Abstract
Tumours are highly complex tissues composed of carcinoma cells and surrounding stroma, which is constructed by various different types of mesenchymal cells and an extracellular matrix (ECM). Carcinoma-associated fibroblasts (CAFs), which consist of both fibroblasts and myofibroblasts, are frequently observed in the stroma of human carcinomas, and their presence in large numbers is often associated with the development of high-grade malignancies and poor prognoses. Moreover, in human tumour xenograft models, CAFs extracted from the tumour are more capable of promoting tumour growth through their interactions with carcinoma cells when compared to those isolated from non-cancerous stroma. Taken together, these observations strongly suggest that CAFs actively contribute to tumour progression. In this review we highlight the emerging roles of these cells in promoting tumourigenesis, and we discuss the molecular mechanisms underlying their tumour-promoting capabilities and their cellular origin.
Keywords: Carcinoma-associated fibroblasts (CAFs), Stromal myofibroblasts, Wound healing, Fibrosis, TGF-β, SDF-1
1. Introduction
It has long been established that cancer consists of transformed cells that harbour genetic and/or epigenetic aberrances in oncogenes and/or tumour suppressor genes. Accumulation of these alterations can endow carcinoma cells with greater proliferative, invasive and survival propensities in a cell-autonomous fashion [1]. However, it is widely recognised that in addition to carcinoma cells, tumours contain large numbers of various non-transformed cells and a specialised ECM which are collectively referred to as the stroma. The auxiliary cells found within the stroma include fibroblasts, myofibroblasts, leukocytes, endothelial cells and bone marrow-derived cells, all of which collaborate to create the complexity of the tumour microenvironment.
Recent evidence also suggests that tumourigenesis is dependant upon contextual signals received from the closely apposed tumour-associated stroma [2], [3], [4], [5], [6], [7], [8]. The stroma actively provides continuous support to carcinoma cells throughout the different pathophysiological processes that modulate tumour progression. In normal tissue the stroma may actually act as a barrier in promoting tumourigenesis by constraining tumour cell proliferation. During tumourigenesis however, the stroma overtime evolves to actively support tumour growth in response to molecular signals derived from carcinoma cells and other host cell types.
The development of the tumour and progression towards advanced stages of the disease requires the successful co-evolution of both cancer cells and stromal cells. Within the tumour, the normal architecture of the tissue becomes disordered and the ECM is remodelled by mesenchymal cells such as myofibroblasts found within the stroma.
Myofibroblasts express characteristics common to smooth muscle cells and pericytes and are the main cell population found in CAFs. Stromal fibroblasts exposed to medium that has been conditioned by carcinoma cells can differentiate into myofibroblasts, and are more competent in promoting tumour growth [9], [10], [11]. It is likely that carcinoma cells not only initiate the conversion of stromal fibroblasts into myofibroblasts but also help to maintain their activated phenotype in vivo. The presence of myofibroblastic CAFs within the carcinoma can corroborate the evolution of the normal stroma towards a tumour-promoting microenvironment.
2. Myofibroblasts, a hallmark of activated fibroblasts, in promoting tumourigenesis
Myofibroblasts were initially identified by Gabbiani et al. [12] in granulation tissue during wound healing. The physiological roles of myofibroblasts during wound healing have been extensively studied. These cells express stress fibres that aid healing by contracting the wound, bringing the edges of the damaged tissue closer together. They also stimulate angiogenesis and epithelial growth by increasing their deposition of ECM proteins and secreting various growth factors and cytokines [8], [13], [14]. Whilst the wound-healing process is normally complete after the first few weeks following injury, an inappropriate repair program is chronically sustained in pathological, fibrotic diseases, such as hypertrophic scars, keloids, scleroderma, rheumatoid arthritis and idiopathic tissue fibroses. Importantly, myofibroblasts extracted from patients suffering from these diseases stably maintain a transforming growth factor-β (TGF-β) autocrine signalling pathway, which continues to mediate their activated fibroblastic properties during propagation in vitro [15], [16], [17]. The stability of such autocrine signalling may be programmed by the improper tissue repair response that is constantly activated in these diseases although this molecular mechanism is poorly understood.
