Abstract
The cerebellum has long been implicated in time perception, particularly in the subsecond range. The current set of studies examines the role of the cerebellum in suprasecond timing, using analysis of behavioral data in subjects with cerebellar lesions. Eleven cerebellar lesion subjects and 17 controls were tested on temporal estimation, reproduction and production, for times ranging from 2 to 12 s. Cerebellar patients overproduced times on both the reproduction and production tasks; the effect was greatest at the shortest duration. A subset of patients also underestimated intervals. Cerebellar patients were significantly more variable on the estimation and reproduction tasks. No significant differences between normal and cerebellar patients were found on temporal discrimination tasks with either sub- or suprasecond times. Patients with damage to the lateral superior hemispheres or the dentate nuclei showed more significant impairments than those with damage elsewhere in the cerebellum, and patients with damage to the left cerebellum had more significant differences from controls than those with damage to the right. These data suggest that damage to the middle-to-superior lobules or the left hemisphere is especially detrimental to timing suprasecond intervals. We suggest that this region be considered part of a network of brain structures including the DLPFC that is crucial for interval timing.
Keywords: cerebellum, time perception, lesion-based
A seminal series of experiments by Ivry and colleagues (Ivry, Keele, & Diener, 1988; Ivry & Keele, 1989) demonstrated that patients with cerebellar damage exhibited difficulty on tasks that required accurate timing. Although some additional studies (for review, see Ivry & Spencer, 2004) have supported the claim that the cerebellum is involved in timing, not all investigators have confirmed these effects. We address this on-going controversy here.
In the study of time perception, a distinction has been made between intervals under a second and those that range from a few seconds to minutes (Gibbon, Malapani, Dale, & Gallistel, 1997; Ivry, 1996). On some accounts, different brain mechanisms and anatomic structures are assumed to mediate the processing of sub- and supra-second time intervals (Lewis & Miall, 2003). The cerebellum has been widely implicated in perceiving, and especially producing, intervals less than a second in duration (for review, see Ivry & Spencer, 2004; Ivry, Spencer, Zelaznik, & Diedrichsen, 2002). Evidence for this comes from imaging (Penhune, Zatorre, & Evans, 1998; Schubotz, Friederici, & Yves von Cramon, 2000), lesion (Casini & Ivry, 1999; Spencer, Zelaznik, Diedrichsen, & Ivry, 2003; Spencer & Ivry, 2005), and transcranial magnetic stimulation (TMS) studies (Fierro et al., 2007; Koch et al., 2007; Lee et al., 2007). The case is not closed, however, as the authors of some imaging studies conclude that cerebellar activation is not specific to timing (Rao et al., 1997; Rao, Mayer, & Harrington, 2001). Further, at least one group of investgigators has questioned the effect of cerebellar lesions on timing subsecond intervals (Harrington, Lee, Boyd, Rapcsak, & Knight, 2004). They found no effect of patient status (lesion patient or control) on duration discrimination, or on the timekeeping component of variability on a tapping task with intervals of less than one second. Noting that many previous studies of the role of the cerebellum in timing had included patients with degenerative diseases involving, but not restricted to the cerebellum, these investigators questioned the claim that the cerebellum is crucial to timing.
Two reports involving patients with focal lesions of the cerebellum have examined its role in timing suprasecond intervals. One of these used a motor timing task in which the subject’s experience of time is operationalized by the latency to make a response, and the other used a perceptual timing task, which did not require a motor response to define the time interval. Some (e.g., Bueti, Walsh, Frith, & Rees, 2008; Lewis & Miall, 2003) but not all (e.g., Keele, Pokorny, Corcos, & Ivry, 1985; Merchant, Zarco, & Prado, 2008; Robertson, et al., 1999) investigators have proposed that different mechanisms may be responsible for each type of timing. Using a reproduction (motor timing) task with intervals ranging from eight to 21 seconds, Malapani and colleagues (Malapani, Dubois, Rancurel, & Gibbon, 1998) showed an increase in variability in patients with lesions restricted to the cerebellar hemispheres as compared to those with lesions of the midline cerebellum and vermis. Mangels, Ivry, & Shimizu (1998) compared perceptual timing in patients with cerebellar lesions and patients with prefrontal lesions with an adaptive psychophysical procedure, Parametric Estimation by Sequential Testing (PEST; Pentland, 1980); standard durations were 400 ms and 4 s. Cerebellar patients were found to have greater variability than control subjects at both times, but had a bias at the point of subjective equality (PSE) only at 4 s. These data suggested that the cerebellum is important for timing intervals both above and below a second. In contrast, studies involving a “virtual lesion” of the cerebellum induced by TMS demonstrated disruption of timing only for intervals of less than 1 s (Koch et al., 2007; Lee et al., 2007).
The current set of experiments seeks to further elucidate the role of the cerebellum in timing. To this end, we presented patients with focal, unilateral cerebellar lesions with two perceptual timing tasks (temporal estimation and temporal discrimination) and two motor timing tasks (temporal production and reproduction).
EXPERIMENT 1
Method
Subjects
Patients included 11 subjects with lesions of the cerebellum 10 vascular, 1 resection of a benign tumor). Seventeen healthy age-matched individuals served as controls. The mean age of the patients and controls was 59 ± 11 years and 56 ± 11 years, respectively. Patients had suffered their cerebellar lesion an average of 5.9 ± 3.9 years previously. No subject had a history of substance abuse or psychiatric illness. Patient demographic data are described in Table 1. Brain imaging was available for all patients (4 MRI and 7 CT scans). To facilitate the investigation of the anatomic basis of performance, the lesions were rendered on a common anatomic space (Colin27 http://imaging.mrc-cbu.cam.ac.uk/downloads/Colin/) using MRICro (http://www.sph.sc.edu/comd/rorden/mricro.html) or MRICron (http://www.sph.sc.edu/comd/rorden/mricron/) by a neurologist (HBC) who was naïve with respect to the behavioral data (See Figure 1).
Table 1.
Patient demographic data. Patient number refers to the tile number in Figure 1.
| Patient no. | Nature of lesion | Age | Education (yrs) | Sex | Handedness | Time post incident (yrs) | MMSE |
|---|---|---|---|---|---|---|---|
| 1 | Stroke | 53 | 12 | M | R | 6 | 24 |
| 2 | Hemorrhage | 61 | 12 | F | R | 3 | 22 |
| 3 | Stroke | 66 | 12 | M | L | 7 | 23 |
| 4 | Stroke | 60 | 18 | M | R | 4 | 29 |
| 5 | Stroke | 46 | 13 | M | R | 3 | 29 |
| 6 | Stroke | 77 | 11 | F | R | >10 | 25 |
| 7 | Stroke | 71 | 12 | F | R | 8 | 25 |
| 8 | Stroke | 66 | 12 | M | R | 7 | 29 |
| 9 | Stroke | 65 | 12 | F | R | 1 | 23 |
| 10 | Tumor resection | 43 | 18 | M | A | 15 | 30 |
| 11 | Stroke | 44 | 16 | F | R | 5 | 27 |
Figure 1.
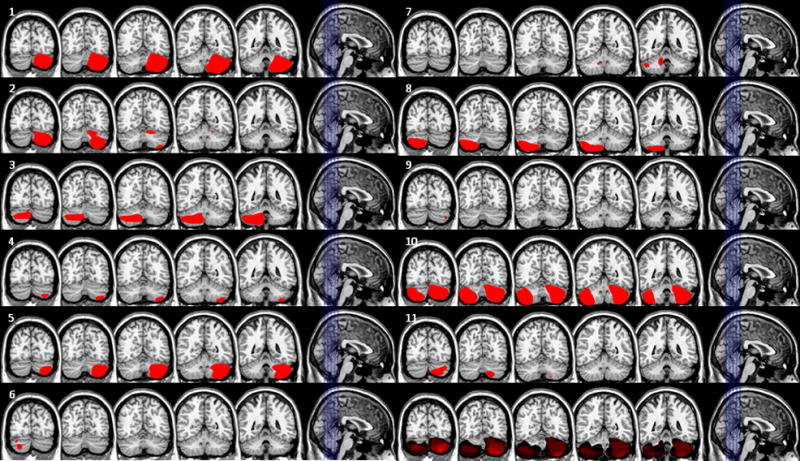
Patient lesions. Each tile represents one patient. Slices are depicted in the leftmost image in each tile. The number in the upper left of each tile refers to the patient number in Table 1. The bottom right tile shows an overlap of all lesions.
