Abstract
Thyroid transcription factor-1 (TTF-1) is a transcription factor that plays a role in the development and physiology of the thyroid and lungs. Expression of TTF-1 is used as a marker of lung and thyroid clinically. Commercially available clones of TTF-1 monoclonal antibodies, 8G7G3/1 and SPT24, have been reported to have different sensitivities for the detection of neoplasms of different origins. Although they are used extensively in daily practice, a comprehensive comparative study of these antibodies in a wide variety of neoplasms is lacking. We examined TTF-1 expression in primary tumors of the lung, prostrate, pancreas, stomach, salivary glands, breast, bladder, colon and squamous cell carcinoma of the head and neck and compared the results obtained with both TTF-1 clones. The SPT24 clone detected more primary lung tumors of all histologic subtypes. Importantly, the SPT24 clone detected a significantly higher number of squamous cell carcinomas and carcinoid tumors of the lung. Among non-pulmonary primary tumors, a significant number of invasive urothelial carcinoma of the bladder (5.1%) was TTF-1 positive. Additionally, a small proportion of prostate (1.2%), stomach (0.9%), salivary gland (1.8%), and colon (2.5%) carcinomas were positive with both clones. Notably, both clones stained the same non-pulmonary tumors with similar intensity and distribution. Carcinomas of the pancreas, breast and squamous cell carcinomas of the head and neck were negative with both clones. In summary, the SPT24 clone detected a higher number of pulmonary non-small cell tumors of all histologic subtypes while both clones stained a similar proportion of non-pulmonary tumors.
Keywords: Thyroid transcription factor-1, SPT24 clone, 8G7G3/1 clone, tissue microarrays
Introduction
Thyroid transcription factor-1, also known as Nkx2.1 or thyroid-specific enhancer binding-protein, is a 38 kDa DNA-binding protein containing 371 amino acids. It is encoded by a gene located on chromosome 14q13 and is preferentially expressed in the thyroid and lung. In the thyroid, TTF-1 is expressed in follicular cells and C-cells in developing and adult thyroid gland where it activates thyroglobulin and thyroperoxidase gene transcription (1-4). In the lung, the human TTF-1 protein has been detected during development as early as 11 weeks of gestation and is thought to play a role in the regulation of surfactant B production (5).
TTF-1 expression is employed widely as a marker of lung and thyroid tumors (6-8). In carcinomas of the lung, TTF-1 expression has been reported to be positive in nearly all small cell carcinomas, in the majority of adenocarcinomas and non-mucinous bronchio-alveolar carcinomas; however, TTF-1 is negative in squamous cell carcinomas and mucinous bronchio-alveolar carcinomas (6). As the lung is one of the most common sites of metastasis, TTF-1 is used as a reliable marker to distinguish between primary and secondary lung tumors, especially when dealing with an adenocarcinoma or a large-cell carcinoma (8-10).
Although, TTF-1 expression is thought to be quite specific for thyroid and lung, TTF-1 immunostaining has been reported be positive in cases of colonic adenocarcinoma (11), ovarian epithelial neoplasms (12), and uterine tumors (13, 14).
Recently, new clones of TTF-1 antibodies have become commercially available and their sensitivity and specificity in the detection of different types of lung and non-lung tumors has been partially reported. The most widely used in diagnostic laboratories is the clone 8G7G3/1 by Dako (15). Subsequently, SPT24 was introduced to the market and reported to be less specific for lung tissue since it was positive in occasional cases of metastatic colonic adenocarcinoma (11). Here, we performed a comprehensive comparative study of TTF-1 expression utilizing both clones in a series of primary tumors of the lung, as well as tumors of the prostrate, pancreas, stomach, salivary glands, breast and squamous cell carcinoma of the head and neck.
