Abstract
Transient receptor potential A1 (TRPA1) forms non-selective cation channels implicated in acute inflammatory pain and nociception. The mechanism of ligand activation of TRPA1 may involve either covalent modification of cysteine residues or conventional reversible ligand-receptor interactions. For certain electrophilic prostaglandins, covalent modification has been considered as the main mechanism involved in their stimulatory effect on TRPA1. Because some non-steroidal anti-inflammatory drugs (NSAIDs) are structural analogs of prostaglandins, we examined several non-electrophilic NSAIDs on TRPA1 activation using electrophysiological techniques and intracellular Ca2+ measurements and found that a selected group of NSAIDs can act as TRPA1 agonists. Extracellularly applied flufenamic, niflumic, and mefenamic acid, as well as flurbiprofen, ketoprofen, diclofenac, and indomethacin, rapidly activated rat TRPA1 expressed in Xenopus oocytes and human TRPA1 endogenously expressed in WI-38 fibroblasts. Similarly, the NSAID ligands activated human TRPA1 inducibly expressed in HEK293 cells, but the responses were absent in uninduced and parental HEK293 cells. The response to fenamate agonists was blocked by TRPA1 antagonists, AP-18, HC-030031, and ruthenium red. At subsaturating concentrations, the fenamate NSAIDs also potentiate the activation of TRPA1 by allyl isothiocyanate, cinnamaldehyde, and cold, demonstrating positive synergistic interactions with other well-characterized TRPA1 activators. Importantly, among several thermosensitive TRP channels, the stimulatory effect is specific to TRPA1 because flufenamic acid inhibited TRPV1, TRPV3 and TRPM8. We conclude that fenamate NSAIDs are novel class of potent and reversible direct agonists of TRPA1. This selective group of TRPA1-stimulating NSAIDs should provide a structural basis for developing novel ligands that noncovalently interact with TRPA1 channels.
Keywords: NSAID, TRP channel, pain, cancer, sensory neurons
Introduction
Members of the Transient Receptor Potential (TRP) superfamily of cation channels are implicated in diverse cellular functions. Among them, TRPA1 is highly expressed in a subset of primary sensory neurons in dorsal root ganglia that primarily project unmyelinated C-fibers and it plays a pivotal role in regulating nociceptor excitability [19,34,42]. Studies of TRPA1 knockout mice have demonstrated the importance of this channel in behavioral responses to conditions that elicit inflammatory pain as well as nociceptive responses to chemical and mechanical stimuli. Despite some initial controversy [5,19,25,42], recent data also support the role of TRPA1 in sensing noxious cold [9,22].
Consistent with an essential role for polymodal sensing of environmental changes, TRPA1 has been shown to be activated by a long list of pungent chemicals and environmental irritants, including the ingredients of mustard (allyl isothiocyanate, AITC), cinnamon (cinnamaldehyde, CA), ginger (gingerol), garlic (allicin and diallyl sulfides), and α,β-unsaturated aldehydes such as acrolein and iodoacetamide [3–5,19,24,27]. The mechanism of activation for most of these drugs involves covalent modification of cysteine residues at the N-terminus of the TRPA1 protein by highly electrophilic thiol-reactive compounds. However, different cysteine residues may be involved for different compounds and TRPA1 from different animal species [6,11,28,43]. On the other hand, non-electrophilic substances such as Δ9-tetrahydrocannabinol, 2-aminoethoxydiphenyl borate (2APB), and icilin can also activate TRPA1 via conventional, reversible ligand-receptor interaction mechanisms [11,28]. Furthermore, ligands that lead to stimulation of phospholipase C can also indirectly activate TRPA1 [3]. This may occur due to a rise in intracellular Ca2+ or in combination with a transient increase in arachidonic acid derivatives.
Calcium is thought to directly activate TRPA1, although the precise cytoplasmic site of action for Ca2+ on the channel protein remains controversial [8,46,49]. Interestingly, due to the fact that noxious cold causes intracellular Ca2+ concentration ([Ca2+]i) increases in some cells used for heterologous expression, it has been suggested that Ca2+ may also underlie the cold-induced activation of TRPA1 [49]. However, for both ectopically expressed TRPA1 in HEK293 and CHO cells, as well as the native TRPA1 channel in dorsal root ganglia neurons, noxious cold induced activation can be detected in the absence of Ca2+ on both sides of the plasma membrane and in excised membrane patches, arguing that TRPA1 can also be activated by cold in a Ca2+-independent fashion [22,40]. Therefore, multiple mechanisms appear to exist for the activation of TRPA1, making it one of the critical nociceptive and acute inflammatory therapeutic targets, on par with TRPV1, for inflammatory, mechanical, chemical and thermal pain.
A number of arachidonic acid derivatives, including several electrophilic prostaglandins, have been shown to activate TRPA1 [3,29,30,43,44]. Covalent modification is thought to be the main mechanism involved in the direct activation of TRPA1 channels by these arachidonic acid derivatives because the nonelectrophilic precursors of these prostaglandins failed to show the same effect. However, it was not known whether the overall structure of eicosanoids also contributed to channel regulation. Because many non-steroidal anti-inflammatory drugs (NSAIDs) are structural analogs of arachidonic acid and because TRPA1 is involved in inflammatory pain, we sought to determine if NSAIDs could exert their action, at least in part, through regulation of TRPA1 activity. We found that several NSAIDs, especially those in the fenamate (N-arylanthranilic acids) group, such as flufenamic acid (FFA), niflumic acid (NFA) and mefenamic acid (MFA), but also some members of arylalkanoic acids (diclofenac and indomethacin) and 2-arylpropionic acids (flurbiprofen and ketoprofen), are able to activate TRPA1. The stimulatory effects are detected in Xenopus oocytes that ectopically expressed rat TRPA1 and an HEK293 cell line that inducibly expressed human TRPA1, as well as in WI-38 fibroblasts that endogenously expressed human TRPA1. This adds another group of well-known, clinically relevant drugs as functional regulators of TRPA1 channels.
Materials and Methods
cRNA Synthesis and TRP Channel Expression in Xenopus Oocytes
TRPA1 cDNA was obtained by RT-PCR using total RNA prepared from rat dorsal root ganglia and the sequence confirmed by DNA sequencing. The cDNAs for rat TRPA1, murine TRPV1, murine TRPV3, and murine TRPM8 were placed in the pAGA3 vector [12] and linearized using Xho1. Complementary RNAs were synthesized using mMessage mMachine reagents and protocols obtained from Ambion (Austin, TX). The resulting cRNAs were dissolved in diethylpyrocarbonate-treated H2O. Sexually mature female Xenopus laevis of older than 2.5 years of age were purchased from Xenopus Express, Inc. (Plant City, FL). For oocyte isolation, small pieces of ovarian lobe were dissected out from anesthetized frogs and shaken gently at 19°C for 90 min in the sterile OR2 solution containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.4, and supplemented with 1 mg/ml collagenase (Worthington Biochem, Lakewood, NJ). Denuded, healthy looking oocytes of more than 1.5 mm in diameter were selected and injected in a volume of 50 nl/cell with a total of 5 ng of cRNA. The injected oocytes were incubated at 19°C for 2–5 days in the sterile ND96 solution containing: 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.6, supplemented with 275 µg/ml pyruvic acid and 20 µg/ml gentamycin. The solution was changed daily.
