Abstract
Background. In vivo comparison of cardiac radiofrequency ablation lesions between standard and magnetically steered 4 mm tip catheters has never been reported.
Methods. High and low right atrium (RA) free wall, isthmus, right ventricle (RV) free wall and outflow tract lesions were studied macroscopically and microscopically five days after lesion formation in seven pigs. Shape, size, thrombus formation, and ablation parameters were compared. The effect of minimal, medium and high wall contact was assessed by a contact measurement utility for magnetic catheters.
Results. All 14 RA free wall lesions were transmural with a similar epicardial and endocardial surface area. In the RV, the epicardial area usually appeared to be smaller than the endocardial area with standard catheters. Isthmus lesions were difficult to assess transmurality. There was no difference in endocardial area: standard 39 mm2 (range 16 to 82 mm2) vs. magnetic 36 mm2 (range 23 to 111 mm2). If the catheter tip was perpendicular to the tissue, magnetic lesions were more often round or oval, while standard lesions were more often elongated (p<0.05). When the catheter tip was parallel to tissue, lesions always tended to be elongated. Microscopic characteristics were similar. The contact utility was not useful. Average impedance (p<0.0001) and energy delivered (p<0.05) were less with magnetic catheters.
Conclusion. Lesions from magnetically steered catheters are transmural of similar size, but with less variability than standard catheter lesions when the tip is perpendicular to the tissue. Magnetic lesions are associated with lower impedance and energy delivery. This suggests a more stable tip-to-tissue contact. (Neth Heart J 2010;18:66–71.)
Keywords: Catheter Ablation/instrumentation, Equipment Design, Myocardium/pathology, Magnetics
Radiofrequency (RF) ablation lesions are created by resistive heating as RF current passes through tissue. Lesion size depends on tissue temperature which relates to controllable and noncontrollable factors.1 Controllable factors include catheter tip size (tip surface area), tip orientation, power settings, application time, and temperature cut-off. Non-controllable factors include tip cooling by blood flow and tissue contact. These factors have been studied extensively.1-6
A magnetic navigation system (Niobe, Stereotaxis Inc., St. Louis, MO, USA) allows the use of floppy magnetic tip catheters, steered and advanced remotely, using an applied magnetic field and an external advancer system. These catheters may have advantages in positioning in difficult anatomy, and also in maintaining position during ablation. Standard stiff catheters pose difficulties in maintaining tip contact when the heart is moving during the cardiac and respiratory cycle. Movement during ablation may cause elongated ‘brush’ lesions. Catheter flexibility during the cardiorespiratory cycle may allow more stable contact.
A difference between in vitro and in vivo models may be the effect of cardiac and respiratory motion on catheter tissue contact. The nature of the magnetic catheter's floppy shaft and the unique way Stereotaxis directs the catheter against the tissue meant we needed to compare the efficacy of standard versus magnetic catheters using the same type of ablation tip and energy settings in an in vivo model, as there is no other model mimicking cardiorespiratory movement. The magnetic system is integrated with an electroanatomical mapping system (CARTO RMT, Biosense Webster, Diamond Bar, CA, USA). The integrated system has a contact indicator which attempts to indicate the contact which the catheter makes with the tissue surface.
The purpose of this study was to prospectively compare the size of lesions produced by standard and magnetically steered 4 mm tip catheters. We also assessed the impact of the CARTO RMT tissue contact utility when forming lesions.
Methods
Introduction
Eight Yorkshire pigs (mean weight 40 kg) underwent ablation with a predetermined protocol (figure 1), with sacrifice five days postprocedure. This protocol was approved by the Animal Ethics Committee at the Erasmus University, and conformed to the guidelines in the ‘Guide for the Care and Use of Laboratory Animals.’ (NIH publication No. 85-23, revised 1996).
Figure 1.
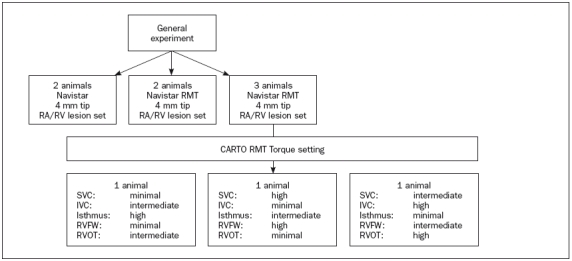
Flow chart of the study. The first four animals were a comparison between a standard (Navistar) catheter and a magnetically steered catheter of the same size, but with magnetic properties (Navistar RMT). The last three animals were submitted to various torque contact settings. IVC=inferior vena cava, RA=right atrium, RMT=remote, RV=right ventricle, RVFW=right ventricular free wall, RVOT=right ventricular outflow tract, SVC=superior vena cava.
