Abstract
HIV-1 infection affects white matter circuits linking frontal, parietal, and subcortical regions that subserve visuospatial attention processes. Normal perception requires the integration of details, preferentially processed in the left hemisphere, and the global composition of an object or scene, preferentially processed in the right hemisphere. We tested whether HIV-related callosal white matter degradation contributes to disruption of selective lateralized visuospatial and attention processes. A hierarchical letter target detection paradigm was devised, where large (global) letters were composed of small (local) letters. Participants were required to identify target letters among distractors presented at global, local, both or neither level. Attention was directed to one (global or local) or both levels. Participants were 21 HIV-1 infected and 19 healthy control men and women who also underwent Diffusion Tensor Imaging (DTI). HIV-1 participants showed impaired hierarchical perception owing to abnormally enhanced global facilitation effects but no impairment in attentional control on local-global feature selection. DTI metrics revealed poorer fiber integrity of the corpus callosum in HIV-1 than controls that was more pronounced in posterior than anterior regions. Analysis revealed a double dissociation of anterior and posterior callosal compromise in HIV-1 infection: Compromise in anterior but not posterior callosal fiber integrity predicted response conflict elicited by global targets, whereas compromise in posterior but not anterior callosal fiber integrity predicted response facilitation elicited by global targets. We conclude that component processes of visuospatial perception are compromised in HIV-1 infection attributable, at least in part, to degraded callosal microstructural integrity relevant for local-global feature integration.
Keywords: HIV-1 infection, visuospatial, global, local, attention, interhemispheric, corpus callosum, diffusion tensor imaging
Introduction
Human immunodeficiency virus (HIV-1) enters the brain soon after initial infection and remains there throughout the course of HIV-1 disease (Gartner, 2000; Major, Rausch, Marra, & Clifford, 2000). The virus infection induces oxidative stress by enhancing the production of cytotoxic markers associated with synaptic changes and neuronal cell death in the central nervous system (Archeampong et al., 2007; Hauser et al., 2007; Moroni & Antinori, 2003). HIV-1 also affects white matter circuits linking frontal, parietal and specific subcortical regions (Chang et al., 2008a; Meyerhoff et al., 1999; Pfefferbaum et al., 2006, 2009) that subserve visuospatial and attention processes (Devinsky & D'Esposito, 2003). Despite evidence of cognitive impairment in HIV-1 infection in motor speed, memory, and visuoconstruction, which have been related to cerebral white matter damage (Chen et al., 2009; Cloak, Chang, & Ernst, 2004; Paul et al., 2007; Ragin, Storey, Cohen, Epstein, & Edelman, 2004, Ragin et al., 2005; Wu et al., 2006), little is known about the neural substrates affected by HIV-1 infection and contributing to impairment of visuospatial perception and attention.
A paradigm widely used to study visuospatial functions is a target detection task that uses a hierarchical letter scheme involving large (global) letters that are made of smaller (local) letters, modeling the hierarchical structure of visual world scenes (Fink et al., 1997; Navon, 1977). These multilevel scenes can be decomposed into component features and then integrated into more complex stimuli, objects and scenes – a concept originated from investigations of the visual cortex (Pandya & Sanides, 1973; Felleman, Burkhalter, & Van Essen, 1997). Global–local processing starts on a perceptual level (Fink, Marshall, Halligan, & Dolan, 1999; Mevorach, Humphreys, & Shalev, 2006, Mevorach, Shalev, Allen, & Humphreys, 2009; Mordkoff & Miller, 1993) that can be facilitated by redundant target information (Müller-Oehring, Schulte, Raassi, Pfefferbaum, & Sullivan 2007, Müller-Oehring, Schulte, Fama, Pfefferbaum, & Sullivan 2009; Schulte, Mueller-Oehring, Rosenbloom, Pfefferbaum, & Sullivan, 2005). Depending on task requirements, processing of local features (details or parts), the global composition, or both levels is modulated by attentional allocation, interference processing, and response control (Han & He, 2003; Han & Jiang, 2006; Müller-Oehring et al., 2007; Qin & Han, 2007; Yoshida, Yoshino, Takahashi, & Nomura, 2007).
Asymmetries between right and left parietal lobe function have been described for global and local processing, with preferentially right parietal activation for global visuospatial attention (Corbetta, Miezin, Shulman, & Petersen, 1993), arousal, and vigilance (Paus et al. 1997), and left parietal lobe activation for local visuospatial attention and feature processing (Kimchi & Merhav, 1991; Sergent, 1982; van Kleeck, 1989; for a review). Lesion studies of lateralized temporo-parietal cortical damage (Delis, Robertson, & Efron, 1986; Robertson & Lamb, 1991) and electrophysiological studies (e.g., Yamaguchi, Yamagata, & Kobayashi, 2000; Yoshida et al., 2007) have confirmed this right-global and left-local hemispheric specialization. Normal perception requires the integration of these two stimulus features, achieved through transfer of information between the hemispheres via the corpus callosum (Barnett, Kirk, & Corballis, 2007; Corballis, Barnett, Fabri, Paggi, & Corballis, 2004; Engel, König, Kreiter, & Singer, 1991; Gazzaniga, 1987, 2000; Gazzaniga, Bogen, & Sperry, 1965; Stephan, Marshall, Penny, Friston, & Fink, 2007).
Local-global visuospatial processing in HIV-1 has been investigated using a hierarchical letter task in which attention was implicitly manipulated by varying target probabilities to favor local, global, or neither level (Martin et al., 1995; Olesen, Schendan, Amick, & Cronin-Golomb, 2007). HIV-1 infected individuals exhibited greater cost effects than controls for global and local targets (Martin et al., 1995) or for local targets only (Olesen et al., 2007) when attention was implicitly biased away from the target level, but performed similarly to controls in the unbiased condition. Local-global processing deficits in HIV-1 were restricted to controlled (biased) attentional processes but did not affect automatic (non-biased) attentional processes (Martin et al., 1995), possibly reflecting HIV-1-related functional compromise in parietal visuospatial attention systems (Olesen et al., 2007). Consistent with this interpretation, functional neuroimaging studies have indicated reduced efficiency in fronto-parietal attention networks as evidenced by less activation in fronto-parietal regions but greater activation in adjacent or contralateral brain regions in HIV-1 patients than controls during an attentionally challenging task (Chang et al., 2004, Chang, Yakupov, Nakama, Stokes, & Ernst, 2008b; Ernst, Chang, Jovicich, Ames, & Arnold 2002).
In addition to HIV-1-related impairment of lateralized local-global functions, white matter microstructure compromise observed with diffusion tensor imaging (DTI) occurs in HIV-1 involving callosal fiber tracts connecting bilateral frontal (Filippi, Ulug, Ryan, Ferrando, & van Gorp 2001; Thurnher et al., 2005), temporal and parietal (Pfefferbaum et al., 2007; Wu et al., 2006) and occipital cortical regions (Filippi et al., 2001; Pfefferbaum et al., 2009; Wu et al., 2006), despite the effectiveness of antiretroviral treatment (Gongvatana et al., 2009).
To examine the functional implications and neural substrates of HIV-1 infection-related compromise on component local-global processes, we devised a hierarchical letter task that permitted examination of attention, interference, and response control based on global versus local information. We then related behavioral measures from this task to DTI measures of the integrity of tissue microstructure of the corpus callosum. We assumed that HIV-1-related impairment in attentional control would be related to compromised integrity in anterior and middle callosal fibers connecting frontal and parietal cortices. Attentional control is required when local and global information is incongruent (i.e., a global E made up from local Ts) (Hibi, Takeda, & Yagi, 2002; Müller-Oehring et al., 2007; Navon, 1977; Proverbio, Minniti, & Zani, 1998) and is associated with conflicting responses (Müller-Oehring et al., 2007; Volberg & Hübner, 2006). Because we had observed earlier (Müller-Oehring et al., 2007, 2009) that redundant target information at both local and global processing levels results in response facilitation, an effect attributable to perceptual preattentive processing (Martin, Sorensen, Robertson, Edelstein, & Chirurgi, 1992, Martin et al., 1995; Sorensen, Martin, & Robertson, 1994; Tzelgov, Henik, & Berger, 1992), we assumed that HIV-1-related impairment in local-global facilitation would be related to compromise in posterior callosal microstructural integrity connecting occipito-temporal visual processing areas.
