Abstract
O-GlcNAcylation is an essential posttranslational modification in metazoa. Modulation of O-GlcNAc levels with small molecule inhibitors of O-GlcNAc hydrolase (OGA) is a useful strategy to probe the role of this modification in a range of cellular processes. Here we report the discovery of novel, low molecular weight and drug-like O-GlcNAcase inhibitor scaffolds by high-throughput screening. Kinetic and X-ray crystallographic analyses of the binding modes with human/bacterial O-GlcNAcases identify some of these as competitive inhibitors. Comparative kinetic experiments with the mechanistically related human lysosomal hexosaminidases reveal that three of the inhibitor scaffolds show selectivity towards human OGA. These scaffolds provide attractive starting points for the development of non-carbohydrate, drug-like OGA inhibitors.
Keywords: O-GlcNAc, Posttranslational modification, Inhibitor, Crystal structure
1. Introduction
The posttranslational modification of intracellular proteins with O-linked N-acetylglucosamine (O-GlcNAc) was discovered 25 years ago by Torres and Hart [1], breaking the dogma that protein glycosylation was restricted to the endoplasmic reticulum, Golgi apparatus, cell surface and extracellular matrix [2]. O-GlcNAc modified proteins are found mostly in the nucleoplasm, cytoplasm and to some extent in the mitochondria, and are not further extended by glycosylation. Similar to protein phosphorylation, the O-GlcNAc modification can dynamically and inducibly be transferred to, and removed from, proteins, and has been shown to be involved in signalling processes [3–5]. The O-GlcNAc modification is carried out by an enzyme called O-GlcNAc transferase (OGT) that utilises the nucleotide sugar donor UDP-GlcNAc, produced through the hexosamine biosynthetic pathway, to exclusively modify serine/threonine residues. OGT was first discovered in human, rat and the nematode Caenorhabditis elegans [6,7], subsequently described in Arabidopsis thaliana [8] and very recently in Giardia and Cryptosporidium parvum [9]. The O-GlcNAc modified protein can be transformed back into its native state by O-GlcNAc hydrolysis by O-GlcNAcase (OGA). OGA has been characterised in human, mouse, rat, Drosophila and C. elegans and was shown to be encoded by a single gene [10–14]. In all metazoa, O-GlcNAc modified proteins have been found in all functional classes [15,16,5]. Dynamic interplay between protein O-GlcNAcylation and phosphorylation has been observed on a number of proteins [17]. Specific Ser/Thr residues show reciprocal, same-site occupancy by phosphorylation or O-GlcNAcylation (e.g. c-myc and oestrogen receptor-β or tau-protein) or alternatively adjacent Ser/Thr residues can be occupied by O-GlcNAcylation and phosphorylation (e.g. p53-protein) [17].
For the past decade PUGNAc and streptozotocin (STZ), both inhibitors of the human OGA (hOGA), have been extensively used to raise cellular O-GlcNAc levels to study the function of protein O-GlcNAcylation [18,19]. Recently, it was discovered that NAG-thiazoline, a transition state analogue, can also be used in cell-based assays to potently inhibit hOGA [20]. However, all three compounds have disadvantages. PUGNAc and NAG-thiazoline were originally identified as potent inhibitors of human lysosomal hexosaminidases (HexA/B), members of the glycoside hydrolase family 20 (GH20) [21,22]. Recent kinetic and structural data obtained from hOGA and bacterial homologues (GH84 family members) have revealed that GH84 and GH20 perform hydrolysis via substrate-assisted catalysis and the active site architecture is structurally conserved [23–25]. Thus it is not surprising that hOGA and HexA/B are equally potently inhibited by NAG-thiazoline and PUGNAc. PUGNAc is a promiscuous inhibitor, targeting multiple enzymes from functionally related glycoside hydrolase families, including retaining α- and β-glucosaminidases from GH89 and GH3, respectively [26,27]. Consequently, in cell-based studies PUGNAc shows off-target effects, as shown recently by Macauley et al. [28]. The authors also used a 600-fold selective thiazoline derivative (NButGT) to inhibit hOGA in 3T3-L1 adipocytes. Whilst both compounds increased cellular O-GlcNAcylation levels, only the non-selective PUGNAc caused insulin resistance in 3T3-L1 adipocytes [28]. STZ, another weak inhibitor of hOGA, carries a reactive N-methylnitrosourea moiety that has been shown to be toxic to eukaryotic cells by DNA alkylation [29–31] and nitric oxide release [32]. STZ was shown to be particularly toxic to pancreatic β cells that secrete insulin, and it has since been used extensively to create animal models of type I diabetes [33]. However, several studies have shown that STZ toxicity does not correlate with hOGA inhibition, suggesting that STZ-dependent β-cell toxicity occurs independent of hOGA inhibition [34–36,31].