The Oral Trails task was administered to all cerebellar lesion subjects. In this task subjects are asked to alternate the letters of the alphabet with their corresponding numbers (e.g., “a -1 - b - 2, etc). As subjects are required to maintain their location in the letter or number sequence while retrieving the next item, the test is regarded as a measure of working memory.
The research was approved by the Institutional Review Board of the University of Pennsylvania.
Procedure
Participants completed tasks in the following order: estimation, production, reproduction, and, finally, the four blocks of the temporal discrimination task. The order of tasks was fixed because we had an insufficient number of subjects to completely counterbalance task sequence. No practice trials were administered for any of the tasks. Tasks are described below.
Tasks
Duration estimation task
At the onset of each trial, a fixation point was presented in the center of the screen for 1 s. A 4 cm x 4 cm red square was presented in the center of the computer screen for one of 6 durations: 2, 4, 6, 8, 10, or 12 s. At the offset of the stimulus, subjects were prompted by the word “respond” to indicate how long they believed the stimulus was present; subjects were told to respond with whatever precision they desired (that is, seconds, tenths of a second, etc.). Stimuli for each of the six durations were presented five times in random sequence. Thus, there were 30 trials in each block. Subjects were not told the range of stimulus durations and were not given feedback regarding accuracy. For this and all other tasks stimuli were presented on a Macintosh PowerBook G4 using PsyScope v. 1.2.5 (Cohen, MacWhinney, Flatt, & Provost, 1993). Temporal resolution of stimulus presentation was approximately 16 ms.
Duration production task
Subjects initiated each trial with a key press. At the onset of each trial, a fixation point was presented in the center of the screen for 1 s. The fixation point was replaced by a number (2, 4, 6, 8, 10, or 12) that indicated the duration of the interval to be generated, in seconds. The subjects initiated the stimulus onset by depressing the space bar on the keyboard. When the subjects believed the required interval had elapsed, they pressed the space bar a second time to terminate the trial. Depressing the space bar generated the red square used in the estimation task. Each duration was presented five times for a total of 30 trials in each administration of the task. Different durations were presented randomly. Subjects were not given feedback regarding accuracy.
Time reproduction task
Subjects initiated each trial with a key press. At the onset of each trial, a fixation point was presented in the middle of the screen for 1 s. Following this, the fixation point extinguished and was replaced by a red square. The stimulus persisted for a fixed duration (2, 4, 6, 8, 10, or 12 s). After the prescribed duration, the stimulus extinguished and subjects initiated the reproduction stimulus by pressing the space bar causing the red square to appear; subjects pressed the space bar a second time when they believed the target interval had been reached. Five trials at each of the six durations were presented randomly, for a total of 30 trials. Subjects were not told the range of stimulus durations and were not given feedback regarding accuracy.
Temporal Discrimination with Sub- and Supra-second Intervals
The temporal discimintation task was performed utilizing the PEST algorithm (Pentland, 1980). Four blocks of trials, each assessing a different interval, were presented in random order. At the onset of each trial, subjects were presented with a fixation point for 1 s, followed by the presentation of a red square for one of the following target intervals: 300, 600, 2000, and 8000 ms. (standard duration). When the target interval was reached, the red square extinguished for 1 s. A second red square was then presented for a variable duration of time (comparison duration) as determined by the adaptive staircase procedure of the PEST algorithm. Subjects were required to indicate on the keyboard whether they judged the second stimulus to be longer (by pressing the “L” key) or shorter (by pressing the “S” key) than the first stimulus. Subjects were not told the range of stimulus durations and were not given feedback regarding accuracy. Each interval (300, 600, 2000, 8000 ms) was assessed in a separate block of 60 trials. Stimuli were presented using the Matlab Psychophysics toolbox extensions (Brainard, 1997) on a Macintosh Powerbook G4. Temporal resolution of stimulus presentation was approximately 16 ms.
Data Analysis
Data from the PEST procedure were analyzed as previously described (Wiener & Coslett, 2008). The point of subjective equality (PSE), or the duration at which participants responded “longer” 50% of the time, and difference limen (DL), or the time interval between those durations at which participants responded “longer” 25% and 75% of the time, were subjected to the analysis described below.
For the estimation, production, and reproduction tasks, two dependent variables (DVs) were analyzed: proportional error ([mean − target duration]/target duration) and coefficient of variation (CV, standard deviation/mean). Both of these measures are or approximate ratios of the target duration and thus allow for comparison of performance at each interval independent of duration. All tasks were subjected to analysis in a mixed-model ANOVA with duration (2 to 12 s) as a within-subject factor and group (patient or control) as a between-subject factor. Polynomial contrasts were applied to the duration factor to look for trends in estimation and production of these intervals. Dependent variables for the temporal discrimination task were proportional error ([PSE − target duration])/target duration) and CV (DL/PSE). Two patients and four controls did not complete all blocks of the PEST and were thus excluded from this analysis.
Although earlier studies suggested that the lateral hemispheres of the cerebellum are responsible for time perception (Ivry et al., 1988; Malapani et al., 1998), Harrington et al (2004) suggested that the middle to superior lobules of the cerebellar hemisphere and dentate nuclei were particularly relevant to timing (Harrington, Lee et al., 2004). To test this hypothesis, we partitioned cerebellar lesion patients into those with damage including either the middle-to-superior portions of the cerebellar hemispheres or the dentate nuclei (N=7) and those with damage that predominantly involved the midline cerebellum or inferior portion of the cerebellar hemispheres (N=4). Both groups were compared to controls in analyses such as those described above.
We also divided patients into those with damage predominantly to the left (N=4) or the right cerebellum (N= 6). The patient whose lesion was the result of a tumor resection had extensive bilateral damage and was thus excluded from this analysis. Each of these groups was compared to controls in the same manner as described for the overall analysis.
To address the question of whether the three tasks (production, estimation, and reproduction) that utilized the same time intervals relied upon similar mechanisms, we ran correlation analyses on the average variability (CV) between each task. High correlations for variability between any two tasks suggests that performance of these tasks relies upon similar mechanisms (Keele, Pokorny, Corcos, & Ivry, 1985; Merchant, Zarco, & Prado, 2008; Robertson, et al., 1999).
In order to determine whether performance of a subset of patients was particularly poor on each task, we performed a K-means cluster analysis on the z-score of average variability (CV) for all four tasks, to look for two groups of subjects: high and low variability. Cluster analysis was performed for patients and controls separately. Where appropriate, the resulting groups were subjected to ANOVA as above with the additional between-subject factor of cluster group (high or low variability, patient or control).
Results
Temporal Production task
Proportional error data for this task are depicted in Figure 2. ANOVA on proportional errors showed significant main effects for group (F(1,26) = 4.8, p < 0.05) and duration (F(5,130) = 9.2, p < 0.05; linear contrast F(1,26) = 10.5, p < 0.05). Cerebellar patients overproduced durations as compared to controls. Further, productions were proportionally longer for the shorter durations than the longer ones. A significant interaction was found for duration x group (F(5,130) = 6.8, p < 0.05; linear contrast F(1,26) = 7.7, p < 0.05). Relative to controls, cerebellar lesion patients overproduced shorter intervals (2 s, patient v controls: t(26) = 2.65, p = 0.014; 4 s, patient v controls: t(26) = 2.6, p = 0.015; 10 s, patient v controls: t(26) = 1.34, p = 0.2; 12 s, patient v controls: t(26) = 1.32, p = 0.2).