Methods
Participants
The study sample comprised 19 normal healthy controls (CTL) (8 women, 11 men) and 21 HIV-1 positive patients (HIV-1) (5 women, 16 men) (Table 1). Participants in both groups had normal or corrected to normal visual acuity. Study groups did not significantly differ in sex distribution (Chi-square=1.52, ns). All subjects underwent a panel of blood tests to determine HIV-1 status. HIV-1 infected participants had average CD4 T-cell counts of 519 ± 170 (range = 279 to 920), and viral loads of 13,096 ± 23,516 (range = 49 to 100,001 units). Six HIV-1 infected men had had an acquired immunodeficiency syndrome (AIDS)-defining event or low CD4 T-cell counts (<200) in the course of their illness; one was also infected with hepatitis C. Of the 21 HIV-1 participants, 15 received HAART medication, 3 received other HIV-1 medication, and 3 were without pharmacological treatment at the time of testing.
Table 1.
| Subject table | ||||
|---|---|---|---|---|
| ANOVA | ||||
| HIV (16m, 5w) | CTL (11m, 8w) | F | P | |
| Age | 42.7 (10.3) | 41.5 (8.8) | 0.17 | 0.69 |
| Body Mass Index | 25.5 (5.7) | 25.7 (3.9) | 0.04 | 0.85 |
| Handedness * | 26.5 (11.8) | 22.7 (12.2) | 0.99 | 0.32 |
| Education (years) | 14.4 (3.0) | 15.5 (2.1) | 1.78 | 0.19 |
| Sozioeconomic Status (SES) | 32.6 (14.5) | 29.9 (15.3) | 0.29 | 0.59 |
| Verbal Intelligence NART IQ | 110.7 (8.3) | 114.2 (5.5) | 2.06 | 0.16 |
| Global Functioning (GAF) | 69.6 (11.6) | 77.2 (10.1) | 3.34 | 0.078 |
| Depressive Symptoms (BDI) | 12.1 (9.3) | 2.4 (2.2) | 15. 4 | 0.0001 |
| CD4+ Count | 519 (169.8) | - | - | |
| Viral Load | 13,096 (26,516) | - | - | |
Crovitz & Zener, 1962. Right handedness = 14 to 32; left handedness = 50 to 70.
Participants received a Structural Clinical Interview for DSM-IV diagnosis (American Psychiatric Association, 1994) by trained clinicians to rule out non-target psychiatric and neurological disease. Additional interviews and questionnaires assessed global functioning (GAF; First, Spitzer, Gibbon, & Williams 1998), depression (BDI; a quantitative measure of depressive symptoms; Beck, Steer, & Brown 1996); socioeconomic status (SES; a two-factor scale based on education and occupation; Hollingshead & Redlich, 1958); handedness (Crovitz & Zener, 1962); and body mass index (height/weight in cm/kg2; an index of nutritional status). General cognitive status was assessed with the National Adult Reading Test (NART, Nelson, 1982), a retrospective estimator of premorbid verbal intelligence. Means, SD, and statistical significance of these and other demographic values are presented in Table 1. No group differences were found for age, body mass index (BMI), handedness (Crovitz score), verbal intelligence (NART IQ), education, and socioeconomic status (SES). On average both groups had an education beyond high school. HIV-1 infected participants showed a trend for lower global functioning (GAF) and expressed more depressive symptoms (BDI) than healthy controls. HIV-1 infected individuals with lower CD4+ counts reported lower socioeconomic status (r = .52, p = 0.019). Furthermore, lower global functioning scores in HIV-1 infected individuals were associated with higher depression scores (r = -.56, p = 0.010). No patient was clinically demented. Written informed consent was obtained from all participants and the Institutional Review Boards of Stanford University and SRI International approved the study in accordance with the ethical standards established in the 1964 Declaration of Helsinki.
Analyses of DTI corpus callosum data from HIV patients and their clinical and demographic characteristics (Pfefferbaum et al., 2009), and local-global behavioral data from most control participants (Müller-Oehring et al., 2007, 2009) have been published.
Global-local paradigm
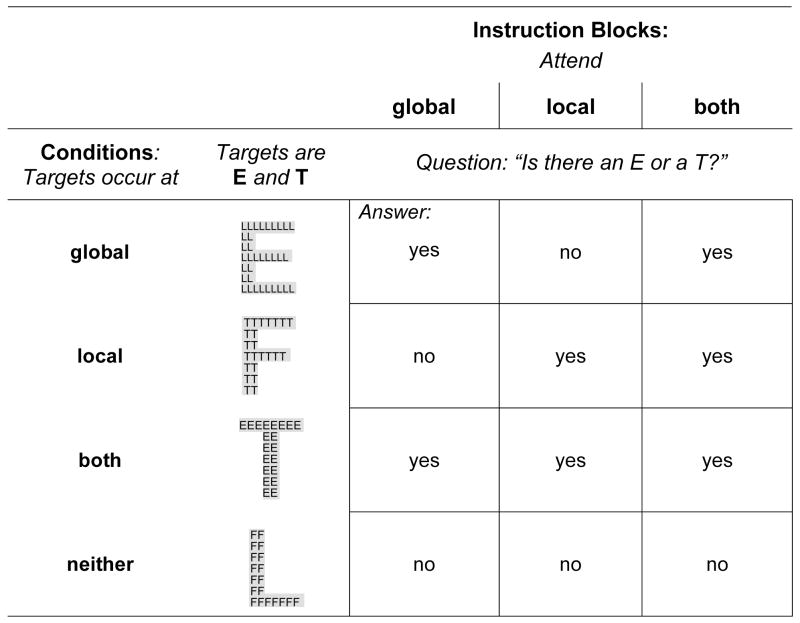
Hierarchical stimuli were large letters that were made up of tiny letters (e.g., a global F made out of local Es) (Figure 1). Target letters were E and T, nontargets F and L. Hierarchical letters were presented on a white background. The tiny or local letters were black; the large or global letters had a light gray background to enhance their salience. Hierarchical letters were presented in two selective attention blocks and one divided attention block. In the selective attention blocks, subjects attended either to the global or local spatial scale. In the divided attention block, subjects simultaneously attended to both spatial scales. Stimuli were the same for each block; only the attention instruction differed.
Figure 1. Design of the local-global paradigm.
3 attention blocks (1. Attend the global level, 2. Attend the local level, 3. Attend both levels) with 4 randomly intermixed conditions (global targets, local targets, both: global and local targets, neither global nor local targets) were repeatedly presented. Target letters were Es and Ts, non-target letters Fs and Ls. Subjects answered the question: “Is there an E or a T?” by pressing a Yes key for targets (E and T) and a No key for non-targets (F and L) at the attended level.
Four specific component process effects were calculated:
-
Precedence: selective (SA) or divided attention (DA) block with targets at one level, i.e.,
- precedence (SA) = (global targets/global instruction) – (local targets/local instruction);
- precedence (DA) = (global targets/both instruction) – (local targets/both instruction);
- Interference: selective and divided attention blocks with targets at both levels: congruency = incongruent trials (e.g. global T, local E) – congruent trials (global E, local E);
-
Response conflict for selective attention:
- global instruction block: inhibition = (local targets) – (no targets at either level),
- local instruction block: inhibition = (global targets) – (no targets at either level);
-
Response facilitation for selective attention:
- global instruction block: inhibition = (global targets) – (targets at both level),
- local instruction block: inhibition = (local targets) – (targets at both level);
The design comprised four target conditions: a target letter appeared on the global, local, both levels, or not at all; and three attention conditions: attend to global, local, or to both levels (Figure 1). Subjects answered the question, “Is there an E or T?” by pressing a YES button with the index finger of their dominant hand when a target letter appeared at the attended level, and a NO button with the middle finger of the same hand when a non-target appeared at the attended level. Stimuli remained on the screen until the subject pressed the button initiating the onset of the next trial. Reaction times (RTs) and errors were collected for each trial. A total of 288 stimuli were presented. Each of the three attention blocks comprised 32 stimuli with 8 stimuli in each of the four target conditions, and each block was presented three times. Total task duration was approximately 9 minutes (3min/block). All subjects performed a practice trial for each attention block before testing. Four global-local processing effects measured were (1) precedence of spatial level, (2) congruency of information at two spatial levels, (3) response conflict from target information at the unattended level, and (4) response facilitation from additional target information at the unattended level.