hOGA has so far resisted production in pure recombinant form with yields required for protein crystallography. Two bacterial apparent OGA orthologues from Clostridium perfringens and Bacteroides thetaiotaomicron have been studied by protein crystallography to obtain insight in the active site and the catalytic mechanism [24,37,25]. Recently, a series of NAG-thiazoline derivatives, as well as GlcNAcstatins, a glucoimidazole-based class of inhibitors, were described to inhibit hOGA potently and selectively [28,38–40]. Crystal structures of the two bacterial OGAs in complex with these compounds have been used to identify and iteratively improve protein–ligand interactions. However, all currently known OGA inhibitors have in common a carbohydrate scaffold, that is inherently non-drug-like when the Lipinski rules are considered [41] and not synthetically easily accessible for further optimization by medicinal chemistry. Thus, identification of novel, more drug-like, and synthetically accessible inhibitors of the GH84 enzymes could facilitate further efforts towards the identification of potent, cell permeable and metabolically stable OGA inhibitors. Ideally, such compounds would be selective for GH84 enzymes versus GH20 enzymes or could easily be modified to improve selectivity towards hOGA. A possible approach to identify molecules with these properties is by high-throughput screening. Here we report the result of a screen, together with kinetic and structural studies of the hits, resulting in the discovery of novel, drug-like scaffolds that competitively inhibit hOGA.
2. Results and discussion
2.1. Identification of novel OGA inhibitors from a high-throughput screen
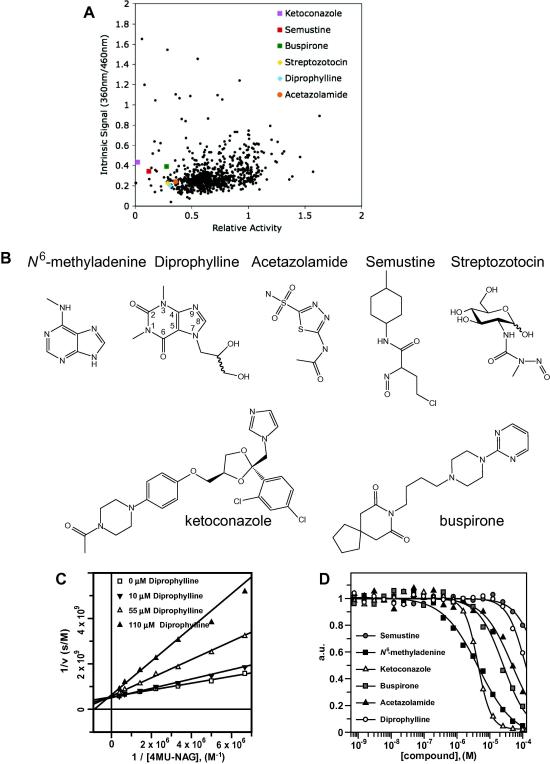
In order to identify new human O-GlcNAcase inhibitors, a high-throughput screening assay was performed based on the release of 4-methylumbelliferol (4MU) from the pseudo substrate 4MU-GlcNAc, initially using the bacterial OGA homologue from C. perfringens (CpOGA) which has recently been shown to be a good model of hOGA [24]. The CpOGA protein was screened against the library from Prestwick Chemicals Inc. that contains 880 off-patent small molecules. 85% of these compounds are currently marketed drugs. These compounds were dissolved in 100% dimethyl sulfoxide (DMSO) to generate library stocks with a final a concentration of 2 mg/ml. With an average molecular weight of 377 Da, screening assays were performed at an average concentration of 30.2 μM, with a final DMSO concentration of 1%. PUGNAc was used at a final concentration of 100 μM as a positive control. Probable false positives were identified by plotting the relative enzymatic activity against the intrinsic absorbance of the compound at the fluorescence excitation wavelength. After subtracting the intrinsic fluorescent signal from the compounds, the relative activity of CpOGA in presence of the compounds in the screen was plotted against the real fluorescent signal from the reaction product (Fig. 1A). Surprisingly, a large number of compounds clustered around a relative activity of 0.6 (Fig. 1A) and not, as expected, around 1.0. This may be caused by the fact that those compounds are real inhibitors with much smaller molecular weights than the average 377 Da and were thus screened at effective concentrations higher than 30.2 μM. Only compounds that showed inhibition >60% at the assumed concentration of 30.2 μM (equal to a relative activity smaller than 0.4) were considered for further analysis and prioritised by the presence of chemical features likely to be compatible with the GH84 active site.
Fig. 1.
(A) Prestwick library screen against CpOGA. The scatter-plot shows on the horizontal axis the remaining activity of CpOGA in presence of the compounds (measured by the liberation of 4MU) and on the vertical axis the intrinsic absorbance/fluorescence of the compounds at the excitation and emission wavelength (360 nm/460 nm). Points in the lower left corner indicate compounds that do not absorb at the excitation wavelength, yet do exhibit reduced fluorescence and thus reduce CpOGA activity. Six compounds are labelled in coloured dots, according to the legend in the graph. (B) Chemical structures of compounds selected with the aid of the Prestwick screening data: (a) N6-methyladenine, (b) diprophylline, (c) acetazolamide, (d) semustine, (e) streptozotocin (STZ), (f) ketoconazole and (g) buspirone. (C) Diprophylline is a competitive inhibitor of CpOGA. Steady-state kinetic data (triplicates) using 0.2 nM CpOGA, 0–25 μM substrate (4MU-GlcNAc), 0–110 μ inhibitor and 7 min reaction time were fitted using the standard equation for competitive inhibition in the GraFit program [52]. (D) Dose–response curves of the selected compounds against hOGA. Data obtained with the 4MU-GlcNAc assay (2 nM hOGA, 80 μM 4MU-GlcNAc, 0.7 nM–100 μM inhibitor, 60 min reaction time) from buspirone, diprophylline and acetazolamide, semustine, N6-methyladenine and ketoconazole were fitted using the standard IC50 equation in the GraFit program [52].