Figure 2.
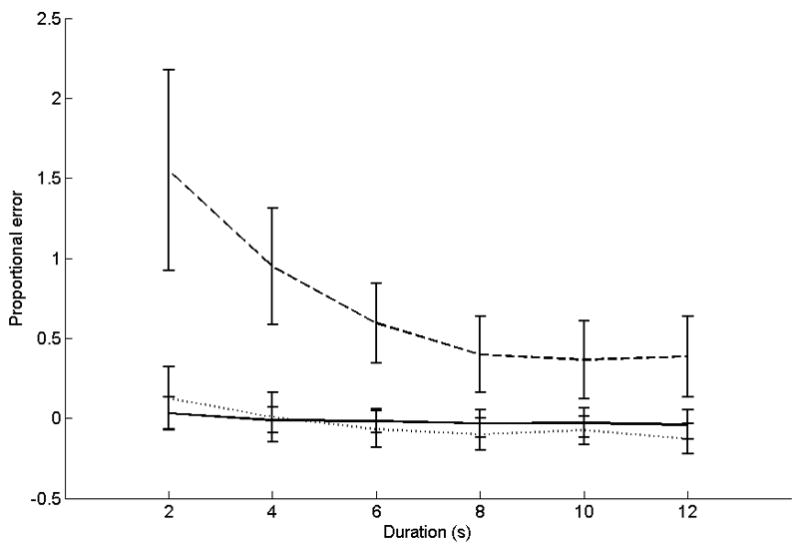
Accuracy data for the temporal production task. The x-axis is target duration. The y-axis is proportional error ((production − target duration)/target duration). Solid lines represent control subjects. Dashed lines represent patients with middle-to-superior cerebellar damage or damage that includes the dentate. Dotted lines represent patients with damage predominantly outside these areas.
Variability (CV) was assessed with an ANOVA that included the factors described above. CV data for this task are shown in Table 2. There was a significant effect of duration (F(5,130) = 6.1, p < 0.05; linear contrast F(1,26) = 12.3, p < 0.05), such that productions of shorter durations were proportionally more variable than longer ones. There was no main effect of group (F(1,26) = 2.2, ns).
Table 2.
Experiment 1: Coefficient of Variation. Coefficient of variation (CV) for each duration tested for control subjects, cerebellar patients with damage to the superior to middle lobules, and cerebellar patients with damage to inferior lobules.
| Duration (s) | |||||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | ||
| Prod | Control | 0.19 ±0.04 | 0.13 ±0.02 | 0.13 ±0.02 | 0.12 ±0.01 | 0.11 ±0.02 | 0.10 ±0.01 |
| Superior | 0.25 ± 0.05 | 0.14 ±0.04 | 0.24 ± 0.08 | 0.14 ±0.04 | 0.19 ±0.06 | 0.16 ±0.04 | |
| Inferior | 0.21 ±0.05 | 0.12 ±0.03 | 0.17 ±0.04 | 0.13 ±0.04 | 0.08 ± 0.02 | 0.11 ±0.03 | |
| Reprod | Control | 0.21 ±0.04 | 0.15 ±0.02 | 0.10 ±0.01 | 0.08 ±0.01 | 0.08 ± 0.02 | 0.09 ±0.01 |
| Superior | 0.34 ±0.08 | 0.25 ± 0.09 | 0.23 ± 0.08 | 0.26 ±0.12 | 0.20 ± 0.09 | 0.24 ± 0.09 | |
| Inferior | 0.24 ±0.11 | 0.21 ±0.04 | 0.14 ±0.04 | 0.20 ± 0.04 | 0.12 ±0.01 | 0.17 ±0.05 | |
| Estim | Control | 0.21 ±0.05 | 0.13 ±0.03 | 0.11 ±0.03 | 0.11 ±0.02 | 0.10 ±0.01 | 0.11 ±0.02 |
| Superior | 0.33 ±0.13 | 0.27 ± 0.04 | 0.19 ±0.01 | 0.14 ±0.05 | 0.22 ± 0.06 | 0.20 ±0.07 | |
| Inferior | 0.18 ±0.07 | 0.21 ±0.05 | 0.12 ±0.00 | 0.16 ±0.02 | 0.23 ± 0.07 | 0.21 ±0.08 | |
Effect of lesion location
Patients with lesions including the middle-to-superior cerebellar hemispheres or the dentate nuclei showed a pattern of differences from controls that was similar to the overall analysis. For proportional errors, significant main effects were shown for group (F(1,22) = 9.9, p < 0.05) and duration (F(5,110) = 13.4, p < 0.05; linear contrast F(1,22) = 15.5, p < 0.05). Patients with lesions to these areas overproduced time intervals relative to controls. Shorter intervals were proportionally longer than longer intervals. A significant interaction was found for duration x group (F(5,110) = 10.8, p < 0.05; linear contrast F(1,22) = 12.5, p < 0.05). Patients overproduced times at shorter durations to a greater degree than controls (2 s, patients vs controls: t(22) = 2.6, p = 0.015; 4 s, patients vs controls: t(22) = 2.7, p = 0.013; 10 s, patients vs controls: t(22) = 0.8, ns. 12 s, patients vs controls: t(22) = 0.9, ns).
When CV data were compared between middle-to-superior or dentate lesion patients and controls, a significant main effect was found for duration (F(5,110) = 4.4, p < 0.05; linear contrast F(1,22) = 6.5, p < 0.05). Shorter durations were produced more variably than controls, overall. Patients with inferior cerebellar lesions did not differ from controls.
Effect of lesion side
When compared to controls, left-sided lesion patients significantly overproduced shorter intervals, as evidenced by the interaction of group and duration (F(5,95) = 8.8, p < 0.05; linear contrast F(1,19) = 9.2, p < 0.05). There was also a significant effect of duration (F(5,95) = 10.8, p < 0.05; linear contrast F(1,19) = 11.4, p < 0.05), such that shorter intervals were overproduced more than longer intervals. Left-sided lesion patients did not differ from controls in the CV analysis. Analysis of right-sided patients as compared to controls showed significant effects of duration (F(5,105) = 8.1, p < 0.05; linear contrast F(1,21) = 14.1, p < 0.05) and duration x group (F(5,105) = 4.3, p < 0.05; linear contrast F(1,21) = 6.6, p < 0.05) on proportional errors. Trends were similar to those for left-sided cerebellar patients. CV analysis showed no significant effects between right-sided patients and controls.
Temporal Reproduction Task
Proportional errors for this task are illustrated in Figure 3. ANOVA on proportional errors showed significant effects for duration (F(5,130) = 4.1, p < 0.05; linear contrast F(1,26) = 5.2, p < 0.05) and the interaction of duration x group (F(5,130) = 2.4, p < 0.05; linear contrast F(1,26) = 5.2, p < 0.05). Shorter intervals were over-reproduced to a greater degree than longer intervals; this effect was significantly greater for cerebellar patients than controls. Variability data for this task are shown in Table 2. ANOVA on CV data showed significant main effects for duration (F(5,130) = 5.5, p < 0.05; linear contrast F(1,26) = 12.3, p < 0.05) and group (F(1,26) = 7.1, p < 0.05). Shorter intervals were proportionally more variable than longer intervals, and patients’ responses were more variable than those of controls.
Figure 3.