Initial procedure
All animals received preprocedural sedation with ketamine and midazolam. Maintenance anaesthesia was instituted with midazolam and fentanyl supplemented by isoflurane and an oxygen/nitrous oxide inhalation mixture. An endotracheal tube was introduced and the animals were ventilated with a tidal volume of 13 ml/kg. Body temperature was maintained at 37 to 38 °C.
An introducer sheath was inserted into the right femoral vein by direct cut-down and the ablation catheter under study was advanced to the heart. All animals received a single dose of 150 IU/kg of heparin sulphate at the beginning of the procedure, followed after one hour by an additional 500 IU/h.
Magnetic navigation
Magnetic navigation was undertaken as previously described using the Niobe system.7 The Niobe I magnetic navigation system (Stereotaxis Inc.), with a combined field strength of 0.08 Tesla, was combined with a monoplane angiography system (AXIOM Artis, Siemens, Erlangen, Germany). The magnets and generated field were controlled via a workstation (Navigant, Stereotaxis Inc.) to allow for changes in the direction of a stable magnetic field within the heart of the patient. Magnets in the distal shaft of the ablation catheters align the catheter tip with the field. Remote catheter advancement and retraction was performed using a catheter advancer system (Cardiodrive™, Stereotaxis Inc.). Remote control of the system was performed from the control room.
Ablation: standard vs. magnetic
In the first four animals ablation was performed using either a standard Navistar 4 mm tip, or a magnetic Navistar RMT 4 mm tip catheter (both Biosense Webster, Diamond Bar, CA, USA). RF lesions were produced in the high right atrial and low right atrial free wall, right ventricular free wall, right ventricular outflow tract, and inferior vena cava-tricuspid annulus (IVC-TA) isthmus, in the same sequence in each animal. RF energy was delivered using an EP Shuttle generator (Stockert GmbH, Freiburg, Germany) with maximum set values of 58 °C, 50 Watts and 60 seconds. Continuous recording prior to, during, and after ablation was made from the ablation catheter. During ablation, recordings were made of the ablation site radiologically, as well as on the Stereotaxis system when used. Tissue contact was determined by the physician on the basis of the radiographic images.
Ablation: contact indicator use
The Stereotaxis-CARTO RMT system determines contact by assessing the difference between the magnetic field orientation determined by the Stereotaxis system, and the catheter orientation determined by the CARTO RMT system. As the difference between applied magnetic field orientation and localised catheter orientation increases, so the contact indicator value increases on a scale from 0 to 4. When advancing a catheter perpendicularly into a wall, the contact meter only rises when the catheter prolapses.
In three animals ablation was performed at the same sites, using the same generator settings, while trying to achieve predetermined contact indicator settings for each position. Attempts to change the contact indicator setting were made by modifying the magnetic vector and/or advancing the catheter into the wall. The attained contact was recorded.
Lesion formation characteristics
During each RF application continuous measurements were taken of steady-state power, energy, temperature and impedance.
Sacrifice
After five days, the animals were sedated as described previously, and anaesthetised with pentobarbitol 12 mg/kg after intubation. The chest was opened and the heart exposed. The animals were euthanised using an overdose of pentobarbitol and the heart fibrillated using direct current, explanted and opened.
Macroscopic lesion assessment
Epicardial and endocardial surfaces of the heart were examined macroscopically and all lesions photographed. Qualitative assessment included a description of shape (round, oval or elongated), cavitation and surface thrombus, and transmurality. Collateral damage to other structures was noted. Quantitative assessment of endocardial surface area was made by two independent observers, using planimetric software (Clemex Vision PE, Clemex Technologies Inc., Longueuil, Canada).
Microscopic lesion assessment
Lesions were dissected and fixed in 10% formalin. Tissue was embedded in paraffin. A section was obtained perpendicular to the surface at the maximum diameter and stained with haematoxylin and eosin, and resorcin-fuchsin as an elastin stain. Lesions were analysed using the planimetric software for diameter, maximum depth, area of necrosis and inflammation, and presence of thrombus.
