Abstract
Hybrid imaging of positron emission tomography (PET) together with computed tomography (CT) is rapidly emerging. In cardiology, this new advanced hybrid imaging modality allows quantification of cardiac perfusion in combination with assessment of coronary anatomy within a single scanning session of less than 45 minutes. The near-simultaneous anatomical evaluation of coronary arteries using CT and corresponding functional status using PET provides a wealth of complementary information in patients who are being evaluated for (suspected) coronary artery disease, and could help guide clinical patient management in a novel manner. Clinical experience gained with this recently introduced advanced hybrid imaging tool, however, is still limited and its implementation into daily clinical practice remains largely unchartered territory. This review discusses principles of perfusion PET, its diagnostic accuracy, and potential clinical applications of cardiac PET-CT in patients with ischaemic heart disease. (Neth Heart J 2010;18:90–8.)
Keywords: Coronary Artery Disease, Positron-Emission Tomography, Tomography, X-Ray Computed
Positron emission tomography (PET) is the unrivalled imaging modality to quantitatively and noninvasively track biochemical pathways in vivo. Perfusion is among those physiological processes that can accurately be evaluated.1 More than any other technique, cardiac PET has greatly improved our understanding of human coronary vasomotor function in both physiological and pathophysiological conditions.2 Furthermore, it has been demonstrated that the diagnostic accuracy of myocardial perfusion imaging (MPI) with PET for detecting obstructive coronary artery disease (CAD) surpasses that of single photon emission computed tomography (SPECT).3,4 Nonetheless, owing to its limited availability and high costs, perfusion PET has predominantly been confined to the sideline in the clinical arena.
In recent years, however, instrumentation hardware of PET has been fused with computed tomography (CT). In a short period of time, these hybrid devices have gained great popularity, predominantly in clinical oncology, which has led to an exponential growth of the numbers of scanners installed worldwide. This growth has been paralleled by the expanding clinical applications of cardiac CT, such as coronary artery calcium scoring (CAC) and contrast coronary angiography (CTCA).5 The near simultaneous anatomical evaluation of the coronary arteries with CT and their functional status with PET offers a wealth of complementary information in patients who are being evaluated for (suspected) CAD, and could help guide clinical patient management in a novel manner. The clinical experience gained with these recently introduced advanced hybrid imaging tools, however, is limited and implementation of such imaging facilities into daily clinical practice remains largely unchartered territory. This review discusses the principles of perfusion PET, its diagnostic accuracy, and the potential clinical applications of cardiac PET-CT in patients with ischaemic heart disease.
Principles of positron emission tomography
PET is a nuclear medicine technique that uses positron emitting radionuclides, which are labelled to biological compounds of interest, to generate images of physiological processes. Positron emitters do not exist in nature and have to be produced artificially with the use of a particle accelerator, usually a cyclotron. Once released from the nucleus, as schematically depicted in figure 1, the positrons travel for a short distance through tissue (in the order of millimetres) where they annihilate with an encountering electron, producing two photons which emit in the opposite direction.6 When opposing detectors of the PET scanner record these photons within a narrow temporal window (in the order of nanoseconds), it is assumed that this coincidence event is the result of an annihilation that has taken place along the line connecting these two detectors (line of response). Following the registration of these events, counts from all lines of response (sinograms) are reconstructed to provide a three-dimensional map of the radioactivity distribution.
Figure 1.
