Summary
The regulatory pathways necessary for the maintenance of adult hematopoietic stem cells (HSCs) remain poorly defined. By using loss-of-function approaches, we report a selective and cell-autonomous requirement for the p300/CBP-binding transcriptional coactivator Cited2 in adult HSC maintenance. Conditional deletion of Cited2 in the adult mouse results in loss of HSCs causing multilineage bone marrow failure and increased lethality. In contrast, conditional ablation of Cited2 after lineage specification in lymphoid and myeloid lineages has no impact on the maintenance of these lineages. Additional deletion of Ink4a/Arf (encoding p16Ink4a and p19Arf) or Trp53 (encoding p53, a downstream target of p19Arf) in a Cited2-deficient background restores HSC functionality and rescues mice from bone marrow failure. Furthermore, we show that the critical role of Cited2 in primitive hematopoietic cells is conserved in humans. Taken together, our studies provide genetic evidence that Cited2 selectively maintains adult HSC functions, at least in part, via Ink4a/Arf and Trp53.
Keywords: STEMCELL
Introduction
Adult hematopoiesis depends on rare multipotent bone marrow (BM)-resident hematopoietic stem cells (HSCs) (Orkin and Zon, 2008). HSCs may remain quiescent, self-renew, undergo apoptosis, or differentiate into multiple blood lineages. Tight regulation of these fates is essential to maintain the adult HSC pool, and studies in mice have revealed some of the key regulators of HSC maintenance. To identify novel regulators of adult HSC maintenance, we and others employed comparative global gene expression approaches. These studies identified the p300/CBP-binding transcriptional coactivator Cited2 as a candidate regulator of adult HSCs (Gomes et al., 2002; Mansson et al., 2007; Zhong et al., 2005), but functional validation remains to be performed.
CITED2 mutations are found in patients with congenital heart disease (Sperling et al., 2005), lending clinical significance in trying to understand CITED2 function. Cited2 physically interacts with the histone acetyltransferase p300/CBP (Bhattacharya et al., 1999), coactivates DNA-binding transcription factors (Bamforth et al., 2001; Chou et al., 2006; Glenn and Maurer, 1999; Tien et al., 2004), and represses HIF-1-mediated transcription (Bhattacharya et al., 1999). Cited2 has oncogenic properties (Sun et al., 1998) and controls proliferation of mouse embryonic fibroblasts (MEFs) via polycomb group genes Bmi-1 and Mel18 and the tumor suppressor Ink4a/Arf (Kranc et al., 2003). Cited2 deletion in mice is embryonic lethal, causing multiple developmental defects (Bamforth et al., 2001; Yin et al., 2002), including impaired fetal liver hematopoiesis (Chen et al., 2007). Severe fetal liver malformations (Qu et al., 2007) precluded defining a cell-autonomous role for Cited2 in HSC function and hematopoiesis, although these findings suggest a potential role for Cited2 in fetal HSC regulation. In this study, we use a conditional knockout strategy to establish a requirement for Cited2 in adult HSCs. Further, we demonstrate a role for CITED2 in human hematopoiesis by RNA interference in CD34+ cord blood (CB) cells.
Results
Cited2 Is Essential for Sustaining Multilineage Hematopoiesis
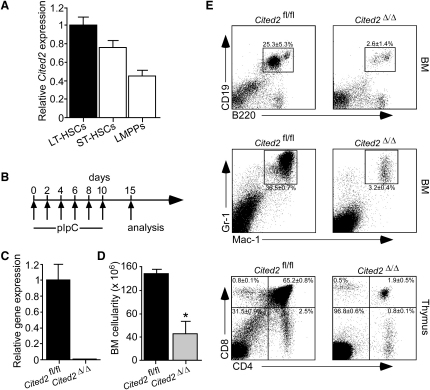
Cited2 expression analysis indicated that it is highly expressed in long-term HSCs (LT-HSCs; Lin−Sca-1+c-kit+(LSK)CD34−Flt3− cells), less abundantly in short-term HSCs (ST-HSCs; LSKCD34+Flt3− cells), and profoundly downregulated in lymphoid-primed multipotent progenitors (LMPPs; LSKCD34+Flt3+ cells) (Figure 1A). To investigate a functional requirement for Cited2 in adult hematopoiesis, we generated Cited2fl/fl Mx1-Cre conditional knockout mice (MacDonald et al., 2008), in which treatment with poly(I)-poly(C) (pIpC) induces efficient gene deletion in hematopoietic cells (Kuhn et al., 1995). We treated Cited2fl/fl Mx1-Cre and Cited2fl/fl mice with pIpC (Figure 1B) and refer to these as Cited2Δ/Δ and Cited2fl/fl mice, respectively. After Cre-mediated recombination, a lacZ expression cassette comes under the control of the endogenous Cited2 promoter (MacDonald et al., 2008), and efficient gene deletion was demonstrated by abundant lacZ expression in Cited2Δ/Δ BM cells (Figure S1A available online). Furthermore, Cited2 mRNA was undetectable in Cited2Δ/Δ BM cells (Figure 1C). Within 6 to 15 days after initiation of pIpC treatment, most Cited2Δ/Δ mice became moribund and were sacrificed, in contrast to control mice, which survived normally (Figure S1B). BM analysis revealed severely reduced cellularity in Cited2Δ/Δ mice (Figure 1D) and strikingly reduced frequencies of mature myeloid (Mac-1+Gr-1+) and B-lymphoid (CD19+B220+) cells in Cited2Δ/Δ BM, as compared to control mice (Figure 1E). Conditional loss of Cited2 also reduced T cell frequencies (Figure 1E). These data support an essential role for Cited2 in sustaining adult multilineage hematopoiesis.