The precedence effect indicates which level - global or local - was processed faster and was calculated for selective and divided attention conditions. For selective attention conditions, mean RTs to local targets in the local attention block were compared with mean RTs to global targets in the global attention block. For divided attention conditions, mean RTs to local targets were compared with mean RTs to global targets in divided attention blocks requiring attention at both target levels.
The congruency effect is indicated by shorter RTs to congruent than incongruent stimuli. When target letters (E, T) appeared at local and global levels, they were either congruent (local E – global E, local T – global T) or incongruent (local E – global T, local T – global E). Because both letters (E and T) required a YES response, the response to the question “Is there an E or T?” remained the same independent of the congruence of the stimulus. Congruency effects were calculated for selective (attend global, attend local) and divided attention (attend both, global and local) conditions.
Response conflict usually occurs for stimuli where information at each level is associated with a different response (see Hübner and Malinowski, 2002). We tested response conflict between information provided from attended and unattended levels. Thus, when target letters (E or T) appeared at the unattended level, processing of unattended targets would lead to response conflict, as the correct response was NO. Such conflicting trials were compared with non-conflicting trials, i.e., with non-target letter (F or L) at either level. Difference in reaction time between conflicting and non-conflicting trials indexes response conflict elicited by unattended targets and were calculated for selective attention (attend global, attend local) conditions.
Response facilitation indicates faster responses to trials where target letters (E or T) appear both at the attended level and unattended level compared to trials where the non-attended level consists of nontarget letters (F or L). Differences in reaction time between trials where targets appear at both levels relative to those where targets appear at only one level index the amount of response facilitation elicited by unattended targets. Facilitation effects were calculated for selective attention conditions, for the global attention block by comparing RTs to global targets with RTs to targets at both spatial scales and for the local attention block by comparing RTs to local targets with RTs to targets at both spatial scales.
Stimuli were presented in the center of a 21-inch computer screen. Global stimuli were 9.5 cm high and 7.0 cm wide; local stimuli were 1.0 cm by 0.5 cm. With a subject-monitor distance of approximately 55 cm, global stimuli were ± 5.0° visual angle vertically and ± 3.5° visual angle horizontally. Local stimuli measured 1.0° by 0.5° visual angle.
Magnet Resonance Imaging
MR Image Acquisition
MR imaging was performed on a 1.5 Tesla GE clinical whole body system. A dual-echo fast spin-echo (FSE) coronal structural sequence was acquired (47 contiguous, 4mm thick slices; TR/TE1/TE2=7500/14/98ms; matrix=256×192). DTI was performed with the same slice location parameters as the dual-echo FSE, using a single-shot, spin-echo, echo-planar imaging technique (47 contiguous, 4mm thick slices, TR/TE=10,000/103ms, matrix= 128×128, in-plane resolution=1.875mm2, b-value = 860 s/mm2). Diffusion was measured along six noncollinear directions (6 NEX) with alternating signs to minimize the need to account for cross-terms between imaging and diffusion gradients (Neeman, Freyer, & Sillerud, 1991). For each slice, six images with no diffusion weighting (b=0 s/mm2) were also acquired.
Image processing
The structural data were passed through the FSL Brain Extraction Tool (Smith, 2002) to extract the brain. Eddy current-induced image distortions in the diffusion-weighted images for each direction were minimized by alignment with an average made of all 12 diffusion-weighted images using a 2-D 6-parameter affine correction on a slice-by-slice basis (Woods, Grafton, Holmes, Cherry, & Mazziotta, 1998). The DTI data were then aligned using the FSE data by a non-linear 3D warp (3rd-order polynomial), which provided in-plane and through-plane alignment. On a voxel-by-voxel basis, fractional anisotropy (FA) and apparent diffusion coefficient (ADC), the latter decomposed into its longitudinal and (λL = λ1) and transverse (λT = [λ2 +λ3]/2) components, were computed. FA ranged from 0 to 1, and diffusivity was expressed in units of 10-3 mm2/s.
Warping to common coordinates
To achieve common anatomical coordinates across subjects, a population-average FA template (Sullivan, Rohlfing, & Pfefferbaum, 2008) was constructed from the FA data of 120 control subjects (20-81 years old) with group-wise affine registration (Learned-Miller, 2006) followed by iterative nonrigid averaging. Each subject's FA data set was registered to the population FA template with a 9-parameter affine transformation followed by nonrigid alignment using a multi-level, 3rd-order B-spline, with 5-mm final control point spacing (Rohlfing & Maurer, 2003).
Fiber tracking
A more detailed description of our fiber tracking procedures appears elsewhere (Sullivan et al., 2008). The fiber tracking routine (Mori, Crain, Chacko, & van Zijl, 1999; Xu, Mori, Solaiyappan, van Zijl, & Davatzikos 2002) applies a target-source convention that restricts fibers to ones originating in source voxels and passing through target voxels. For the corpus callosum, six geometrically defined targets, modified to reflect documented (Pandya & Seltzer, 1986) callosal anatomical projections (Sullivan, Adalsteinsson, & Pfefferbaum, 2006) were identified on the midsagittal population FA template. Sources were defined as 5.625 mm thick planes: a) 9.375 mm bilateral to the corpus callosum subtending the entire anterior-posterior extent of the brain. For each subject the targets and sources were mapped from the population FA template to that subject's native image space and passed to the fiber tracking routine (Gerig, Corouge, Vachet, Krishnan, & MacFall, 2005; www.cs.unc.edu). Tracking parameters specified minimum FA (.17), 37° maximum angular deviation between voxels, and minimum (11.25 mm) and maximum (45 mm) fiber length, with essentially no limit on the number of fibers (other than the number of source pixels). We refer hereafter to the group of fibers coursing through each target region as “fiber bundles” (Figure 3). For each fiber bundle, mean FA, λL, and λT of voxels comprising the bundle were the units of analysis. The mean FA, ADC, longitudinal diffusivity (λL=λ1) and transverse diffusivity (λT=(λ2 + λ3)/2) for each fiber bundle were the units of analysis.
Figure 3.
Corpus callosum microstructural fiber integrity in HIV-1 infected individuals (HIV) and controls (CTL) measured with diffusion tensor imaging (DTI): Mean raw values and standard errors (SE) for fractional anisotropy (FA) (lower left panel), longitudinal diffusivity (λL) (upper right panel), and transverse diffusivity (λT) (lower right panel) for six callosal sectors: Genu, premotor, motor, parietal, temporal and splenium (upper left panel; figure was taken from Pfefferbaum et al., 2009).
Statistical analysis
Analyses of variance (ANOVA) and χ2 tests were used for group (HIV, CTL) and sex (men vs. women) comparisons of demographic data. Reaction time (RT) analysis of global and local information processing was based on correct responses. First, a series of ANOVAs used group as between subjects variable, and the repeated measures component tested for the four specific effects of global-local processing: precedence, congruency, response conflict, and response facilitation. The alpha level was set to 0.05 for all hypotheses tested. Second, a series of ANOVAs tested for fiber bundle integrity (FA, longitudinal and transverse diffusivity) using group as between subjects variable and 6 callosal sectors as repeated measures variable. The alpha level was set to 0.05, one-tailed, assuming lower FA and higher longitudinal and transverse diffusivity in HIV-1 than CTL, as demonstrated in a larger sample (Pfefferbaum et al., 2009) from which the current sample was drawn. Third, relations of local-global specific effects with clinical variables (CD4 count, viral load) were tested for the HIV-1 group, and relations with age and callosal microstructure (FA, longitudinal and transverse diffusivity in 6 callosal sectors) were tested for both groups with two-tailed Pearson product moment correlations. Applying family-wise Bonferroni correction for 6 comparisons of callosal sectors (genu, premotor, motor, parietal, temporal, and splenium), p-values ≤ .008 were considered significant. Third, for significant correlations, the predictive value and regional specificity of callosal microstructure on special effects of global-local processing was tested using linear regression analysis (SPSS 15.0). Additionally, we tested for differences between correlations of the two groups (Walker & Lev, 1953).
Results
1. Error frequency
Subject groups did not differ in the number of errors committed (F(1,38) = 0.61, p = 0.44). Overall error rate was low: less than 2% in CTL (5.7 ± 3.3) and less than 2.5% in HIV-1 (6.9 ± 5.4). HIV-1 patients (842 ± 135 ms) had overall slower response times than controls (753 ± 109 ms) (F(1,38) = 5.28, p = 0.027). Table 2 shows mean reaction time data for HIV-1 and control groups and t-statistics testing for the presence of each special effect in each group. Table 3 provides a results summary of between-group statistical differences for each special effect.