Material and Methods
Tissue samples
Tissue microarrays were constructed from archival paraffin embedded tissue samples from patients with pulmonary and non-pulmonary primary tumors. Metastatic tumors of the lung were studied by whole-block tissue sections. Cases were retrieved by diagnosis search from the surgical pathology files of Rhode Island Hospital and The Miriam Hospital/Lifespan Academic Medical Center, Providence, RI. The study was performed in accordance with the guidelines and approval from the Institutional Review Committee for Research on Human Subjects. Primary lung tumor microarrays were composed of 374 cases, including 185 adenocarcinomas, 97 squamous cell carcinomas, 47 large cell carcinomas, 23 carcinoid tumors and 22 unclassified tumors. Non-lung primary tumors included 160 prostate, 120 colon, 110 gastric and 110 pancreatic ductal adenocarcinomas, 98 invasive urothelial carcinomas of the bladder, 56 salivary gland tumors, 38 squamous cell carcinomas of head and neck and 34 invasive ductal carcinomas of the breast. None of the patients with non-lung tumors had a history of lung cancer, based on chart review.
Immunohistochemistry
Tissue microarrays and tissue sections were immunostained using monoclonal antibodies to TTF-1, clones 8G7G3/1 (dilution 1:100, Dako North America, Inc. Cartinteria, CA) and SPT24 (dilution 1:25, Novocastra Laboratories Ltd., Newcastle Upon Tyne, NE12 8EW, United Kingdon). Both antibodies are IgG1 kappa isotype. Staining was performed using the Ventana Discovery System (Ventana. Tucson, AZ). Antigen retrieval was performed using the Cell Conditioning Solution (CC1) kit from Ventana following the manufacturer protocol. Reduction of endogenous peroxidase activity, background blocking and chromogen system was performed by using the Discovery DAB Map kit (RUO) from Ventana following the manufacturer protocol. Counter-stain was done with hematoxylin. Thyroid microarrays constructed with tumor and normal tissue were used as positive controls. The immunohistochemical stains were scored using a two-tiered scoring system: positive or negative. A positive score was based on moderate to strong nuclear staining. AM and LJW independently scored each of the sections without knowledge of the histologic diagnosis or staining pattern with the other marker. There was a high correlation between the two scores and in the few discrepant cases; a consensus was reached after joint review.
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism Software. P values were calculated using Fisher's exact test.
Results
Pulmonary primary tumors
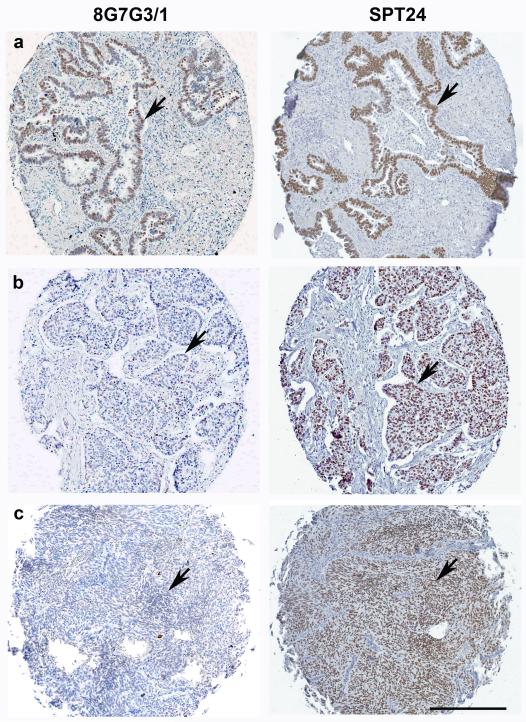
A total of 374 cases of non-small cell carcinoma of the lung was evaluated (Table 1). Staining was limited to the nuclei where it was present as a finely granular diffuse pattern in the majority of the cells. Notably, SPT24 detected a higher number of positive cases through all histologic subtypes of lung tumors. Expression of TTF-1 in adenocarcinomas was similar using both antibody clones, with a trend towards higher staining with the SPT24 clone (P=0.08) (Figure 1a). The differential staining was highest in squamous cell carcinoma and carcinoid tumors. In squamous cell carcinomas, the SPT24 clone detected 14 of 97 (16.8%) cases as opposed to the 8G7G3/1 clone which detected 1 of 97 (1%) cases(P=0.003) (Figure 1b). Staining of carcinoid tumors was also significantly higher with SPT24 clone, which detected 14 of 23 (60.8%) cases as opposed to the 8G7G3/1 clone which detected 4 of 23 (17.4%) cases (P=0.003)(Figure 1c). The proportion of large cell carcinomas and poorly differentiated carcinomas stained similar with both antibody clones.