Myofibroblasts derived from fibrotic tissues are more capable of promoting tumourigenesis through their interaction with carcinoma cells compared with control fibroblasts derived from normal tissues. Activated fibroblasts extracted from the inflamed synovium of a rheumatoid arthritis patient, when injected along with human breast cancer cells into a recipient mouse, promote carcinoma growth by elevating the expression of cyclooxygenase-2 (COX-2) by carcinoma cells, an enzyme responsible for inflammation-associated tumourigenesis [18].
Several animal models provide evidence that fibrotic and wounded tissues have the potential to develop into a tumour, suggesting the presence of precursory oncogenic factors in both afflicted tissues. In an avian model, Rous sarcoma virus (RSV)-infected chickens develop sarcomas at the site of wounding through the subsequent inflammatory response [19]. The loss of Notch 1 expression within the epidermis of a conditional Notch 1flox/flox mouse causes a wound-like microenvironment which consists of infiltrating myofibroblasts and leukocytes. These cells produce elevated levels of TGF-β, keratinocyte growth factor (KGF), and stromal cell-derived factor 1 (SDF-1: also called CXCL12) [20]. This altered microenvironment, rich in such growth factors and cytokines, potentially promotes chemical carcinogen-induced skin tumourigenesis of Notch 1-expressing keratinocytes in a non-cell-autonomous manner. This study therefore indicates the inherent tumour-suppressive role of stromal Notch signalling in maintaining tissue integrity and homeostasis within the skin. Collectively, these findings illustrate that the altered tissue microenvironment, elicited by wounding and fibrosis, can facilitate tumour incidence and progression.
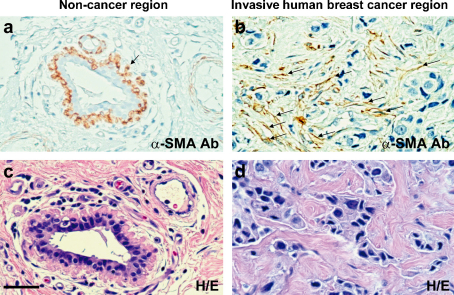
Myofibroblasts are frequently observed in the stroma of various types of human carcinomas including breast (Fig. 1) [21], [22]. Previous studies demonstrate that stromal myofibroblasts within the tumour resemble those present in wounded and fibrotic tissues [23], [24], [25]. Patients whose carcinomas exhibit a greater myofibroblastic stromal reaction resulting in a so-called “desmoplastic stroma”, usually develop higher-grade malignancies associated with poor prognostic outcome [11], [26], [27].
Fig. 1.
Large numbers of myofibroblasts exist in the stroma of human breast tumour. Paraffin sections were prepared from human non-neoplastic breast tissue (a and c) or invasive breast cancer tissue (b and d) dissected from the same individual. These sections were stained with hematoxylin and eosin (H&E) (c and d) or immunostained with an anti-α-SMA antibody (a and b). α-SMA-positive myoepithelial cells surrounding epithelial cells (an arrow in a) are observed in normal tissue (a), whilst α-SMA-positive myofibroblasts (arrows in b) are only found in the tumour-associated stroma (b) (from Ref. [22]).
CAFs have been extracted from a number of different types of human carcinomas including breast, prostate, ovary, pancreas, skin, colon and esophagus [22], [28], [29], [30], [31], [32], [33]. DNA microarray analyses, using either cultured CAFs or tumour-associated stromal tissues microdissected from the regions adjacent to human carcinoma cells, show gene expression profiles distinct to those in their control groups [34], [35], [36], [37]. CAFs isolated from different sample sets show differences in the expression of large varieties of genes encoding for a number of cytokines, growth factors, enzymes, ECM and muscle-related proteins. These differences may result from discrete alterations in different signalling pathways within CAFs that regulate their phenotype and have been modified through their interactions with carcinoma cells.
The ability of tumour stromal fibroblasts to promote carcinogenesis was investigated using a tumour xenograft model in which tumour cells were implanted into immunodeficient mice, along with CAFs, counterpart fibroblasts extracted from the non-cancerous tissue in the same individual, or normal fibroblasts extracted from healthy donors [18], [22], [28], [29], [33]. Those tumours that developed from carcinoma cells injected with CAFs show a faster growth rate, compared to those tumours admixed with either of the control sets of fibroblasts. Considerable numbers of CAFs or control fibroblasts can be observed alongside carcinoma cells in advanced tumour xenografts [22], [38]. CAF-secreted paracrine factors likely act upon these cancer cells and encourage carcinoma growth during tumour development.