Table 2.
| Local-Global Special Effects | HIV | CTL | HIV | CTL | |||
|---|---|---|---|---|---|---|---|
| Precedence | Global | Local | Global | Local | Special Effects Global–Local | ||
| Selective Attention | 735.5 (114.7) |
728.7 (144.7) |
650.6 (110.6) |
623.9 (100.8) |
-3.2 (67.8) |
26.7* (52.6) |
|
| Divided Attention | 1076.2 (190.0) |
967.1 (166.0) |
974.1 (184.6) |
877.9 (161.8) |
109.1*** (107.4) |
96.1 ** (135.9) |
|
| Congruency | INC | CON | INC | CON | INC–CON | ||
| Attend to Global | 825.3 (174.2) |
738.3 (177.3) |
681.3 (150.4) |
637.3 (127.1) |
86.9 *** (108.1) |
43.9 ** (66.8) |
|
| Attend to Local | 762.0 (152.7) |
750.6 (179.5) |
671.4 (118.1) |
653.2 (112.5) |
11.4 (110.8) |
18.2 (65.6) |
|
| Divided Attention | 916.0 (174.1) |
855.6 (158.6) |
825.7 (165.6) |
765.6 (175.7) |
60.4* (105.2) |
60.1* (102.9) |
|
| Response Conflict | Conflict | No Conflict | Conflict | No Conflict | Conflict–No Conflict | ||
| Attend to Global | 813.7 (148.8) |
776.6 (150.8) |
751.5 (135.4) |
703.0 (108.3) |
37.1 * (73.6) |
48.5 *** (53.9) |
|
| Attend to Local |  |
805.1 (162.2) |
791.7 (166.6) |
738.1 (119.3) |
697.3 (109.7) |
13.4 (73.9) |
40.8 ** (56.5) |
| Response Facilitation | 1 Target | 2 Targets | 1 Target | 2 Targets | One–Two Targets | ||
| Attend to Global | 725.5 (114.7) |
718.2 (132.2) |
650.6 (110.6) |
622.4 (100.3) |
7.3 (48.5) |
28.2* (43.3) |
|
| Attend to Local | 728.7 (144.7) |
696.7 (123.2) |
623.9 (100.8) |
622.5 (98.7) |
31.9* (54.7) |
1.47 (30.4) |
|
Local-global Performance in HIV-1 infected individuals (HIV) and controls (CTL).
Mean reaction times and standard deviations (SD) for each condition and for difference reaction times between two conditions to calculate local-global special effects: Precedence = RTglobal − RTlocal; Congruency = RTincongruent − RTcongruent; Response Conflict = RTconflict − RTno conflict; Response Facilitation = RTone target − RTtwo targets.
Target letters were E and T and required a YES response.
Nontarget letters were F and L and required a NO response.
p < 0.05
p < 0.01
p < 0.001 for within-groups t-tests for each special effect.
Figure 2 (A-D) illustrates the four local-global special effects for HIV and CTL groups.
Table 3.
| Selective Attention | |||||||
|---|---|---|---|---|---|---|---|
| Group | Special Effect | Group × Special Effect | Selective Attention (to global, to local) | Group × Selective Attention | Special Effect × Selective Attention | Group × Special Effect × Selective Attention | |
| A. Precedence | |||||||
| F | 3.93 | 0.01 | 1.69 | 1.25 | 0.74 | 93.1 | 1.63 |
| p | 0.055 | 0.94 | 0.20 | 0.27 | 0.39 | 0.0001** | 0.21 |
| B. Congruency | |||||||
| F | 6.85 | 18.9 | 0.96 | 0.75 | 1.19 | 5.16 | 1.24 |
| p | 0.013* | 0.0001** | 0.33 | 0.39 | 0.28 | 0.029* | 0.27 |
| C. Response Conflict | |||||||
| F | 3.14 | 16.8 | 1.29 | 0.09 | 0.38 | 1.76 | 0.46 |
| p | 0.084 | 0.0001** | 0.26 | 0.77 | 0.54 | 0.19 | 0.50 |
| D. Response Facilitation | |||||||
| F | 5.89 | 8.59 | 0.16 | 1.99 | 0.07 | 0.02 | 9.39 |
| p | 0.02* | 0.006** | 0.69 | 0.17 | 0.79 | 0.90 | 0.004** |
| Divided Attention | |||||||
| A. Precedence | |||||||
| F | 3.34 | 28.3 | 0.11 | ||||
| p | 0.075 | 0.0001** | 0.74 | ||||
| B. Congruency | |||||||
| F | 3.15 | 13.8 | 0.00 | ||||
| p | 0.084 | 0.001** | 0.99 | ||||
Summary statistic for behavioral data for each special effect: Upper table: Precedence, congruency, response conflict, and response facilitation under selective attention conditions. Mixed measures ANOVAs with between-subjects factor: group (HIV-1, controls), and within-subject factors: special effect and selective attention (to global, to local) (for congruency, response conflict, and response facilitation). Left: For precedence and congruency effects, ANOVAs were also carried out for the divided attention condition.
p < 0.05, two-tailed.
p< 0.01, two-tailed.
2. Analyses of specific local-global processing effects
2.1 Precedence: Which is processed faster, local or global information?
The precedence effect indicates which level - global or local - is processed faster and was calculated for selective and divided attention conditions.
For selective attention, mean RTs to global targets in the global attention block were compared with mean RTs to local targets in the local attention block (RTglobal versus RTlocal). Local information was processed faster than global information in controls (26.7 ms) but not HIV-1 (-3.2 ms) (Table 2). This group difference did not reach statistical significance (F(1,38) = 2.39, p = 0.13) in an ANOVA with group (HIV-1, CTL) as between-subjects factor and precedence (local, global) as within-subject factor.
To explore the effect of attentional selection on local-global processing speed in HIV-1 and controls, we analyzed response time to local and global targets while participants selectively attended to either the local or the global level. Thus, this analysis included response times to targets at the unattended spatial level. A significant precedence-by-attention interaction (Table 3) indicated global precedence effects, i.e., faster response times to global than local targets, when attention was directed to the global level (HIV: 88 ± 68 ms, CTL: 101 ± 68 ms; t(38) = 0.59, p = 0.56), and local precedence effects, i.e., faster response times to local than global targets, when attention was directed to the local level (HIV: 76 ± 80 ms, CTL: 114 ± 58 ms; t(38) = 1.69, p = 0.10). This precedence-by-attention interaction was similar in HIV-1 and control groups (p = 0.21) (Table 3) (Figure 2A).
Figure 2.
Mean reaction times (RT) and standard errors (SE) for HIV-1 infected and control participants for the local-global special effects: A. Precedence effects and B. Congruency effects (incongruent=INC, congruent=CON) for selective (local, global) and divided (local+global) attention conditions. C. Response conflict and D. Response facilitation for selective (local, global) attention conditions. See Table 2 for reaction times and difference values for each local-global special effect.
For divided attention, mean RTs to local targets were compared with mean RTs to global targets in divided attention blocks requiring attention at local and global target levels simultaneously. Both groups exhibited local-over-global precedence effects (HIV: 109.1 ms, CTL: 96.1 ms) (Table 2). The observed local precedence effects during divided attention conditions did not differ between groups (p = 0.74) (Table 3).
An ANOVA conducted to compare precedence effects (local target, global target) for selective and divided attention between the two groups (HIV-1, CTL) showed that HIV-1 and controls did not differ in local-global precedence processing (group-by-precedence interaction F(1,38) = 0.09, p = 0.77), attention (group-by-attention interaction F(1,38) = 1.19, p = 0.28) or in precedence processing during selective versus divided attention conditions (group-by-attention-by-precedence interaction F(1,38) = 1.51, p = 0.23) (Figure 2A).
2.2 Interference from incongruent local-global information: Selective and divided attention
The congruency effect tests for stimulus-related interference, where incongruent trials (local E – global T; local T – global E) were compared with congruent trials (local E – global E; local T – global T) for selective and divided attention conditions.
For selective attention, HIV-1 and controls showed congruency effects, i.e., longer response times to incongruent than congruent targets, when attending the global level (HIV-1: 86.9 ms; CTL: 43.9 ms), but not when attending the local level (HIV-1: 11.4 ms; CTL: 18.2 ms) (Table 2). An ANOVA with group (HIV-1, CTL) as between-subjects factor and congruency (incongruent, congruent) and selective attention (to local, to global) as within-subject factors revealed that this congruency-by-attention interaction (p = 0.029) was similar for HIV-1 and controls (p = 0.27) (Table 3).