Table 1.
Summary of immunohistochemistry results.
| Total cases | SPT24 | 8G7G3/1 | P | |
|---|---|---|---|---|
| Lung | 374 | |||
| Adenocarcinoma | 185 | 134 (72.4%) | 121 (65.4%) | 0.08 |
| Large Cell | 47 | 22(46.8%) | 17(36.2%) | 0.201 |
| Carcinoid | 23 | 14(60.8%) | 4(17.4%) | 0.003 |
| Squamous Cell | 97 | 14(16.8%) | 1(1.0%) | 0.003 |
| Unclassified | 22 | 10(45.5%) | 7(31.8%) | 0.26 |
| Bladder | 98 | 5 (5.1%) | 5 (5.1%) | NS |
| Colon | 120 | 3 (2.5%) | 3 (2.5%) | NS |
| Prostate | 160 | 2(1.2%) | 2(1.2%) | NS |
| Stomach | 110 | 1(0.9%) | 1(0.9%) | NS |
| Salivary Gland | 56 | 1(1.8%) | 1(1.8%) | NS |
| Squamous cell carcinoma of head and neck | 38 | 0(0%) | 0(0%) | NS |
| Pancreatic adenocarcinomas | 110 | 0(0%) | 0(0%) | NS |
| Breast | 34 | 0(0%) | 0(0%) | NS |
NS: not significant
Figure 1.
TTF-1 expression in lung primary tumors. (a) Neoplastic epithelial cells of adenocarcinomas are positive for TTF-1 with either 8G7G3/1 or SPT24 clones (arrows). (b) Nuclear staining of tumor cells in squamous cell carcinoma was noted using SPT24 clone (arrow, right), while the same case was negative with the 8G7G3/1 clone (arrow, left). (c) Diffusely positive TTF-1 expression in a case of carcinoid tumor was detected by SPT24 clone (arrow, right); the same case was negative with the 8G7G3/1 cone (arrow, left). Bar 200 μ.
In addition to primary tumors of the lung, we conducted a small pilot study of metastatic lung carcinomas to other organs which was heavily weighted towards poorly differentiated tumors (17 poorly differentiated carcinomas unclassified, 5 adenocarcinomas and 1 squamous carcinoma). The metastatic tumors studied included 9 metastases to lymph nodes, 4 brain, 4 adrenal, 4 liver and 2 bone. There was no statistically significance in the difference of staining between the two clones (results not shown).