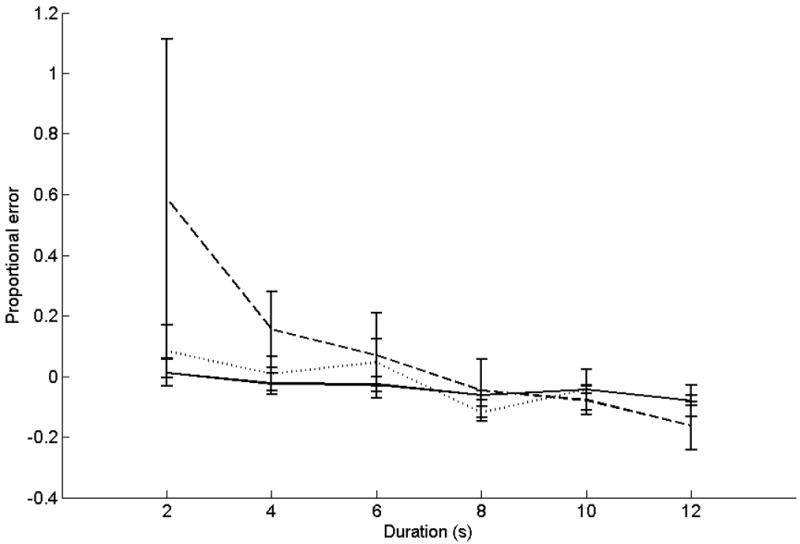
Accuracy data for the reproduction task.
The x-axis is target duration. The y-axis is proportional error ((reproduction − target duration)/target duration). Solid lines represent control subjects. Dashed lines represent patients with middle-to- superior cerebellar damage or damage that includes the dentate. Dotted lines represent patients with damage predominantly outside these areas.
Effect of lesion location
Proportional error results for patients with middle-to-superior or dentate lesions closely mirrored those for the overall analysis. Significant effects were found for the main effect of duration (F(5,110) = 5.0, p < 0.05; linear contrast F(1,22) = 6.2, p < 0.05) and the interaction of duration x group (F(5,110) = 3,3, p < 0.05; linear contrast F(1,22) = 4.2, p = 0.053). Trends were the same as those described in the overall analysis. CV results showed a main effect for group (F(1,22) = 8.0, p < 0.05) and duration (F(5,110) = 3.9, p < 0.05; linear contrast F(1,22) = 8.0, p < 0.05). Patients were more variable in responses than controls, and reproductions at shorter durations were proportionally more variable than those at longer durations, overall.
Proportional error did not differ between patients with inferior cerebellar lesions and controls. However, CV analysis between these groups showed a main effect of group (F(1,19) = 4.7, p < 0.05).
Effect of lesion side
For proportional errors, analysis of left-sided lesion patients vs controls showed a significant effect of group (F(1,19) = 6.8, p < 0.05), duration (F(5,95) = 8.6, p < 0.05, linear contrast F(1,19) = 9.8, p < 0.05), and the interaction between group and duration (F(5,95) = 6.7, p < 0.05; linear contrast F(1,95) = 7.6, p < 0.05). Analysis of CV between left-sided lesion patients and controls showed a significant effect of group (F(1,19) = 10.3, p < 0.05) and duration (F(5,95) = 4.3, p < 0.05; linear contrast F(1,19) = 5.1, p < 0.05), with patients more variable than controls. Right-sided cerebellar patients also showed a significant effect of group for the CV measure (F(1,21) = 5.6, p < 0.05), with patients more variable than controls.
Temporal Estimation task
Proportional errors for this task are presented in Figure 4. ANOVA on proportional errors showed a significant main effect for duration (F(5,130) = 11.2, p < 0.05; linear contrast F(1,26) = 11.1, p < 0.05). Paired t-tests showed the 2-s duration was overestimated out of proportion to the others, by both controls and patients (2 s v 4 s: t(27) = 4.5, p < 0.001; 2 s v 6 s: t(27) = 3.8, p = 0.001; 2 s v 8 s: t(27) = 3.6, p = 0.001; 2 s v 10 s: t(27) = 3.9, p = 0.001; 2 s v 12 s: t(27) = 3.6, p = 0.001). The main effect of group was not significant (F(1,26) = 2.7, ns). No significant interactions were found. CV data for this task are shown in Table 2. ANOVA on CVs showed significant main effects for duration (F(5,130) = 3.1, p < 0.05; linear contrast F(1,26) = 4.1, p < 0.05), and group (F(1,26) = 6.7, p < 0.05). Lesion patients were more variable in their estimations than controls, and shorter durations were estimated proportionally more variably than longer durations. No significant interactions were found.
Figure 4.
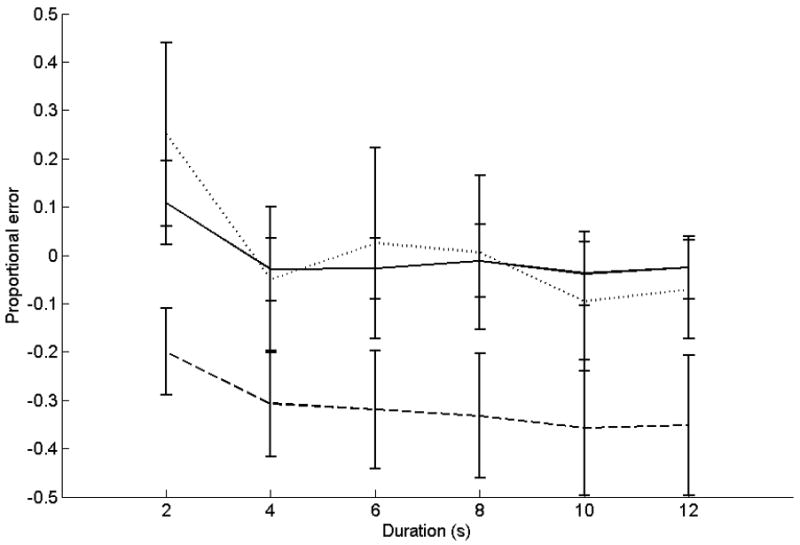
Temporal Estimation. Accuracy data for the temporal estimation task. The x-axis is target duration. The y-axis is proportional error ((estimation − target duration)/target duration). Solid lines represent control subjects. Dashed lines represent patients with middle-to-superior cerebellar damage or damage that includes the dentate. Dotted lines represent patients with damage predominantly outside these areas.
Effect of lesion location
For middle-to-superior and dentate lesion patients, main effects of duration (F(5,110) = 5.5, p < 0.05; linear contrast F(1,22) = 5.4, p < 0.05) and group (F(1,22) = 5.7, p < 0.05; linear contrast F(1,22) = 6.7, p < 0.05) were found when comparing proportional error to controls. Estimations of shorter times were proportionally longer than the others. Examination of the means suggested that the 2-second interval was estimated to be longer than the other durations, so we performed individual mixed-model ANOVAs with group as the between-subjects measure, and individually comparing the 2-second interval with each of the other intervals as a within-subjects measure. Each ANOVA showed a significant difference between 2 s and the other time interval (Fs ranged from 5.3 to 5.9, p < 0.05). CV data showed significant main effects of group (F(1,22) = 6.2, p < 0.05) and duration (F(5,110) = 3.3, p < 0.05; linear contrast F(1,22) = 6.1, p < 0.05). Patients responded more variably than controls, and shorter durations were estimated more variably than longer durations proportionally, overall.
Proportional error and CV analysis between patients with inferior cerebellar lesions not including the dentate and controls showed no group effects.
Effect of lesion side
Left-sided lesion patients were more variable than control subjects, as evidenced by the CV measure for estimation (F(1,19) = 8.8, p < 0.05). There was a significant effect of duration in this analysis also (F(5,95) = 6.1, p < 0.05; linear contrast F(1,19) = 4.6, p < 0.05). Patients with lesions of the left side of the cerebellum showed no differences from controls in proportional error of estimation. Right-sided lesion patients showed no significant differences from controls.
Temporal Discrimination Task
Proportional errors and CV data for this task are shown in Table 3. There were no group effects for the PEST procedure. ANOVA of the proportional error of PSE showed a significant effect of duration (F(3,60) = 4.3, p < 0.05; linear contrast F(1,20) = 4.0, p = 0.058), such that all subjects overestimated the shorter durations as compared to the longer durations. ANOVA of CV data showed a significant effect of duration (F(3,60) = 3.5, p < 0.05; linear contrast F(1,20) = 7.6, p < 0.05): shorter durations were judged more variably than longer durations by both groups of subjects.