2.2. Selected screening hits competitively inhibit CpOGA in the micromolar range
A number of compounds that inhibit CpOGA at the assumed screening concentration of 30.2 μM to more than 60% were selected from the Prestwick screen hits (Fig. 1A). Streptozotocin (STZ), a known hOGA and CpOGA inhibitor [31], was identified from the screen. The most potent compound was ketoconazole, followed by semustine (Fig. 1A). Buspirone, STZ and diprophylline show similar inhibition under the screening conditions, followed by acetazolamide (Fig. 1A). All these compounds were commercially available. 6-Methylaminopurine, a derivative of the weak (30% inhibition) hit 6-benzylaminopurine, was also purchased, given the recently published purine derivatives that inhibit chitinases, enzymes that are mechanistically related to OGA [42,43,24]. The molecular weights of the resulting selected compounds (Fig. 1B) were used to calculate the true screening concentration (Table 1). The compounds were then evaluated for CpOGA inhibition using a dose–response experiment (Table 1). Ketoconazole appears to be the most potent CpOGA inhibitor with an IC50 of 2 μM. N6-methyladenine inhibits with an IC50 of 21 μM, whilst semustine, buspirone and diprophylline inhibit with an IC50 of 39 μM, 50 μM and 60 μM, respectively. The dose–response curve of acetazolamide yields an IC50 of 80 μM. The ranking of the levels of inhibition of these compounds is the same from the screening and IC50 data.
Table 1.
Screening data, inhibition constants and binding efficiency obtained for the selected compounds.
| Compound | MW (Da) | Screening (μM) | CpOGA IC50 (μM) | CpOGA Ki (μM) | hOGA IC50 (μM) | HexA/B IC50 (μM) | Selectivity (HexA/B/hOGA) | hOGA BEIa,b |
|---|---|---|---|---|---|---|---|---|
| Acetazolamide | 222 | 90 | 80 ± 14 | n.d. | 47.0 ± 0.1 | 32 ± 2 | 0.7 | 21 |
| Buspirone | 386 | 52 | 50 ± 15 | n.d. | 25.0 ± 0.1 | 47 ± 3 | 1.9 | 13 |
| Diprophylline | 254 | 79 | 60 ± 10 | 25 ± 2 | 121 ± 10 | 200 ± 30 | 1.6 | 16 |
| Ketoconazole | 531 | 38 | 2.0 ± 0.4 | 15 ± 5 | 4.3 ± 0.4 | 11 ± 1 | 2.6 | 11 |
| Semustine | 248 | 81 | 39 ± 6 | 23 ± 7 | 262 ± 6 | 2500 | 9 | 16 |
| N6-methyladenine | 149 | – | 21 ± 4 | 14 ± 1 | 4.0 ± 0.3 | 300 | 75 | 34 |
| Streptozotocin | 265 | 87 | 30c | n.d. | 1500d | 45 000 | 30 | 11 |
| NAG-thiazoline | 219 | – | – | n.d. | 0.07d | 0.07d | 1.0 | 32 |
| PUGNAc | 353 | – | – | 0.005e | 0.05d | 0.05d | 1.0 | 21 |
BEI Binding Efficiency Index (BEI) [46] calculated for hOGA inhibition, BEI = −log(Ki)/M,with M being the mass of the compound in kDa.
The Cheng–Prusoff equation (Ki = IC50/1 + ([S]/Km)) was used to convert the IC50 values to anabsolute inhibition constant (Ki).
Data taken from [31].
Ki value from [20].
Ki value from [24].
Further kinetic experiments addressed the inhibition mode of the four most potent compounds (diprophylline, ketoconazole, semustine and N6-methyladenine) against CpOGA. Steady state kinetics were measured to determine the inhibition mode and the corresponding inhibition constant, Ki. Concentrations of the inhibitors were chosen according to the previously determined IC50 values (Table 1). For all four compounds, Lineweaver–Burk analysis indicated that the pseudo-substrate 4MU-GlcNAc and the compounds compete for binding to the same CpOGA active site (Fig. 1C, Table 1). The absolute inhibition constants against CpOGA measured for diprophylline (Ki = 25 μM), semustine (Ki = 23 μM) and N6-methyladenine (Ki = 14 μM) are in good agreement with the IC50 data (Table 1). Interestingly, N6-methyladenine is the most potent CpOGA inhibitor identified, despite having the lowest molecular weight (Table 1).