Non-pulmonary primary tumors
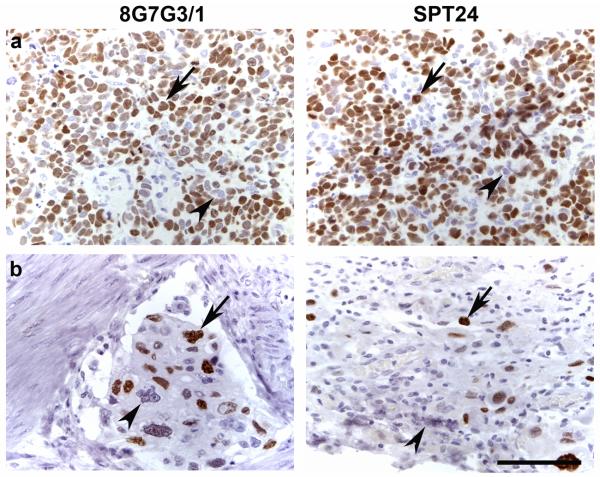
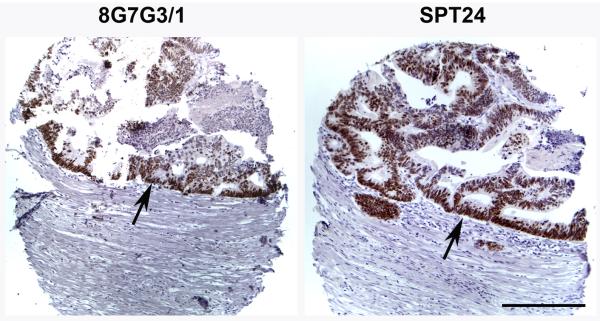
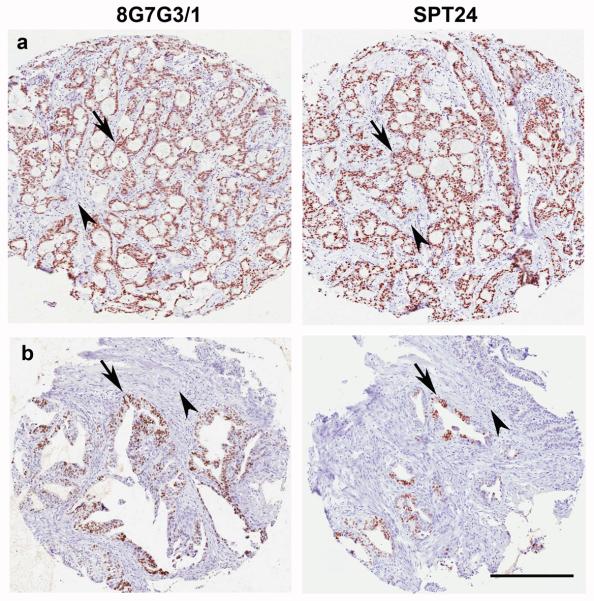
TTF-1 expression was present in a small subset of non-lung primary tumors of the urinary bladder, colon, stomach and salivary gland, but not in squamous cell carcinomas of the head and neck, pancreatic adenocarcinomas or invasive mammary carcinomas (Table 1). The non-lung primary tumor type that showed the highest number of positive cases was invasive urothelial carcinoma of the urinary bladder (5 of 98 cases, 5.1%; Figure 2). Three of these cases contained more than 70% positive cells (Figure 2a), while 2 cases were focally positive staining 20% of the cells (Figure 2b). The intensity and distribution of positive cells was similar with both antibodies. Three cases of colon cancer were positive using both antibody clones. As illustrated in figure 3, the intensity and extent of stain was also similar when using either SPT24 or 8G7G3/1 clones (Figure 3). Single cases of salivary gland adenoid cystic carcinoma and gastric adenocarcinoma were positive. While the salivary gland tumor stained homogeneously (Figure 4a), the staining in the gastric adenocarcinoma case was focally positive (30%; Figure 4b).
Figure 2.
TTF-1 expression in invasive urothelial carcinoma of the bladder. (a) Three of 98 bladder cancer cases showed diffuse positive staining of the majority of the tumor cells (arrows) while some tumor cells remained negative (arrowheads). (b) Two of 98 cases of bladder cancer showed focal positive staining (arrows), while the majority of tumor cells were negative (arrowheads). Note that the intensity and number of positive cells is similar with either antibody clone (a and b). Bar 50 μ.
Figure 3.
TTF-1 expression in colon adenocarcinoma. Epithelial cells stained positive for TTF-1 with both antibody clones (arrows). Bar 200 μ.
Figure 4.
TTF-1 expression in a case of salivary gland tumor and gastric adenocarcinoma. (a) Epithelial cells of a case of adenoid cystic carcinoma of the salivary gland stained diffusely positive with either antibody clone (arrows). Stromal cells were negative (arrowheads). (b) Focal nuclear staining of epithelial cells (arrows) was seen in a single case of gastric adenocarcinoma. Stromal cells were negative (arrowheads). Note similar intensity and number of positive cells with either antibody clone (a and b). Bar 200 μ.