Figure 1.
Conditional Deletion of Cited2 Results in Multilineage Bone Marrow Failure
(A) Relative expression of Cited2 mRNA in LT-HSC, ST-HSC, and LMPP populations sorted from WT C57BL/6J mice. Data are mean ± SEM (n = 3).
(B) Cited2fl/flMx1-Cre and Cited2fl/fl mice received six injections of pIpC on alternate days and analyzed 5 days after the last injection.
(C) Relative expression of Cited2 mRNA in total BM cells from Cited2Δ/Δ and control mice (mean ± SEM; n = 3).
(D) Total number of BM nucleated cells obtained from two tibias and two femurs of Cited2Δ/Δ and control mice. The results are presented as mean number of cells ± SD (n = 5). ∗p < 0.0001.
(E) Top and middle: Frequencies of B-lymphoid and myeloid cells, respectively, in BM from Cited2Δ/Δ and control mice. Bottom: FACS plot showing CD4 and CD8 staining in thymi from Cited2Δ/Δ and control mice. Data are shown as mean frequency ± SD (n = 3).
Mx1-Cre mediates gene deletion in both hematopoietic and nonhematopoietic tissues (Kuhn et al., 1995), so we assessed the contribution of Cited2 deletion in nonhematopoietic tissues to morbidity. We transplanted wild-type (WT) BM cells into Cited2fl/fl Mx1-Cre and Cited2fl/fl mice, and 12 weeks after transplantation, recipients received pIpC. We observed no lethality in either cohort of mice (Figure S1C), indicating that BM failure in Cited2Δ/Δ mice is the primary cause of mortality.
Cited2 Is Dispensable for the Maintenance of Committed Blood Lineages
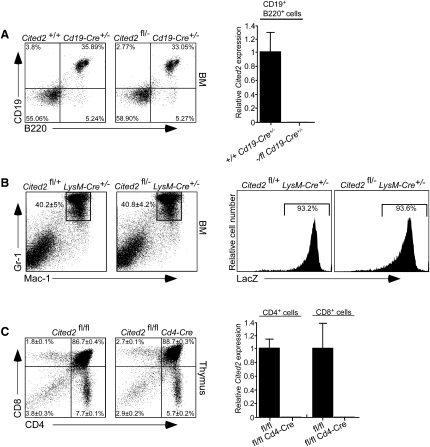
The multilineage defects observed in Cited2Δ/Δ mice could reflect a requirement for Cited2 in the maintenance of committed hematopoietic lineages. To test this hypothesis, we used Cd19-Cre, LysM-Cre, and Cd4-Cre strains to delete Cited2 in B cell, myeloid, and T cell lineages, respectively. Cd19-Cre efficiently excised Cited2 in CD19+B220+ cells but did not affect their frequency in the BM (Figure 2A). Likewise, efficient deletion of Cited2 in the myeloid compartment led to lacZ expression in the majority of Mac-1+Gr-1+ cells, but did not alter the frequency of these cells in the BM (Figure 2B). Cd4-Cre efficiently excised Cited2 in T cells but did not change their frequency in the thymus (Figure 2C). Therefore, Cited2 is expendable for the maintenance of these committed lineages.
Figure 2.
Cited2 Is Dispensable for the Maintenance of Mature Lymphoid and Myeloid Lineages
(A) Left: FACS plots showing CD19 and B220 staining of BM cells obtained from Cited2+/+Cd19-Cre+/− and Cited2fl/−Cd19-Cre+/− mice. Results from representative animals are shown (n = 3). Right: Relative expression of Cited2 mRNA in CD19+B220+ cells sorted from peripheral blood (PB) of Cited2+/+Cd19-Cre+/− and Cited2fl/−Cd19-Cre+/− mice. Data are mean ± SEM (n = 3).
(B) Left: FACS plot showing Mac-1 and Gr-1 staining of BM cells obtained from Cited2fl/+LyzM-Cre+/− and Cited2fl/−LyzM-Cre+/− mice. Data are shown as mean frequency ± SD (n = 3). Right: LacZ staining of BM Mac-1+Gr-1+ cells from Cited2fl/+LyzM-Cre+/− and Cited2fl/−LyzM-Cre+/− mice.