Table 3.
Experiment 1: Temporal Discrimination. Proportional error (prop error) and coefficient of variation (CV) for each duration tested for control subjects, cerebellar patients with damage to the superior to middle lobules, and cerebellar patients with damage to inferior lobules.
| Duration(ms) | |||||
|---|---|---|---|---|---|
| 300 | 600 | 2000 | 8000 | ||
| Control | Prop Error | 0.13 ±0.05 | −0.02 ± 0.07 | −0.04 ± 0.03 | −0.04 ± 0.02 |
| CV | 0.30 ± 0.04 | 0.26 ± 0.03 | 0.20 ± 0.02 | 0.22 ± 0.03 | |
| Superior to Middle | Prop Error | 0.32 ± 0.38 | 0.21 ± .025 | −0.16 ± 0.09 | 0.08 ± 0.06 |
| CV | 0.31 ± 0.07 | 0.33 ± 0.07 | 0.20 ± 0.03 | 0.17 ± 0.07 | |
| Inferior | Prop Error | 0.18 ± 0.21 | 0.12 ± 0.12 | −0.64 ±0.50 | −0.18 ± 0.06 |
| CV | 0.24 ± 0.08 | 0.14 ± 0.05 | 0.23 ± 0.08 | 0.17 ± 0.09 | |
Effect of lesion location
Results for proportional errors when comparing patients with middle-to-superior cerebellar lesions mirrored those in the overall analysis. No group effects were significant for the PEST procedure. CV analysis revealed a significant main effect of duration (F(3,51) = 4.3, p < 0.05; linear contrast F(1,17) = 8.7, p < 0.05). Shorter durations were judged more variably than longer durations by both groups of subjects.
For patients with inferior lesions not including the dentate, no significant effects were found for the proportional error or CV analysis between these two groups.
Effect of lesion side
Left and right-sided lesion patients were not significantly different from controls on either measure.
Cluster analysis
No clusters were found for the patient groups for the production, reproduction, and estimation tasks. In this analysis all patients but one were classified to the same cluster. However, in the temporal discrimination task, two clusters, high and low variability, were identified in both the patient and control groups. ANOVA was performed on raw CV scores with duration as a within-subject variable and cluster group (patient- high variability, patient-low variability, control- high variability, and control- low variability) as a between subject factor. The main effects of cluster group (F(3,18) = 13.6, p < 0.05) and duration (F(3,54) = 3.2, p < 0.05) were significant. Post-hoc analysis (least-squares difference, LSD) showed significant differences between the high and low variability groups (high-variability patient vs low-variability patient, p < 0.001; high-variability control vs low-variability control, p < 0.001), but neither the high variability groups (patient vs control, p = 0.13) nor the low variability groups (patient vs control, p = 0.33) differed from each other. There was no interaction between cluster and duration, suggesting that high variability groups were more variable at every duration.
Correlation Analysis
Correlation analyses showed a strong correlation between production and reproduction CV in the patient group (R2 = 0.87, p < 0.001). A significant correlation was found between production and estimation in the control group (R2 = 0.49, p = 0.048).
Significant negative correlations were found between the oral trail making task and production (R2 = −0.758, p = 0.007) and reproduction (R2 = −0.65, p = 0.03) proportional error, and estimation CV (R2 = −0.729, p = 0.01).
Discussion
Participants with cerebellar lesions involving middle-to-superior and dentate nucleus exhibited several abnormalities. First, they underestimated and overproduced intervals of greater than one second. There are multiple possible accounts of such a finding (cf. Weiner and Coslett, 2008). One possible explanation is that lesions involving the middle-to-superior cerebellum and/or dentate nucleus cause the putative “clock” mechanism to run more slowly. On this account, during an estimation task, subjects accumulate a smaller number of “beats” while monitoring the stimulus; when converting the number of beats to an interval measured in seconds, subjects consult memory stores that reflect a long-standing (that is, premorbid) calibration. Conversely, when asked to produce an interval, subjects consult the same memory stores to determine the number of beats that correspond to the desired interval. As each beat for a “slow clock” corresponds to a longer absolute interval, subjects will produce a longer interval; similarly, while estimating an interval, subjects will employ a clock for which beats are longer in absolute terms; as a consequence, subjects will count fewer beats and the interval will be underestimated. The slow clock account is also consistent with the lack of an effect of group on the reproduction and temporal discrimination tasks. Because the same time basis is used for both the standard and the comparison time in the discrimination task, or the standard and the reproduced time in the reproduction task, there should be no differences between patients and controls on these tasks.
A second possibility is that the deficits are attributable to impaired long-term memory. In the estimation and production tasks, participants must rely upon long-term memory for a representation for each interval. In contrast, long-term memory is not necessary for the reproduction or temporal discrimination tasks as the comparison stimulus is provided for each trial; consistent with the long term deficit account, no main effect of group was found. Another observation consistent with this account is that despite the fact that subjects were tested years after their lesion, they had not accommodated to the deficit. As work in animals (reviewed in Meck, 1996) suggests that subjects adapt relatively quickly to changes in clock speed with the result that estimations typically become accurate despite the alteration in clock speed. Subjects adapt less well to deficits in timing that are thought to reflect memory (Meck, 1996).
Finally, the pattern of deficit exhibited by cerebellar subjects could reflect an impairment in memory retrieval. Indeed, the right dorsolateral prefrontal cortex (DLPFC), which has connections with the left superior cerebellar lobule, has been implicated in retrieval of long-term memories for visual stimuli (Rossi, et al., 2001). Poor retrieval of temporal values for the seconds-values tested here would produce a similar pattern of results.
A second significant finding from Experiment 1 is that subjects with cerebellar lesions demonstrate increased variability in responses. This deficit has been reported in a number of investigations of timing in subjects with brain lesions (e.g., Coslett et al, 2007, Nichelli, Clark, Hollnagel, & Grafman, 1995). Increased variability may reflect a noisy or inconsistent timing mechanism, or possibly variability in factors unrelated to timing, such as motor performance.
The preponderance of data from earlier studies points to an effect of cerebellar lesions on timing millisecond intervals. We did not find such an effect in the temporal discrimination task. To address this, we further broke down the patient and control groups into high and low variability groups to determine if a more fine-grained approach would shed light on more subtle effects. This did not produce differential effects for cerebellar patients; high variability patients were no more variable in responding than high variability controls. Thus, we find no clear evidence of sub-second timing abnormalities in subjects with cerebellar lesions. The lack of an effect on the duration discrimination assessing millisecond timing must be interpreted with caution, however. The temporal discrimination task is the only test on which cerebellar lesion subjects did not differ from controls by any measure, even with suprasecond stimuli. Thus, it is possible that the failure to demonstrate an abnormality on this task is attributable to task demands rather than the interval to be timed.
Also of relevance in this context is the fact that on several tasks, there was a group by duration interaction reflecting the fact that cerebellar lesion subjects were more impaired than controls with shorter intervals, particularly 2 second stimuli. This finding is consistent with our previous findings from patients with hemispheric brain lesions (Coslett, Shenton, Dyer, & Wiener, 2007), coincides with a breakpoint in CV function found by Getty (1975), and raises the possibility that the cerebellum is, as suggested by a number of investigators, most relevant for timing of short intervals but that the distinction between “short” and “long” intervals is continuous across intervals of up to several seconds rather than sharply defined. In this context it should be noted that although a number of investigators have supported the claim that different mechanisms underlie the timing of short and long intervals, there has been disagreement as to when the interval divides short from long (Gibbon et al., 1997; Lewis & Miall, 2003; Rammsayer, 1992, 1999). Furthermore, some studies have found a distinction between modes of processing at times of up to 3.5 seconds (Kagerer, Wittmann, Szelag, & Steinbuchel, 2002). Thus while the current results do speak to a role for the cerebellum in timing longer intervals, they also support a model in which the cerebellum more directly or more strongly influences the timing of short intervals, with the line of demarcation possibly up to 3 seconds or greater for some tasks.