Discussion
The expression of TTF-1 by immunohistochemistry was first shown by Lazzaro et al. in fetal rat lung, thyroid gland and neuro-hypophysis. The antibody used in that study was developed against peptides El, A2 and Fl, spanning amino acid residues 2-14, 92-104 and 110-122 from the TTF-1 coding region, respectively (16). Subsequently, Holzinger et al. produced and characterized the 8G7G3/1 monoclonal antibody to TTF-1 and reported positive nuclear staining in pulmonary adenocarcinomas and small cell carcinomas of the lung, but negative staining in breast and colon carcinomas (15). Thereafter, TTF-1 was considered a very specific marker for adenocarcinoma and small cell carcinoma of lung in histological as well as cytological specimens and gained popularity among pathologists as a useful marker of lung and thyroid (8, 10). Concomitantly, several studies began to challenge the specificity of TTF-1. For example, the expression of TTF-1 in extrapulmonary small cell carcinomas raised initial concern (9, 17). More recently, Alkushi et al. reported a TTF-1 positive case of uterine papillary serous carcinoma in their study of cervical and uterine adenocarcinoma (18). TTF-1 staining has also been observed in ependymoma (19), glioblastoma multiforme (20), mixed mullerian tumor of ovary (21), serous, mixed serous and endometroid carcinoma of ovary (12), clear cell carcinoma of the ovary (22), endocervical adenocarcinoma (13), mucinous carcinoma of the ovary (14), primary and metastatic colonic adenocarcinoma (11, 23), atrophic gastritis and ciliated metaplasia (24), prostate adenocarcinoma (25), melanoma (26), and in nephroblastoma (27).
Penman et al. studied primary and metastatic colonic adenocarcinomas with 8G7G3/1 or SPT24 TTF-1 clones and described different detection capability, with SPT24 being more sensitive for lung adenocarcinomas. However, only the SPT24 clone detected a few adenocarcinomas of colonic origin (11). Recently, Wong et al. reported two cases of TTF-1 positive colonic adenocarcinoma using the 8G7G3/1 clone (23). In our study, 3/120 (2.5%) primary colonic adenocarcinoma were positive for TTF-1 with SPT24 or 8G3G7/1 clones. Interestingly, both antibodies stained the tumors with similar extents and intensities.
We noted positive staining in 5 of 98 (5.1%) cases of invasive urothelial carcinoma and in one of 56 (1.8%) cases of salivary gland carcinoma with both TTF-1 clones. This is the first report of TTF-1 expression in urothelial carcinoma and salivary gland carcinoma in the English literature. Two cases (2.1%) of prostatic ductal adenocarcinoma were positive for TTF-1 with both antibody clones. Lim et al. described a TTF-1 positive prostate adenocarcinoma with predominant ductal differentiation (25).
Differences in the results of immunohistochemical TTF-1 expression by different TTF-1 clones have been emphasized in few other studies. For instance, 14 of 28 glioblastoma multiforme tumors stained with TTF-1 clone SPT24, while all of these cases were negative using the 8G7G3-1 TTF-1 clone (20). Recently, Zhang et al. concluded that the SPT24 clone was the most sensitive primary antibody for TTF-1 using the Refine/Bond Max Autostainer. In their study, TTF-1 reactivity could be detected in all major histologic subtypes of gynecologic tumors, in up to 26% of all cases tested on routine surgical specimens, and in 6.4% of cases on tissue microarray. In addition to malignant tumors, this study also reports positive TTF-1 staining in benign tumors and in benign tubal and endometrial epithelia (14).