CAFs secrete elevated levels of the cytokine SDF-1 that stimulates carcinoma cell proliferation in vivo, acting through the CXCR4 receptor expressed on the surface of carcinoma cells [22], [39]. Other studies have also shown that CAFs secrete high levels of TGF-β1 that increases CXCR4 expression on human prostate preneoplastic cells [39], [40], [41]. These studies highlight the importance of stroma-derived SDF-1/CXCR4 and TGF-β paracrine signalling in promoting tumourigenesis. Understanding the molecular crosstalk which occurs between CAFs and carcinoma cells is essential and may in the future provide novel therapeutic targets for the treatment of cancer.
Myofibroblasts derived from both tumour and fibrotic tissues share similar molecular and cellular traits. The cellular processes that occur within both the tumour and the diseased tissues are perhaps best epitomised by the statement that “tumours are wounds that do not heal” [23], [24], [25].
TGF-β autocrine signalling plays a central role in forming and maintaining the myofibroblastic phenotype of cells involved in fibrosis. However, it is unclear if such a TGF-β autocrine signalling pathway is also responsible for mediating this phenotype in tumour-associated myofibroblasts, and if these cells possess specific genetic alteration(s) that are not shared with those present in fibrotic tissue. This issue warrants further consideration and will be addressed in the following section.
3. Somatic genetic and epigenetic alterations in tumour-associated stromal cells
In human epithelial carcinomas, cellular transformation results from genetic aberrances that lead to tumour development and progression. Although it is now widely accepted that tumour-associated stromal cells can influence tumourigenesis, the mechanism(s) by which they acquire these tumour-promoting properties are not known. The presence of somatic genetic alterations observed in tumour-associated stromal cells has been controversial [5], [42], [43], and the functional relevance of these proposed alterations to their phenotypes remains unclear.
Genetic alterations, such as chromosomal loss of heterozygosity (LOH) and somatic mutations, have been reported in stromal regions microdissected from various human carcinomas including those of breast [44], [45], [46], [47], ovarian [48], colon [45], bladder [49], and head and neck [50]. Indeed, analyses performed on microdissected regions of stromal tissues from within the tumour identify somatic TP53 mutations within 25.6% of the hereditary group, and 19.4% of the sporadic group of breast cancer patients [47]. There is also a higher risk of regional lymph node metastases occurring in these patients. Moreover, tumour-associated stromal regions microdissected from prostate tumours, when raised in epithelial cell-specific oncogene-driven transgenic mice with a TP53+/− background, display frequent loss of the wild type TP53 allele [51]. A complementary study finds that, when human breast carcinoma cells are implanted into p53+/− recipient mice, the host stromal cells isolated from the developing tumour lose their wild type TP53 allele resulting in p53−/− tumour-associated stromal cells [52].
Although loss of p53 activity within stromal cells appears instrumental in the development of a tumour-promoting stroma, it is unclear if this can trigger the differentiation of fibroblasts and/or progenitor cells into myofibroblasts. Furthermore, although aberrations in p53 signalling are often associated with a higher incidence of mesenchymal tumours in patients with Li–Fraumeni syndrome and in p53-mutant mice, it is unclear why carcinosarcomas, which are malignant biphasic epithelial and mesenchymal tumours, very rarely (∼0.2%) develop in human breast carcinoma patients [53], [54].
The above studies detected genetic alterations within the stroma of paraffin-fixed tumour tissues. Conversely, genome-wide genetic analyses, including single nucleotide polymorphism (SNP) and comparative genomic hybridisation (CGH) arrays using freshly frozen tissues, fail to detect any such significant somatic genetic alterations within the stroma of human breast tumours. This is highlighted in one important study that detected a LOH event on chromosome 22 in only 1 out of 35 human breast and ovarian carcinoma-associated stroma samples [55].
Specifically in CAFs, such analyses have also failed to detect substantial genetic alterations [34], [55], [56]. Our own observations indicate that CAFs themselves are non-neoplastic as they typically senesce in vitro, and show no detectable chromosomal abnormalities and no cellular phenotypes characteristic of malignant cell types [22]. Nonetheless, there is a possibility that only a small number of CAFs may actually possess genetic alterations, and these cells may be difficult to detect in a large heterogenous fibroblast population. Other studies, however, have reported the presence of epigenetic modifications, such as DNA methylation within the genome of CAFs that may give rise to their tumour-promoting phenotype [57], [58].