Cerebellar lesion subjects were significantly different from controls on the production and reproduction tasks, both of which are tests of motor timing; furthermore, for both these tasks, there was a group by duration interaction reflecting the fact that patients were more inaccurate at shorter intervals than longer ones. An alternative hypothesis is that cerebellar lesions lead to poorer motor processing at shorter intervals. In support of this, variability in the production and reproduction tasks was correlated in patients, but not controls. To address the possibility that motor deficits underlie the impairment exhibited by cerebellar lesion subjects, a motor control task was performed that places minimal demands on timing systems. If motor deficits underlie the abnormalities exhibited by cerebellar subjects in Experiment 1, one would expect the cerebellar lesion group to differ from controls on this task. Furthermore, one might expect those subjects who were most impaired in the timing tasks to exhibit the greatest abnormality on the motor control task.
EXPERIMENT 2
Method
Subjects
Lesion subjects were the same subjects as in Experiment 1. Control subjects included 15 individuals with no neurological or psychiatric history. Mean age of patients and controls was 59 ± 11 years and 57 ± 11 years, respectively.
Task
Participants performed a cued reaction time task, in which a red square was presented for 2, 4, 6, 8, 10 or 12 s. Participants were instructed to press the space bar as quickly as possible after the square disappeared. Durations were presented randomly, and every duration was presented 10 times for a total of 60 trials in one block.
Analysis
For each individual, reaction time latencies were averaged for each duration. Also, intra-individual variability was calculated as standard deviation divided by mean reaction time to produce a coefficient of variation score. A mixed-model ANOVA was run for each dependent variable in which patient status (lesion patient vs. control) was the between-subject variable and duration was the within-subject variable. As post-hoc tests, individual t-tests were applied between lesion patients and controls at the shortest and longest durations.
Further, correlations between patient performance on the cued RT task and patient performance on the two motor tasks, temporal production and reproduction, from Experiment 1, were performed. Mean RT latency was analyzed against mean proportional error on the temporal tasks, and mean standard deviation of the RT task was analyzed against mean CV of the temporal tasks.
Results
Data for the cued reaction time experiment are shown in Table 4. Patients were slower to respond than controls, as evidenced by a significant main effect of group on latency (F(1,24) = 7.9, p < 0.05). There was also a significant effect of duration (F(5,120) = 13.1, p < 0.05), such that all participants responded less quickly to squares of shorter durations (2 s vs 12 s: t(25) = 5.0, p < 0.05). There was also a significant interaction between duration and group (F(5,120) = 2.4, p < 0.05), such that the effect of duration seen in control subjects was exaggerated in patients.
Table 4.
Experiment 2 Latency & variability data for the six durations tested in the cued reaction time task.
| 2000 | 4000 | 6000 | 8000 | 10000 | 12000 | ||
|---|---|---|---|---|---|---|---|
| Patient | Latency (ms) | 630.3 ± 74.0 | 494.2 ± 49.2 | 498.3 ± 84.4 | 497.2 ± 86.7 | 443.3 ± 49.0 | 448.9 ± 49.4 |
| Variability (Std Dev) | 208.6 ± 55.3 | 128.1 ± 23.7 | 131.0 ± 35.8 | 152.8 ± 68.6 | 134.0 ± 34.3 | 118.6 ± 31.1 | |
| Control | Latency (ms) | 408.4 ± 15.5 | 361.8 ± 14.6 | 335.7 ± 14.1 | 328.4 ±11.1 | 328.0 ± 11.6 | 336.6 ± 12.7 |
| Variability (Std Dev) | 105.8 ± 24.2 | 94.7 ± 24.4 | 81.7 ± 21.0 | 70.2 ± 20.4 | 78.9 ± 17.9 | 86.0 ± 22.1 | |
Variability was also assessed with an ANOVA. There was a main effect of duration (F(5,120) = 4.3, p < 0.05), such that responses to shorter durations were more variable than those to longer durations (2 s vs 12 s: t(25) = 3.0, p < 0.05). Patients were not more variable than controls, however, as there was no main effect of group on standard deviation of responses (F(1,24) = 2.0, ns).
For cerebellar lesion subjects mean reaction time latency did not correlate with latency for any timing measure analyzed (production, estimation, or reproduction). However, mean reaction time variability correlated with mean CV for both production (R2 = 0.74, p < 0.01) and reproduction (R2 = 0.89, p < 0.001) in patients. Mean reaction time variability did not correlate with mean CV for estimation in patients.
Discussion
We believe that the relatively minor abnormalities exhibited by the cerebellar lesion subjects are unlikely to explain the timing deficits exhibited by these subjects in Experiment 1 for several reasons. First, response latency on the reaction time task did not correlate with performance on the timing tasks, meaning that those patients who were the slowest to respond were not those who overproduced or over-reproduced to the greatest extent. Second, differences in latency on the RT task were modest, whereas differences on the timing tasks were on a scale of seconds. It is not clear how a 200 ms delay in motor response would explain the several order of magnitudes larger differences observed on timing tasks.
GENERAL DISCUSSION
Participants with cerebellar lesions exhibited significant impairments in accuracy as well as increased variability on several timing tasks involving stimuli in the suprasecond range. Based on findings from imaging (Penhune et al., 1998; Schubotz et al., 2000), lesion subjects (Casini & Ivry, 1999; Spencer et al., 2003; Spencer & Ivry, 2005), and TMS studies (Fierro et al., 2007; Koch et al., 2007; Lee et al., 2007), it has been argued that the cerebellum is relevant to timing of subsecond intervals only (for review, see Ivry & Spencer, 2004; Ivry et al., 2002). In contrast, timing of intervals in the seconds-to-minutes range has often been attributed to areas of the brain concerned with working memory (e.g., DLPFC: Jech, Dusek, Wackermann, & Vymazal, 2005; Jones, Rosenkranz, Rothwell, & Jahanshahi, 2004; Koch, Oliveri, Torriero, & Caltagirone, 2003; Lewis & Miall, 2006; Lewis & Miall, 2006; Rao et al., 2001; Rubia & Smith, 2004), motor areas (e.g., the basal ganglia: Lustig, Matell, & Meck, 2005; Matell & Meck, 2004) and supplementary motor area (Ferrandez et al., 2003; Macar, Anton, Bonnet, & Vidal, 2004; Macar, Vidal, & Casini, 1999). The current results argue for a cerebellar role in timing longer intervals as well as subsecond intervals.
Although the findings must be interpreted with caution given the relatively small sample size, our data speak to the anatomic basis of temporal processing in the cerebellum in two respects. First, like Harrington et al (2004), we found that damage to the middle-to-superior lobules is especially detrimental to timing. Second, we found that patients with left cerebellar damage were more impaired than patients with right cerebellar damage. This finding is consistent with a number of functional imaging studies that have reported activation restricted to the left cerebellum in time perception tasks (Bueti et al., 2008; Harrington, Boyd et al., 2004; Jantzen, Steinberg, & Kelso, 2005; Smith, Taylor, Lidzba, & Rubia, 2003). The dentate nucleus, the major source of outflow from the cerebellar hemisphere, projects contralaterally to the DLPFC (areas 9 and 46) in primate cortex (Middleton & Strick, 1994; Middleton & Strick, 2001), and, to a lesser extent, premotor areas and the SMA (Akkal, Dum, & Strick, 2007). The cerebellum also receives input from these areas (Glickstein, May, & Mercier, 1985), creating what are believed to be closed-circuit loops (Schmahmann, 1996; Strick, Dum, & Fiez, 2009). Recent imaging studies (e.g., Stoodley & Schmahmann, 2009, Habas, et al., 2009) suggest that the superior cerebellum is involved in a network that includes the DLPFC and lobules V and VI of the cerebellum). Additionally, lobule VII, crus I and II of the cerebellum and the SMA appear to be linked in a putative sensorimotor network.