Statistics
Data were expressed as median, with ranges. Comparisons among groups were performed with a twotailed analysis of variance. Comparisons of simple proportions between two groups were made with a Fisher's exact test. A p value of ≤0.05 was considered statistically significant.
Results
Animal data
One animal developed VF during the first RF application and died. All other animals completed the protocol without complications. In total, 35 lesions were created at the high and low RA free wall, isthmus, and the RV free wall and outflow tract in the seven surviving pigs. In four animals we compared 19 lesions using standard and magnetic catheters – one RV free wall lesion could not be found. In three animals the contact measurement utility was assessed, with one high, medium, and low contact ablation made at each of the five predetermined sites (15 lesions).
Macroscopic lesion assessment
Standard vs. magnetically steered catheters
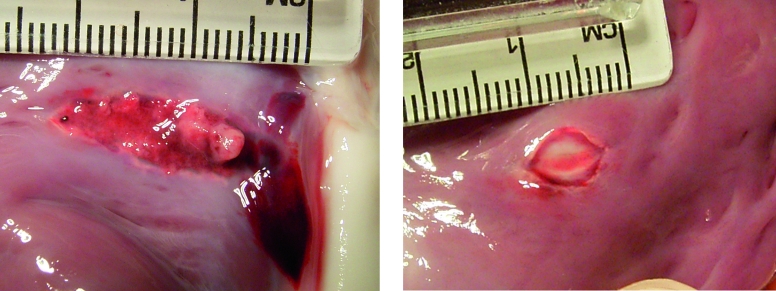
All RA free wall lesions were transmural with similar epicardial and endocardial surface area. In the RV, the epicardial surface area appeared smaller than the endocardial surface area with both catheters. Transmurality of isthmus lesions was difficult to assess due to the complex anatomy of this region. There was no significant difference in endocardial surface area between standard and magnetic catheters: 39 mm2 (range 16 to 82 mm2) vs. 36 mm2 (range 23 to 111 mm2). Additional lesions from the three other animals were included in the analysis of table 1. After exclusion of the isthmus, and with perpendicular applications to RA and RV, lesions from magnetic catheters were significantly more often round or oval (8) than those from standard catheters (3) which were often elongated (p<0.05) (figure 2). With the catheter tip parallel to the tissue, as in the isthmus, or sometimes in the RVOT, lesions tended to be elongated, although less so with the magnetic catheters.
Table 1.
Macroscopic and microscopic assessment of the lesions.
| Magnetic | Standard | p | |
|---|---|---|---|
| Macroscopic assessment endocardium | |||
| Total number | 24 | 9 | |
| Area (mm2) | |||
| - Observer 1 | 43 (21–83) | 38 (20–164) | NS |
| - Observer 2 (quantitative analysis)* | 48(18–120) | 39 (16–82) | NS |
| Perpendicular to RA/RV | 8 | 7 | |
| - Round/oval* | 5 / 3 | 1 / 2 | 0.05 |
| - Elongated* | 0 | 4 | 0.05 |
| Microscopic assessment mid-lesion | |||
| Total number | 24 | 9 | |
| Thrombus (no.) | 15/24 | 7/9 | NS |
| Number for analysis | 21 | 9 | |
| - Diameter (mm) | 10.59 (5.55–18.6) | 8.90 (3.76–15.7) | NS |
| - Maximal depth (mm) | 3.64 (1.78–8.13) | 3.78 (1.32–7.38) | NS |
| - Necrotic area (mm2) | 17.44 (4.59–57.01) | 7.05 (2.32–50.17) | NS |
| - Inflammatory area (mm2) | 2.84 (4.70–14.34) | 3.26 (0.41–10.33) | NS |
| - Total area (mm2) | 21.46 (5,26–69,87) | 10.22 (2.70–59.80) | NS |
| - Thrombotic area (mm2) | 0.25 (0–5.02) | 0.33 (0–5.87) | NS |
The median and range are given. NS=not significant, RA=right atrium, RV=right ventricle.*Isthmus lesions were excluded.
Figure 2.
Macroscopic aspect of lesions in the right ventricle with a standard catheter (left) and a magnetically steered catheter (right).
