interference. If vulnerability to interference from prior-list items is indicative of faulty inhibition in STM, then our results indicate that: (a) the inhibitory mechanism is modality-specific, or at least subject to fractionation under brain damage; and (b) it is not just semantic STM patients who suffer from the inhibitory deficit. Furthermore, if, as has been suggested, the vulnerability of semantic STM patients to interference in this and related tasks indicates that the root of the STM deficit is faulty inhibition, then based on our results, the same thinking should apply to phonological STM deficits.
However, there is another way of explaining the current results that does not necessitate a role for inhibition and that is consistent with traditional, decay-based accounts of the STM deficit. The following sections outline this alternative account, termed the “reactivation hypothesis”, beginning with the exposition and defense of its underlying assumptions.
Information Persistence
The operative assumption in Hamilton and R. Martin’s (2005 in Hamilton and R. Martin’s (2007) explanation of ML’s perseverative errors in immediate serial recall is that in order for previously presented words to interfere with current processing, the memory traces of these prior words must persist in memory. Persistence is nearly always described as though items remain “in STM,” activated above some baseline across trials, strongly enough to compete with the items in the current list. Although this is intuitively appealing, alternative mechanisms are possible. Specifically, in discussing perseverative errors in immediate serial recall, Page and Norris (1998; see also Estes, 1991) note that the time course of the phenomenon actually implies that long-term memory (LTM) mechanisms influence such across-trial errors (for recent evidence from patients with conduction aphasia see Baldo, Kolosterman, & Dronkers, 2008). Moreover, in connectionist models of priming, long-term influences, sometimes called “incremental learning,” have been operationalized in terms of small weight changes between features, or changes to baselines or thresholds (e.g., Cree, McRae, & McNorgan, 1999; Damian & Als, 2005; Howard, Nickels, Coltheart, & Cole-Virtue, 2006; Hsaio, Schwartz, Schnur, & Dell, 2009; Oppenheim, Dell & Schwartz, in press; Plaut & Booth, 2000; Vitkovitch & Humphreys, 1991; Vitkovitch, Kirby, & Tyrrell, 1996; Wheeldon & Monsell, 1994). Following these accounts, it is possible that interference in serial recall, as shown by patients like ML, is not the persistent activation of prior list items in STM; rather, list items may persist across trials by virtue of subtle and incremental changes to their lexical representations following encoding. The reactivation hypothesis extends this line of reasoning to the item-recognition task, with the assumption that the encoding of memory-set items alters their lexical representations in a manner that makes them more likely to be retrieved or reactivated in the presence of a related probe.
Activation and Reactivation in Short-term/Working Memory
The reactivation hypothesis aligns with a view of STM as the temporarily maintained active representations in LTM (Anderson, 1983; Cowan, 1995, 2003; Engle, Kane, & Tuholski, 1999; McElree, 2001, 2006; N. Martin & Saffran, 1997; O’Reilly, Braver, & Cohen, 1999; Ruchkin, Grafman, Cameron, & Berndt, 2003). Contrary to R. Martin et al. (1994), we do not envision separate “storage” buffers to which semantic or phonological information is copied or sent; rather, lexical and other long-term representations, including task-relevant goals, contextual cues, and, perhaps, rules, are “in” STM when attention provides sufficient boost to their baseline activation. A combination of behavioral, neuropsychological, neurophysiological, and neuroimaging evidence supports such a conceptualization of STM/working memory (see Postle, 2006).
It is assumed that in our item recognition task, the probe, possibly by virtue of remaining on screen, is processed to the point of activating its own lexical representation and priming its semantic and phonological neighbors. When the neighbor is a prior-list item, there is a reasonable probability that the priming activation, acting on the incrementally strengthened long-term representation, will suffice to reactivate that item. At that point, the contents of STM will include the current list items, the reactivated item, and the probe. The (semantic or phonological) similarity between the probe and the reactivated prior list item creates conflict (e.g., between a “yes” and “no” decision), which triggers further inspection of these two items (see Figure 4). We assume that such inspection involves the allocation of attention in the form of biasing activation (Desimone & Duncan, 1995) but acknowledge (and discuss later on) the possibility that inhibition could also be involved. The critical point is that this deeper inspection of the contents of STM is what adds time to the latency to arrive at a critical “no” decision in the Related-1 and Related-2 conditions.