(C) Left: FACS plots showing distribution of T cell subsets in the thymi of Cited2fl/fl and Cited2fl/flCd4-Cre mice. Data are shown as mean frequency ± SD (n = 3). Right: Relative expression of Cited2 mRNA in CD4+ and CD8+ cells sorted from PB of Cited2fl/fl and Cited2fl/flCd4-Cre mice. Data are mean ± SEM (n = 3).
Cited2 Is Required for the Maintenance of Adult HSCs
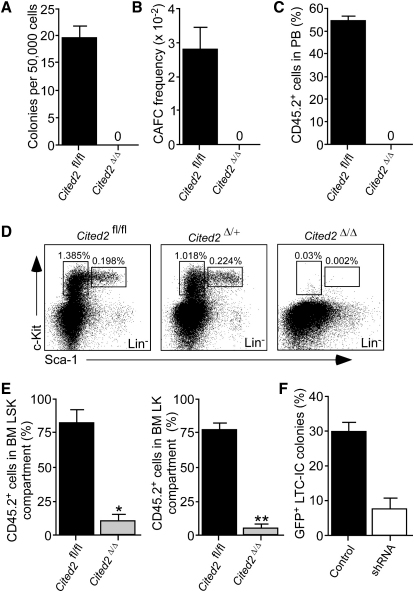
Next, we addressed the impact of Cited2 deletion on HSC and progenitor cell activity. In colony-forming cell (CFC) assays, Cited2Δ/Δ BM cells failed to generate colonies in methylcellulose (Figure 3A). To evaluate HSC activity in vitro, we performed limiting dilution cobblestone area-forming cell (CAFC) assays and found that Cited2Δ/Δ BM completely lacked CAFCs (Figure 3B). To assess HSC activity in vivo, we transplanted CD45.2+ BM cells from Cited2Δ/Δ and Cited2fl/fl control mice (with or without WT CD45.1+ BM competitors) into irradiated congenic CD45.1+ recipients. Without CD45.1+ BM competitors, Cited2Δ/Δ BM cells did not rescue recipient mice from lethal irradiation (data not shown). Furthermore, CD45.2+ Cited2Δ/Δ BM cells transplanted with CD45.1+ BM competitor cells did not contribute to multilineage hematopoiesis (Figure 3C). Immunophenotypic analysis of Cited2Δ/Δ BM revealed a near complete loss of cells in the LSK compartment (Figure 3D) that contains LT-HSCs, ST-HSCs, and LMPPs. The frequency of Lin−Sca-1−c-Kit+ (LK) myeloid progenitor cells was also profoundly decreased in Cited2Δ/Δ mice. To exclude the effects of pIpC-induced Cre-mediated toxicity on hematopoietic stem and progenitor cells (HSPCs), we compared the immunophenotypic and functional properties of HSPCs from Cited2+/+ Mx1-Cre and Cited2fl/fl mice and found no apparent differences (Figures S2A–S2D). These data indicate that pIpC-induced Cre activity does not phenocopy Cited2 deletion in HSPCs.
Figure 3.
Cited2 Maintains HSCs in a Cell-Autonomous Manner
Cited2fl/flMx1-Cre and control mice were treated with pIpC as shown in Figure 1B.
(A) CFC assay performed on total BM cells from Cited2Δ/Δ and control mice. The graph shows the mean number of CFC colonies ± SD counted on day 10 (n = 3 per group).
(B) CAFC assay. The graph shows the mean number of cobblestone areas ± SEM counted at week 5 (n = 3).
(C) Competitive repopulation assay. CD45.2+ BM cells from Cited2Δ/Δ or control mice were mixed with CD45.1+ WT competitor BM cells and transplanted into irradiated CD45.1+ WT recipients. After 16 weeks, the contribution of CD45.2+ cells was analyzed. Data are mean percentage of CD45.2+ cells in PB of recipient mice ± SD (n = 6 per group).
(D) Frequencies of the BM LSK and Lin−Sca-1−c-Kit+ (LK) cells from Cited2Δ/Δ, Cited2+/Δ, and Cited2fl/fl control mice. The data are representative of four independent experiments.
(E) BM cells from untreated Cited2fl/flMx1-Cre and Cited2fl/fl mice were mixed with CD45.1+ WT competitive BM and transplanted into irradiated recipients. Eight weeks after tansplantation, the mice were treated with five doses of pIpC. Five days after last pIpC administration, the percentage of test CD45.2+ cells was measured in BM LSK and LK compartments. Data are mean ± SD (n = 3). ∗p < 0.001; ∗∗p < 0.0002.
(F) LTC-IC assay. Human CD34+ CB cells transduced with shRNA and control lentiviruses were cocultured with MS5 stromal cells. After 5 weeks, medium was replaced with complete methylcellulose. The graph shows the mean percentage of GFP+ LTC-IC colonies in cultures ± SD (n = 2) scored 2 weeks after adding methylcellulose.