2.3. N6-methyladenine is an efficient, selective, micromolar hOGA inhibitor
The objective of this study was to identify new chemical scaffolds that are inhibitors of hOGA. Thus, inhibition studies of the Prestwick screen-derived CpOGA inhibitors (Fig. 1B) were carried out using recombinant hOGA protein (Fig. 1D). Both ketoconazole and N6-methyladenine inhibit hOGA with IC50 values of 4 μM (Table 1). Buspirone and acetazolamide inhibit hOGA with IC50 values of 25 and 47 μM, whilst diprophylline and semustine are weak hOGA inhibitors with inhibition constants of 120 and 260 μM.
Compared to the inhibition constants obtained against CpOGA (Table 1), only the inhibition constant for semustine is significantly higher for hOGA than for CpOGA. The IC50 values determined for acetazolamide, buspirone and ketoconazole against hOGA protein are in agreement with the inhibition constants determined for CpOGA (Table 1). However, N6-methyladenine appears to more potently inhibit the human enzyme (IC50 = 4 μM for hOGA, vs. 21 μ for CpOGA, Table 1). Since the active site architecture of CpOGA and hOGA are closely related [24], it is assumed that the inhibitors bind to hOGA competitively, as determined for CpOGA. Therefore, four compounds that competitively inhibit hOGA with an IC50 < 50 μM have been identified from screening the Prestwick Chemical library with the bacterial homologue CpOGA.
Ligand efficiency is a powerful tool to evaluate the usefulness of a scaffold as a starting point for further medicinal chemistry [44–46]. The binding efficiency index (BEI) [46] was calculated for all drug-like O-GlcNAcase scaffolds and compared to the BEI of well known potent hOGA inhibitors NAG-thiazoline and PUGNAc (Table 1). Interestingly, while the small compound N6-methyladenine is a weaker inhibitor of hOGA than PUGNAc and NAG-thiazoline in absolute terms, in terms of ligand efficiency it, with a BEI of 34 (NAG-thiazoline and PUGNAc show a BEI of 32 and 21, respectively), is a more efficient binder.
To investigate whether the compounds identified from the Prestwick library screen display selectivity for hOGA over the closely related lysosomal hexosaminidases A/B (HexA/B), the enzymatic activity of HexA/B in the presence of each the compounds was analysed (Table 1). Ketoconazole, acetazolamide and buspirone were determined to have inhibition constants between IC50 = 10 μM and 50 μM (Table 1). Comparison of these inhibition constants with those determined for the hOGA enzyme shows no selectivity for the human O-GlcNAcase (Table 1). Similarly, dipro-phylline, with an inhibition constant of IC50 = 200 μM for HexA/B, is not selective for hOGA. Interestingly, a 75-fold selectivity is observed for N6-methyladenine (IC50 = 300 μM for HexA/B vs. 4 μM for hOGA, Table 1).
2.4. Diprophylline mimics substrate binding in the OGA active site
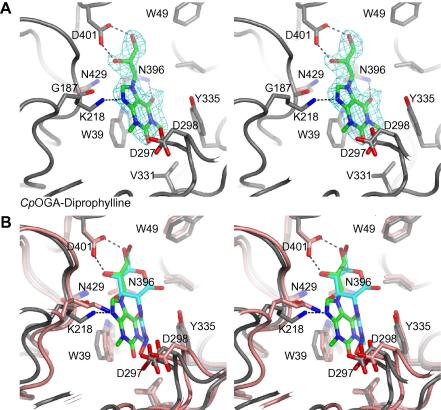
To determine the binding mode of the competitive OGA inhibitors, these compounds were soaked into CpOGA crystals. Ordered electron density could only be obtained for diprophylline. Diprophylline is a xanthine derivative that consists of two moieties. The xanthine moiety is methylated on the N1 and N3 ring atoms (Fig. 1B). Furthermore, the N7 atom is modified with a (2,3-dihydroxypropyl) moiety that possesses a stereo centre at the carbon of the secondary alcohol (Fig. 1B). The electron density for the inhibitor was located in the active site of CpOGA, where it competes with substrate binding, in agreement with the competitive inhibition mode determined from kinetic studies. Diprophylline interacts with three active site residues via hydrogen bonds (Fig. 2A). The 2-OH and 3-OH hydroxyl groups from the 2,3-dihydroxypropyl moiety hydrogen bond to the carboxyl group of residue Asp401, which is conserved between bacterial (CpOGA) and hOGA (Fig. 2A). This interaction mimics the interaction of GlcNAc 4/6-OH with Asp401 (Fig. 2A and B). The xanthine moiety is sandwiched between the two aromatic residues Trp394 and Tyr335, where it participates in π-stacking interactions (Fig. 2A). Furthermore, Lys218 and Asn396 hydrogen bond to the N9 and O6 atoms from the theophylline moiety, respectively (Fig. 2A). The electron density unambiguously represented the S-enantiomer of diprophylline (Fig. 2A).