Figure 4.
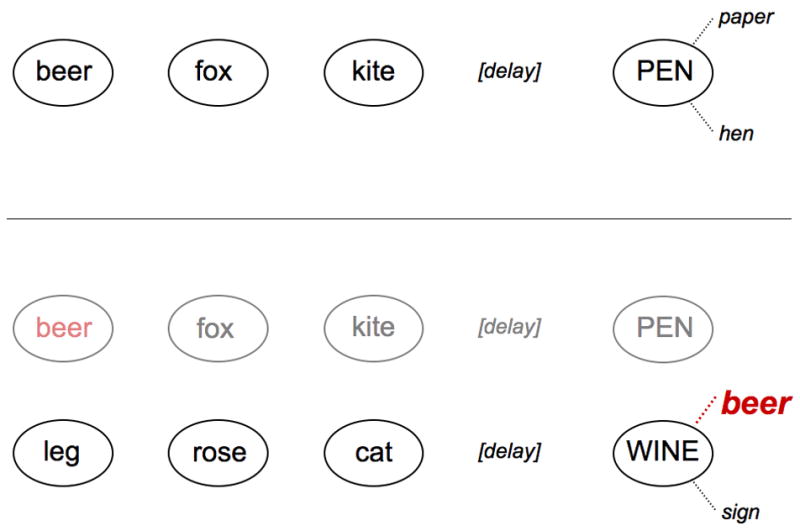
Diagram of reactivation hypothesis in two sample trials in the item-recognition task. The top panel illustrates that presentation of the probe elicits the activation of related LTM representations. The bottom panel illustrates (a) the first trial is no longer in STM (depicted in lighter font) and (b) presenting the probe has reactivated a prior list item (e.g., BEER).
The Nature of the Deficit in Patients
The foregoing explanation is meant to apply to healthy participants and patients alike; that is, we assume that patients do not differ from controls with respect to the encoding of memory set items; the associated, incremental strengthening of the lexical representations; or the likelihood that they will reactivate in the presence of a related probe. This is a reasonable assumption for patients such as ML, and those in the current study, for whom tests of single word semantic and phonological processing are largely normal.5 For patients like these, the traditional account of the STM disorder invokes failure to maintain lexical features in a state of temporary activation within specialized (semantic or phonological) buffers (R. Martin, Shelton, & Yaffee, 1994; Vallar, DiBetta, & Silvieri, 1997) or within the lexical representations themselves (Dell, Schwartz, N. Martin, Saffran, & Gagnon, 1997; N. Martin, Dell, Saffran, & Schwartz, 1994; N. Martin & Saffran, 1997). The latter view is most compatible with the present framework and has the potential to explain the effects in patients. To see how, let us pick up the explanation from the previous section. To arrive at a correct rejection decision, the participant must compare the probe to the contents of STM, which, to repeat, includes the items in the current memory set (all different from the probe) and the reactivated prior-set lure (semantically or phonologically related to the probe). The feature overlap between lure and probe delays the decision by creating conflict and the need for further processing of the contents of STM. For the STM patients, this inspection takes longer, and may be more prone to failure, because relevant features of the lure are inadequately maintained during the inspection process. The size of the semantic STM span is indicative of the problem in maintaining semantic features; the size of the phonological STM span is indicative of the problem in maintaining phonological features. By these span measures, patients’ maintenance deficits were asymmetric (greater for semantic than phonological information, or the reverse), thereby allowing for eventual rejection of the related lure on the basis of the less affected dimension. Nevertheless, we hypothesize that the inability to maintain a clear representation of those features of the lure that are relevant to its overlap with the probe adds decision time proportional to the maintenance deficit for features of that type, as indexed by the span measures. This explains the key aspects of the data, namely the specific and inverse relationship observed between STM composite scores and interference in the item recognition task.