Two-electrode Voltage Clamp
cRNA-injected oocytes were placed in a RC-3Z Oocyte Recording Chamber (Warner Instruments, Hamden, CT) and perfused with a nominally Ca2+-free bath solution that contained 100 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, and 5 mM Hepes, pH 7.4. The oocytes were impaled with two intracellular glass electrodes filled with 3 M KCl connected to an OC-725C Oocyte Clamp amplifier (Warner Instruments). Voltage commands were made from the Pulse+Pulsefit program (HEKA Instruments, Southboro, MA) via an ITC-18 Computer Interface (Instrutech Co. Port Washington, NY). Oocytes were clamped at −20 mV, stepped to −100 mV for 20 ms, followed by a voltage ramp of 200 ms from −100 mV to +100 mV once every second. Currents were recorded at the sampling rate of 1 kHz. Experiments were performed at 22–24°C unless indicated otherwise. Temperature changes were made using a CL-100 Bipolar temperature controller connected to a SC-20 dual in-line solution heater/cooler (Warner Instruments).
Mammalian Cell Culture and Measurement of Intracellular Ca2+ Concentrations
The human lung fibroblast WI-38 cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA) and maintained in Eagle's Minimum Essential Medium supplemented with 10% heat-inactivated fetal bovine serum, 50 units/ml of penicillin and 50 µg/ml of streptomycin at 37°C in 95% air 5% CO2. HEK293 cells were grown in Dulbecco’s minimal essential medium containing 4.5 mg/ml glucose and the same supplements as WI-38 cells. The HEK293 cell line that expresses human TRPA1 in an inducible manner (HEK-A1 cells) was generously provided by Dr. A. Patapoutian (The Scripps Research Institute) and maintained in the same medium as wild type HEK293 cells but supplemented with 5 µg/ml blasticidin and 50 µg/ml hygromycin B. The expression of TRPA1 was induced by including in the culture medium 1 µg/ml doxycycline. For the induced cells, ruthenium red (5 µM) was also added to minimize the constitutive TRPA1 channel activity and cells were used 14–18 hrs later. Stable HEK293 cell lines expressing murine TRPV3 or TRPM8 were produced by transfection of cDNA for TRPV3 in pcDNA3 vector or that for TRPM8 in pIRESneo vector into HEK293 cells and selection using 400 µg/ml G418 as described [37]. The TRPV3 cell line is clonal selected while the TRPM8 cell line is polyclonal. Murine TRPV1 in pIRES2EGFP vector was transiently expressed in HEK293 cells as previously described [12].
To measure [Ca2+]i changes, cells were seeded in wells of 96-well plates at ~100,000 cells/well and grown for 14–18 hrs to reach confluency. To prevent cell loss from subsequent washing, the wells were treated with 20 µg/ml polyornithine (MW >30,000, Sigma, St Louis, MO) for >15 min and rinsed once with Hank’s balanced salt solution without Mg2+ and Ca2+. To load the Ca2+ indicator dye, cells were washed once with an extracellular solution (ECS) containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, and 15 Hepes, pH 7.4 and then incubated in 50 µl ECS supplemented with 2 µM Fluo4-AM and 0.05% Pluronic F-127 at 37°C for 60 min. Probenecid (2 mM) was included in all solutions to prevent the leakage of Fluo4 from the cells. At the end of the incubation, cells were washed three times with ECS and placed in 80 µl of the same solution. Fluorescence changes were measured using a fluid handling integrated fluorescence plate reader, FlexStation (Molecular Devices, Sunnyvale, CA). Drugs were diluted into ECS at 2x or 3 x the desired final concentrations and delivered to the sample plate by the integrated robotic 8-channel pipettor at the preprogrammed time points. The Fluo4 fluorescence was read at excitation of 494 nm and emission of 525 nm from the bottom of the plate at 0.67 Hz. Experiments were performed at 20–25°C.
For cells transiently expressing TRPV1, cells were seeded on polyornithine-treated glass coverslips one day after transfection. After 16 hrs, cells were loaded with 2 µM Fura2-AM in ECS supplemented with 0.05% Pluronic F-127 at 37°C for 60 min and then washed three times with ECS. [Ca2+]i changes were monitored using the InCyt Fluorescence Imaging System (Intracellular Imaging Inc. Cincinnati, Ohio) attached to a Nikon TE200 fluorescence microscope at 22°C. Transfected cells were identified by the green fluorescence excited at 470 nm. Fura2 fluorescence images were acquired by alternating excitation at 340 and 380 nm and detection of the emitted fluorescence at 510 nm. Drugs were diluted in the final concentration and applied through perfusion.
Whole-cell recordings of mammalian cells
WI-38 cells were detached from the culture dish by the treatment with trypsin (0.05% in Hank’s balanced salt solution without Mg2+ and Ca2+ and supplemented with 0.53 mM EDTA) for 5–10 min. The treatment was terminated by the addition of the culture medium and cells were collected and maintained in an Eppendorf tube at 37°C until use. Before patching, an aliquot of cells was allowed to settle on a polyornithine-treated glass coverslip placed in a recording chamber for 3–5 min before the start of perfusion. HEK-A1 cells were seeded on polyornithine-treated glass coverlips one day before patching. Recording pipettes were pulled from micropipette glass (World Precision Instruments Inc, Sarasota, FL) to 2–4 MΩ when filled with a pipette solution containing (in mM): 117 CsCl, 9 EGTA, 1.8 MgCl2, 14 Tris-creatine phosphate, 4 Mg-ATP, 0.3 Tris-GTP, 9 HEPES, pH 7.4 and placed in the bath solution containing (in mM): 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, 10 HEPES, pH 7.4. Isolated cells were voltage-clamped in the whole-cell mode using an EPC10 amplifier with data collected at a sampling rate of 5 kHz using an analog-to-digital converter under the control of PatchMaster (HEKA Instruments). Voltage ramps of 100 ms of −100 mV to +100 mV from the holding potential of −60 mV were applied every 0.5 s. The patched cell was continuously superfused by the bath solution through an 8-channel SmartSquirt perfusion system (AutoMate Scientific, Inc. Berkeley, CA, USA). Drugs were diluted in the final concentration and applied for 10–40 sec as desired. For dose response studies, the application of FFA was 10 sec and the maximal current densities at −100 and +100 mV were used for calculations. All recordings were performed at room temperature (~23°C)
Drugs and Chemicals
Acetylsalicylic acid (aspirin), BAPTA, FFA, NFA, MFA, probenecid, nifedipine, HC-030031, and ruthenium red were purchased from Sigma (St. Louis, MO). AITC, CA, and (S)-(+)-ibuprofen were from Acros Organics (Geel, Belgium). Diclofenac sodium salt, (±)-flurbiprofen, indomethacin, ketorolac tromethamine salt, and (S)-naproxen were from Cayman Chemical Co. (Ann Arbor MI). Acrolein was from Alfa Aesar (Ward Hill, MA). Ketoprofen was from MP Biomedicals Inc. (Solon, OH). Fluo4-AM and Pluronic F-127 were obtained from Invitrogen (Carlsbad, CA). AP-18 was purchased from Enzo Life Sciences International, Inc. (Plymouth Meeting, PA). FFA, NFA, MFA, AITC, CA, acrolein, ibuprofen, diclofenac, flurbiprofen, indomethacin, ketorolac, ketoprofen, AP-18, and HC-030031 were prepared as 0.1 M stock solutions in DMSO. Working solutions were made freshly before experiments by adding stock solution to bathing solution with calculated final concentrations. AP-18 and HC-030031 were added to cells >30 min before the addition of agonists.