The rapid kinetics of HSC loss upon acute deletion of Cited2 suggest a survival defect. To test this, we deleted Cited2 in cultured LSK cells and demonstrated that the rate of apoptosis was markedly increased in Cited2Δ/Δ cells, as compared to WT cells (Figure S2E). Thus, decreased survival of LSK cells underpins the multilineage BM failure observed in Cited2Δ/Δ mice.
Cited2 Functions in a Cell-Autonomous Manner in HSCs
To independently examine whether loss of Cited2Δ/Δ HSCs is caused by Cited2 deletion specifically in the hematopoietic system, we mixed CD45.2+ BM cells from untreated Cited2fl/fl Mx1-Cre or Cited2fl/fl mice with CD45.1+ WT BM competitor cells and transplanted them into irradiated recipients. Eight weeks after transplantation, the mice received pIpC and five days after the last dose the percentage of the donor-derived CD45.2+ cells was analyzed in the BM. The percentage of CD45.2+ Cited2Δ/Δ cells in LSK and LK compartments was significantly reduced compared to CD45.2+ Cited2fl/fl cells (Figure 3E). These data indicate a cell-autonomous requirement for Cited2 in HSC maintenance.
CITED2 Is a Regulator of Primitive Hematopoietic Cell Function in Human Cord Blood
The high evolutionary conservation of Cited2 in mammals (Bhattacharya et al., 1999) suggests a conserved role for Cited2 in HSC function. We generated a lentivirus expressing short-hairpin RNA (shRNA) targeting human CITED2 (Figures S3A–S3D) and performed assays to enumerate LTC-ICs, the most primitive human progenitors assessable in vitro. CB CD34+ cells transduced with shRNA and control lentiviruses were cocultured on stromal cells. CITED2 knockdown in CD34+ cells led to a severe reduction in cellularity over time, compared to CD34+ cells transduced with a control lentivirus (Figure S3E). Furthermore, CITED2 knockdown in CD34+ cells strikingly impaired primitive hematopoietic cell activity, as judged by LTC-IC assays (Figure 3F). Thus, our data indicate that CITED2 is a conserved regulator of primitive hematopoietic cell function in mammals. Furthermore, with this Mx1-Cre-independent model system, we corroborate the data obtained in our conditional mouse model.
Intact Ink4a/Arf and Trp53 Are Required for the Loss of Cited2Δ/Δ HSCs
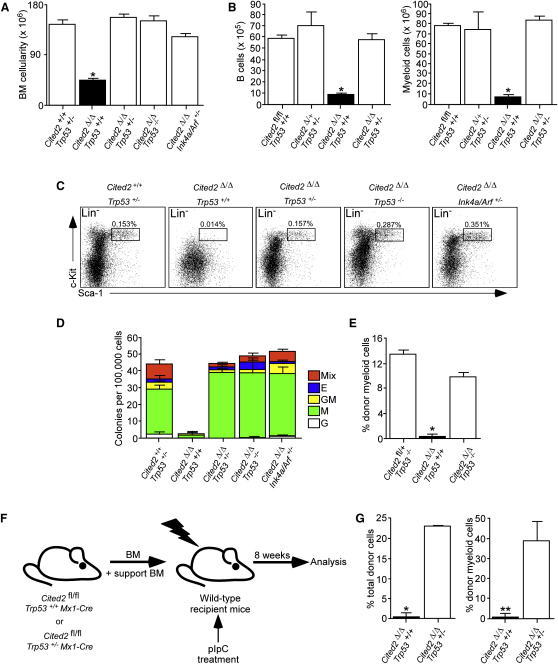
We previously showed that Cited2 null MEFs senesce prematurely and have increased levels of p16Ink4a and p19Arf (Kranc et al., 2003), whereas ectopic expression of Cited2 represses p16Ink4a and p19Arf, enhancing MEF proliferation. Deletion of Ink4a/Arf or Trp53 (encoding p53, a downstream target of p19Arf), rescued defective proliferation in Cited2−/− MEFs (Figure S4A; Kranc et al., 2003). Ink4a/Arf and Trp53 are essential in maintaining HSC function (Akala et al., 2008), so we hypothesized their involvement in the loss of Cited2Δ/Δ HSCs. Consistent with this, Cited2 deletion in LSK cells resulted in an increased expression of p19Arf and p53 proteins and a p53 target gene Cdkn1a (Figures S4B–S4D). Next, we generated Cited2fl/fl Mx1-Cre Trp53+/−, Cited2fl/fl Mx1-Cre Trp53−/−, Cited2fl/fl Mx1-Cre Ink4a/Arf +/−, and control mice and treated them with pIpC. Q-PCR confirmed that Cited2 was not expressed in Cited2Δ/Δ BM cells, regardless of Ink4a/Arf and Trp53 status (Figure S4E). Deletion of one Ink4a/Arf allele or one or two alleles of Trp53 restored total BM cellularity in Cited2Δ/Δ mice to the levels observed in Cited2fl/fl control mice (Figures 4A and 1D). Ablation of one allele of Trp53 also rescued B cell and myeloid development in Cited2Δ/Δ BM (Figure 4B). Furthermore, deletion of one allele of Ink4a/Arf or one or two alleles of Trp53 restored BM Cited2Δ/Δ LSK cells (Figure 4C). BM cells from Cited2Δ/Δ Trp53+/−, Cited2Δ/Δ Trp53−/−, and Cited2Δ/Δ Ink4a/Arf +/−, but not Cited2Δ/Δ, mice efficiently generated multilineage colonies in methylcellulose (Figure 4D). After confirming that Cited2Δ/Δ Trp53+/−, Cited2Δ/Δ Trp53−/−, and Cited2Δ/Δ Ink4a/Arf +/− cells from primary colonies lacked Cited2 expression, we demonstrated efficient generation of secondary colonies (data not shown).