For divided attention, both groups showed congruency effects, i.e., longer response times to incongruent that congruent local-global target information (HIV-1: 60.4 ms; CTL: 60.1 ms) (Table 2). Groups did not differ from each other in processing congruency, i.e., stimulus-related interference, during divided attention (p = 0.99) (Table 3).
An ANOVA conducted to compare congruency effects for selective and divided attention between the two groups (HIV-1, CTL) showed that HIV-1 and controls did not differ in attention (group-by-attention interaction F(1,38) = 0.32, p = 0.58) and congruency processing during selective and divided attention (group-by-congruency-by-attention interaction F(1,38) = 0.26, p = 0.61) (Figure 2B).
2.3 Response conflict from unattended targets: Selective attention
Response conflict tests for response-related interference by using stimuli where information at each level is associated with a different response. Conflicting trials with the letters F or L (nontargets) at the attended level (NO response) but with the concurrent presence of target letters E or T at the unattended level (YES response) were compared with non-conflicting trials, i.e., with nontarget letters (F or L) at either level (both levels: NO response), and was calculated for selective attention conditions.
Response conflict from local targets was observed in HIV-1 (37.1 ms) and controls (48.5 ms), whereas response conflict from global targets was observed in controls (40.8 ms) but less in HIV-1 (13.4 ms) (Table 2). Specifically, only 13 out of 21 HIV-1 participants, but 17 out of 19 controls showed response conflict from global targets (χ2 = 4.04, p = 0.044). An ANOVA with group (HIV-1, CTL) as between subjects factor and response conflict (conflict vs. no conflict) and selective attention (to local, to global) as within subject factors yielded significant response conflict (p < 0.0001) with faster RTs to non-conflicting than conflicting trials. Response conflict did not significantly differ between groups (p = 0.69) or interact with group and attention (p = 0.90) (Table 3) (Figure 2C).
2.4 Response facilitation from unattended targets: Selective attention
The response facilitation effect tests for reaction time gain from redundant target information using stimuli containing two targets (E or T), i.e., one at the local and one at the global level, compared to stimuli containing only one target at the attended level. Facilitation effects were calculated for selective attention conditions.
Response facilitation from additional global targets was observed in HIV-1 (31.9 ms) but not controls (1.5 ms), whereas response facilitation from additional local targets was observed in controls (28.2 ms) but less in HIV-1 (7.3 ms) (Table 2). An ANOVA with group (HIV-1, CTL) as between-subjects factor and response facilitation (two targets vs. one targets) and attention (to local, to global) as within-subject factors revealed a significant interaction between group, response facilitation and selective attention (p = 0.004) (Table 3). Follow-up paired-samples t-tests examining response facilitation in each group showed response facilitation from global (t(20) = 2.66, p = 0.015) but not local targets (t(20) = 0.69, p = 0.50) in HIV-1 and the opposite pattern in CTL, i.e., response facilitation from additional local (t(18) = 2.84, p = 0.011) but not global targets (t(18) = 0.21, p = 0.84) (Table 2). Independent sample t-tests showed that groups specifically differed in response facilitation from global (t(38) = 2.25, p = 0.031) but not local targets (t(38) = 1.44, p = 0.16) (Figure 2D).
Finally, we tested whether local-global processing speed (precedence) was related to interference (congruency, response conflict) and facilitation processing during selective attention conditions in HIV-1 and controls. In neither group were precedence effects related to interference, neither stimulus- (congruency) nor response-related (response conflict). However, we found a significant relationship between local-global precedence and response facilitation: In HIV-1, less pronounced local precedence effects, i.e., relatively greater global processing advantages, correlated with greater response facilitation from additional global targets (r = -.59, p = 0.005, two-tailed). Controls, by contrast, showed a trend for the opposite relationship with greater local precedence correlating with greater response facilitation from local targets (r = .44, p = 0.06, two-tailed).
3. Relations between local-global processing effects and clinical parameters
In HIV-1 infected individuals, higher CD4 T-cell counts correlated significantly with a more pronounced local precedence effect under divided (r = .55, p < 0.01, two-tailed) but not selective attention conditions (r = .25, p = 0.27).
4. Local-global test performance associations with callosal fiber FA and diffusivity
Figure 3 displays raw values for callosal fiber integrity indexed as orientational diffusion coherence (fractional anisotropy, FA) and magnitude of diffusion, quantified separately for longitudinal (λL) and transverse diffusivity (λT) in HIV-1 infected participants and controls. Consistent with the larger group from which the current sample was drawn (Pfefferbaum et al., 2009), HIV-1 infected individuals exhibited modestly lower FA than controls (F(1,38) = 2.01, p = 0.08), and higher diffusivity of λL (F(1,38) = 5.37, p = 0.013) and λT (F(1,38) = 3.74, p = 0.031). HIV-1 participants had a higher diffusivity in posterior than anterior callosal sectors (group-by-sector interactions: λL: F(1,38) = 6.46, p = 0.008; λT: F(1,38) = 1.94, p = 0.09). Follow-up t-tests indicated group differences in parietal (λL: t(38) = 2.06, p = 0.023; λT: t(38) = 1.61, p = 0.06), temporal (λL: t(38) = 3.30, p < 0.001; λT: t(38) = 2.92, p < 0.003) and splenium sectors (λL: t(38) = 3.29, p < 0.001; λT: t(38) = 2.61, p < 0.007).
We next tested whether callosal microstructural integrity predicted components of local-global processing in HIV-1. Applying family-wise Bonferroni correction for 6 comparisons (i.e., callosal sectors), p-values ≤ 0.008 were considered significant. Scatter plots, regression lines, correlation coefficients, and p-values for significant associations of callosal regional FA and λL with local-global performance are displayed in Figure 4.
Figure 4.
Correlations between specific effects of local-global processing (left panel: response conflict, right panel: response facilitation) and DTI metrics (fractional anisotropy (FA) or longitudinal (λL) diffusivity) for genu and temporal callosal sections. CTL: controls; HIV: HIV-1 infection.
Local-global precedence and callosal microstructural integrity
In the HIV-1 group, global processing advantages during selective attention were related to higher longitudinal diffusivity in the temporal callosal section (λL r = -.57, p = 0.007), a relationship that was not observed in control participants who, on average, exhibited local precedence effects (λL r = -.39, p = 0.10). Correlations, however, were not significantly different between HIV-1 and CTL (z = 0.48, p = 0.30) (r to zr transformation (zr = 0.5 loge (1+r)/(1−r)), Walker and Lev, 1953).
Congruency and callosal microstructural integrity
In neither group were congruency effects significantly related to measures of callosal microstructural integrity.
Response conflict and callosal microstructural integrity
In the HIV-1 group but not CTL group, lower genu fiber integrity correlated significantly with less interference from conflicting global information (HIV: FA r = .64, p < 0.002; CTL: FA r = .05, p = 0.85). Correlations differed significantly between HIV-1 and CTL (z = 2.061, p < 0.02).
Response facilitation and callosal microstructural integrity
In the HIV-1 group but not CTL group, greater temporal callosal fiber diffusivity (λL, λT) was associated with more response facilitation from additional global targets (HIV: λL r = .78, p < 0.0001; λT r = .55, p = 0.01; CTL: λL r = .26, p = 0.28; λT r = .28, p = 0.25). Correlations differed significantly between HIV-1 and CTL for longitudinal diffusivity (λL z = 2.27, p < 0.015; λT z = 0.96, p = 0.17).
To estimate the statistical contribution of regional callosal fiber integrity to the variance of local-global specific effects in HIV-1, we conducted a series of multiple regression analyses.
For precedence effects (selective attention), temporal λL explained 27% of the total variance (F(1,19) = 7.14, p = 0.015). With CD4 count as additional predictors, the variables together accounted for 40% of the total variance (F(2,18) = 5.97, p = 0.01), with λL (p = 0.005) contributing independently and CD4 count showing a trend for an independent contribution (p = 0.068). For precedence effects (divided attention), temporal λL and CD4 count together explained 31% of the total variance (F(1,19) = 4.09, p = 0.034) with CD4 count (p = 0.01) contributing independently over the contribution of λL (p = 0.62).