Fig. 2.
(A) Stereo figure of diprophylline in the active site of CpOGA. The CpOGA active site is shown with residues contributing to diprophylline binding and/or surrounding the compound, as sticks with grey carbon, red oxygen and blue nitrogen atoms. Diprophylline is depicted in sticks with green carbon, red oxygen, blue nitrogen atoms. Hydrogen bonds between the ligand and the protein are indicated by black dashed lines. Unbiased ∣Fo∣ − ∣FC∣, ϕcalc electron density (2.75 σ) for diprophylline is shown in cyan. (B) Stereo view of the superimposed crystallographically determined complexes of CpOGA with diprophylline (colour scheme as in panel (A) and STZ (PDB entry 2VUR, depicted in sticks with cyan carbon, red oxygen and blue nitrogen atoms). Hydrogen bonds between the protein and diprophylline are indicated by black dashed lines.
The overall active site of the diprophylline-CpOGA complex is in the same arrangement observed for the unliganded [24] and the STZ-CpOGA complex [31] (Fig. 2B). Interestingly, the loop carrying the catalytic acid Asp298 and Asp297 is in the ‘open’ conformation. The peptide bond of Asp297–Asp298 is in the same conformation as described for the apo structure [24] and the STZ-liganded structure [31], thus the backbone carbonyl from Asp297 points into the active site and the side chain of Asp298 points out of the active site. Comparison of the STZ-CpOGA complex with the diprophylline-CpOGA complex reveals only minor differences in terms of the protein conformation. The loop carrying Asp297 and Asp298 has moved closer into the active site (maximum atomic shift = 1.2 Å), but neither Asp297 nor Asp298 interact with the compound. Superposition reveals that the O6 carbonyl group of diprophylline occupies the same position as the carbonyl oxygen of STZ (positional shift = 0.35 Å), and accepts a similar hydrogen bond from Nδ2 of (the conserved) Asn396 at the back of the active site (Fig. 2A and B).
3. Concluding remarks
Screening a bacterial homologue of hOGA against a small commercial library from Prestwick Chemicals Inc. has resulted in the discovery of the first inhibitors of human O-GlcNAc that do not exhibit a sugar-like scaffold (Fig. 1B). Four of the these molecules competitively inhibit the GH84 enzymes. Interestingly, the small molecule N6-methyladenine, is a potent hOGA inhibitor (IC50 = 4 μM and shows 75-fold selectivity for the hOGA enzyme over the lysosomal hexosaminidases. This compound could be a lead for chemical modification to increase both the selectivity and potency of inhibition. Since no structural data was obtained, interactions of N6-methyladenine with the GH84 active site remain unknown. Interestingly, recent studies have described the binding of xanthine-like compounds to the active site of Aspergillus fumigatus chitinase 1 B (AfChiB) [47] and a virtual screening-based approach that resulted in the synthesis of a derivative with micromolar inhibition [43]. A similar strategy could be applied to N6-methyladenine, which binds with a BEI of 34 to the hOGA active site.
Diprophylline, another xanthine-based molecule, was identified as a micromolar inhibitor for hOGA and the binding mode was structurally determined. Only the S-isoform of diprophylline binds to the GH84 active site and interacts with several residues conserved between hOGA and CpOGA (Fig. 2A and B). Diprophylline is an interesting lead that could be further exploited by structure-based design to generate more potent derivatives that may inhibit hOGA in vivo.
In summary, this study shows that it is possible to identify hOGA inhibitors with scaffolds different from a sugar core, with promising properties in terms of synthetic accessibility, potency and selectivity. This will stimulate future work, both in terms of a medicinal chemistry exploration of these scaffolds, and the identification of more potent inhibitors by screening campaigns on larger libraries.
4. Materials and methods
4.1. Cloning, expression and purification
CpOGA and hOGA protein were expressed and purified following the protocol described previously [24,39,31,40].
4.2. Determination of the CpOGA-diprophylline complex structure
CpOGA crystals were produced as described previously [24]. Precipitant was carefully removed and solid diprophylline was added straight to the drop. After 30 min the crystal was removed and cryo-protected in mother liquor containing 15% glycerol. Diffraction data were collected to 2.25 Å at the ESRF, Grenoble on ID14-3, and processed with the HKL suite [48], resulting in a data set with 99.9% completeness (100% in the highest resolution shell) with an overall Rmerge of 0.071 (0.535 in the highest resolution shell). Refinement was initiated using a native CpOGA structure (PDB-code 2CBI), immediately revealing well defined ∣Fo∣ − ∣Fc∣, ϕcalc electron density for the inhibitor, which was built with the help of a structure and topology generated by PRODRG [49]. Further model building with COOT [50]) and refinement with REFMAC [51] then yielded the final model with good statistics (R, Rfree: 19.8, 24.7).