The biological relevance of genetic and/or epigenetic alterations within the tumour stroma and the cellular and molecular mechanisms that underlie the tumour-promoting phenotype of CAFs have yet to be elucidated. The detection of somatic alterations in some studies, whilst not in others, may be in part due to the different methods used to acquire and prepare tissues for analysis, the use of paraffin-fixed tissues instead of frozen tissues, or the different types of genomic analyses performed. Future independent analyses are required in order to clarify these issues.
4. Heterogeneity of tumour stromal fibroblasts
Within a murine renal fibrosis model, significant numbers of myofibroblasts found in affected tissues are derived from epithelial cells, endothelial cells and circulating bone marrow cells [59]. In tumour tissue, myofibroblasts are also proposed to originate from different cell types including pre-existing fibroblasts, preadipocytes, smooth muscle cells, endothelial cells, epithelial cells and bone marrow-derived progenitors [60], [61], [62], [63], [64], [65]. Bone marrow-derived cells are a significant cellular source of myofibroblasts found in the tumour stroma. Nearly 40% of the total stromal myofibroblast population within the tumour, 28 days after implantation of pancreatic carcinoma cells, is shown as bone marrow-derived in one tumour xenograft murine model [60], and approximately 25% in 16–18-week-old pancreatic tumours, spontaneously arising in another transgenic mouse model [61].
Whilst epithelial cells are the major cellular origin of activated fibroblasts during fibrosis, it is unclear if normal or malignant epithelial cells convert into myofibroblasts in tumour. The tumour microenvironment is rich in the cytokine TGF-β. In vitro studies have shown that fibroblasts cultured in the presence of TGF-β most often undergo transdifferentiation into myofibroblasts. This supports the notion that myofibroblast differentiation of residual fibroblasts may also occur within the stroma of tumour in response to TGF-β paracrine signalling, although this has yet to be proven.
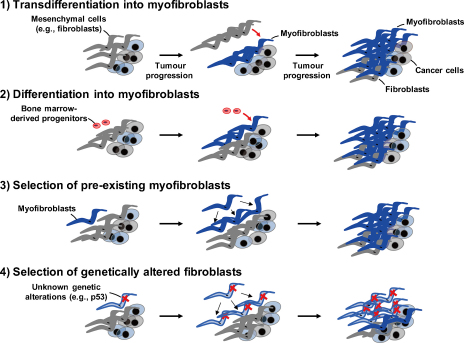
In 2006 we proposed three tentative models for the origins of CAF myofibroblasts within the tumour [6]. In light of recent studies and emerging data, we have now revised our models as illustrated in Fig. 2; (1) populations of residual mesenchymal cells (e.g., stromal fibroblasts) might transdifferentiate into myofibroblasts without the acquisition of any genetic alterations, thus mimicking the scenario that occurs during wound healing. Myofibroblasts found within the tumour are therefore essentially the same as myofibroblasts involved in wound healing and fibrosis; (2) specialised circulating progenitor cell types, such as fibrocytes and mesenchymal stem cells (MSCs), are recruited into the tumour stroma where they are able to differentiate into myofibroblasts. These first two models suggest that tumour-induced education of the nearby stromal cells occurs through myofibroblast differentiation; (3) a rare population of pre-existing myofibroblasts may be clonally expanded in the tumour but do not acquire any additional alterations; (4) acquisition of genetic alterations (e.g., p53 loss) within a small population of fibroblasts and/or progenitors may allow for their clonal selection and expansion. Such genetic alterations may or may not be relevant to myofibroblast differentiation. These last two models effectively rely upon the clonal selection of a small number of altered stromal cells.
Fig. 2.
Four alternative models for cellular origins and evolution of myofibroblasts in the stroma of tumour. (1) Transdifferentiation into myofibroblasts. Populations of residual mesenchymal cells (e.g., stromal fibroblasts) might transdifferentiate into myofibroblasts without acquiring any significant genetic alterations; (2) differentiation into myofibroblasts. Stromal myofibroblasts are recruited from specialised circulating bone marrow-derived progenitor cell types, such as fibrocytes and MSCs, which differentiate into myofibroblasts within the tumour stroma; (3) selection of pre-existing myofibroblasts. A small population of pre-existing myofibroblasts may be clonally expanded in the tumour without acquiring any further phenotypic alterations; (4) selection of genetically altered fibroblasts. Acquisition of genetic alterations (e.g., p53 loss) may allow for the clonal selection from a small population of fibroblasts or progenitors that have undergone such alterations. The resulting fibroblasts may or may not then differentiate into myofibroblasts.