For response conflict, genu FA explained 42% (F(1,19) = 13.45, p < 0.002), and with genu λT adding only 1% to the total variance (F(2,18) = 6.81, p < 0.006); here, genu FA (p = 0.039) contributed independently over λT (p=0.48).
For response facilitation, λL of the temporal callosal section explained alone 61% (F(1,19) = 30.26, p < 0.0001), with λT adding only 3% to the total variance (F(2,18) = 16.01, p < 0.0001), and λL (p= 0.001) contributing independently over λT (p = 0.27).
5. Summary of results
Behaviorally, the HIV-1 group performed similarly to the control group in most visuospatial local-global processes tested but differed from controls in response facilitation, specifically, while the HIV-1 group exhibited response facilitation from additional global targets, controls showed the opposite pattern, i.e., response facilitation from additional local targets. This pattern of facilitation was related to precedence effects in HIV-1, i.e., those HIV-1 participants who exhibited more global processing advantages also showed more response facilitation from global targets. Response facilitation from global targets, and also less pronounced local precedence effects, both were related to posterior (temporal) microstructural compromise of the corpus callosum in HIV-1. Despite unaffected response-related interference (response conflict) in HIV-1 as a group, we found a within-group relationship between poorer anterior callosal integrity and reduced global response conflict. Finally, group differences in correlations between anterior and posterior callosal microstructural integrity and response conflict and response facilitation effects indicate an HIV-1-specific dissociation of anterior (genu) callosal fiber integrity with response conflict and posterior (temporal) callosal fiber integrity with response facilitation.
Discussion
Our results provide novel evidence to support the assumption that lateralized local-global processes require transcallosal integration to enable hierarchical perception via occipito-temporal connectivity and response control via prefrontal connectivity (Han et al., 2002; Volberg & Hübner, 2004). This conclusion was drawn from combined behavioral and DTI data showing that moderate regional callosal compromise in HIV-1 predicts impairment in these component local-global processes. Specifically, DTI data indicated moderate compromise in callosal white matter of the HIV-1 group and was more pronounced in posterior than anterior callosal regions. The pattern of sparing and compromise is consistent with that observed in the larger sample (Pfefferbaum et al., 2009) from which the current sample was drawn, and with another report (Wu et al., 2006). Behavioral data indicated that HIV-1 infected participants were as accurate as controls in the local–global task, but responded slower, which may represent general cognitive slowing of processing speed (Foley, Ettenhofer, Wright, & Hinkin, 2008; Grant et al., 1987; Wilkie et al., 2000). Correlational data indicated a double dissociation of anterior and posterior callosal compromise in HIV-1 affecting different components of visuospatial processing: Lower integrity of posterior callosal fibers connecting occipital regions predicted enhanced response facilitation from additional global targets, whereas lower integrity of anterior callosal fibers connecting prefrontal regions predicted less interference from conflicting global targets, i.e., less response conflict.
This pattern of callosal structure - visuospatial function relationships provides insights into specific local-global processes compromised in HIV-1, yet, it also supports the assumption that interhemispheric pathways play a role in hierarchical visuospatial processing. Although functional imaging (Han, Liu, Yund, & Woods, 2000; Han et al., 2002; Malinowski, Hübner, Keil, & Gruber, 2002; Yamaguchi et al., 2000) and lesion studies (Robertson & Lamb, 1991; Robertson, Lamb, & Zaidel, 1993) provide support for hemispheric specialization of local-global processes, this does not imply that either hemisphere could do the task alone but rather that the hemispheres interact under guidance of an allocation of processing demands (Banich, 1998; Daselaar & Cabeza, 2005; van Kleeck, 1989; Volberg & Hübner, 2004).
Local-global facilitation and posterior callosal microstructural integrity in HIV-1
Response facilitation occurred when an additional target speeded up responses even though it was irrelevant to the task. In HIV-1 patients, additional global targets speeded responses more than additional local targets, whereas controls showed the opposite pattern. Lower integrity of posterior callosal fibers connecting left temporal cortex involved in processing of local features and right temporal cortex involved in processing of global features (Han et al., 2002; Yamaguchi et al., 2000; Yoshida et al., 2007), predicted the amount of response facilitation from global targets in HIV-1. A possible explanation is that the posterior system functions of feature perception and integration (Kasamatsu, Polat, Pettet, & Norcia, 2001; Polat & Bonneh, 2000; Sterkin, Sterkin, & Polat, 2008) are enhanced when the specific visuospatial functions of each hemisphere are effectively integrated and degraded by posterior callosal fiber compromise in HIV-1. We can only speculate why this compromise shifts facilitation effects from local in controls to global in HIV-1. One possibility is that the faster processed local information inhibited processing of global information via callosal connectivity in healthy subjects (Chiarello & Maxfield, 1996; Kinsbourne, 1973, 1981). In HIV, posterior callosal compromise may have reduced such interhemispheric inhibition by local features and consequently enhanced global facilitation.
Local-global inhibition and anterior callosal microstructural integrity in HIV-1
Local-global inhibition, evidenced by longer response times to incongruent conflicting than congruent nonconflicting global–local information, was not affected in HIV-1. This diagnostic group showed normal stimulus-related global–local interference elicited by incongruent hierarchical information. In addition, HIV-1 did also not differ from controls in response-related interference, i.e., when an unattended target slowed responses even though it was irrelevant to the task (response conflict). These results differ from others reporting enhanced interference and deficient response inhibition in HIV-1 (e.g., Hardy et al., 2006; Hinkin, Castellon, Hardy, Granholm, & Siegle, 1999). These differential findings may be explained by disease status, antiretroviral therapy, substance abuse, and psychological distress which all influence the brain's structural integrity and individual cognitive performance level (Larussa et al., 2006; Martin et al., 1998; Pfefferbaum et al., 2007, 2009; Sacktor et al., 2006). In the present study, the majority of patients were asymptomatic and treated with antiretroviral therapy. Deficits in frontal executive functions (Castellon, Hinkin, & Myers, 2000; Chang et al., 2002; Hinkin et al., 1999; Schulte, Müller-Oehring, Javitz, Pfefferbaum, & Sullivan, 2008) have been attributed to a disruption of fronto-striatal circuitry in HIV-1 (Melrose, Tinaz, Castelo, Courtney, & Stern, 2008). Studies that reported such frontostriatal circuitry compromise have included HIV-1 participants in a more severe stage of the disease (e.g., Castelo, Sherman, Courtney, Melrose, & Stern, 2006; Chang et al., 2001; Hall et al., 1996). Thus, the virus may affect frontal executive control functions later during the course of the disease. The lack of marked deficits in frontal executive control functions in our sample may reflect their relative good clinical status, lack of alcohol dependency comorbidity and use of pharmacological treatment.
Nonetheless, despite unaffected local-global inhibition effects in HIV-1, we found a within-group relationship between compromised anterior callosal integrity and reduced global response conflict. Individuals with asymptomatic HIV-1 may recruit attentional brain reserves by drawing on bilateral fronto-parietal attention systems to achieve normal performance. Such compensatory brain recruitment has been observed in functional MRI studies in HIV-1 who showed the same levels of performance as controls but invoked additional frontal and parietal brain regions to achieve normal performance (Chang et al., 2001, 2004, 2008b; Ernst et al., 2009). More demonstrative white matter compromise, however, may attenuate efficient recruitment of bilateral brain reserves and reduce normal attentional and executive control functions. Thus, DTI-measured anterior callosal microstructural compromise may detect subtle deficits in prefrontal functions such as interference processing and conflict resolution in HIV-1 (Schulte et al., 2008). Finally, compromised fiber systems in HIV-1 likely contribute to abnormal functional asymmetry.
Together these results demonstrate, at least under selective attention conditions, a shift in local-global processing in HIV-1 favoring the global composition of a stimulus as evidenced by global facilitation effects, which were further related to a less lateralized local-global processing pattern in HIV-1. With high attentional load, however, as under divided attention conditions, less pronounced local precedence effects in HIV-1 were predicted by disease severity, expressed as lower CD4 t-cell counts, and greater posterior callosal longitudinal diffusivity, indicative for axonal compromise in HIV-1. Thus, slowing of local relative to global processing speed was attributable, at least in part, to disease severity and poorer posterior callosal pathway integrity in HIV-1. Finally, that HIV-1 patients showed impaired local-global facilitation effects but normal interference and response conflict argues against impaired attentional and executive control on local-global feature selection in asymptomatic HIV-1 patients and rather for a modification of local-global feature perception and integration. Consequently, HIV-1 related visuospatial compromise may only become apparent under conditions that demand interhemispheric integration of lateralized functions drawing on callosal microstructural integrity.