4.3. Inhibitor library screening
Purified CpOGA protein was screened against a commercial library (Prestwick Chemicals Inc. France) containing 880 off-patent small molecules (85% of which are marketed drugs). The compounds were stored in 100% dimethyl sulfoxide (DMSO) at a concentration of 2 mg/ml – CpOGA hydrolyses 4MU-GlcNAc without significant loss of activity at up to 4% DMSO. 0.5 μl aliquots of the compounds from the library were pipetted into 96 well-plates. 44.5 μl of the standard reaction mixture containing CpOGA protein at a final concentration of 0.2 nM (in 50 μl final reaction volume) was added to the compounds. 5 μl of the fluorescent substrate 4MU-NAG was added in a 10-fold concentration (32 μM) to initiate the reaction after a 5 min incubation time of the CpOGA enzyme with the compound. The reaction was stopped after 7 min at RT (20 °C) using standard procedure and the fluorescent signal was measured using the standard procedure described previously [24,31,39,40]. Hits were selected using several criteria: the compounds had to inhibit CpOGA greater than 60% at the concentration screened and to posses a chemical scaffold with chemical features compatible with binding to the active site of GH84 enzymes.
4.4. Inhibition measurements of CpOGA, hOGA and human HexA/B
Further kinetic experiments to determine the mode of inhibition were carried out according to the procedure described previously [40]. Ketoconazole, acetazolamide, buspirone, diprophylline, N6-methyladenine, streptozotocin and semustine were purchased from Sigma. IC50 measurements with CpOGA, hOGA and a mixture of human hexosaminidase A/B activities (Sigma A6152) against the compounds were performed using the fluorogenic 4MU-NAG substrate and standard reaction mixtures as described previously with some changes [39,31,40]. Standard reaction mixtures (50 μl) contained 0.2 nM CpOGA, 2 nM hOGA or 50 μ units unit/ml HexA/B in McIlvaine buffer (0.2 M Na2HPO4 mixed with 0.1 M citric acid to pH 5.7) supplemented with 0.1 mg/ml BSA. IC50 determinations were carried out using substrate concentrations corresponding to the Km established for CpOGA (2.9 μM), hOGA (80 μM) and HexA/B (230 μM). The reactions were run at room temperature for 7 min (CpOGA), 60 min (hOGA) or 15 min (HexA/B). Determination of the Ki for CpOGA was performed by steady-state kinetics using the parameters described for CpOGA IC50 determinations with a series of inhibitor concentrations (0–110 μM. The reactions were stopped by the addition of 100 μl 3 M glycine–NaOH, pH 10.3. The fluorescence of the released 4MU was quantified as described previously [39,31,40]. Ki experiments were performed in triplicate and IC50 determinations with single data points for a series of 18 concentrations between 0.7 nM and 100 μM.
Acknowledgments
We thank the European Synchrotron Radiation Facility, Grenoble, for the time at beamline ID14-3. We would like to thank Adel Ibrahim (University of Dundee, Cloning Service, DSTT) for cloning of the human OGA construct and Sharon Shepherd and Mark Dorward for protein expression and purification. We would like to thank Willem van Heugten for help with crystallographic refinement. This work was supported by a Wellcome Trust Senior Fellowship and a Lister Institute for Preventive Medicine Research Prize. H.C.D. is supported by the College of Life Sciences Alumni Studentship. The coordinates and structure factors have been deposited with the PDB (PDB entry 2x0y).
References
- 1.Torres C.R., Hart G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes – evidence for O-linked GlcNAc. J. Biol. Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 2.Hart G.W., Haltiwanger R.S., Holt G.D., Kelly W.G. Nucleoplasmic and cytoplasmic glycoproteins. Ciba Found. Symp. 1989;145:102–112. doi: 10.1002/9780470513828.ch7. di. [DOI] [PubMed] [Google Scholar]
- 3.Roquemore E.P., Chevrier M.R., Cotter R.J., Hart G.W. Dynamic O-GlcNAcylation of the small heat shock protein α b-crystallin. Biochemistry. 1996;35(11):3578–3586. doi: 10.1021/bi951918j. [DOI] [PubMed] [Google Scholar]
- 4.Chou C.F., Smith A.J., Omary M.B. Characterization and dynamics of o-linked glycosylation of human cytokeratin 8 and 18. J. Biol. Chem. 1992;267(6):3901–3906. [PubMed] [Google Scholar]