Results
Fenamate NSAIDs activate rat TRPA1 expressed in Xenopus oocytes
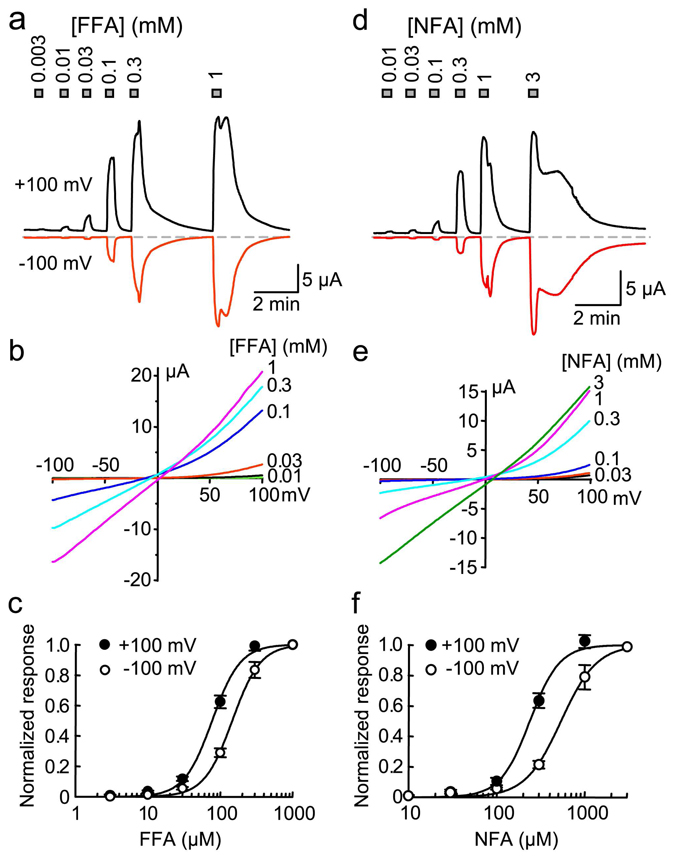
FFA, MFA and NFA are fenamate NSAIDs, capable of inhibiting cyclooxygenases (COXs), but also known as blockers of chloride channels at higher concentrations [18]. Both inhibitory and facilitatory effects of these drugs have been documented for TRPC channels [16]. In Xenopus oocytes injected with cRNA for rat TRPA1 but not uninjected oocytes, bath application of the TRPA1 agonists, AITC or CA, evoked current development (data not shown, but see Fig. 2), demonstrating that the TRPA1 channel is expressed and functional. Upon bath application of FFA, TRPA1-expressing oocytes displayed an immediate increase in currents at both positive and negative potentials in a concentration-dependent manner. Uninjected oocytes did not display any response to FFA (data not shown). The EC50 of FFA for the current activation at +100 mV was 78 ± 4 µM (Hill co-efficient: 2.2 ± 0.2) and at −100 mV was 147 ± 4 µM (Hill co-efficient: 2.2 ± 0.1, n = 8) (Figs. 1a–c). The threshold of activation was about 10 µM (Fig. 1a). Similarly, NFA activated TRPA1 with EC50 values of 240 ± 12 µM (Hill co-efficient: 2.6 ± 0.3) at +100 mV and 540 ± 27 µM (Hill co-efficient: 2.1 ± 0.2, n = 6) at −100 mV (Figs. 1d–f). The threshold of NFA-evoked activation was around 10 to 30 µM (Fig. 1d).
Figure 2. Positive synergistic effects between fenamate NSAIDs and other TRPA1 agonists and noxious cold on the activation of rat TRPA1 expressed in Xenopus oocytes.
a – c, representative traces of currents at +100 and −100 mV for TRPA1-expressing oocytes stimulated with 30 µM FFA, 3 µM AITC (a), 3 µM CA (b), or cold (14°C, c) alone or in combinations as indicated. I–V curves obtained from voltage ramps are shown below the traces. d, summary of relative peak responses (means ± SEM, n = 5–6) as compared to 30 µM FFA, NFA, or MFA at +100 mV, with the molecular structures shown. All responses were normalized to those elicited by the fenamate alone for the same cell. For the combined stimulations, horizontal lines indicate the predicted response if the effects were simply additive. In all cases, the co-application of the fenamate NSAIDs with AITC, CA, or cold elicited a much larger response than the sum of those evoked by each drug alone.
Figure 1. Rat TRPA1 expressed in Xenopus oocytes is activated by flufenamic and niflumic acid.
a, current development at +100 mV (upward deflections) and −100 mV (downward deflections) in a TRPA1-expressing oocyte in response to increasing concentrations of FFA. The durations of the drug applications are indicated by the gray bars with the FFA concentrations indicated. b, current-voltage (I–V) relationships at different FFA concentrations obtained from the same cell as in (a) by voltage ramps. c, concentration response curves at +100 and −100 mV for FFA. Data points are means ± SEM from 8 cells and are fitted with Hill equation (solid lines). d–f, similar to a–c but NFA was used. For (f), n = 6.