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA010723, AA017347, AA005965, AA017168). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. The authors thank Carla Raassi, B.A., Anne O'Reilly, Ph.D., Stephanie Sassoon, Ph.D., Andrea Spadoni, Ph.D., and Marya Schulte, Ph.D. for help with recruiting and screening study participants and assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American-Psychiatric-Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Acheampong E, Parveen Z, Mengistu A, Ngoubilly N, Wigdahl B, Lossinsky AS, Pomerantz RJ, Mukhtar M. Cholesterol-depleting statin drugs protect postmitotically differentiated human neurons against ethanol- and human immunodeficiency virus type 1-induced oxidative stress in vitro. Journal of Virology. 2007;81:1492–1501. doi: 10.1128/JVI.01843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. The missing link: the role of interhemispheric interaction in attentional processing. Brain and Cognition. 1998;36:128–157. doi: 10.1006/brcg.1997.0950. Review. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Kirk IJ, Corballis MC. Bilateral disadvantage: lack of interhemispheric cooperation in schizophrenia. Consciousness and Cognition. 2007;16:436–444. doi: 10.1016/j.concog.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- Castellon SA, Hinkin CH, Myers HF. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. Journal of the International Neuropsychological Society: JINS. 2000;6:336–347. doi: 10.1017/s1355617700633088. [DOI] [PubMed] [Google Scholar]
- Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66:1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T. Adaptation of the attention network in human immunodeficiency virus brain injury. Annals of Neurology. 2004;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T. Greater than age-related changes in brain diffusion of HIV patients after 1 year. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2008a;3(4):265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Nakama H, Stokes B, Ernst T. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. Journal of Neuroimmune Pharmacology. 2008b;3:95–104. doi: 10.1007/s11481-007-9092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, Bullitt E, Shen D, Lin W. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.04.030. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Maxfield L. Varieties of interhemispheric inhibition, or how to keep a good hemisphere down. Brain and Cognition. 1996;30:81–108. doi: 10.1006/brcg.1996.0006. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Chang L, Ernst T. Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. Journal of Neuroimmunology. 2004;157:147–152. doi: 10.1016/j.jneuroim.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Corballis MC, Barnett KJ, Fabri M, Paggi A, Corballis PM. Hemispheric integration and differences in perception of a line-motion illusion in the divided brain. Neuropsychologia. 2004;42:1852–1857. doi: 10.1016/j.neuropsychologia.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group test for assessing hand- and eye-dominance. American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Daselaar S, Cabeza R. Age-related changes in hemispheric organization. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. Oxford: Oxford University Press; 2005. [Google Scholar]
- Delis DC, Robertson LC, Efron R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychologia. 1986;24:205–214. doi: 10.1016/0028-3932(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Devinsky O, D'Esposito M. Neurology of Cognitive and Behavioral Disorders. Oxford: Oxford University Press; 2003. [Google Scholar]
- Engel AK, König P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Annals of Neurology. 2009;65:316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Burkhalter A, Van Essen DC. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. The Journal of Comparative Neurology. 1997;379:21–47. doi: 10.1002/(sici)1096-9861(19970303)379:1<21::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion Tensor Imaging of Patients with HIV and Normal-appearing White Matter on MR Images of the Brain. AJNR. American Journal of Neuroradiology. 2001;22:277–283. [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Frith CD, Frackowiak RS, Dolan RJ. Hemispheric specialization for global and local processing: the effect of stimulus category. Proceedings. Biological Sciences. 1997;264:487–494. doi: 10.1098/rspb.1997.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Dolan RJ. Hemispheric asymmetries in global/local processing are modulated by perceptual salience. Neuropsychologia. 1999;37:31–40. doi: 10.1016/s0028-3932(98)00047-5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Foley J, Ettenhofer M, Wright M, Hinkin CH. Emerging issues in the neuropsychology of HIV infection. Current HIV/AIDS Reports. 2008;5:204–211. doi: 10.1007/s11904-008-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Perceptual and attentional processes following callosal section in humans. Neuropsychologia. 1987;25:119–133. doi: 10.1016/0028-3932(87)90048-0. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. Review. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Bogen JE, Sperry RW. Observations on visual perception after disconnexion of the cerebral hemispheres in man. Brain. 1965;88:221–236. doi: 10.1093/brain/88.2.221. [DOI] [PubMed] [Google Scholar]
- Gerig G, Corouge I, Vachet C, Krishnan KR, MacFall JR. Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study. 13th Proceedings of the International Society for Magnetic Resonance in Medicine; Miami, FL. 2005. [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, Jacobus J, Woods SP, Jernigan TL, Ellis RJ, Frank LR, Grant I. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. Journal of neurovirology. 2009;15(2):187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Atkinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, McCutchan JA. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Annals of Internal Medicine. 1987;107:828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- Hall M, Whaley R, Robertson K, Hamby S, Wilkins J, Hall C. The correlation between neuropsychological and neuroanatomic changes over time in asymptomatic and symptomatic HIV-1-infected individuals. Neurology. 1996;46:1697–1702. doi: 10.1212/wnl.46.6.1697. [DOI] [PubMed] [Google Scholar]
- Han S, He X. Modulation of neural activities by enhanced local selection in the processing of compound stimuli. Human Brain Mapping. 2003;19:273–281. doi: 10.1002/hbm.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Jiang Y. Neural correlates of within-level and across-level attention to multiple compound stimuli. Brain Research. 2006;1076:193–197. doi: 10.1016/j.brainres.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Han S, Liu W, Yund EW, Woods DL. Interactions between spatial attention and global/local feature selection: an ERP study. Neuroreport. 2000;11:2753–2758. doi: 10.1097/00001756-200008210-00029. [DOI] [PubMed] [Google Scholar]
- Han S, Weaver JA, Murray SO, Kang X, Yund EW, Woods DL. Hemispheric asymmetry in global/local processing: effects of stimulus position and spatial frequency. Neuroimage. 2002;17:1290–1299. doi: 10.1006/nimg.2002.1255. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. Journal of Neurochemistry. 2007;100:567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi Y, Takeda Y, Yagi A. Global interference: the effect of exposure duration that is substituted for spatial frequency. Perception. 2002;31:341–348. doi: 10.1068/p3282. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13:306–316. doi: 10.1037//0894-4105.13.2.306. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social Class and Mental Illness. New York: John Wiley and Sons; 1958. [Google Scholar]
- Hubner R, Malinowski P. The effect of response competition on functional hemispheric asymmetries for global/local processing. Perception & Psychophysics. 2002;64:1290–1300. doi: 10.3758/bf03194772. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Polat U, Pettet MW, Norcia AM. Colinear facilitation promotes reliability of single-cell responses in cat striate cortex. Experimental Brain Research. 2001;138:163–172. doi: 10.1007/s002210100675. [DOI] [PubMed] [Google Scholar]
- Kimchi R, Merhav I. Hemispheric processing of global form, local form, and texture. Acta Psychologica. 1991;76:133–147. doi: 10.1016/0001-6918(91)90042-x. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The control of attention by interaction between the cerebral hemispheres. In: Kornblum S, editor. Attention and Performace IV. New-York: Academic Press; 1973. pp. 239–256. [Google Scholar]
- Kinsbourne M. Cognitive deficit and the unity of brain organization: Goldstein's perspective updated. Journal of Communication Disorders. 1981;14:181–194. doi: 10.1016/0021-9924(81)90035-6. [DOI] [PubMed] [Google Scholar]
- Larussa D, Lorenzini P, Cingolani A, Bossolasco S, Grisetti S, Bongiovanni M, Moretti F, et al. Highly active antiretroviral therapy reduces the age-associated risk of dementia in a cohort of older HIV-1-infected patients. Italian Registry Investigative Neuro AIDS (IRINA) AIDS Research and Human Retroviruses. 2006;22:386–392. doi: 10.1089/aid.2006.22.386. [DOI] [PubMed] [Google Scholar]
- Learned-Miller EG. Data driven image models through continuous joint alignment. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2006;28:236–250. doi: 10.1109/TPAMI.2006.34. [DOI] [PubMed] [Google Scholar]
- Major EO, Rausch D, Marra C, Clifford D. HIV-associated dementia. Science. 2000;288:440–442. doi: 10.1126/science.288.5465.439d. [DOI] [PubMed] [Google Scholar]