Our results demonstrate that the extent of TTF-1 expression depends on the type of TTF-1 clone used only when testing primary lung tumors. Although not statistically significant, we found a trend towards higher TTF-1 positivity in pulmonary adenocarcinomas with the SPT24 clone. Furthermore, a significantly higher number of carcinoids and squamous cell carcinoma was positive for TTF-1 with the SPT24 clone. This is important as previous studies performed with the 8G7G3/1 clone reported inconsistent expression of TTF-1 in carcinoids and minimal to absent expression in squamous cell carcinomas. Oliveira et al. reported 95% (19 of 20) of lung carcinoids were variably positive for TTF-1 using 8G7G3/1 clone (28). Pelosi et al. reported 5.0% TTF-1 positive squamous cell carcinomas with the 8G3G7/1 clone while Kaufman et al. reported all of the squamous cell carcinomas were negative with the same TTF-1 clone (9, 29).
The relatively high positivity on squamous cell carcinomas of the lung (14 of 97) with the SPT24 clone is interesting. Currently, there is no clinically useful marker to separate primary from metastatic squamous cell carcinoma involving the lung. Although further testing of additional non-pulmonary squamous cell carcinoma cases is mandated, negativity of all our squamous cell carcinomas of the head and neck (0 of 38) suggests a relative specificity of the TTF-1 SPT24 clone towards squamous neoplasms originating in the lungs.
Immunohistochemistry for TTF-1 is also frequently used to establish the pulmonary origin of a metastatic carcinoma to an extrapulmonary site. We studied a relatively small set of metastatic lung carcinomas to other organs and saw no statistically significant difference in staining between the two antibody clones. It is possible that a larger number of metastatic tumors, including more squamous cell carcinomas and carcinoid tumors, are needed in order to detect sensitivity differences between the two clones as seen in the primary lung tumors.
Although certain previous reports suggest that the SPT24 clone is more sensitive than the 8G3G7/1 clone in detecting non-pulmonary tumors, the discrepancies with our results could be explained due to technical differences such as antibody dilutions, antigen retrieval, detection systems, etc. For example, Penman et al. reported three out of six TTF-1 positive colon cancer cases with the SPT24 clone and none with the 8G3G7/1 clone, yet they used a different staining system, antigen retrieval and antibody dilution (11). Furthermore, Zhang et al. show that detection of TTF-1 expression in gynecologic tumors can vary as a result of different antigen retrieval and detection system used (14). Importantly, by applying the technical conditions presented here we detect exactly the same number of TTF-1 positive extrapulmonary tumors with both antibody clones while showing a different sensitivity limited to the lung. One could speculate that that the higher detection rate of SPT24 clone in primary lung tumors is due to its higher lung specificity. However, this assumption could prove to be wrong if comparable numbers of TTF-1 expressing tumors of non-pulmonary origins are tested. Unfortunately, the low prevalence of extrapulmonary TTF-1 positive tumors prevented us from proving or refuting that hypothesis.
In summary, we report a comprehensive comparative study of TTF-1 expression by two monoclonal antibodies in pulmonary and extrapulmonary tumors. Our results show that the TTF-1 SPT24 clone has a higher detection rate of all histologic subtypes of non-small cell pulmonary primary tumors with a clear advantage over 8G7G3/1 in squamous cell carcinomas and carcinoid tumors. Consistent with previous results, our study suggests that caution should be used when interpreting TTF-1 immunostain results, since a small proportion of non-pulmonary primary tumors are positive with both SPT24 or 8G7G3/1 antibody clones. Importantly, this study adds bladder urothelial carcinoma and salivary gland tumors to the already extensive list of non-pulmonary and non-thyroid primary tumors that express TTF-1.
Acknowledgements
These findings were presented in part at the United States and Canadian Academy of Pathology Annual Meeting 2009, Boston, MA.
Disclosure of funding: This project was supported by the Molecular Pathology Core of the COBRE Center for Cancer Research Development, NIH #P20 RR17695, awarded by the National Center for Research Resources, Institutional Development Award (IdeA) Program.
Footnotes
Disclosure/conflict of interest
The authors state no conflict of interest.