The reactivation hypothesis and inhibition models of interference resolution
We offered the reactivation hypothesis as an alternative toHamilton and R. Martin’s (2007) inhibitory account, which, if it were to account for current findings, would need to postulate separate inhibitory deficits for semantic and phonological information and reject the traditional view that STM deficits are due to faulty maintenance or rapid decay. However, we recognize that a parsimonious account is not always a correct one; it is altogether possible that inhibition and inhibitory deficits make some contribution to the present findings. For example, once the prior-trial lure is reactivated, inhibition might play a role in resolving the conflict it engenders (cf. Hasher & Zacks, 1988; Hulme, Maughan, & Brown, 1991; Lustig, May, & Hasher, 2001; May, Hasher, & Kane, 1999; Rosen & Engle, 1997). And if this inhibitory process helps to speed a correct “no” decision, damage to this process in aphasic patients could contribute to the exaggerated interference effects. In other words, the effects in patients could be caused by a combination of modality-specific maintenance deficits and failure to inhibit a strong competitor, regardless of modality.
The notion that maintenance and inhibition mechanisms may be distinct processes that operate in concert at different stages of interference resolution is compatible with recent work in neuroscience implicating the left inferior prefrontal cortex in interference resolution (e.g., Desimone & Duncan, 1995; D’Esposito et al., 1999; Feredoes, Tononi, & Postle, 2006; Jonides et al., 1998; Thompson-Schill et al., 1998, 2002). Concurrent mechanisms are also compatible with multi-component accounts of interference resolution (e.g., Badre & Wagner, 2005; Gough, Nobre, & Devlin, 2005; see also Jonides & Nee, 2006), including a recent account that postulates a role for time-related decay in a similar item-recognition task in healthy young adults (Berman, Jonides, & Lewis, 2009). Even though time-induced decay may differ qualitatively relative to the observed maintenance deficits in STM patients, the findings of Berman et al. suggest a predictive role of decay for performance, independent of the effects of inhibition.
Conclusions and future directions
The present study presents results from a large group of both semantic and phonological STM patients that demonstrate a specific and inverse relation between STM span and interference in item recognition. Framed within the reactivation hypothesis, these results argue that defective maintenance of semantic and/or phonological information in STM compromises the resolution of conflict between the current probe and reactivated related lure.
A lesson to be drawn from this study is that the dichotomous categorization of patients as having either semantic or phonological STM deficits may complicate interpretations of patterns of interference effects in these groups. An example of the risks of such a dichotomous categorization of patient deficits can be seen in Table 8: under semantic interference, 3 out of 9 patients showing exaggerated effects were categorized as presenting with a phonological STM deficit; conversely, under phonological interference, one out of 6 patients showing exaggerated effects were categorized as presenting with a semantic STM deficit. Based on these findings one could conclude, incorrectly as it turns out, that there was no relation between the type of STM deficit and the type of interference susceptibility. Although we included this analysis to maintain consistency and allow comparisons with previous work (e.g., N. Martin & Ayala, 2004; R. Martin et al., 1994), it should be understood that most patients exhibit concurrent semantic and phonological STM deficits, albeit to different degrees, and that dichotomization can lead to oversimplification or, as in this case, distortion of the underlying relationships.
We believe that a goal for future research should be to clarify the respective contributions of maintenance and inhibition deficits to enhanced interference resolution in item recognition and related tasks. Evidence from lesion mapping can be useful in this regard. The maps presented in Figure 1 showed frontal involvement even in patients with predominantly phonological short-term memory deficits, contrary to what the literature suggests (Shallice & Vallar, 1990; but see also Kinsbourne, 1972, for an example of a phonological STM patient with a fronto-temporal lesion). This should be replicated and, ideally, extended with voxel-based lesion mapping methods (e.g., Bates, Wilson, Saygin, Dick, Sereno, & Knight, 2003; Kimberg, Coslett, & Schwartz, 2007; Schnur, Schwartz, Kimberg, Hirshorn, Coslett, & Thompson-Schill, 2009; Schwartz, Kimberg, Walker, Faseyitan, Brecher, Dell & Coslett, in press). A better characterization of lesion location in conjunction with behavioral measures may allow for differential predictions regarding the sources of interference in these patients and a better conceptualization of the contribution of STM deficits to these effects.