- Malinowski P, Hübner R, Keil A, Gruber T. The influence of response competition on cerebral asymmetries for processing hierarchical stimuli revealed by ERP recordings. Experimental Brain Research. 2002;144:136–139. doi: 10.1007/s00221-002-1057-1. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Robertson LC, Novak RM, Mullane KM, Pursell KJ. Global-local analysis in HIV-1 infection. Neuropsychology. 1995;9:102–109. [Google Scholar]
- Martin EM, Pitrak DL, Pursell KJ, Andersen BR, Mullane KM, Novak RM. Information processing and antiretroviral therapy in HIV-1 infection. Journal of the International Neuropsychological Society: JINS. 1998;4:329–335. [PubMed] [Google Scholar]
- Martin EM, Sorensen DJ, Robertson LC, Edelstein HE, Chirurgi VA. Spatial attention in HIV-1 infection: a preliminary report. The Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4:288–293. doi: 10.1176/jnp.4.3.288. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behavioral Brain Research. 2008;188:337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Mevorach C, Humphreys GW, Shalev L. Effects of saliency, not global dominance, in patients with left parietal damage. Neuropsychologia. 2006;44:307–319. doi: 10.1016/j.neuropsychologia.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Mevorach C, Shalev L, Allen HA, Humphreys GW. The left intraparietal sulcus modulates the selection of low salient stimuli. Journal of Cognitive Neuroscience. 2009;21:303–315. doi: 10.1162/jocn.2009.21044. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Moroni M, Antinori S. HIV and direct damage of organs: disease spectrum before and during the highly active antiretroviral therapy era. AIDS. 2003;17(Suppl 1):51–64. [PubMed] [Google Scholar]
- Müller-Oehring EM, Schulte T, Raassi C, Pfefferbaum A, Sullivan EV. Local-global interference is modulated by age, sex and anterior corpus callosum size. Brain Research. 2007;1142:189–205. doi: 10.1016/j.brainres.2007.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Schulte T, Fama R, Pfefferbaum A, Sullivan EV. Global-local interference is related to callosal compromise in alcoholism: a behavior-DTI association study. Alcoholism, Clinical and Experimental Research. 2009;33:477–489. doi: 10.1111/j.1530-0277.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D. Forest before trees: the precedence of global features in visual perception. Cognitive Psychology. 1977;9:353–383. [Google Scholar]
- Neeman M, Freyer JP, Sillerud LO. A simple method for obtaining cross-term-free images for diffusion anisotropy studies in NMR microimaging. Magnetic Resonance in Medicine. 1991;21:138–143. doi: 10.1002/mrm.1910210117. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Windsor, Canada: Nelson Publishing Company; 1982. [Google Scholar]
- Olesen PJ, Schendan HE, Amick MM, Cronin-Golomb A. HIV infection affects parietal-dependent spatial cognition: evidence from mental rotation and hierarchical pattern perception. Behavioral Neuroscience. 2007;121:1163–73. doi: 10.1037/0735-7044.121.6.1163. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Sanides F. Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. Zeitschrift für Anatomie und Entwicklungsgeschichte. 1973;139:127–161. doi: 10.1007/BF00523634. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two Hemispheres- One Brain: Functions of the Corpus Callosum. New York: Alan R. Liss, Inc; pp. 47–74. [Google Scholar]
- Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, Ernst T, Singer E, Richards T, Jarvik GJ, Price R, Meyerhoff DJ, Kolson D, Ellis RJ, Gonzalez G, Lenkinski RE, Cohen RA, Navia BA. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. The Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans AC. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. Journal of Cognitive Neuroscience. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, et al. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal Fiber Bundle Compromise in HIV Infection without Dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Bonneh Y. Collinear interactions and contour integration. Spatial Vision. 2000;13:393–401. doi: 10.1163/156856800741270. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Minniti A, Zani A. Electrophysiological evidence of a perceptual precedence of global vs. local visual information. Brain Research Cognitive Brain Research. 1998;6:321–334. doi: 10.1016/s0926-6410(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Qin J, Han S. The role of parietal cortex in global/local processing of hierarchical stimuli: a transcranial magnetic stimulation study. Neuroreport. 2007;18:1921–1924. doi: 10.1097/WNR.0b013e3282f1c9d2. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Storey P, Cohen BA, Epstein LG, Edelman RR. Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR. American Journal of Neuroradiology. 2004;25:195–200. [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. Journal of Neurovirology. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR. Neuropsychological contributions to theories of part/whole organization. Cognitive Psychology. 1991;23:299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Zaidel E. Interhemispheric relations in processing hierarchical patterns: evidence from normal and commisurotomized subjects. Neuropsychology. 1993;7:325–342. [Google Scholar]
- Rohlfing T, Maurer CR. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Transactions on Information Technology in Biomedicine. 2003;7:16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky R, Robertson K, Wong M, Musisi S, Ronald A, Katabira E. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67:311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- Schulte T, Mueller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: evidence from a Stroop Match-to-Sample task. Biological Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Javitz H, Pfefferbaum A, Sullivan EV. Callosal compromise differentially affects conflict processing and attentional allocation in alcoholism, HIV, and their comorbidity. Brain Imaging and Behavior. 2008;2:27–38. doi: 10.1007/s11682-007-9014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J. The cerebral balance of power: confrontation or cooperation? Journal of experimental psychology Human perception and performance. 1982;8:253–272. doi: 10.1037//0096-1523.8.2.253. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen DJ, Martin EM, Robertson LC. Visual attention in HIV-1 infection. Neuropsychology. 1994;8:424–432. [Google Scholar]
- Stephan KE, Marshall JC, Penny WD, Friston KJ, Fink GR. Interhemispheric integration of visual processing during task-driven lateralization. Journal of Neuroscience. 2007;27:3512–3522. doi: 10.1523/JNEUROSCI.4766-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterkin A, Sterkin A, Polat U. Response similarity as a basis for perceptual binding. Journal of Vision. 2008;8:17.1–12. doi: 10.1167/8.7.17. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.007. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC. Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. AJNR. American Journal of Neuroradiology. 2005;26:2275–2281. [PMC free article] [PubMed] [Google Scholar]
- Tzelgov J, Henik A, Berger J. Controlling Stroop effects by manipulating expectations for color words. Memory & Cognition. 1992;20:727–735. doi: 10.3758/bf03202722. [DOI] [PubMed] [Google Scholar]
- van Kleeck MH. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: new data and a meta-analysis of previous studies. Neuropsychologia. 1989;27:1165–1178. doi: 10.1016/0028-3932(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Volberg G, Hubner R. On the role of response conflicts and stimulus position for hemispheric differences in global/local processing: an ERP study. Neuropsychologia. 2004;42:1805–1813. doi: 10.1016/j.neuropsychologia.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Volberg G, Hübner R. Hemispheric differences for the integration of stimulus levels and their contents: evidence from bilateral presentations. Perception & Psychophysics. 2006;68:1274–1285. doi: 10.3758/bf03193727. [DOI] [PubMed] [Google Scholar]
- Walker H, Lev J. Statistical Inference. New York: Holt; 1953. [Google Scholar]
- Wilkie FL, Goodkin K, van Zuilen MH, Tyll MD, Lecusay R, Edwin T. Cognitive Effects of HIV-1 Infection. CNS Spectrums. 2000;5:33–51. doi: 10.1017/s1092852900013249. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. American Journal of Neuroradiology. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- Xu D, Mori S, Solaiyappan M, van Zijl PC, Davatzikos C. A framework for callosal fiber distribution analysis. Neuroimage. 2002;17:1131–1143. doi: 10.1006/nimg.2002.1285. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Yamagata S, Kobayashi S. Cerebral asymmetry of the “top-down” allocation of attention to global and local features. Journal of Neuroscience. 2000;20:RC72. doi: 10.1523/JNEUROSCI.20-09-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Yoshino A, Takahashi Y, Nomura S. Comparison of hemispheric asymmetry in global and local information processing and interference in divided and selective attention using spatial frequency filters. Experimental Brain Research. 2007;181:519–529. doi: 10.1007/s00221-007-0948-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Han S. Global perception depends on coherent work of bilateral visual cortices: transcranial magnetic stimulation (TMS) studies. Science in China. Series C, Life sciences/Chinese Academy of Sciences. 2007;50:557–565. doi: 10.1007/s11427-007-0067-4. [DOI] [PubMed] [Google Scholar]