Positive synergistic interactions among agonists have been well-characterized for TRPV channels. For example, capsaicin, acid, heat, 2APB all interact in a synergistic manner with each other on TRPV1 [7,12]. We tested whether FFA, NFA, and the related MFA, had positive synergistic effects with other TRPA1 agonists, AITC and CA, as well as noxious cold. Low concentrations of fenamates (30 µM) and AITC or CA (3 µM) were bath applied. For noxious cold, the bath temperature was decreased to 14°C. As exemplified in Fig. 2, AITC alone evoked slowly activating currents (Fig. 2a) that were 45 ± 14% and 51 ± 10% of that evoked by 30 µM FFA at +100 and −100 mV, respectively, in TRPA1-expressing oocytes. When applied together, FFA and AITC evoked much larger responses than the simple addition of the two individual responses, reaching 300 ± 52% and 350 ± 62% of that evoked by FFA alone at +100 and −100 mV, respectively. Similarly, the amplitudes of CA-activated currents were 44 ± 16% and 45 ± 8% of that evoked by FFA, but those stimulated by FFA and CA together were 223 ± 25% and 234 ± 43% of that evoked by FFA at +100 and −100 mV, respectively (Fig. 2b). For cold, lowering the temperature of the nominally Ca2+-free bath solution to 14°C elicited a transient desensitizing current that was followed by a steady-state current equivalent to 30 ± 8% (+100 mV) and 60 ± 6% (−100 mV) of that evoked by FFA at 22°C. The addition of FFA at 14°C boosted the current to 226 ± 86% (+100 mV) and 315 ± 85% (−100 mV) of that evoked by the same concentration of the drug at 22°C (Fig. 2c). Likewise, positive synergistic effects on channel activation were observed between NFA and AITC, CA, or cold, as well as between MFA and AITC, CA, or cold (Fig. 2d).
Specificity of the stimulatory effect for NSAIDs on thermosensitive TRP channels
NSAIDs are composed of several groups of structurally different molecules. To learn whether the stimulatory effect on TRPA1 is limited to the fenamates or more widespread, we examined several additional NSAIDs, including diclofenac, indomethacin, flurbiprofen, ketoprofen, ketorolac, and naproxen. At 300 µM, only flurbiprofen, diclofenac and indomethacin caused current increases in TRPA1-expressing oocytes; ketoprofen appeared to be a very weak activator; whereas ketorolac and naproxen did not have an effect on oocytes expressing rat TRPA1 (Fig. 3), indicating that the stimulatory effect on TRPA1 is limited to certain NSAIDs.
Figure 3. Effects of several other NSAIDs on TRPA1 expressed in Xenopus oocytes.
TRPA1-expressing oocytes were exposed to 300 µM diclofenac, indomethacin, flurbiprofen, ketoprofen, ketorolac, or naproxen in the bath while currents were recorded using voltage ramps from −100 to +100 mV. Shown are representative current traces at +100 and −100 mV for uninjected oocytes (gray dashed lines, cntl) and oocytes injected with rat TRPA1 cRNA (black solid lines). Structures of the drugs are shown next to the traces. I–V curves obtained from voltage ramps at the peak of the response to diclofenac (D), indomethacin (I), flurbiprofen (F), and ketoprofen (K) are shown after subtraction of the basal currents.
Next, we examined the specificity of fenamate NSAIDs on thermosensitive TRP channels using murine TRPV1, TRPV3 or TRPM8 individually expressed in Xenopus oocytes. For these studies, TRPV1 and TRPV3 were activated by 2APB (100 µM) whereas TRPM8 was activated by menthol (100 µM). However, rather than having any stimulatory effect, FFA inhibited these channels. As shown in Figs. 4a–d, when applied together with 2APB, FFA (100 µM) suppressed the 2APB-activated current in oocytes expressing TRPV1 by 57% and 75% (n = 6) and similarly inhibited 2APB-evoked current in oocytes that expressed TRPV3 by 57% and 67% (n = 5) at +100 and −100 mV, respectively. The inhibition at positive potentials is somewhat less pronounced than at negative potentials. The inhibition to menthol-stimulated current in oocytes that expressed TRPM8 was 16% at +100 mV and 30% at −100 mV (n = 7). A similar inhibitory effect was also observed with NFA and MFA (data not shown). Furthermore, in Fluo4-loaded HEK293 cells that stably expressed TRPV3 or TRPM8 or Fura2-loaded HEK293 cells that transiently expressed TRPV1, we observed no significant increase in Fluo4 fluorescence or Fura2 ratio upon application of FFA from 3.7 to 100 µM (Figs. 4e–h), indicating that in this broad concentration range, the fenamate NSAID does not activate these TRP channels and thereby causes [Ca2+]i increase. At 300 µM, a slow fluorescence increase was detected in cells that expressed TRPM8 (Figs. 4g and 4h). Therefore, FFA may be a very weak activator of TRPM8; however, the concentration required is much higher (at least 10-fold) than that needed to activate TRPA1. For all these TRP channels, [Ca2+]i increases were elicited by their corresponding agonists and the responses were inhibited by high concentrations of FFA (30 to 300 µM). Therefore, the stimulatory effect of fenamate NSAIDs on TRPA1 appears to be specific to this member of the TRP family among temperature sensitive TRP channels.
Figure 4. Inhibitory effect of FFA on TRPV1, TRPV3, and TRPM8.
a–d, Xenopus oocytes expressing murine TRPV1 (a), TRPV3 (b) or TRPM8 (c) were stimulated with the corresponding agonists, 2APB (100 µM, a and b) or menthol (100 µM, c) in the absence and presence of 100 µM FFA. a–c show representative I–V curves obtained using voltage-ramps under conditions as indicated. Note the strong constitutive basal activity of TRPM8-expressing cells. The recording temperature was 22°C, which is lower than the temperature threshold (<27°C) required for TRPM8 activation. d, means ± SEM of % inhibition at +100 mV and −100 mV caused by FFA. Numbers in the parentheses refer to the numbers of oocytes tested for each group. e–g, effect of FFA on [Ca2+]i of HEK293 cells expressing TRPV1, TRPV3, or TRPM8. Representative traces show Fura2 ratio changes measured by Ca2+ imaging in cells that transiently expressed TRPV1 (e) and Fluo4 fluorescence changes monitored using a fluorescence plate reader in stable TRPV3 (f) and TRPM8 (g) cell lines. FFA and channel ligands were applied as indicated. Cap: capsaicin. h, means ± SEM of maximal changes in Fura2 ratio (for TRPV1) or Fluo4 fluorescence (TRPV3 and TRPM8) at different FFA concentrations. Only 300 µM FFA caused a significant fluorescence increase in TRPM8-expressing cells. n = 3.
Intracellular Ca2+ facilitates FFA-evoked activation of TRPA1
Recently, several studies have shown the critical role of [Ca2+]i rise in the activity of TRPA1 [8,46,49]. In experiments described above for TRPA1-expressing oocytes, a nominally Ca2+-free bath solution was used. Since TRPA1 is Ca2+-permeable, the entry of Ca2+ through the TRPA1 channel can feedback positively to further enhance the channel activity. Indeed, increasing bath Ca2+ concentration to 10 mM increased FFA-activated current by 240 ± 39% at +100 mV and 309 ± 49% at −100 mV (Fig. 5a, n = 4), suggesting that Ca2+-entry can facilitate the current. However, we cannot exclude that some of the increased current may arise from the endogenous Ca2+-activated Cl− currents in oocytes at this FFA concentration [48]. To examine whether [Ca2+]i rise is necessary for FFA-evoked currents, we injected BAPTA into TRPA1-expressing oocytes (50 nl of 100 mM, with the estimated final concentration of ~2.5 mM) to minimize the Ca2+ fluctuation in the cytoplasm. Compared to cells that were not injected with BAPTA, current amplitudes activated by 30 µM FFA in the nominally Ca2+-free bath were decreased ~44% at +100 mV and ~33% at −100 mV, but the activation was still obvious in the TRPA1-expressing oocytes that were injected with BAPTA (Figs. 5b and 5c, n = 8 for uninjected and 10 for BAPTA injected). These results indicate that increases in [Ca2+]i facilitate, but are not necessary for the NSAID-evoked TRPA1 activation.