Acknowledgments
This research was supported by a grant awarded to L.H.F.B. from the Albert Einstein Society, by NIDCD grant R01-DC00191-28 awarded to M.F.S., and by NIH grant R01-DC009209 awarded to S.T.S. The authors would like to acknowledge Drs. Randi Martin and Nadine Martin for their generosity in sharing testing materials, Adelyn Brecher, Paula Sobel, Esther Lee, Susan Lipsett, and Grant Walker for assistance in collecting data, and to Anjan Chatterjee, John Trueswell, Gary Dell, and Nadine Martin for their insightful comments during study development. This research was conducted in partial fulfillment of requirements for a doctoral dissertation by the first author at the University of Pennsylvania, Philadelphia, PA, USA.
Footnotes
In some studies, performance and interference errors on STM tests could be related to the size of the sets from which the stimuli were selected and not to different underlying mechanisms for semantic versus phonological STM impairment. However, the STM assessments used in this study, as well as those studies reviewed in the Introduction, draw items from sets of equal size (e.g., 10 items per set for immediate serial recall).
We are grateful to Drs. N. Martin and R. Martin, who generously provided us materials for STM tests that have proven effective in distinguishing phonological from semantic STM deficits.
A patient’s span is calculated relative to 75%, as this is the accuracy cutoff established which the individual is allowed to advance to the next list length (see above). If accuracy on the subsequent list falls below 75%, span is interpolated from the accuracy achieved on the prior list length.
According to Monsell (1978), positive trials should produce a facilitation effect, where subjects would be putatively faster to respond to Related-YES probes. However, this effect has been only sporadically observed in the literature (cf., Jonides & Nee, 2006), and here we found no effects of relatedness on YES trials. As such, we do not include those data here.
Nevertheless, the patients in this study did vary somewhat in how they performed the various lexical processing tasks. To determine if this could account for the interference effects, we examined the pattern of correlations between the 6 lexical processing measures and the semantic and phonological interference measures. For 4 of 6 measures, results were non significant (p’s > .30). This is further evidence that interference effects in these patients were not due to weakness in the actual lexical representations, such as might lead to faulty encoding or reactivation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JR. A spreading activation theory of memory. Journal of Verbal Learning and Verbal Behavior. 1983;22:261–295. [Google Scholar]
- Awh E, Vogel EK. The bouncer in the brain. Nature Neuroscience. 2008;11(1):5–6. doi: 10.1038/nn0108-5. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cerebral Cortex. 2005;15(12):2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Barde LHF, Schwartz MF, Thompson-Schill SL. The role of left inferior frontal gyrus (LIFG) in semantic short-term memory: A comparison of two case studies. Brain and Language. 2006;99(1–2):71–72. [Google Scholar]
- Baldo JV, Klosterman EC, Dronkers NF. It’s either a cook or a baker: Patients with conduction aphasia get the gist but lose the trace. Brain and Language. 2008;105:134–140. doi: 10.1016/j.bandl.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Berman MG, Jonides J, Lewis RL. In search of decay in verbal short-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:317–333. doi: 10.1037/a0014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. Oxford psychology Series. Vol. 26. New York: Oxford University Press; 1995. Attention and memory: An integrated framework. [Google Scholar]
- Cowan N. Varieties of procedural accounts of working memory retention systems. Behavioral and Brain Sciences. 2003;26(6):731–732. doi: 10.1017/s0140525x03000165. [DOI] [PubMed] [Google Scholar]
- Cowan N, Morey CC. Visual working memory depends on attentional filtering. Trends in Cognitive Sciences. 2006;10(4):139–141. doi: 10.1016/j.tics.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree GS, McRae K, McNorgan C. An attractor model of lexical conceptual processing: simulating semantic priming. Cognitive Science. 1999;23:371–414. [Google Scholar]
- Damian MF, Als LC. Long-lasting semantic context effects in the spoken production of object names. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1372–1384. doi: 10.1037/0278-7393.31.6.1372. [DOI] [PubMed] [Google Scholar]
- Dell G, Schwartz MF, Martin N, Saffran E, Gagnon D. Lexical access in aphasic and nonaphasic speakers. Psychological Review. 1997;104:801–38. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related fMRI. Proceedings of the National Academy of Sciences, USA. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3. Bloomington, MN: Pearson Assessments; 1997. [Google Scholar]