A number of lines of evidence implicate the DLPFC (Jones et al., 2004; Koch et al., 2003; Lewis & Miall, 2006; Weiner et al, in press) in temporal processing. Additionally, a number of studies have suggested that the right DLPFC in particular is important for temporal processing (for review see Lewis & Miall, 2006; Smith et al., 2003). In light of these findings we suggest that the left superior cerebellar hemisphere should be regarded as a component of a fronto-cerebellar network that involves the right DLPFC (Smith et al., 2003).
Several lines of evidence support this hypothesis. Lewis and Miall (2006) proposed that the DLPFC keeps track of time with neurons that change activity in a predictable way over the duration to be timed (Genovesio, Tsujimoto, & Wise, 2006; Matell & Meck, 2004; Niki & Watanabe 1979). In keeping with this account, inhibition of DLPFC activity either by structural brain lesion (Koch, Oliveri, Carlesimo, & Caltagirone, 2002) or transient disruption with TMS (Koch, Oliveri, & Caltagirone, 2009) cause decrements in timing behavior, perhaps by interfering with the activity of these neurons. A second point is the finding that performance on the Oral Trails task correlates strongly with performance on estimation, production and reproduction tasks in subjects with cerebellar lesions. As the DLPFC is assumed to be crucial to working memory (Goldman-Rakic, 1995), disruption of DLPFC by a cerebellar lesion might be expected to not only interfere with timing but working memory as well.
One potential objection to the hypothesis that the deficits in temporal processing for supra-second intervals is attributable to a disruption of a cerebellar-DLPFC network is that cerebellar lesion subjects performed well on the temporal discrimination task for which working memory may be relevant. There are at least three possible explanations for this. One possibility is that that the working memory load for this task is modest; thus, if subjects do not perform the task by maintaining the standard interval as a veridical representation but instead code the interval in more abstract terms such as units (e.g., beats, counts, quarter seconds, etc), the working memory load for the task would be expected to be small. A second possibility appeals to the fact that “working memory deficits” may arise for different reasons. One form of working memory impairment is noise or imprecision in the system such that information is corrupted in a random fashion; such a deficit would be expected to cause an impairment on the temporal discrimination task. An alternative account of a working memory deficit, however, is that the problem reflects a systematic “bias” or alteration in the system (e.g., steep ramping of neural activity). Such a systematic bias would not be expected to generate impairment on the temporal discrimination task because the same bias would be evident in coding both the standard and test stimuli.
An alternative to the hypothesis that the deficits exhibited by cerebellar lesion subjects reflect abnormal function within a cerebellar-DLPFC loop is the possibility that timing mechanisms intrinsic to the cerebellum are impaired. There are several possible accounts. Purkinje cells change their response patterns during acquisition of new learned timing (Kotani, Kawahara, & Kirino, 2003), indicating that these cells are sensitive to the timing of events in the milliseconds range. Timing of these responses relies upon long-term depression of Purkinje cells (Koekkoek, et al., 2003). In light of the Multiple Time Scales (MTS; Staddon, 2005) account of timing, in which decaying memory traces represent time passing, decaying activity in these cells may support accurate timing behavior in the cerebellum. Additionally a recent model suggests that the olivo-cerebellar system, via temporal patterns generated in the inferior olive, is responsible for timing intervals on the order of hundreds of milliseconds using oscillations to keep track of time (Jacobson, Rokni, & Yarom, 2009). While it is unclear whether these putative mechanisms are relevant to timing in the range of seconds, these models provide physiologically plausible examples of neural processes could be relevant to timing deficits in cerebellar lesion subjects.
The SMA has also been linked to timing (Ferrandez et al., 2003; Macar et al., 2004; Macar et al., 1999) and, as noted above, is directly connected to the cerebellum. In contrast to the DLPFC, however, the cerebellar-SMA connections are relatively modest and are less robust than SMA- basal ganglia connections (Akkal, Dum, & Strick, 2007). On the basis of anatomic considerations, it appears likely that interconnections between the cerebellum and DLPFC are critical to timing suprasecond intervals. We speculate that the SMA, perhaps in conjunction with the basal ganglia, may form a partially distinct timing mechanism.
Our findings are consistent with some but not all previous investigations of patients with cerebellar lesions. Our results from the reproduction task may explain why Malapani and colleagues (1998) did not find an effect of cerebellar lesions on temporal reproduction accuracy. We found differences between lesion and control patients only with stimuli that were shorter than the intervals employed in their study (8–21 s). Additionally, like those investigators, we found increased variability at intervals (8–12 s) that were included in their study.
Two previous studies with cerebellar lesion patients have employed an interval comparison procedure using the PEST procedure. Mangels et al (1998) examined perception of 400 ms and 4-s intervals in patients with cerebellar lesions; they found that cerebellar lesion patients were more variable in their responses than control subjects (Mangels et al., 1998). In contrast, Harrington et al (2004) found no differences between cerebellar lesion and normal subjects with subsecond intervals (Harrington, Lee et al., 2004). We found no differences between controls and cerebellar lesion patients on the PEST procedure at any duration. Although the explanation for the discrepancy between studies cannot be stated with certainty, a number of possible factors may be identified. For example, patient groups may have differed with respect to the location and/or size of the lesions. Additional investigations of a larger cohort of subjects will be necessary to explain the discrepancies across studies.
One potential concern regarding the present study is that tasks were administered in a fixed order. This was done because the small number of cerebellar lesion subjects precluded a full counter-balancing of task sequence. We believe that this is an unlikely explanation for our findings for a number of reasons. First, it is not clear, for example, how a familiarity with the range of stimulus duration would lead to inverse patterns of performance on the estimation and production tasks or the finding that the effects were restricted to those subjects with lesions involving the middle to superior portion of the hemisphere or the dentate gyrus.