Figure 5. Ca2+ dependence of FFA-evoked TRPA1 activation.
a, a strong potentiation by an increase in extracellular Ca2+. Bath application of 30 µM FFA activated currents in a TRPA1-expressing oocyte. Increasing Ca2+ to 10 mM strongly enlarged the current at both positive and negative potentials and the effect was reversible after washout of the high Ca2+. b and c, effect of BAPTA injection on FFA-evoked response. Recordings were carried out in a nominally Ca2+-free bath solution. b, representative traces. Left, a control uninjected oocytes showed no response to FFA (30 µM). Middle, FFA-activated current in a TRPA1-expressing oocyte. Right, similar to the middle panel, but the oocyte was injected with BAPTA before the recording. c, means ± SEM of current amplitudes at +100 and −100 mV at the peak of the response to FFA in TRPA1-expressing oocytes uninjected (n = 8) or injected (n =10) with BAPTA. The FFA-activated currents were reduced, but not eliminated, by BAPTA injection.
Activation of endogenous human TRPA1 in WI-38 fibroblasts by NSAIDs
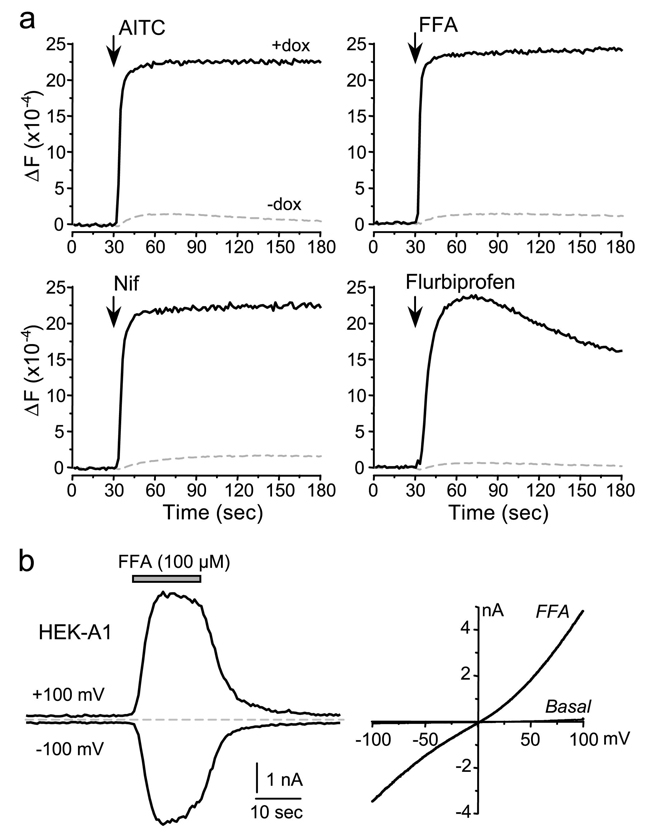
TRPA1 was originally identified from the human lung fibroblast WI-38 cell line and its expression found to be repressed by SV40 transformation and in most mesenchymal tumor cells. [17,41]. However, TRPA1 channel activities in WI-38 cells have not been characterized before. We sought to examine the endogenous TRPA1 function in this fibroblast cell line. Stimulation of Fluo4-loaded WI-38 cells with AITC, CA, or other known TRPA1 agonists, acrolein and nifedipine, caused a rapid fluorescence increase, indicative of [Ca2+]i rise and TRPA1 activation (Fig. 6a). The response to AITC was blocked by TRPA1 antagonists, ruthenium red, AP-18, and HC-030031 (all at 20 µM, Fig. 6b). As a negative control, HEK293 cells, which do not express TRPA1 endogenously, failed to respond to all of these drugs (Fig. 6a). At maximal agonist concentrations, the Ca2+ increase was sustained, but at submaximal concentrations, it showed desensitization (Fig. 6c). The concentration response relationships of TRPA1 agonists were determined by measuring the initial rates of the fluorescence increase upon addition of varying concentrations of the drug. We used the rate (slope) of the rising phase of fluorescence increase instead of the maximal fluorescence change in order to differentiate the potencies of those concentrations that gave rise to the same maximal fluorescence increase but were clearly distinct in the rates in achieving the peak (see Fig. 6c for examples). These measurements yielded EC50 values of 50 ± 5, 85 ± 9, 157 ± 8 and 400 ± 41 µM for AITC, acrolein, nifedipine, and CA, respectively (Fig. 6d and Table 1). These values are higher than those reported in the literature for TRPA1 channels heterologously expressed in mammalian cells and Xenopus oocytes, which are as low as 5, 0.8, 0.4, and 6.5 µM, respectively [1,8,29]. Variations in assay conditions and methods used to differentiate the response levels may contribute to the difference. In whole-cell recordings, we detected AITC-evoked currents in WI-38 cells (Fig. 6e). The current-voltage (I–V) relationship obtained using the voltage ramp protocol at the peak of current activation is near linear and the reversal potential is about 0 mV, which resemble TRPA1 currents measured in oocytes (Fig. 1) and in HEK293 cells that expressed TRPA1 (see Fig. 8b). The mean whole-cell capacitance for the WI-38 cells is 101 ± 10 pF (n = 31), which is about five-fold larger than HEK293 cells (~20 pF).
Figure 6. Increase of [Ca2+]i and membrane current in WI-38 human fibroblasts by TRPA1 agonists.
WI-38 cells, which endogenously express TRPA1, grown in wells of a 96-well plate were loaded with Fluo4-AM and tested for drug-induced Ca2+ response using a fluorescence plate reader. HEK293 cells, which do not express TRPA1, were used as a negative control. a, fluorescence changes induced by 300 µM AITC, CA, acrolein, or nifedipine (Nif) in WI-38 cells (black solid lines) and HEK293 cells (gray dashed lines). b, inhibition of AITC (33 µM)-induced response by TRPA1 antagonists: AP-18, HC-030031, and ruthenium red (RR). c, comparison of time courses of fluorescence changes induced by different concentrations of AITC. At 300 µM, the fluorescence increased faster than at 33 µM, even though the peak fluorescence levels were similar. Also note the similar peak levels in response to 11 and 3.7 µM AITC, despite the different initial rate. d, concentration-response relationships for AITC, CA, acrolein, and nifedipine-induced [Ca2+]i changes in WI-38 cells. Data points are means ± SEM for 4 measurements. Solid lines are fits to the Hill equation. e, AITC-elicited whole-cell current in WI-38 cells. Left, time course of current development at +100 and −100 mV with the duration of AITC application indicated by the gray bar. Right, I–V curves obtained by the voltage ramp protocol at basal and the maximal response to AITC.