References
- 1.Cassel TN, Suske G, Nord M. C/EBP alpha and TTF-1 synergistically transactivate the Clara cell secretory protein gene. Ann N Y Acad Sci. 2000;923:300–302. doi: 10.1111/j.1749-6632.2000.tb05537.x. [DOI] [PubMed] [Google Scholar]
- 2.Civitareale D, Castelli MP, Falasca P, et al. Thyroid transcription factor 1 activates the promoter of the thyrotropin receptor gene. Mol Endocrinol. 1993;7:1589–1595. doi: 10.1210/mend.7.12.8145764. [DOI] [PubMed] [Google Scholar]
- 3.Francis-Lang H, Price M, Polycarpou-Schwarz M, et al. Cell-type-specific expression of the rat thyroperoxidase promoter indicates common mechanisms for thyroid-specific gene expression. Mol Cell Biol. 1992;12:576–588. doi: 10.1128/mcb.12.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno MD, Korfhagen TR, Liu C, et al. GATA-6 activates transcription of surfactant protein A. J Biol Chem. 2000;275:1043–1049. doi: 10.1074/jbc.275.2.1043. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K, Clark JC, Shaw-White JR, et al. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270:8108–8114. doi: 10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 6.Jerome Marson V, Mazieres J, Groussard O, et al. Expression of TTF-1 and cytokeratins in primary and secondary epithelial lung tumours: correlation with histological type and grade. Histopathology. 2004;45:125–134. doi: 10.1111/j.1365-2559.2004.01893.x. [DOI] [PubMed] [Google Scholar]
- 7.Kargi A, Gurel D, Tuna B. The diagnostic value of TTF-1, CK 5/6, and p63 immunostaining in classification of lung carcinomas. Appl Immunohistochem Mol Morphol. 2007;15:415–420. doi: 10.1097/PAI.0b013e31802fab75. [DOI] [PubMed] [Google Scholar]
- 8.Moldvay J, Jackel M, Bogos K, et al. The role of TTF-1 in differentiating primary and metastatic lung adenocarcinomas. Pathol Oncol Res. 2004;10:85–88. doi: 10.1007/BF02893461. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann O, Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology. 2000;36:8–16. doi: 10.1046/j.1365-2559.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 10.Ng WK, Chow JC, Ng PK. Thyroid transcription factor-1 is highly sensitive and specific in differentiating metastatic pulmonary from extrapulmonary adenocarcinoma in effusion fluid cytology specimens. Cancer. 2002;96:43–48. doi: 10.1002/cncr.10310. [DOI] [PubMed] [Google Scholar]
- 11.Penman D, Downie I, Roberts F. Positive immunostaining for thyroid transcription factor-1 in primary and metastatic colonic adenocarcinoma: a note of caution. J Clin Pathol. 2006;59:663–664. doi: 10.1136/jcp.2005.030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham AD, Williams AR, Salter DM. TTF-1 expression in primary ovarian epithelial neoplasia. Histopathology. 2006;48:764–765. doi: 10.1111/j.1365-2559.2006.02365.x. [DOI] [PubMed] [Google Scholar]
- 13.Siami K, McCluggage WG, Ordonez NG, et al. Thyroid transcription factor-1 expression in endometrial and endocervical adenocarcinomas. Am J Surg Pathol. 2007;31:1759–1763. doi: 10.1097/PAS.0b013e3181131e21. [DOI] [PubMed] [Google Scholar]
- 14.Zhang PJ, Gao HG, Pasha TL, et al. TTF-1 expression in ovarian and uterine epithelial neoplasia and its potential significance, an immunohistochemical assessment with multiple monoclonal antibodies and different secondary detection systems. Int J Gynecol Pathol. 2009;28:10–18. doi: 10.1097/PGP.0b013e3181804bc6. [DOI] [PubMed] [Google Scholar]