Contact utility comparison
In 15 lesions where the contact measurement utility was assessed (table 2), it did not appear useful at assessing contact compared with radiographic assessment.
Table 2.
Comparison of lesion shape with different contact utility settings.
| Low | Medium | High | |
|---|---|---|---|
| HRA | Elongated | Elongated | Oval |
| LRA | Elongated | Round | Round |
| RV | Oval | Oval | Oval |
| RVOT | Oval | Oval | Elongated |
| Isthmus | Oval | Elongated | Elongated |
HRA=high right atrium, LRA=low right atrium, RV=right ventricle, RVOT=right ventricular outflow tract.
Microscopic assessment
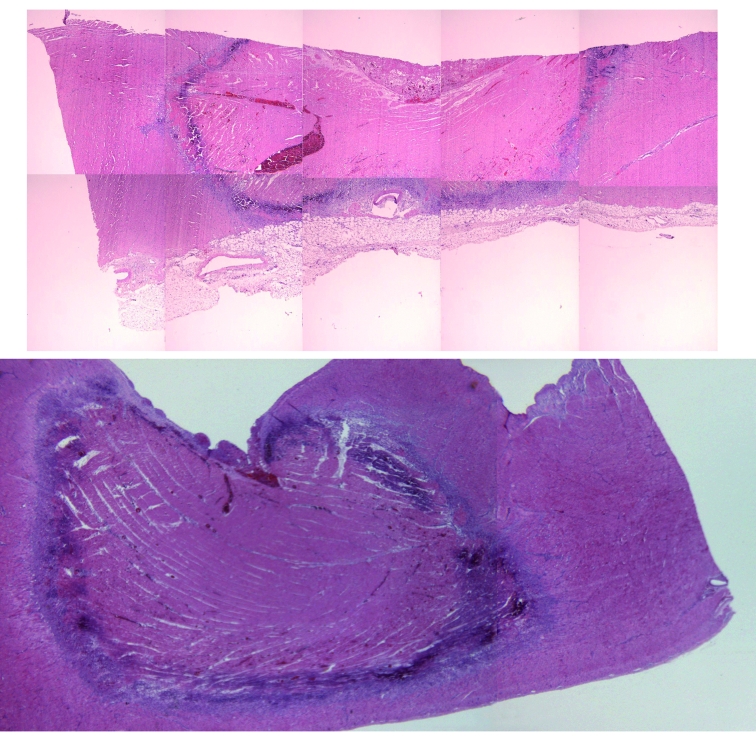
Lesions were transmural in 15 of 24 magnetic cases and in 4 of 9 standard procedures (figure 3). Three lesions from one animal were excluded from quantitative histological analysis for technical reasons. Microscopic assessment did not suggest any significant difference in lesions caused by either catheter. There was no significant difference in maximum diameter or depth, the area of necrosis or inflammation, and the combined area of inflammation and necrosis between catheters. Thrombi were seen in 15 of 24 magnetic lesions and 7 of 9 standard lesions.
Figure 3.
Histological aspect of lesions in the right ventricle, as prepared for planimetric analysis. Haematoxylin eosin stain. Both sections show a large necrotic area, with an inflammatory margin, with a small central thrombus in the upper one. The upper lesion is the result of a magnetically steered application; the lower one from a standard catheter.
Radiofrequency ablation characteristics
The data on steady-state power and impedance during ablation are presented in table 3. Average impedance was significantly lower for lesions created using magnetically steered catheters (p<0.0001) as was average energy used (p<0.05).
Table 3.
Measured ablation parameters.
| Magnetic | Standard | P | |
|---|---|---|---|
| Duration (sec) | 60 (51–60) | 60 (60–60) | NS |
| Temperature (°C) | 53( 51–60) | 53 (49–53) | NS |
| Power (Watt) | 19 (5–37) | 16 (2–42) | NS |
| Impedance (Ohm) | 118 (105–128) | 131 (121–225) | 0.0001 |
| Energy (Joules) | 970 (327–2228) | 1799 (963–2525) | 0.05 |
The median and range are given. NS=not significant.
Discussion
Catheter ablation is successful in treating many arrhythmias. Technologies have evolved to treat more complex arrhythmias and to improve results in other tachycardias, and include catheter designs allowing higher energy delivery, and developments in arrhythmia mapping systems. Magnetic and mechanical remote navigation systems have been incorporated into combined systems.