Table 1.
EC50 and Hill coefficient values for [Ca2+]i increases elicited by TRPA1 agonists in WI-38 and HEK-A1 cells.
| Compounds | EC50 (µM) | nHa |
|---|---|---|
| WI-38 cells | ||
| Flufenamic acid | 24 ± 3 | 1.3 ± 0.2 |
| Niflumic acid | 28 ± 3 | 1.4 ± 0.2 |
| Allyl isothiocyanate | 50 ± 5 | 1.0 ± 0.1 |
| Mefenamic acid | 61 ± 5 | 1.5 ± 0.2 |
| Acrolein | 85 ± 9 | 1.0 ± 0.1 |
| Nifedipine | 157 ± 8 | 1.1 ± 0.1 |
| Diclofenac | 210 ± 22 | 1.3 ± 0.2 |
| Flurbiprofen | 342 ± 6 | 1.5 ± 0.1 |
| Cinnamonaldehyde | 400 ± 41 | 0.9 ± 0.1 |
| Indomethacin | 470 ± 54 | 1.5 b |
| Ketoprofen | >500 | ND |
| HEK-A1 cells | ||
| Flufenamic acid | 57 ± 5 | 1.6 ± 0.1 |
| Allyl isothiocyanate | 109 ± 6 | 0.8 ± 0.1 |
| Nifedipine | 140 ± 23 | 1.0 ± 0.1 |
| Flurbiprofen | 310 ± 66 | 2.0 ± 0.3 |
nH is Hill coefficient
nH was fixed to 1.5 to make the fit.
ND: not determined
Figure 8. Effects of NSAIDs on [Ca2+]i and membrane current in an HEK293 cell line that inducibly expresses human TRPA1.
a, the stable HEK293 cell line were treated with and without doxycycline (+dox or −dox) in wells of a 96 well plate for 16 hrs, washed and then loaded with Fluo4-AM. The responses to TRPA1 agonists were studied using a fluorescence plate reader. Shown are representative traces for induced cells (black solid lines) and non-induced cells (gray dashed lines) in response to 300 µM AITC, FFA, nifedipine (Nif) or flurbiprofen. b, FFA-elicited whole-cell current in a dox-treated HEK-A1 cell. Left, time course of current development at +100 and −100 mV. Right, I–V curves obtained at basal and the maximal response to FFA.
To test whether the NSAIDs also activate the endogenous human TRPA1 channels, we then tested their ability to induce [Ca2+]i increases in WI-38 cells. Consistent with the data obtained from oocytes that expressed rat TRPA1, FFA, NFA, MFA, flurbiprofen, ketoprofen, diclofenac and indomethacin all increased the Fluo4 fluorescence in WI-38 cells but had little effect in HEK293 cells (Fig. 7a). The response to FFA was inhibited by ruthenium red with an IC50 of 2.1 µM (curve not shown but see trace for 20 µM in Fig. 7b) and by preincubation of the cells with AP-18 and HC-030031 (both at 20 µM) (Fig. 7b). The NSAIDs that did not activate TRPA1 in Xenopus oocytes also failed to induce an appreciable Ca2+ response in WI-38 cells, i.e. >5% of that induced by 300 µM FFA. In addition, acetylsalicylic acid (aspirin) and ibuprofen did not act as agonists on these cells. When added at 100 µM 2 min before the agonist, these non-stimulating NSAIDs had no inhibitory effect on the response induced by FFA or AITC (data not shown), indicating that they are also not TRPA1 antagonists. The relative potency of the NSAIDs in stimulating [Ca2+]i increases in WI-38 cells is in good agreement with the data obtained from the oocytes, although the EC50 values are several fold lower in WI-38 cells (Fig. 7c and Table 1). For other TRP channels, it is not uncommon that EC50 values are higher in oocytes than in mammalian cells [12,31]. The absence of Ca2+ in the bath solution for oocyte recordings may also contribute to the lower apparent affinity values, given that Ca2+ facilitates TRPA1 activation.
Figure 7. Effects of NSAIDs on [Ca2+]i and membrane current in WI-38 human fibroblasts.
Similar to Fig. 6, but the WI-38 and HEK293 cells were treated with NSAIDs as indicated. a, FFA, NFA, MFA, flurbiprofen, diclofenac, indomethacin, and ketoprofen evoked Ca2+ responses in WI-38 but not parental HEK293 cells while the other NSAIDs did not have stimulatory effect in either cell type. The final concentration for all drugs was 300 µM. b, inhibition of FFA (33 µM)-induced response by TRPA1 antagonists: AP-18, HC-030031, and ruthenium red (RR). c, concentration-response relationships for FFA-, NFA-, MFA-, flurbiprofen-, diclofenac-, and indomethacin-induced [Ca2+]i changes in WI-38 cells. Data points are means ± SEM for 6 measurements. Solid lines are fits to the Hill equation. d, FFA-elicited whole-cell current in WI-38 cells. Ruthenium red (RR, 30 µM) was absent (solid black lines) or present (gray dots) in the bath. Left, time course of current development at +100 and −100 mV. Right, I–V curves obtained at basal and the maximal response to FFA. e, concentration-response relationships at +100 and −100 mV for FFA-evoked whole-cell currents. Data points are means ± SEM for 5 cells. Solid lines are fits to the Hill equation.
Bath application of FFA also induced currents in the WI-38 cells (Fig. 7d). These currents show desensitization even in the continued presence of the NSAID and they were completely abolished by including ruthenium red (30 µM) in the bath. The I–V relationship of the FFA-evoked currents is indistinguishable from that elicited by AITC in the same cell line (Fig. 6e) and from that elicited by FFA in HEK293 cells inducibly expressing human TRPA1 (Fig. 8b). However, it is obvious that FFA activated the currents faster than AITC despite the three-fold lower concentration used. The concentration-response curves of FFA revealed EC50 values of 44 ± 11 µM (Hill co-efficient: 1.7 ± 0.2) and 55 ± 4 µM (Hill co-efficient: 2.4 ± 0.1) for currents developed at +100 and −100 mV, respectively (n = 5). These values are not different from that obtained for FFA using the Ca2+ assay (Fig. 7c and Table 1).