- 5.Zachara N.E., Hart G.W., signaling Cell. the essential role of o-GlcNAc! Biochim. Biophys. Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Kreppel L., Blomberg M., Hart G. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetra-tricopeptide repeats. J. Biol. Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 7.Lubas W., Frank D., Krause M., Hanover J. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 1997;272:9316–9324. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen S.E., Binkowski K.A., Olszewski N.E. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1996;93(17):9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee S., Robbins P.W., Samuelson J. Molecular characterization of nucleocytoso-lic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum. Glycobiology. 2009;19(4):331–336. doi: 10.1093/glycob/cwn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y., Wells L., Comer F.I., Parker G.J., Hart G.W. Dynamic O-glycosylation of nuclear and cytosolic proteins – cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 11.Comtesse N., Maldener E., Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a β-N-acetylglucosaminidase. Biochem. Biophys. Res. Commun. 2001;283(3):634–640. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 12.Dong D.L.Y., Hart G.W. Purification and characterization of an O-GlcNAc selective N-acetyl-β-d-glucosaminidase from rat spleen cytosol. J. Biol. Chem. 1994;269:19321–19330. [PubMed] [Google Scholar]
- 13.Kelly W.G., Hart G.W. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 1989;57(2):243–251. doi: 10.1016/0092-8674(89)90962-8. [DOI] [PubMed] [Google Scholar]
- 14.Forsythe M.E., Love D.C., Lazarus B.D., Kim E.J., Prinz W.A., Ashwell G., Krause M.W., Hanover J.A. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (o-GlcNAcase) knockout impacts o-GlcNAc cycling, metabolism, and dauer. Proc. Natl. Acad. Sci. USA. 2006;103:11952–11957. doi: 10.1073/pnas.0601931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells L., Vosseller K., Hart G.W. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291(5512):2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 16.Love D.C., Hanover J.A. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci. STKE. 2005;312:1–14. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 17.Hart G.W., Housley M.P., Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 18.Haltiwanger R.S., Grove K., Philipsberg G.A. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-β-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate. J. Biol. Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 19.Liu K., Paterson A.J., Chin E., Kudlow J.E. Glucose stimulates protein modification by o-linked GlcNAc in pancreatic β cells: linkage of o-linked GlcNAc to β cell death. Proc. Natl. Acad. Sci. USA. 2000;97:2820–2825. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macauley M.S., Whitworth G.E., Debowski A.W., Chin D., Vocadlo D.J. O-GlcNAcase uses substrate-assisted catalysis – kinetic analysis and development of highly selective mechanism-inspired inhibitors. J. Biol. Chem. 2005;280:25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 21.Horsch M., Hoesch L., Vasella A., Rast D.M. N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime – novel and potent inhibitors of β-N-acetylglucosaminidase. Eur. J. Biochem. 1991;197:815–818. doi: 10.1111/j.1432-1033.1991.tb15976.x. [DOI] [PubMed] [Google Scholar]
- 22.Knapp S., Vocadlo D., Gao Z., Kirk B., Lou J., Withers S.G. NAG-thiazoline, an N-acetyl-β-hexosaminidase inhibitor that implicates acetamido participation. J. Am. Chem. Soc. 1996;118:6804–6805. [Google Scholar]
- 23.Cetinbas N., Macauley M.S., Stubbs K.A., Drapala R., Vocadlo D.J. Identification of asp174 and asp175 as the key catalytic residues of human o-glcnacase by functional analysis of site-directed mutants. Biochemistry. 2006;45(11):3835–3844. doi: 10.1021/bi052370b. [DOI] [PubMed] [Google Scholar]
- 24.Rao F.V., Dorfmueller H.C., Villa F., Allwood M., Eggleston I.M., van Aalten D.M.F. Structural insights into the mechanism and inhibition of eukaryotic O-GlcNAc hydrolysis. EMBO J. 2006;25:1569–1578. doi: 10.1038/sj.emboj.7601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis R.J., Taylor E.J., Macauley M.S., Stubbs K.A., Turkenburg J.P., Hart S.J., Black G.N., Vocadlo D.J., Davies G.J. Structure and mechanism of a bacterial β-glucosaminidase having O-GlcNAcase activity. Nat. Struct. Mol. Biol. 2006;13:365–371. doi: 10.1038/nsmb1079. [DOI] [PubMed] [Google Scholar]
- 26.Stubbs K.A., Balcewich M., Mark B.L., Vocadlo D.J. Small molecule inhibitors of a glycoside hydrolase attenuate inducible AmpC mediated β-lactam resistance. J. Biol. Chem. 2007 doi: 10.1074/jbc.M700084200. [DOI] [PubMed] [Google Scholar]
- 27.Ficko-Blean E., Stubbs K.A., Nemirovsky O., Vocadlo D.J., Boraston A.B. Structural and mechanistic insight into the basis of mucopolysaccharidosis IIIB. Proc. Natl. Acad. Sci. USA. 2008;105(18):6560–6565. doi: 10.1073/pnas.0711491105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macauley M.S., Bubb A.K., Martinez-Fleites C., Davies G.J., Vocadlo D.J. Elevation of global O-GlcNAc levels in 3T3-L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J. Biol. Chem. 2008;283(50):34687–34695. doi: 10.1074/jbc.M804525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junod A., Lambert A.E., Orci L., Pictet R., Gonet A.E., Renold A.E. Studies of the diabetogenic action of streptozotocin. Proc. Soc. Exp. Biol. Med. 1967;126(1):201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- 30.Bennett R.A., Pegg A.E. Alkylation of DNA in rat tissues following administration of streptozotocin. Cancer Res. 1981;41(7):2786–2790. [PubMed] [Google Scholar]