Figure 4.
Genetic Deletion of Trp53 or Ink4a/Arf Restores HSC Functions and Rescues Bone Marrow Failure in Cited2Δ/Δ Mice
Mice of indicated genotypes were treated with pIpC.
(A) Total BM cellularity from two tibias and two femurs. The results are presented as mean number of cells ± SD (n = 3 per genotype). ∗p < 0.002 versus remaining genotypes.
(B) Graphs show total number of BM CD19+B220+ cells (B cells) and Mac-1+Gr-1+ cells (myeloid cells) in two tibias and two femurs per mouse. Mean values ± SD (n = 4). ∗p < 0.005 versus remaining genotypes.
(C) Frequencies of the BM LSK cells from mice of indicated genotypes. FACS plots are representative of three independent experiments.
(D) CFC assay. Nucleated BM cells were plated in methylcellulose medium. Cultures were assessed on day 10 for granulocyte (CFC-G), macrophage (CFC-M), granulocyte-macrophage (CFC-GM), erythroid (E), and mixed (Mix) colony formation. The data are representative of three independent experiments and are shown as the mean ± SD (n = 2 mice per genotype).
(E) Contribution of donor cells from Cited2fl/+Trp53−/−, Cited2Δ/ΔTrp53+/+, and Cited2Δ/ΔTrp53−/− mice to the myeloid compartment of PB 16 weeks after transplantation. BM cells from mice of the indicated genotypes were mixed with support WT BM cells and transplanted into irradiated recipients. The graph shows the mean (±SD) percentage of CD45.2+ cells in myeloid compartment of recipient mice (n = 3 per group). ∗p < 0.0003 versus remaining genotypes.
(F) Schematic of experimental design.
(G) Contribution of donor cells of the indicated genotypes to PB. Percentage of lacZ+ donor cells was analyzed by flow cytometry in total PB mononuclear compartment and myeloid (Mac-1+Gr-1+) compartment of recipients (n = 5 per group). ∗p < 0.002; ∗∗p < 0.00008.
To examine whether HSCs lacking both Cited2 and Trp53 have long-term repopulating capacity, we transplanted Cited2Δ/Δ Trp53+/+, Cited2Δ/Δ Trp53−/−, and Cited2fl/+ Trp53−/− total BM cells (mixed with WT support BM cells) into irradiated recipients and analyzed peripheral blood (PB) 16 weeks after transplantation. Cited2Δ/Δ Trp53+/+ BM cells failed to repopulate recipients (Figure 4E), whereas BM cells lacking both Cited2 and Trp53 repopulated recipients to a similar extent as those lacking Trp53 with intact Cited2. To corroborate this, we transplanted BM cells from untreated Cited2fl/fl Mx1-Cre Trp53+/+ and Cited2fl/fl Mx1-Cre Trp53+/− mice into irradiated recipients (Figure 4F). After reconstitution, the recipients were treated with pIpC and analyzed 8 weeks after administration of the last dose. We measured the percentage of donor cell chimerism in PB nucleated cells or myeloid cells of recipients by using lacZ as a marker of Cited2-deficient cells. Whereas Cited2Δ/Δ Trp53+/+ cells failed to sustain hematopoiesis, those lacking Cited2 and one allele of Trp53 showed significant donor-derived contribution (Figure 4G). Together, these data provide genetic evidence that the loss of HSCs in Cited2Δ/Δ mice is, at least in part, mediated by Ink4a/Arf and Trp53.
Discussion
In this report, we investigate the requirement for Cited2 in adult HSCs maintenance and committed hematopoietic lineages. By using an inducible conditional knockout approach in adult mice, we demonstrate that Cited2 deletion results in an acute loss of HSCs, at least in part via apoptosis, subsequently causing multilineage BM failure. Specific deletion of Cited2 within the hematopoietic system demonstrates a cell-autonomous requirement for Cited2 in maintaining adult HSC integrity, whereas deleting Cited2 in committed lymphoid and myeloid lineages has no impact. Furthermore, CITED2 knockdown in human CD34+CB reveals a conserved requirement for Cited2 in HSC maintenance. Together, our data provide evidence that Cited2 functions in a cell-autonomous manner to maintain HSCs.