5. The role of tumour stromal fibroblasts in promoting tumour progression
5.1. Tumour stromal fibroblasts boost neoangiogenesis
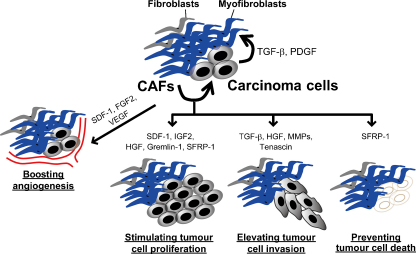
In cancer neoangiogenesis is a pathophysiological process essential for the growth and progression of a tumour. The process of neoangiogenesis is facilitated through the increased expression of various growth factors, cytokines and ECM proteins produced by tumour-associated stromal cells (Fig. 3).
Fig. 3.
Diverse tumour-promoting affects of CAFs. Carcinoma cell-derived TGF-β and PDGFs play central role to induce and maintain CAF myofibroblasts within the stroma of tumour. CAF-derived SDF-1 [22], FGF2 [69] and VEGF [75] boost angiogenesis. SDF-1 [22], IGF2 [76], HGF [77], Gremlin-1 [30], and SFRP-1 [78] also stimulate tumour cell proliferation. Moreover, CAFs enhance tumour invasion through their secretion of TGF-β, tenascin-C [31], tenascin-W [79], HGF [31], and matrix metalloproteinases (MMPs). Furthermore, CAF-produced SFRP1 [78] serves as an anti-apoptotic factor. Taken together, CAFs could promote tumour progression in several aspects through their ability to produce elevated levels of various growth factors, cytokines, ECM proteins and MMPs.
Elevated levels of the proangiogenic chemokine SDF-1 are secreted by stromal fibroblasts found in human breast and prostate carcinomas [22], [34], [39], [66]. SDF-1 boosts neoangiogenesis by recruiting endothelial progenitor cells (EPCs) into the tumour [22]. Moreover, elevated levels of CXCL14 expression are detected in mammary tumour stromal fibroblasts and in tumour stromal regions microdissected from human prostate cancers [34], [67]. The cognate receptor for this ligand has not yet been identified. However, forced expression of CXCL14 in fibroblasts, injected into a murine xenograft model along with prostate carcinoma cells, enhances neoangiogenesis within the tumour [67].
A recent report suggests that tumour stromal fibroblasts also mediate resistance to anti-angiogenic therapy. In a murine model, fibroblast-enriched cell fractions, extracted from refractory lymphomas resistant to anti-VEGF antibody treatment, upregulate their levels of PDGF-C mRNA expression by 200-fold compared to fibroblasts isolated from non-refractory tumours. The authors conclude that the elevated levels of PDGF-C in fibroblasts expressed in the therapy-resistant tumours directly promote neoangiogenesis [68]. In a transgenic mouse model for cervical squamous cell carcinoma induced by infection with human papilloma virus, type 16 E6/E7, stromal PDGF receptor signalling is inhibited by treatment with a specific kinase inhibitor. As a result, tumour growth and neoangiogenesis are significantly inhibited through attenuation of FGF2 and FGF7 expression in tumour stromal fibroblasts, highlighting the importance of stromal PDGF receptor signalling in promoting neoangiogenesis [69]. Collectively, these findings highlight the important role of tumour stromal fibroblasts in promoting neoangiogenesis through secretion of various cytokines and growth factors.
5.2. Tumour stromal fibroblasts promote tumour cell invasion
The tumour invasion-metastasis cascade is a complex multistep process that allows tumour cells to escape the primary tumour mass and colonise distant organs and tissues. The cascade consists of a series of distinct processes which include localised invasion, entrance into the systemic circulation, survival during transportation, extravasation, the establishment of micrometastases in distal tissues and colonisation resulting in the formation of macroscopic metastases [70]. It has long been assumed that dissemination of metastatic carcinoma cells depends largely on their cell-autonomous effects in response to genetic and/or epigenetic alterations that accumulate within them. However, emerging evidence now proposes an additional schema that the interaction of tumour-associated stroma with carcinoma cells facilitates the invasion-metastasis cascade [71].