RF lesions depend on controllable and non-controllable factors, and the Stereotaxis-CARTO RMT system described here uses a radically different way of maintaining catheter tissue contact than standard catheters or the mechanical remote navigation system.8,9 This effects how lesions are formed and may make a previously poorly controllable factor, catheter tip tissue contact, more controllable. This has not yet been shown at a clinically obvious level, while the clinical effects so far seem similar.7,10-16
Comparison of standard vs. magnetic catheters
Ablation lesions formed by magnetically steered catheters have similar characteristics to those from standard catheters. The only significant differences appear to be that the lesions from magnetically steered catheters are more round or oval rather than elongated, suggesting more constant catheter tissue contact. There is less likelihood of ‘brush lesions’ when the catheter tip is perpendicular to the tissue, but these may still occur to a lesser extent when the catheter tip is parallel to the tissue. This may have advantages when it is important to limit the lesion and disadvantages when ‘brush lesions’ are actually advantageous, as in linear lesions.
Tissue contact is the relationship between contact area and contact force. With magnetically steered catheters the contact area appears to be decreased with the tip being more constantly related to a specific area, as shown by this study. However, there have been questions about the tissue contact force generated by floppy magnetically steered catheters and data would suggest that a lower contact force can be applied.11
RF lesion size relates to the generated tissue temperature, which relates to the amount of energy delivered. The energy delivered to the tissue itself relates to the amount of energy delivered by the generator and the proportion of the energy entering the tissue. This again depends on the electrode tissue contact and the degree of external cooling.1 Constant tissue area contact should mean that the energy is more effectively delivered to the underlying tissue. With magnetically steered catheters the tip contact force may be less, meaning that the tip is less deeply buried in the tissue than a standard catheter, allowing for more cooling of the exposed area of the tip. However, the distal tip is applied constantly to the tissue, therefore preventing cooling to this contact area. The question, therefore, is how much one effect predominates over the other. Lower impedances during ablation may relate to different catheter engineering, but lower impedance may also confirm better tissue contact as seen visually and implied by the macroscopic features seen. Lower system impedance may mean that less energy is necessary to cause similar size lesions. Lower average energy may also relate to impaired cooling of the tip and higher tip-tissue temperatures inhibiting maximum energy delivery. Conversely more effective delivery of that lower energy to the tissue may mean that lesion formation is as effective as suggested by the comparative findings.
Thrombus was seen microscopically, but we had no impedance rises during energy application. No visible thrombus was seen macroscopically with magnetically steered catheters.
In vitro models need to be developed which take into account cardiac and respiratory movement.
Contact utility assessment
During catheter contact assessed by radiographic and electrogram review, the contact utility seemed to show average contact most of the time. The tissue contact assessment was perhaps more useful when the tip was not perpendicular to the tissue.
Study limitations
This study looked at a limited number of lesions. The number of lesions formed using a standard catheter was small. It was not feasible to assess lesion volume and the findings are more subjective than in other preparations but we feel more accurately reflect clinical lesions.
Conclusion
Magnetically steered 4 mm tip RF ablation catheters perform as well as comparable standard catheters as assessed by lesion size. The lesions show less ‘brush’ effect and are more predictably oval or round. The contact utility assessment was not useful. Lower impedances and energy requirement are intriguing and need further assessment.
Acknowledgements
Our thanks to the staff of Experimental Cardiology and the Electrophysiology Laboratory for their assistance in this study. We thank Professor W.J. van der Giessen for providing us with useful comments during the writing of this paper.
Disclosures
This study was supported by a grant from Stereotaxis, Inc. A. Thornton has received speaker's fees from Stereotaxis, Inc. L. Jordaens and Erasmus MC have received a research grant from Stereotaxis, Inc.