Genetic evidence indicates that the tumor suppressors Ink4a/Arf and Trp53 regulate multiple HSC fate decisions (Akala et al., 2008; Liu et al., 2009; Oguro et al., 2006). One function of p19Arf is to stabilize p53 (Pomerantz et al., 1998), and the activation of the p19Arf-p53 pathway results in loss of HSCs (Park et al., 2003). We showed that loss of Cited2 increased p19Arf and p53 expression in the LSK compartment. Based on this observation, we used a genetic rescue approach to test whether Ink4a/Arf and Trp53 are required for loss of HSCs lacking Cited2. Our results demonstrated that deletion of Ink4a/Arf or Trp53 restored functionality of HSCs lacking Cited2, implying that Cited2 maintains HSCs, at least in part, via Ink4a/Arf and Trp53. These data support the postulate that deletion of Cited2 in HSCs results in activation of the p19ARF-p53 pathway and thereby leads to their loss.
It is of interest to relate Cited2 to other critical regulators of HSC maintenance. Cited2 is required for Bmi-1 expression in MEFs (Kranc et al., 2003) and myeloid progenitors (Chen et al., 2007). Bmi-1 maintains HSCs (Lessard and Sauvageau, 2003; Park et al., 2003) and directly represses Ink4a/Arf (Bracken et al., 2007), whereas deletion of Ink4a/Arf (Oguro et al., 2006) or Trp53 (Akala et al., 2008) restores Bmi-1−/− HSC function. Genetic evidence indicates distinct roles for Bmi-1 and Cited2 in HSC fate decisions. Whereas Bmi-1 mediates HSC self-renewal, our results are compatible with a requirement for Cited2 in HSC survival. In agreement with this, acute Cited2 deletion in HSCs does not affect the expression of Bmi-1 (data not shown), suggesting that downregulation of Bmi-1 expression is not responsible for the loss of Cited2Δ/Δ HSCs. However, this does not exclude the possibility that Cited2 controls Bmi-1 in other contexts in HSCs. Conditional deletion of Cited2 generates a stem cell phenotype reminiscent of conditional inactivation of Tel/Etv6 and Mcl-1 (Hock et al., 2004; Opferman et al., 2005). Like Tel/Etv6 (Hock et al., 2004), Cited2 appears to be selectively required for HSC maintenance, but dispensable for mature lineages. Mcl-1, however, also plays critical roles in mature T and B cell survival (Opferman et al., 2003), revealing a broader spectrum of hematopoietic function than Cited2. Conditional deletion of Apc and combined deficiency of c-Myc and N-Myc (but not ablation of N-Myc alone) results in loss of HSCs (Laurenti et al., 2008; Qian et al., 2008). Although the expression of Apc and c-Myc is unaltered in Cited2-deficient HSCs, the expression of N-myc is decreased (data not shown). Although this observation alone does not explain the loss of Cited2-deficient HSCs, N-Myc may mediate some functions of Cited2 in HSCs. Finally, Cited2 binds p300 and its paralog CBP (Bhattacharya et al., 1999). Although Cbp is essential for adult HSC maintenance, p300 appears dispensable for HSC maintenance but required for multilineage hematopoietic differentiation (Kung et al., 2000; Rebel et al., 2002). It will be of interest to clarify the roles of Cbp-Cited2 and p300-Cited2 interactions in adult HSC maintenance and hematopoiesis, and the relationship between Cited2 and other critical stem cell regulators remains an open question meriting future investigation.
In conclusion, we provide genetic evidence that Cited2 is an essential and cell-autonomous regulator of adult mammalian HSC maintenance. Our data, together with the sufficiency of Cited2 to maintain undifferentiated embryonic stem cells (Pritsker et al., 2006), suggest that it is a critical master regulator of stem cell fate. Understanding Cited2 functions at the molecular level will offer insights into the similarities and differences in the transcriptional circuitry of embryonic and somatic stem cells.
Experimental Procedures
Mice
We backcrossed Cited2fl/fl and Cited2+/− mice (Bamforth et al., 2001; MacDonald et al., 2008) to C57BL/6J for ten generations to generate coisogenic mice. Mx1-Cre, Cd19-Cre, and LysM-Cre mice were purchased from the Jackson Laboratory. Cd4-Cre mice were purchased from Taconic. Ink4a/Arf +/− and Trp53+/− mice were obtained from B. Hassan and M. van Lohuizen, respectively. All experiments on animals were performed under UK Home Office authorization.
Administration of pIpC
8- to 12-week-old mice received five to six intraperitoneal injections of pIpC (GE Healthcare; 0.2–0.3 mg per dose) every alternate day. Deletion efficiency was determined by Q-PCR or lacZ expression analysis (via a FluoReporter lacZ Flow Cytometry Kit, Invitrogen).