To further confirm that the responses to NSAIDs in WI-38 cells are mediated by the human TRPA1 channel, we examined the effect of AITC, FFA, nifedipine, and flurbiprofen on human TRPA1 expressed in an inducible manner in a stable HEK293 cell line (HEK-A1 cells). Under the same experimental conditions as that used for WI-38 cells, the Ca2+ response patterns and EC50 values obtained from the HEK-A1 cells are similar to that from WI-38 cells (Fig. 8a and Table 1). The FFA-elicited whole-cell currents in HEK-A1 cells are also very similar to that obtained in WI-38 cells, with an exception that the currents did not desensitize as quickly as that in WI-38 cells (Fig. 8b). This could suggest that WI-38 cells possess an additional desensitization mechanism for TRPA1, which is lacking in the HEK293 cells. Overall, the above data confirm that the NSAID agonist responses obtained from WI-38 cells are mediated by TRPA1 and are independent of the cell line.
Discussion
TRPA1 is an important channel expressed in nociceptive neurons and involved in inflammatory pain and nociceptive responses to stimulation by chemicals, mechanical force, and noxious cold [5,9,22,25,36]. In cancer development, TRPA1 expression is down regulated in transformed fibroblasts [17,41], although whether or not TRPA1 channel activity is present in the untransformed fibroblasts was not reported. Since its discovery, TRPA1 has received a lot of attention because of its potential as a therapeutic target for the development of anti-nociceptive drugs. A large number of synthetic and natural chemicals have been found to activate TRPA1 [3–5,8,19,21,27,29,32,35,43]. In a recent study, we showed that TRPA1 is strongly activated by Zn2+ from the cytoplasmic side [14]. While covalent modification of cysteine residues may underlie the mechanism of action on TRPA1 for most, if not all, electrophilic substances, conventional reversible ligand binding mechanism may be at play for non-electrophilic chemicals [11,28]. In both cases, Ca2+ can potentiate the action of TRPA1 activators via binding to a cytoplasmic region(s) of the channel protein [8,46,49]. We have shown here that a selective group of NSAIDs rapidly and reversibly activate TRPA1 heterologously expressed in Xenopus oocytes. Furthermore, these same NSAIDs selectively cause increases in [Ca2+]i in HEK293 cells that inducibly express human TRPA1 and in human fibroblast cells that endogenously express TRPA1. These findings add these NSAIDs, exemplified by flufenamic acid, as a new class of reversible ligands to the list of existing exogenous TRPA1 agonists and in addition, provide the first evidence for TRPA1 channel activities measured as both Ca2+ entry and plasma membrane currents in the WI-38 human fibroblast cell line, which should become a convenient source for studying endogenous TRPA1 function.
There are several possible mechanisms by which the active NSAIDs may function as agonists or allosteric activators of the TRPA1 channel, including direct interaction with a binding site on the receptor-channel or by indirect modulation of activity of a related target, such as COX-1 and COX-2. Most importantly, a number of potent and relatively non-selective COX inhibitors, such as ketorolac and naproxen, fail to activate TRPA1 in both the WI-38 and oocyte systems. Furthermore, they do not act as antagonists at TRPA1. The lack of correlation between COX inhibition and TRPA1 activation is evident since flurbiprofen, diclofenac, indomethacin, and ketoprofen are also more potent COX-1 inhibitors (IC50 values range from 1 to 700 nM, depending on the assay condition and the enzyme source) than the fenamate NSAIDs (IC50 values range from 12 nM to >100 µM), but are much less potent or inactive as activators of TRPA1 [38,39]. In addition, the active NSAIDs that act as TRPA1 agonists span a wide range of COX-1/COX-2 selectivity ratios which is not correlated with either in vitro or cellular IC50 activity for either isoform [33,39]. The fast onset of TRPA1 activation observed for the fenamate class (FFA, NFA and MFA), t1/2 < 3 s in Xenopus oocytes and < 5 s in WI-38 cells, further argues against a mechanism based upon changes in enzyme activity that modulates intracellular concentrations of a membrane lipid or other signaling intermediates. Taken together, the agonist activity on TRPA1 inherent in selected NSAIDs is inconsistent with a mechanism based upon inhibition of the COX isozymes as a mediator for modulating TRPA1 channel function. It is likely that direct interaction with the TRPA1 channel is responsible for the activation properties.
The mechanism of action for activation of TRPA1 by this group of NSAID ligands does not involve covalent modification of cysteines, as is known for acrolein, AITC, CA, and iodoacetamide. The active compounds do not possess electrophilic groups and their actions on TRPA1 have a fast onset and demonstrate rapid and complete reversibility upon washout. More importantly, mutations at the three cysteine residues (Cys621Ser, Cys641Ser and Cys665Ser) of human TRPA1, which eliminated activation by iodoacetamide, did not affect the FFA-evoked currents. The triple cysteine mutant carrying Cys621Ser, Cys641Ser and Cys665Ser mutations had right-shifted concentration-response curves for FFA as well as menthol and 2APB (two other non-electrophilic TRPA1 agonists), suggesting an overall gating defect (HH, unpublished results).
Another class of recently characterized agonists of the TRPA1 receptor include specific endogenous lipid mediators, such as arachidonic acid, its non-metabolizable analog, 5,8,11,14-eicosatetraynoic acid (ETYA), and several electrophilic prostaglandins, such as 15-deoxy-Δ12,14-prostaglandin J2, Δ12-prostaglandin J2, and prostaglandin A2, [3,29,30,43,44]. This property of receptor-channel activation and/or modulation by arachidonic acid derivatives is shared with other thermosensitive TRP channels, including TRPV1, TRPV3, and TRPV4 [13,15,47]. In this regard, the NSAIDs identified in this study might share a common binding site on the TRPA1 channel with specific native lipid products that are endogenous chemical activators of the TRPA1 channel. It is possible that the active NSAIDs act directly on the channel by mimicking the binding of the endogenous ligands, which most likely derive from arachidonic acid, the content of which is greatly increased under inflammatory conditions. Covalent modification has been hypothesized to be involved in the mechanism of channel activation caused by the electrophilic arachidonic acid derivatives, but since the active NSAIDs characterized in this study are non-electrophilic, our data indicates that conventional reversible ligand-receptor binding mechanism must also be involved in TRPA1 channel gating although the mechanism by which NSAIDs activate TRPA1 warrants further investigation.
In the present study, we also characterized the effect of FFA on closely related members of the TRP superfamily and have shown that FFA inhibits TRPV1 and TRPV3, and to a much lesser extent, TRPM8. FFA induced some activation of TRPM8 only at a very high concentration (300 µM). Previous studies have shown that FFA suppressed TRPC3-like channels in rabbit ear artery myocytes [2] and inhibited TRPC5 activity in murine stomach [26]. However, it increased TRPC6-mediated currents in A7r5 smooth muscle cells [20]. FFA is also an antagonist of TRPM2 in a pH-dependent manner [10]. In murine small intestine, NFA suppresses a TRPC4-like channel in interstitial cells of Cajal [23,45]. Importantly, FFA and the related NFA and MFA all activated TRPA1 at concentrations that are not very different from those that inhibit or potentiate other TRP channels. Other structurally unrelated NSAIDs, such as flurbiprofen, ketoprofen, diclofenac and indomethacin, also activate TRPA1. Therefore, among TRP channels, the stimulatory action of the active NSAIDs appears to be relatively specific for TRPA1.