As previously mentioned, the presence of larger numbers of myofibroblasts in the stroma of different human cancers is associated with an increased risk of invasion and metastasis and a poor clinical prognosis [26], [27], [72]. Human colorectal carcinoma cells that interact with stromal cells at the invasive front also increase their expression of nuclear β-catenin which can activate Wnt signalling in these cells [73]. Moreover, tumour stromal fibroblasts extracted from human colon adenocarcinomas and TGF-β-primed stromal fibroblasts upregulate expression of tenascin-C and HGF, both of which co-operate to promote the invasive propensity of human colon carcinoma cells within a collagen gel matrix [31].
Interestingly, higher levels of cytokines, such as TGF-β, a critical mediator of the epithelial-to-mesenchymal transition (EMT), are often produced by the tumour-associated stroma [74]. It is therefore possible that the activated stroma contributes to induction of EMT in nearby carcinoma cells, although at present little is known about the affects of the stroma upon EMT and the invasion-metastasis cascade.
6. Perspective
In cancer the stroma is able to facilitate disease progression through its interaction with carcinoma cells. Whilst CAFs undoubtedly contribute to tumourigenesis, more research is required in order to shed light on their molecular interactions with carcinoma cells and to further understand the stromal signalling pathways that are actively involved.
As neoplastic cells evolve into a more malignant cell population during tumour progression, tumour-associated stromal cells may evolve alongside them supporting their phenotypic conversion throughout the disease process. Moreover, CAF paracrine signalling may act upon cancer cells, facilitating their invasion into healthy tissue and their subsequent colonisation of distal organs and tissues. Further research is therefore required in order to fully understand the molecular mechanisms underlying the potential role of CAFs in promoting invasion and metastasis. It is also necessary to investigate how CAFs retain their ability to promote carcinoma growth in a cell-autonomous fashion, and if particular somatic genetic alteration(s) not only are responsible for the maintenance of this stable phenotype but also mediate the differentiation of cells into myofibroblasts.
Future research will focus on identifying the selective pressures and determining the cellular and molecular mechanisms which drive forward the evolution of the tumour-promoting stroma. Delineating the molecular crosstalk between stromal cells and carcinoma cells may provide invaluable insights into the pathophysiological processes involved in cancer. The development of new mouse models, multiscale mathematical models, improved in vivo imaging techniques and the identification of new molecules important in regulating stroma-tumour interactions will further our understanding of cancer in the future. Such advancements in research may lead to the development of novel therapeutic approaches to treating cancer by targeting the interactions between the stroma and carcinoma cells that mediate tumourigenesis.
Acknowledgements
This work was supported by Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research (M.S.) and Cancer Research UK (CR-UK) grant number C147/A6058 (A.O.).
References
- 1.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Bissell M.J., Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller M.M., Fusenig N.E. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 4.Bhowmick N.A., Neilson E.G., Moses H.L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polyak K., Haviv I., Campbell I.G. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Orimo A., Weinberg R.A. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 7.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 9.Guo X., Oshima H., Kitmura T., Taketo M.M., Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 10.Noma K., Smalley K.S.M., Lioni M., Naomoto Y., Tanaka N., El-Deiry W. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–1993. doi: 10.1053/j.gastro.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellermann M.G., Sobral L.M., da Silva S.D., Zecchin K.G., Graner E., Lopes M.A. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Gabbiani G., Ryan G.B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 13.Serini G., Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga J. Scleroderma and Smads: dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 2002;46:1703–1713. doi: 10.1002/art.10413. [DOI] [PubMed] [Google Scholar]