- Engle RW, Kane MJ, Tuholski SW. Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In: Miyake A, Shah P, editors. Models of working memory. Cambridge, UK: Cambridge University Press; 1999. pp. 102–134. [Google Scholar]
- Estes WK. On types of item coding and sources of recall in short-term memory. In: Hockley WE, Lewandowsky S, editors. Relating theory and data: In honor of Bennet B. Murdock. Hillsdale, N. J: Erlbaum; 1991. pp. 155–173. [Google Scholar]
- Feredoes E, Tononi G, Postle BR. Direct evidence for a prefrontal contribution to the control of proactive interference in verbal working memory. Proceedings of the National Academy of Sciences. 2006;103(51):19530–19534. doi: 10.1073/pnas.0604509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freedman ML, Martin RC. Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology. 2001;18:193–226. doi: 10.1080/02643290126002. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Sheridan MA, Cooney J, D’Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21(5):532–539. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulations. The Journal of Neuroscience. 2005;25(35):8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Proactive interference in a semantic short-term memory deficit: Role of semantic and phonological relatedness. Cortex. 2007;43:112–123. doi: 10.1016/s0010-9452(08)70449-0. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin R. Dissociations among tasks involving inhibition: A single-case study. Cognitive, Affective and Behavioral Neuroscience. 2005;5(1):1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and new view. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1988. [Google Scholar]
- Hedden T, Yoon C. Individual differences in executive processing predict susceptibility to interference in verbal working memory. Neuropsychology. 2006;20:511–528. doi: 10.1037/0894-4105.20.5.511. [DOI] [PubMed] [Google Scholar]
- Howard D, Nickels L, Coltheart M, Cole-Virtue J. Cumulative semantic inhibition in picture naming: Experimental and computational studies. Cognition. 2006;3(100):464–482. doi: 10.1016/j.cognition.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Hsaio EY, Schwartz MF, Schnur TT, Dell GS. Temporal characteristics of semantic perseverations induced by blocked-cyclic picture naming. Brain and Language. 2009;108(3):133–144. doi: 10.1016/j.bandl.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C, Maughan S, Brown G. Memory for familiar and unfamiliar words: Evidence for a long-term memory contribution to short-term span. Journal of Memory and Language. 1991;30:685–701. [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences, USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Bleckley KM, Conway ARA, Engle RW. A controlled-attention view of working memory capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Hambrick DZ, Engle RW. Variation in working memory capacity as variation in executive attention and control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. New York: Oxford University Press; 2007. [Google Scholar]
- Kertesz A. The Western Aphasia Battery. New York: Grune and Stratton; 1982. [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Kinsbourne N. Behavioral analysis of the repetition deficits in conduction aphasia. Neurology. 1972;22:1126–1132. doi: 10.1212/wnl.22.11.1126. [DOI] [PubMed] [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology: General. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- Martin N, Ayala J. Measurements of auditory-verbal STM span in aphasia: Effects of item, task, and lexical impairment. Brain and Language. 2004;89:464–483. doi: 10.1016/j.bandl.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Martin N, Dell GS, Saffran EM, Schwartz MF. Origins of paraphasias in deep dysphasia: Testing the consequences of a decay impairment to an interactive spreading activation model of lexical retrieval. Brain and Language. 1994;47:609–660. doi: 10.1006/brln.1994.1061. [DOI] [PubMed] [Google Scholar]
- Martin N, Saffran EM. A computational account of deep dysphasia: Evidence from a single case study. Brain and Language. 1992;43:240–274. doi: 10.1016/0093-934x(92)90130-7. [DOI] [PubMed] [Google Scholar]
- Martin N, Saffran EM. Language and auditory-verbal short-term memory impairments: Evidence for common underlying processes. Cognitive Neuropsychology. 1997;14:641–682. [Google Scholar]
- Martin N, Schwartz MF, Kohen FP. Assessment of the ability to process semantic and phonological aspects of words in aphasia: A multi-measurement approach. Aphasiology. 2005;20(2–4):154–166. [Google Scholar]
- Martin RC, Freedman M. Short-term retention of lexical-semantic representations: Implications for speech production. Memory. 2001;9(456):261–280. doi: 10.1080/09658210143000173. [DOI] [PubMed] [Google Scholar]