In summary, our data suggest that the cerebellum is implicated in interval timing across intervals lasting as long as 12 seconds, although perhaps more strongly in shorter intervals. Sorting patients on the basis of lesion location suggests that the deficit is related to damage to the superior portions of the cerebellar hemisphere or dentate gyrus, particularly on the left. We suggest that this region be considered part of a network of brain structures including the DLPFC that is crucial for interval timing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: Targets of basal ganglia and cerebellar output. Journal of Neuroscience. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Bueti D, Walsh V, Frith C, Rees G. Different brain circuits underlie motor and perceptual representations of temporal intervals. Journal of Cognitive Neuroscience. 2008;20(2):204–214. doi: 10.1162/jocn.2008.20017. [DOI] [PubMed] [Google Scholar]
- Casini L, Ivry RB. Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology. 1999;13(1):10–21. doi: 10.1037//0894-4105.13.1.10. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. Psyscope: A new graphic interactive environment for designing psychology experiments. Behavioral Research, Methods, and Computers. 1993;25:257–271. [Google Scholar]
- Coslett HB, Shenton J, Dyer T, Wiener M. Cognitive timing: neuropsychology and anatomic basis. Brain Research. 2009;1254:38–48. doi: 10.1016/j.brainres.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. Journal of Neuroscience. 1997;17(24):9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandez AM, Hugueville L, Lehericy S, Poline JB, Marsault C, Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: an fMRI study. NeuroImage. 2003;19(4):1532–1544. doi: 10.1016/s1053-8119(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Fierro B, Palermo A, Puma A, Francolini M, Panetta ML, Daniele O, et al. Role of the cerebellum in time perception: a TMS study in normal subjects. Journal of the Neurological Sciences. 2007;263(1–2):107–112. doi: 10.1016/j.jns.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. Journal of Neurophysiology. 2006;95(5):3281–3285. doi: 10.1152/jn.01011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty DJ. Discrimination of short temporal intervals: A comparison of two models. Perception & Psychophysics. 1975;18(1):1–8. [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel C. Toward a neurobiology of temporal cognition: Advances and challenges. Current Opinion in Neurobiology. 1997;7(2):170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Glickstein M, May JG, Mercier BE. Corticopontine projection in the macaque: the distribution of labeled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. Journal of Comparative Neurology. 1985;235:342–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Grecius MD. Distinct cerebellar contributions to intrinsic connectivity networks. Journal of Neuroscience. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, et al. Neural representation of interval encoding and decision making. Cognitive Brain Research. 2004;21(2):193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain: A Journal of Neurology. 2004;127(3):561–574. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- Ivry R, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73(1):167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol. 1996;6(6):851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989 Spr;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14(2):225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JAS. Functional MRI reveals the existence of modality and coordination-dependent timing networks. NeuroImage. 2005;25(4):1031–1042. doi: 10.1016/j.neuroimage.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Jech R, Dusek P, Wackermann J, Vymazal J. Cumulative blood oxygenation-level-dependent signal changes support the ‘time accumulator’ hypothesis. Neuroreport: For Rapid Communication of Neuroscience Research. 2005;16(13):1467–1471. doi: 10.1097/01.wnr.0000175616.00936.1c. [DOI] [PubMed] [Google Scholar]
- Jones CRG, Rosenkranz K, Rothwell JC, Jahanshahi M. The right dorsolateral prefrontal cortex is essential in time reproduction: an investigation with repetitive transcranial magnetic stimulation. Experimental Brain Research. 2004;158:366–372. doi: 10.1007/s00221-004-1912-3. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Wittmann M, Szelag E, Steinbuchel N. Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia. 2002;40(3):357–366. doi: 10.1016/s0028-3932(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Keele SW, Pokorny RA, Corcos DM, Ivry R. Do perception and motor production share common timing mechanisms: a correctional analysis. Acta Psychol (Amst) 1985;60(2–3):173–191. doi: 10.1016/0001-6918(85)90054-x. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philosophical Transactions of the Royal Society B. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Carlesimo GA, Caltagirone C. Selective deficit of time perception in a patient with right prefrontal cortex lesion. Neurology. 2002;59:1658–1659. doi: 10.1212/01.wnl.0000032504.45792.8f. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003;60(11):1844–1846. doi: 10.1212/wnl.60.11.1844. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo E, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Experimental Brain Research. 2007;179(2):291–299. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- Koekkoek SKE, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJH, De Zeeuw CI. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science. 2003;301:1736–1739. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- Kotani S, Kawahara S, Kirino Y. Purkinje cell activity during learning a new timing in classical eyeblink conditioning. Brain Research. 2003;994:193–202. doi: 10.1016/j.brainres.2003.09.036. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Egleston PN, Brown WH, Gregory AN, Barker AT, Woodruff PWR. The role of the cerebellum in subsecond time perception: evidence from repetitive transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2007;19(1):147–157. doi: 10.1162/jocn.2007.19.1.147. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall R. Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Current Opinion in Neurobiology. 2003;13(2):250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence for neuroimaging. Current Opinion in Neurobiology. 2003;13:250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Remembering the time: a continuous clock. Trends in Cognitive Sciences. 2006;10(9):401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. A right hemispheric prefrontal system for cognitive time measurement. Behavioural Processes. 2006;71(2–3):226–234. doi: 10.1016/j.beproc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. The precision of temporal judgement: milliseconds, many minutes, and beyond. Philosophical Transactions of the Royal Society B. 2009;364:1897–1905. doi: 10.1098/rstb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Matell MS, Meck WH. Not “just” a coincidence: frontal-striatal interactions in working memory and interval timing. Memory. 2005;13(3–4):441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- Macar F, Anton J-L, Bonnet M, Vidal F. Timing functions of the supplementary motor area: An event-related fMRI study. Cognitive Brain Research. 2004;21(2):206–215. doi: 10.1016/j.cogbrainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Macar F, Vidal F, Casini L. The supplementary motor area in motor and sensory timing: evidence from slow brain potential changes. Experimental Brain Research. 1999;125(3):271–280. doi: 10.1007/s002210050683. [DOI] [PubMed] [Google Scholar]
- Malapani C, Dubois B, Rancurel G, Gibbon J. Cerebellar dysfunctions of temporal processing in the seconds range in humans. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 1998;9(17):3907–3912. doi: 10.1097/00001756-199812010-00026. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res. 1998;7(1):15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Cognitive Brain Research. 2004;21(2):139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Merchant H, Zarco W, Prado L. Do we have a common mechanism for measuring time in the hundreds of millisecond range? Evidence from multiple-interval timing tasks. Journal of Neurophysiology. 2008;99(2):939–949. doi: 10.1152/jn.01225.2007. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichelli P, Clark K, Hollnagel C, Grafman J. Duration processing after frontal lobe lesions. Annals of the New York Academy of Sciences. 1995;769:183–190. doi: 10.1111/j.1749-6632.1995.tb38139.x. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during time behavior in the monkey. Brain Research. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, Evans AC. Cerebellar contributions to motor timing: A PET study of auditory and visual rhythm reproduction. Journal of Cognitive Neuroscience. 1998;10(6):752–765. doi: 10.1162/089892998563149. [DOI] [PubMed] [Google Scholar]
- Pentland A. Maximum likelihood estimation: the best PEST. Perception and Psychophysics. 1980;28:377–379. doi: 10.3758/bf03204398. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Effects of benzodiazepine-induced sedation on temporal processing. Human Psychopharmacology: Clinical and Experimental. 1992;7(5):311–318. [Google Scholar]
- Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1999;52B(3):273–286. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. Journal of Neuroscience. 1997;17(14):5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nature Neuroscience. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Zelaznik HN, Lantero DA, Bojczyk KG, Spencer RM, Doffin JG, Schneidt T. Correlations for timing consistency among tapping and drawing tasks: Evidence against a single timing process for motor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1316–1330. doi: 10.1037//0096-1523.25.5.1316. [DOI] [PubMed] [Google Scholar]
- Rossi S, Cappa SF, Babiloni C, Pasqualetti P, Miniussi C, Carducci F, Babiloni F, Rossini PM. Prefrontal cortex in long-term memory: an “interference” approach using magnetic stimulation. Nature Neuroscience. 2001;4(9):948–952. doi: 10.1038/nn0901-948. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiologiae Experimentalis. 2004;64(3):329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar connection to cognitive processing. Human Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Friederici AD, Yves von Cramon D. Time Perception and Motor Timing: A Common Cortical and Subcortical Basis Revealed by fMRI. NeuroImage. 2000;11(1):1–12. doi: 10.1006/nimg.1999.0514. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Lidzba K, Rubia K. A right hemispheric frontocerebellar network for time discrimination of several hundreds of milliseconds. NeuroImage. 2003;20:344–350. doi: 10.1016/s1053-8119(03)00337-9. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300(5624):1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Ivry RB. Comparison of patients with Parkinson’s disease or cerebellar lesions in the production of periodic movements involving event-based or emergent timing. Brain & Cognition. 2005;58(1):84–93. doi: 10.1016/j.bandc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Staddon JER. Interval timing: memory, not a clock. Trends in Cognitive Sciences. 2005;9(7):312–314. doi: 10.1016/j.tics.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of human imaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strick PM, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Wiener M, Coslett HB. Disruption of temporal processing in a subject with probable frontotemporal dementia. Neuropsychologia. 2008;46:1927–1939. doi: 10.1016/j.neuropsychologia.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub P, Coslett HB. The image of time: A voxel-wise meta-analysis. NeuroImage. doi: 10.1016/j.neuroimage.2009.09.064. (in press) [DOI] [PubMed] [Google Scholar]