- 16.Kim K.K., Wei Y., Szekeres C., Kugler M.C., Wolters P.J., Hill M.L. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori Y., Chen S.J., Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964–1978. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 18.Hu M., Peluffo G., Chen H., Gelman R., Schnitt S., Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci USA. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieweke M.H., Thompson N.L., Sporn M.B., Bissell M.J. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990;248:1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- 20.Demehri S., Turkoz A., Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16:55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sappino A.P., Skalli O., Jackson B., Schurch W., Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988;41:707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- 22.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak H.F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 24.Ronnov-Jessen L., Petersen O.W., Bissell M.J. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Schafer M., Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 26.Maeshima A.M., Niki T., Maeshima A., Yamada T., Kondo H., Matsuno Y. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–2554. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- 27.Cardone A., Tolino A., Zarcone R., Borruto Caracciolo G., Tartaglia E. Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med. 1997;39:174–177. [PubMed] [Google Scholar]
- 28.Olumi A.F., Grossfeld G.D., Hayward S.W., Carroll P.R., Tlsty T.D., Cunha G.R. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang R.F., Moore T., Arumugam T., Ramachandran V., Amos K.D., Rivera A. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sneddon J.B., Zhen H.H., Montgomery K., van de Rijn M., Tward A.D., West R. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci USA. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Wever O., Nguyen Q.D., Van Hoorde L., Bracke M., Bruyneel E., Gespach C. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C., Fu L., Fu J., Hu L., Yang H., Rong T.H. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res. 2009;15:4017–4027. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
- 33.Yang G., Rosen D.G., Zhang Z., Bast R.C., Jr., Mills G.B., Colacino J.A. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allinen M., Beroukhim R., Cai L., Brennan C., Lahti-Domenici J., Huang H. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Mercier I., Casimiro M.C., Wang C., Rosenberg A.L., Quong J., Minkeu A. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Micke P., Kappert K., Ohshima M., Sundquist C., Scheidl S., Lindahl P. In situ identification of genes regulated specifically in fibroblasts of human basal cell carcinoma. J Invest Dermatol. 2007;127:1516–1523. doi: 10.1038/sj.jid.5700714. [DOI] [PubMed] [Google Scholar]
- 37.Dakhova O., Ozen M., Creighton C.J., Li R., Ayala G., Rowley D. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15:3979–3989. doi: 10.1158/1078-0432.CCR-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang F., Tuxhorn J.A., Ressler S.J., McAlhany S.J., Dang T.D., Rowley D.R. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- 39.Ao M., Franco O.E., Park D., Raman D., Williams K., Hayward S.W. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 40.San Francisco I.F., DeWolf W.C., Peehl D.M., Olumi A.F. Expression of transforming growth factor-beta 1 and growth in soft agar differentiate prostate carcinoma-associated fibroblasts from normal prostate fibroblasts. Int J Cancer. 2004;112:213–218. doi: 10.1002/ijc.20388. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal E., McCrory A., Talbert M., Young G., Murphy-Ullrich J., Gladson C. Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol Carcinogen. 2004;40:116–121. doi: 10.1002/mc.20024. [DOI] [PubMed] [Google Scholar]
- 42.Haviv I., Polyak K., Qiu W., Hu M., Campbell I. Origin of carcinoma associated fibroblasts. Cell Cycle. 2009;8:589–595. doi: 10.4161/cc.8.4.7669. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg R.A. Coevolution in the tumor microenvironment. Nat Genet. 2008;40:494–495. doi: 10.1038/ng0508-494. [DOI] [PubMed] [Google Scholar]
- 44.Moinfar F., Man Y.G., Arnould L., Bratthauer G.L., Ratschek M., Tavassoli F.A. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- 45.Wernert N., Locherbach C., Wellmann A., Behrens P., Hugel A. Presence of genetic alterations in microdissected stroma of human colon and breast cancers. Anticancer Res. 2001;21:2259–2264. [PubMed] [Google Scholar]
- 46.Kurose K., Gilley K., Matsumoto S., Watson P.H., Zhou X.P., Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 47.Patocs A., Zhang L., Xu Y., Weber F., Caldes T., Mutter G.L. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 48.Tuhkanen H., Anttila M., Kosma V.M., Yla-Herttuala S., Heinonen S., Kuronen A. Genetic alterations in the peritumoral stromal cells of malignant and borderline epithelial ovarian tumors as indicated by allelic imbalance on chromosome 3p. Int J Cancer. 2004;109:247–252. doi: 10.1002/ijc.11733. [DOI] [PubMed] [Google Scholar]
- 49.Paterson R.F., Ulbright T.M., MacLennan G.T., Zhang S., Pan C.X., Sweeney C.J. Molecular genetic alterations in the laser-capture-microdissected stroma adjacent to bladder carcinoma. Cancer. 2003;98:1830–1836. doi: 10.1002/cncr.11747. [DOI] [PubMed] [Google Scholar]