- Martin RC, He T. Semantic short-term memory and its role in sentence processing: A replication. Brain and Language. 2004;89:76–82. doi: 10.1016/S0093-934X(03)00300-6. [DOI] [PubMed] [Google Scholar]
- Martin RC, Lesch MF. Associations and dissociations between language impairment and list recall: Implications for models of short-term memory. In: Gathercole S, editor. Models of short-term memory. Hove, UK: Erlbaum Publishing; 1996. pp. 149–178. [Google Scholar]
- Martin RC, Lesch M, Bartha M. Independence of input and output phonology in word processing and short-term memory. Journal of Memory and Language. 1999;41:3–29. [Google Scholar]
- Martin RC, Romani C. Verbal working memory and sentence comprehension: A multiple-components view. Neuropsychology. 1994;8(4):506–523. [Google Scholar]
- Martin RC, Shelton JR, Yaffee LS. Language processing and working memory: Neuropsychological evidence for separate phonological and semantic capacities. Journal of Memory and Language. 1994;33:83–111. [Google Scholar]
- Martin RC, Wu D, Freedman M, Jackson EF, Lesch M. An event-related fMRI investigation of phonological versus semantic short-term memory 2003 [Google Scholar]
- May CP, Hasher L, Kane MJ. The role of interference in memory span. Memory and Cognition. 1999;27:759–767. doi: 10.3758/bf03198529. [DOI] [PubMed] [Google Scholar]
- McElree B. Working memory and focal attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:817–835. [PMC free article] [PubMed] [Google Scholar]
- McElree B. Accessing recent events. In: Ross BH, editor. The psychology of learning and motivation. Vol. 46. San Diego: Academic Press; 2006. [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: Time course of recognition. Journal of Experimental Psychology: General. 1989;118:346–373. [Google Scholar]
- Miller EK, Lin L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. Journal of Neuroscience. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Recency, immediate recognition, and reaction time. Cognitive Psychology. 1978;10:465–501. [Google Scholar]
- Oppenheim GM, Dell GS, Schwartz MF. The dark side of incremental learning: A model of cumulative semantic interference during lexical access in speech production. Cognition. doi: 10.1016/j.cognition.2009.09.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Braver TS, Cohen JD. A biologically-based computational model of working memory. In: Miyake A, Shah P, editors. Models of working memory. Cambridge, UK: Cambridge University Press; 1999. pp. 102–134. [Google Scholar]
- Page MPA, Norris D. The primacy model: A new model of immediate serial recall. Psychological Review. 1998;105:761–781. doi: 10.1037/0033-295x.105.4.761-781. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Booth JR. Individual and developmental differences in semantic priming: Empirical and computational support for a single-mechanism account of lexical processing. Psychological Review. 2000;107:786–823. doi: 10.1037/0033-295x.107.4.786. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology: General. 1997;126:211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Grafman J, Cameron K, Berndt RS. Working memory retention systems: A state of activated long-term memory. Behavioral and Brain Sciences. 2003;26:709–728. doi: 10.1017/s0140525x03000165. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: A strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of Physical Medicine and Rehabilitation. 2005;86:1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB. Anterior temporal involvement in semantic word retrieval: VLSM evidence from aphasia. Brain. doi: 10.1093/brain/awp284. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences. 2009;106:322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Vallar G. The impairment of auditory-verbal short-term storage. In: Vallar G, Shallice T, editors. Neuropsychological impairments of short term memory. Cambridge, Englad: Cambridge University Press; 1990. pp. 11–53. [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Kurtz KJ, Gabrieli JDE. Effects of semantic and associative relatedness on automatic priming. Journal of Memory and Language. 1998;38:440–458. [Google Scholar]
- Vallar G, Di Betta AM, Silveri MC. The phonological short-term store-rehearsal system: Patterns of impairment and neural correlates. Neuropsychologia. 1997;35:795–812. doi: 10.1016/s0028-3932(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Vitkovitch M, Humphreys GW. Perseverant responding in speeded picture naming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:664–680. [Google Scholar]
- Vitkovitch M, Kirby A, Tyrrell L. Patterns of excitation and inhibition in picture naming. Visual Cognition. 1996;3:61–80. [Google Scholar]
- Vogel EK, McCullough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Wheeldon LR, Monsell S. Inhibition of spoken word production by priming a semantic competitor. Journal of Memory and Language. 1994;33:332–356. [Google Scholar]