Murine CAFC Assay
Stromal layers were prepared from the BM of C57BL/6J mice, irradiated at 15 Gy, and subcultured in 96-well flat-bottom plates at a density of 2 × 104 cells per well. After 1 to 7 days, cultures were seeded at 2-fold dilutions (2.9 × 105−18,125 per well) of nucleated BM cells from each genotype. CAFCs were scored at week 5.
CFC Assays
H4434 and M3434 media (StemCell Technologies) were used to enumerate human and mouse colony-forming cells, respectively. Two replicates were used per group in each experiment. Colonies were tallied at day 10–14.
Q-PCR
RNA extraction and Q-PCR reactions were performed as previously described (Mansson et al., 2007). For specific TaqMan Assays-on-Demand probes used, see Supplemental Experimental Procedures. Reactions were run on an Applied Biosystems 7500 Fast Real-Time PCR System in normal mode for 50 cycles. All experiments were performed in triplicate. Differences in input cDNA were normalized with a combination of Hprt, Gapdh, Actb, Ubc, and B2m expression with qBase 1.3.5 software (http://medgen.ugent.be/qbase/).
Lentiviral Transductions
CITED2 shRNA was subcloned from the pLKO.1 puro vector (Open Biosystems) into the pLKO.1 GFP vector (gift from J. Larsson). Lentivirus production and transduction of human CD34+ CB cells are described in Supplemental Experimental Procedures.
Human Long-Term Cultures on Stroma and LTC-IC Assays
CB CD34+ cells (StemCell Technologies) were isolated by MiniMACS (Miltenyi Biotec) selection. After transduction, 3 × 104 cells were cultured on MS5 stromal cells in Long-Term Culture medium (see Supplemental Experimental Procedures). Cultures were demidepopulated weekly for analysis. LTC-IC numbers were enumerated by overlaying MS5 stromal cocultures at week 5 with H4434 medium, followed by counting colonies 2 weeks later.
FACS
All samples were analyzed on a CyAn ADP flow cytometer (Dako). Sorts were performed on FACSAriaIIu (BD) or MoFlow (Dako) cell sorters. Antibodies are described in Supplemental Experimental Procedures.
Competitive Repopulation Assay
CD45.2+ test donor BM cells were mixed with CD45.1+ competitor BM cells in a 1:1 ratio and injected intravenously into lethally irradiated (9 Gy) B6.SJL CD45.1+ recipients. The competitor cell number was 5 × 105 cells in all experiments.
Statistical Analysis
Statistical significance was determined via two-tailed Student's t tests assuming unequal variance.
Acknowledgments
K.R.K. is a Beit Memorial Fellow for Medical Research. H.S. is an EMBO long-term fellow. N.P.R. is a MRC Career Development Fellow. The authors' laboratories are supported by John Fell OUP Research Fund (K.R.K.), Leukaemia Research Fund (K.R.K., T.E., S.E.J.), Medical Research Council (S.E.J., T.E.), and Wellcome Trust (K.R.K., S.B.).
Published online: December 3, 2009
Footnotes
Supplemental Data include Supplemental Experimental Procedures and four figures and can be found with this article online at http://www.cell.com/cell-stem-cell/supplemental/S1934-5909(09)00574-8.
Supplemental Data
References
- Akala O.O., Park I.K., Qian D., Pihalja M., Becker M.W., Clarke M.F. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- Bamforth S.D., Braganca J., Eloranta J.J., Murdoch J.N., Marques F.I., Kranc K.R., Farza H., Henderson D.J., Hurst H.C., Bhattacharya S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 2001;29:469–474. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Michels C.L., Leung M.K., Arany Z.P., Kung A.L., Livingston D.M. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken A.P., Kleine-Kohlbrecher D., Dietrich N., Pasini D., Gargiulo G., Beekman C., Theilgaard-Monch K., Minucci S., Porse B.T., Marine J.C. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Haviernik P., Bunting K.D., Yang Y.C. Cited2 is required for normal hematopoiesis in the murine fetal liver. Blood. 2007;110:2889–2898. doi: 10.1182/blood-2007-01-066316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.T., Wang H., Chen Y., Danielpour D., Yang Y.C. Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene. 2006;25:5547–5560. doi: 10.1038/sj.onc.1209552. [DOI] [PubMed] [Google Scholar]
- Glenn D.J., Maurer R.A. MRG1 binds to the LIM domain of Lhx2 and may function as a coactivator to stimulate glycoprotein hormone alpha-subunit gene expression. J. Biol. Chem. 1999;274:36159–36167. doi: 10.1074/jbc.274.51.36159. [DOI] [PubMed] [Google Scholar]
- Gomes I., Sharma T.T., Edassery S., Fulton N., Mar B.G., Westbrook C.A. Novel transcription factors in human CD34 antigen-positive hematopoietic cells. Blood. 2002;100:107–119. doi: 10.1182/blood.v100.1.107. [DOI] [PubMed] [Google Scholar]