- 50.Weber F., Xu Y., Zhang L., Patocs A., Shen L., Platzer P. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA. 2007;297:187–195. doi: 10.1001/jama.297.2.187. [DOI] [PubMed] [Google Scholar]
- 51.Hill R., Song Y., Cardiff R.D., Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 52.Kiaris H., Chatzistamou I., Trimis G., Frangou-Plemmenou M., Pafiti-Kondi A., Kalofoutis A. Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res. 2005;65:1627–1630. doi: 10.1158/0008-5472.CAN-04-3791. [DOI] [PubMed] [Google Scholar]
- 53.Tokudome N., Sakamoto G., Sakai T., Sarumaru S., Okuyama N., Hori F. A case of carcinosarcoma of the breast. Breast Cancer. 2005;12:149–153. doi: 10.1007/BF02966829. [DOI] [PubMed] [Google Scholar]
- 54.Bolton B., Sieunarine K. Carcinosarcoma: a rare tumour of the breast. Aust N Z J Surg. 1990;60:917–919. doi: 10.1111/j.1445-2197.1990.tb07501.x. [DOI] [PubMed] [Google Scholar]
- 55.Qiu W., Hu M., Sridhar A., Opeskin K., Fox S., Shipitsin M. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40:650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter K., Omura N., Hong S.M., Griffith M., Goggins M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008;7:882–888. doi: 10.4161/cbt.7.6.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang L., Gonda T.A., Gamble M.V., Salas M., Seshan V., Tu S. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68:9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu M., Yao J., Cai L., Bachman K.E., van den Brule F., Velculescu V. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 59.Kalluri R., Weinberg R.A. The basics of epithelial–mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishii G., Sangai T., Oda T., Aoyagi Y., Hasebe T., Kanomata N. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 61.Direkze N.C., Hodivala-Dilke K., Jeffery R., Hunt T., Poulsom R., Oukrif D. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 62.Mishra P.J., Mishra P.J., Humeniuk R., Medina D.J., Alexe G., Mesirov J.P. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeisberg E.M., Potenta S., Xie L., Zeisberg M., Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 64.Radisky D.C., Kenny P.A., Bissell M.J. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ronnov-Jessen L., Petersen O.W., Koteliansky V.E., Bissell M.J. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tait L.R., Pauley R.J., Santner S.J., Heppner G.H., Heng H.H., Rak J.W. Dynamic stromal–epithelial interactions during progression of MCF10DCIS.com xenografts. Int J Cancer. 2007;120:2127–2134. doi: 10.1002/ijc.22572. [DOI] [PubMed] [Google Scholar]
- 67.Augsten M., Hagglof C., Olsson E., Stolz C., Tsagozis P., Levchenko T. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc Natl Acad Sci USA. 2009;106:3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crawford Y., Kasman I., Yu L., Zhong C., Wu X., Modrusan Z. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Pietras K., Pahler J., Bergers G., Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fidler I.J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 71.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 72.Surowiak P., Suchocki S., Gyorffy B., Gansukh T., Wojnar A., Maciejczyk A. Stromal myofibroblasts in breast cancer: relations between their occurrence, tumor grade and expression of some tumour markers. Folia Histochem Cytobiol. 2006;44:111–116. [PubMed] [Google Scholar]
- 73.Le N.H., Franken P., Fodde R. Tumour-stroma interactions in colorectal cancer: converging on beta-catenin activation and cancer stemness. Br J Cancer. 2008;98:1886–1893. doi: 10.1038/sj.bjc.6604401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinberg R.A. Garland Science; New York: 2007. The biology of cancer. [Google Scholar]
- 75.Fukumura D., Xavier R., Sugiura T., Chen Y., Park E.C., Lu N. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 76.Zhu C.Q., Popova S.N., Brown E.R., Barsyte-Lovejoy D., Navab R., Shih W. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc Natl Acad Sci USA. 2007;104:11754–11759. doi: 10.1073/pnas.0703040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tokunou M., Niki T., Eguchi K., Iba S., Tsuda H., Yamada T. c-MET expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am J Pathol. 2001;158:1451–1463. doi: 10.1016/S0002-9440(10)64096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joesting M.S., Perrin S., Elenbaas B., Fawell S.E., Rubin J.S., Franco O.E. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65:10423–10430. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- 79.Degen M., Brellier F., Kain R., Ruiz C., Terracciano L., Orend G. Tenascin-W is a novel marker for activated tumor stroma in low-grade human breast cancer and influences cell behavior. Cancer Res. 2007;67:9169–9179. doi: 10.1158/0008-5472.CAN-07-0666. [DOI] [PubMed] [Google Scholar]