The potency for the fenamate NSAID agonists for activation of TRPA1 is equivalent or stronger than that of the commonly used TRPA1 agonists, AITC, CA and acrolein. The EC50 values we measured from the [Ca2+]i assay, which were derived from the rate of initial response rather than the maximal fluorescence increase, were not different from those obtained by current measurements in patch clamp studies. Recently, submicromolar EC50 values were reported for some dihydropyridines for the activation of TRPA1 [8]. In our hands, the value for nifedipine is 156 µM. Given that the EC50 values for CA also differ by more than 60-fold between the two studies, the divergence between the reported potencies of these drugs can be assigned to the different methods and cell lines used. Therefore, the fenamate NSAIDs appear as potent as some of the dihydropyridines in terms of stimulating TRPA1.
In practice, the potent fenamate NSAIDs are more convenient to use than the cysteine modifying drugs, such as AITC, CA, or acrolein, because of the quick onset of action and capacity for rapid reversibility of the response. These advantageous properties offer the potential for fenamates to become excellent research tools for studying TRPA1 channels [14]. Furthermore, the finding that FFA still activates TRPA1 in oocytes injected with BAPTA suggests that [Ca2+]i rise is not absolutely required for its action, although it potentiates the drug action.
In summary, our results show that fenamate and several other NSAID ligands can be used to probe the functional properties of TRPA1. The fact that non-electrophilic NSAIDs selectively activate TRPA1 suggests that the action of structurally related arachidonic acid derivatives on this channel can also be achieved through allosteric binding, in addition to covalent modification of the channel protein. Since TRPA1 is colocalized with TRPV1 in dorsal root ganglia and functions as a polymodal receptor involved in pain pathways, the study of NSAIDs action on TRPA1 and other thermosensitive TRP channels should provide important new insights on pain mechanisms and pain management. In addition, a detailed understanding of the effects of different NSAIDs on members of the TRP channel family may offer the potential to develop a class of analgesic drugs for inflammatory pain.
Acknowledgements
We thank Drs. Ardem Patapoutian and Michael Bandell for the HEK-TRPA1 cell line, Dr. Jun Chen for helpful discussion of the project, Ms. Dina Chuang-Zhu for technical assistance. This work was supported by American Heart Association Grant-in-Aid 0755277B and US NIH grants RO1-NS042183, RO1-DK081654, R21-NS056942, and P30-NS045758.
The abbreviations used
- 2APB
2-aminoethoxydiphenyl borate
- AITC
allyl isothiocyanate
- CA
cinnamaldehyde
- [Ca2+]i
intracellular Ca2+ concentration
- COX
cyclooxygenase
- ECS
extracellular solution
- FFA
flufenamic acid
- MFA
mefenamic acid
- NFA
niflumic acid
- NSAIDs
non-steroidal anti-inflammatory drugs
- TRP
transient receptor potential
References
- 1.Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert AP, Pucovsky V, Prestwich SA, Large WA. TRPC3 properties of a native constitutively active Ca2+- permeable cation channel in rabbit ear artery myocytes. J Physiol. 2006;571:361–369. doi: 10.1113/jphysiol.2005.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 4.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang XF, Kort ME, Huth JR, Sun C, Miesbauer LJ, Cassar SC, Neelands T, Scott VE, Moreland RB, Reilly RM, Hajduk PJ, Kym PR, Hutchins CW, Faltynek CR. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J Neurosci. 2008;28:5063–5071. doi: 10.1523/JNEUROSCI.0047-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]; 8 Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels: Novel targets of 1,4-dihydropyridines. Channels (Austin) 2008;2:429–438. doi: 10.4161/chan.2.6.7126. [DOI] [PubMed] [Google Scholar]
- 9.Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci. 2008;28:7863–7875. doi: 10.1523/JNEUROSCI.1696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill K, Benham CD, McNulty S, Randall AD. Flufenamic acid is a H-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004;47:450–460. doi: 10.1016/j.neuropharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 13.Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, Zhu MX. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208:201–212. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol. 2009;5:183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- 17.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 18.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 19.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 20.Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- 21.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited nonselective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koizumi K, Iwasaki Y, Narukawa M, Iitsuka Y, Fukao T, Seki T, Ariga T, Watanabe T. Diallyl sulfides in garlic activate both TRPA1 and TRPV1. Biochem Biophys Res Commun. 2009;382:545–548. doi: 10.1016/j.bbrc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 25.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 26.Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- 27.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 29.Maher M, Ao H, Banke T, Nasser N, Wu NT, Breitenbucher JG, Chaplan SR, Wickenden AD. Activation of TRPA1 by farnesyl thiosalicylic acid. Mol Pharmacol. 2008;73:1225–1234. doi: 10.1124/mol.107.042663. [DOI] [PubMed] [Google Scholar]
- 30.Materazzi S, Nassini R, Andrè E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Rang HP, Bevan S, James IF. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br J Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meseguer V, Karashima Y, Talavera K, D'Hoedt D, Donovan-Rodríuez T, Viana F, Nilius B, Voets T. Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J Neurosci. 2008;28:576–586. doi: 10.1523/JNEUROSCI.4772-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niforatos W, Zhang XF, Lake MR, Walter KA, Neelands T, Holzman TF, Scott VE, Faltynek CR, Moreland RB, Chen J. Activation of TRPA1 channels by the fatty acid amide hydrolase inhibitor 3'-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597) Mol Pharmacol. 2007;71:1209–1216. doi: 10.1124/mol.106.033621. [DOI] [PubMed] [Google Scholar]
- 36.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2008;283:10026–10036. doi: 10.1074/jbc.M707306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouellet M, Falgueyret JP, Percival MD. Detergents profoundly affect inhibitor potencies against both cyclo-oxygenase isoforms. Biochem J. 2004;377:675–684. doi: 10.1042/BJ20030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riendeau D, Charleson S, Cromlish W, Mancini JA, Wong E, Guay J. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can J Physiol Pharmacol. 1997;75:1088–1095. [PubMed] [Google Scholar]
- 40.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 41.Schenker T, Trueb B. Down-regulated proteins of mesenchymal tumor cells. Exp Cell Res. 1998;239:161–168. doi: 10.1006/excr.1997.3896. [DOI] [PubMed] [Google Scholar]
- 42.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, Uchida K, Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 44.Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- 45.Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am J Physiol Cell Physiol. 2002;283:C1637–C1645. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- 46.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 48.White MM, Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2+-activated Cl− channels in Xenopus oocytes. Mol Pharmacol. 1999;37:720–724. [PubMed] [Google Scholar]
- 49.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]