- 15.Holzinger A, Dingle S, Bejarano PA, et al. Monoclonal antibody to thyroid transcription factor-1: production, characterization, and usefulness in tumor diagnosis. Hybridoma. 1996;15:49–53. doi: 10.1089/hyb.1996.15.49. [DOI] [PubMed] [Google Scholar]
- 16.Lazzaro D, Price M, de Felice M, et al. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 17.Agoff SN, Lamps LW, Philip AT, et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol. 2000;13:238–242. doi: 10.1038/modpathol.3880044. [DOI] [PubMed] [Google Scholar]
- 18.Alkushi A, Irving J, Hsu F, et al. Immunoprofile of cervical and endometrial adenocarcinomas using a tissue microarray. Virchows Arch. 2003;442:271–277. doi: 10.1007/s00428-002-0752-4. [DOI] [PubMed] [Google Scholar]
- 19.Zamecnik J, Chanova M, Kodet R. Expression of thyroid transcription factor 1 in primary brain tumours. J Clin Pathol. 2004;57:1111–1113. doi: 10.1136/jcp.2004.017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galloway M, Sim R. TTF-1 staining in glioblastoma multiforme. Virchows Arch. 2007;451:109–111. doi: 10.1007/s00428-007-0432-5. [DOI] [PubMed] [Google Scholar]
- 21.Agaimy A, Wunsch PH. Unexpected and potentially misleading TTF-1 expression: a word of caution. Virchows Arch. 2006;449:603–605. doi: 10.1007/s00428-006-0298-y. [DOI] [PubMed] [Google Scholar]
- 22.Kubba LA, McCluggage WG, Liu J, et al. Thyroid transcription factor-1 expression in ovarian epithelial neoplasms. Mod Pathol. 2008;21:485–490. doi: 10.1038/modpathol.2008.4. [DOI] [PubMed] [Google Scholar]
- 23.Wong NA, Kamel H, Sheffield EA, et al. Positive immunostaining for thyroid transcription factor-1 in colorectal adenocarcinoma using the 8G7G3/1 monoclonal antibody. J Clin Pathol. 2008;61:1070–1071. doi: 10.1136/jcp.2008.058552. [DOI] [PubMed] [Google Scholar]
- 24.Rau T, Dimmler A, Hafner M, et al. Aberrant expression of TTF-1 and forkhead factor HFH-4 in atrophic gastritis and ciliated metaplasia suggests gastric broncho-pulmonary transdetermination. J Pathol. 2005;206:383–387. doi: 10.1002/path.1795. [DOI] [PubMed] [Google Scholar]
- 25.Lim TK, Teo C, Giron DM, et al. Thyroid transcription factor-1 may be expressed in ductal adenocarcinoma of the prostate:a potential pitfall. J Clin Pathol. 2007;60:941–943. doi: 10.1136/jcp.2007.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plaza JA, Suster D, Perez-Montiel D. Expression of immunohistochemical markers in primary and metastatic malignant melanoma: a comparative study in 70 patients using a tissue microarray technique. Appl Immunohistochem Mol Morphol. 2007;15:421–425. doi: 10.1097/PAI.0b013e318032ea5d. [DOI] [PubMed] [Google Scholar]
- 27.Bisceglia M, Ragazzi M, Galliani CA, et al. TTF-1 expression in nephroblastoma. Am J Surg Pathol. 2009;33:454–461. doi: 10.1097/PAS.0b013e318185d21b. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira AM, Tazelaar HD, Myers JL, et al. Thyroid transcription factor-1 distinguishes metastatic pulmonary from well-differentiated neuroendocrine tumors of other sites. Am J Surg Pathol. 2001;25:815–819. doi: 10.1097/00000478-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Pelosi G, Fraggetta F, Pasini F, et al. Immunoreactivity for thyroid transcription factor-1 in stage I non-small cell carcinomas of the lung. Am J Surg Pathol. 2001;25:363–372. doi: 10.1097/00000478-200103000-00011. [DOI] [PubMed] [Google Scholar]