- Hock H., Meade E., Medeiros S., Schindler J.W., Valk P.J., Fujiwara Y., Orkin S.H. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–2341. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranc K.R., Bamforth S.D., Braganca J., Norbury C., van Lohuizen M., Bhattacharya S. Transcriptional coactivator Cited2 induces Bmi1 and Mel18 and controls fibroblast proliferation via Ink4a/ARF. Mol. Cell. Biol. 2003;23:7658–7666. doi: 10.1128/MCB.23.21.7658-7666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R., Schwenk F., Aguet M., Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Kung A.L., Rebel V.I., Bronson R.T., Ch'ng L.E., Sieff C.A., Livingston D.M., Yao T.P. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000;14:272–277. [PMC free article] [PubMed] [Google Scholar]
- Laurenti E., Varnum-Finney B., Wilson A., Ferrero I., Blanco-Bose W.E., Ehninger A., Knoepfler P.S., Cheng P.F., MacDonald H.R., Eisenman R.N. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell. 2008;3:611–624. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J., Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Liu Y., Elf S.E., Miyata Y., Sashida G., Liu Y., Huang G., Di Giandomenico S., Lee J.M., Deblasio A., Menendez S. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald S.T., Bamforth S.D., Chen C.M., Farthing C.R., Franklyn A., Broadbent C., Schneider J.E., Saga Y., Lewandoski M., Bhattacharya S. Epiblastic Cited2 deficiency results in cardiac phenotypic heterogeneity and provides a mechanism for haploinsufficiency. Cardiovasc. Res. 2008;79:448–457. doi: 10.1093/cvr/cvn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson R., Hultquist A., Luc S., Yang L., Anderson K., Kharazi S., Al-Hashmi S., Liuba K., Thoren L., Adolfsson J. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Oguro H., Iwama A., Morita Y., Kamijo T., van Lohuizen M., Nakauchi H. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. J. Exp. Med. 2006;203:2247–2253. doi: 10.1084/jem.20052477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman J.T., Letai A., Beard C., Sorcinelli M.D., Ong C.C., Korsmeyer S.J. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Opferman J.T., Iwasaki H., Ong C.C., Suh H., Mizuno S., Akashi K., Korsmeyer S.J. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- Orkin S.H., Zon L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.K., Qian D., Kiel M., Becker M.W., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Pomerantz J., Schreiber-Agus N., Liegeois N.J., Silverman A., Alland L., Chin L., Potes J., Chen K., Orlow I., Lee H.W. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- Pritsker M., Ford N.R., Jenq H.T., Lemischka I.R. Genomewide gain-of-function genetic screen identifies functionally active genes in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:6946–6951. doi: 10.1073/pnas.0509861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z., Chen L., Fernald A.A., Williams B.O., Le Beau M.M. A critical role for Apc in hematopoietic stem and progenitor cell survival. J. Exp. Med. 2008;205:2163–2175. doi: 10.1084/jem.20080578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Lam E., Doughman Y.Q., Chen Y., Chou Y.T., Lam M., Turakhia M., Dunwoodie S.L., Watanabe M., Xu B. Cited2, a coactivator of HNF4alpha, is essential for liver development. EMBO J. 2007;26:4445–4456. doi: 10.1038/sj.emboj.7601883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebel V.I., Kung A.L., Tanner E.A., Yang H., Bronson R.T., Livingston D.M. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc. Natl. Acad. Sci. USA. 2002;99:14789–14794. doi: 10.1073/pnas.232568499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling S., Grimm C.H., Dunkel I., Mebus S., Sperling H.P., Ebner A., Galli R., Lehrach H., Fusch C., Berger F. Identification and functional analysis of CITED2 mutations in patients with congenital heart defects. Hum. Mutat. 2005;26:575–582. doi: 10.1002/humu.20262. [DOI] [PubMed] [Google Scholar]
- Sun H.B., Zhu Y.X., Yin T., Sledge G., Yang Y.C. MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc. Natl. Acad. Sci. USA. 1998;95:13555–13560. doi: 10.1073/pnas.95.23.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien E.S., Davis J.W., Vanden Heuvel J.P. Identification of the CREB-binding protein/p300-interacting protein CITED2 as a peroxisome proliferator-activated receptor alpha coregulator. J. Biol. Chem. 2004;279:24053–24063. doi: 10.1074/jbc.M401489200. [DOI] [PubMed] [Google Scholar]
- Yin Z., Haynie J., Yang X., Han B., Kiatchoosakun S., Restivo J., Yuan S., Prabhakar N.R., Herrup K., Conlon R.A. The essential role of Cited2, a negative regulator for HIF-1alpha, in heart development and neurulation. Proc. Natl. Acad. Sci. USA. 2002;99:10488–10493. doi: 10.1073/pnas.162371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.F., Zhao Y., Sutton S., Su A., Zhan Y., Zhu L., Yan C., Gallaher T., Johnston P.B., Anderson W.F. Gene expression profile of murine long-term reconstituting vs. short-term reconstituting hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:2448–2453. doi: 10.1073/pnas.0409459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.