- 31.Pathak S., Dorfmueller H.C., Borodkin V.S., Van Aalten D.M.F. Chemical dissection of the link between streptozotocin, O-GlcNAc, and pancreatic cell death. Chem. Biol. 2008;15:799–807. doi: 10.1016/j.chembiol.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroncke K.D., Fehsel K., Sommer A., Rodriguez M.L., Kolb-Bachofen V. Nitric oxide generation during cellular metabolization of the diabetogenic N-methyl-N-nitroso-urea streptozotozin contributes to islet cell DNA damage. Biol. Chem. Hoppe-Seyler. 1995;376(3):179–185. doi: 10.1515/bchm3.1995.376.3.179. [DOI] [PubMed] [Google Scholar]
- 33.Mansford K.R., Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet. 1968;1:670–671. doi: 10.1016/s0140-6736(68)92103-x. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y., Parker G.J., Hart G.W. Streptozotocin-induced β-cell death is independent of its inhibition of O-GlcNAccase in pancreatic min6 cells. Arch. Biochem. Biophys. 2000;383:296–302. doi: 10.1006/abbi.2000.2094. [DOI] [PubMed] [Google Scholar]
- 35.Kaneto H., Xu G., Song K.H., Suzuma K., Bonner-Weir S., Sharma A., Weir G.C. Activation of the hexosamine pathway leads to deterioration of pancreatic β-cell function through the induction of oxidative stress. J. Biol. Chem. 2001;276:31099–31104. doi: 10.1074/jbc.M104115200. [DOI] [PubMed] [Google Scholar]
- 36.Okuyama R., Yachi M. Cytosolic O-GlcNAc accumulation is not involved in β-cell death in HIT-T15 or Min6. Biochem. Biophys. Res. Commun. 2001;287:366–371. doi: 10.1006/bbrc.2001.5607. [DOI] [PubMed] [Google Scholar]
- 37.Ficko-Blean E., Gregg K.J., Adams J.J., Hehemann J.-H., Czjzek M., Smith S.P., Boraston A.B. Portrait of an enzyme, a complete structural analysis of a multimodular beta-N-acetylglucosaminidase from Clostridium perfringens. J. Biol. Chem. 2009;284(15):9876–9884. doi: 10.1074/jbc.M808954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuzwa S., Macauley M., Heinonen J., Shan X., Dennis R., He Y., Whitworth G., Stubbs K., McEachern E., Davies G., Vocadlo D. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 39.Dorfmueller H.C., Borodkin V.S., Schimpl M., Shepherd S.M., Shpiro N.A., van Aalten D.M.F. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-GlcNAcylation levels. J. Am. Chem. Soc. 2006;128:16484–16485. doi: 10.1021/ja066743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorfmueller H.C., Borodkin V.S., Schimpl M., van Aalten D.M.F. GlcNAcstatins are nanomolar inhibitors of human O-GlcNAcase inducing cellular hyper-O-GlcNAcylation. Biochem. J. 2009;420(2):221–227. doi: 10.1042/BJ20090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipinski C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 42.Rao F.V., Andersen O.A., Vora K.A., DeMartino J.A., van Aalten D.M.F. Methylxanthine drugs are chitinase inhibitors: investigation of inhibition and binding modes. Chem. Biol. 2005;12:973–980. doi: 10.1016/j.chembiol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Schuettelkopf A.W., Andersen O.A., Rao F.V., Allwood M., Lloyd C., Eggleston I.M., van Aalten D.M.F. J. Biol. Chem. 2006;281:27278–27285. doi: 10.1074/jbc.M604048200. [DOI] [PubMed] [Google Scholar]
- 44.Kuntz I.D., Chen K., Sharp K.A., Kollman P.A. The maximal affinity of ligands. Proc. Nat. Acad. Sci. USA. 1999;96:9997–10002. doi: 10.1073/pnas.96.18.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopkins A.L., Groom C.R., Alex A. Ligand efficiency: a useful metric for lead selection. Drug Discov. Today. 2004;9:430–431. doi: 10.1016/S1359-6446(04)03069-7. [DOI] [PubMed] [Google Scholar]
- 46.Abad-Zapatero C., Metz J.T. Ligand efficiency indices as guideposts for drug discovery. Drug Discov. Today. 2005;10(7):464–469. doi: 10.1016/S1359-6446(05)03386-6. [DOI] [PubMed] [Google Scholar]
- 47.Rao F.V., Houston D.R., Boot R.G., Aerts J.M.F.G., Hodkinson M., Adams D.J., Sh-iomi K., Omura S., van Aalten D.M.F. Specificity and affinity of natural product cyclopentapeptide inhibitors against A. fumigatus, human, and bacterial chitinases. Chem. Biol. 2005;12:65–76. doi: 10.1016/j.chembiol.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Otwinowski Z., Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 49.Schuettelkopf A.W., van Aalten D.M.F. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Cryst. 2004;D60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 50.Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Cryst. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 51.Murshudov G.N., Vagin A.A., Dodson E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Cryst. 1997;D53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 52.Leatherbarrow R.J. Erithacus Software Ltd.; Horley, U.K.: 2001. GraFit. Version 5. [Google Scholar]