References
- 1.Stagegaard N, Petersen HH, Chen X, Svendsen JH. Indication of the radiofrequency induced lesion size by pre-ablation measurements. Europace. 2005;7:525–34. [DOI] [PubMed] [Google Scholar]
- 2.Kongsgaard E, Steen T, Jensen O, Aass, H, Amlie JP. Temperature guided radiofrequency catheter ablation of myocardium: comparison of catheter tip and tissue temperatures in vitro. Pacing Clin Electrophysiol. 1997;20:1252–60. [DOI] [PubMed] [Google Scholar]
- 3.Haines DE, Watson DD. Tissue heating during radiofrequency catheter ablation: a hermodynamic model and observations in isolated perfused and superfused canine right ventricular free wall. Pacing Clin Electrophysiol. 1989;12:962–76. [DOI] [PubMed] [Google Scholar]
- 4.Petersen HH, Chen X, Pietersen A, Svendsen JH, Haunso S. Tissue temperatures and lesion size during irrigated tip catheter radiofrequency ablation: an in vitro comparison of temperaturecontrolled irrigated tip ablation, power-controlled irrigated tip ablation, and standard temperature-controlled ablation. Pacing Clin Electrophysiol. 2000;23:8–17. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa H, Yamanashi WS, Pitha JV, Arruda M, Wang X, Ohtomo K, et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation. 1995;91:2264–73. [DOI] [PubMed] [Google Scholar]
- 6.Petersen HH, Chen X, Pietersen A, Svendsen JH, Haunso S. Temperature-controlled radiofrequency ablation of cardiac tissue: an in vitro study of the impact of electrode orientation, electrode tissue contact pressure and external convective cooling. J Interv Card Electrophysiol. 1999;3:257–62. [DOI] [PubMed] [Google Scholar]
- 7.Thornton AS, Janse P, Theuns DA, Scholten MF, Jordaens LJ. Magnetic navigation in AV nodal re-entrant tachycardia study: early results of ablation with one- and three-magnet catheters. Europace. 2006;8:225–30. [DOI] [PubMed] [Google Scholar]
- 8.Al-Ahmad A, Grossman JD, Wang PJ. Early experience with a computerized robotically controlled catheter system. J Interv Card Electrophysiol. 2005;12:199–202. [DOI] [PubMed] [Google Scholar]
- 9.Saliba W, Cummings JE, Oh S, Zhang Y, Mazgalev TN, Schweikert RA, et al. Novel robotic catheter remote control system: feasibility and safety of transseptal puncture and endocardial catheter navigation. J Cardiovasc Electrophysiol. 2006;17:1102–5. [DOI] [PubMed] [Google Scholar]
- 10.Ernst S, Ouyang F, Linder C, Hertting K, Stahl F, Chun J, et al. Initial experience with remote catheter ablation using a novel magnetic navigation system: magnetic remote catheter ablation. Circulation. 2004;109:1472–5. [DOI] [PubMed] [Google Scholar]
- 11.Faddis MN, Blume W, Finney J, Hall A, Rauch J, Sell J, et al. Novel, magnetically guided catheter for endocardial mapping and radiofrequency catheter ablation. Circulation. 2002;106:2980–5. [DOI] [PubMed] [Google Scholar]
- 12.Faddis MN, Chen J, Osborn J, Talcott M, Cain ME, Lindsay BD. Magnetic guidance system for cardiac electrophysiology: a prospective trial of safety and efficacy in humans. J Am Coll Cardiol. 2003;42:1952–8. [DOI] [PubMed] [Google Scholar]
- 13.Kerzner R, Sanchez JM, Osborn JL, Talcott M, Cain ME, Lindsay BD. Radiofrequency ablation of atrioventricular nodal reentrant tachycardia using a novel magnetic guidance system compared with a conventional approach. Heart Rhythm.. 2006;3:261–7. [DOI] [PubMed] [Google Scholar]
- 14.Pappone C, Vicedomini G, Manguso F, Gugliotta F, Mazzone P, Gulletta S, et al. Robotic magnetic navigation for atrial fibrillation ablation. J Am Coll Cardiol. 2006;47:1390–400. [DOI] [PubMed] [Google Scholar]
- 15.Thornton AS, Jordaens LJ. Remote magnetic navigation for mapping and ablating right ventricular outflow tract tachycardia. Heart Rhythm.. 2006;3:691–6. [DOI] [PubMed] [Google Scholar]
- 16.Thornton AS, Rivero-Ayerza M, Knops P, Jordaens LJ. Magnetic navigation in left-sided AV reentrant tachycardias: preliminary results of a retrograde approach. J Cardiovasc Electrophysiol. 2007;18:467–72. [DOI] [PubMed] [Google Scholar]