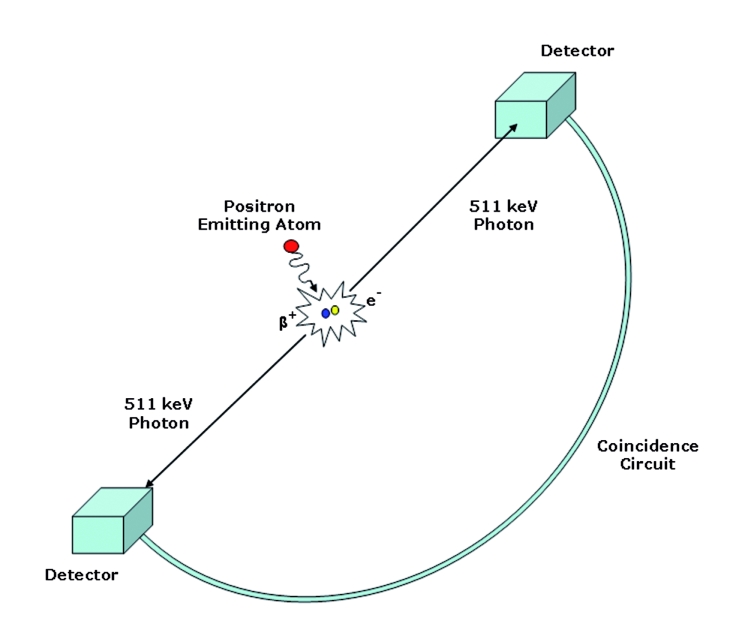
Physics of positron emission, annihilation, and coincidence detection. Adapted from Paolo Camici.6
In comparison with SPECT imaging, PET has some important technical advantages (table 1).2 First, routine correction of radiotracer attenuation is performed with the use of the X-ray CT equipment. As a result, less attenuation artifacts are observed, which reduces the rate of false-positive perfusion defects and hence increases specificity. Second, high spatial resolution of PET (∽5 to 7 mm) in comparison with SPECT (∽12 to 15 mm) enables the detection of small perfusion defects, reducing false-negative results. Sensitivity is further enhanced by the higher extraction fraction of PET perfusion tracers, which improves the detection of subtle regional perfusion differences that can go undetected with SPECT. Third, the short physical half-life of the PET perfusion tracers is accompanied by a low radiation burden and allows acquisition of both rest and stress images within a single scanning session. Finally, a temporal resolution of a few seconds enables tracking of the dynamic flux of radiotracer between arterial blood and myocardium, which makes it possible to quantitively express perfusion in ml • min−1 • g−1 of tissue. Thus, PET not only provides qualitative myocardial perfusion images, but additionally yields absolute levels of perfusion and flow reserve. The latter is of clinical relevance, as will be discussed below.
Table 1.
Characteristics of SPECT vs. PET.
| SPECT | PET | |
|---|---|---|
| Availability | Wide | Limited |
| Attenuation correction | Less accurate | Accurate |
| Spatial resolution | 12–15 mm | 5–7 mm |
| Protocol | 2 days | <1 hour |
| Radiation | >10 mSv | <10 mSv |
| Images | Qualitative | Quantitative |
| Hybrid with CT | Yes | Yes |
PET=positron emission tomography, SPECT=single photon emission computed tomography.
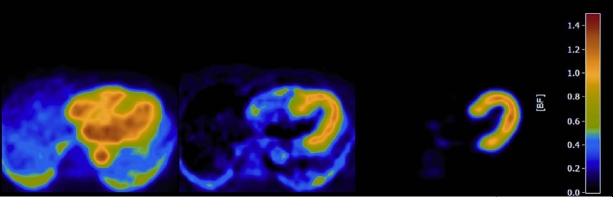
Of the available positron emitting tracers, H215O, 13NH3, and 82Rubidium are the most commonly used for the assessment of perfusion with PET.1 Table 2 lists the characteristics of these tracers. H215O is the only freely diffusible, metabolically inert tracer. Its unique property of complete myocardial extraction from arterial blood renders this tracer ideal to quantify perfusion, as changes in myocardial tracer activity are solely dependent on perfusion and not affected by flow-dependent rates of extraction.7,8 These unique characteristics, however, have long served as a double-edged sword. Being freely diffusible, equilibrium of tracer between blood and myocardium is promptly reached, yielding a low signal gradient between compartments and thus relative tracer distribution images are of little diagnostic value (figure 2A). The latter constraint has excluded its use in clinical practice. Recent advances in both hard and software, however, have enabled the generation of parametric perfusion images (i.e. perfusion is determined at the voxel level) to visually evaluate MPI with H215O for routine diagnostic purposes (figure 2C).9 Currently, clinical trials are being conducted to evaluate the diagnostic accuracy of H215O for the detection of CAD.
Table 2.
Characteristics of oxygen-15-labelled water (H215O), nitrogen-13-labelled ammonia (13NH3), and 82Rubidium chloride for PET perfusion imaging.
| H215O | 13NH3 | 82Rubidium | Comment | |
|---|---|---|---|---|
| Half-life | 123 sec | 9.97 min | 76 sec | Mandatory on-site production of the tracers, given their short physical half-life |
| Production | Cyclotron | Cyclotron | Generator | Generator equipment far lower in costs regarding installment and maintenance |
| Kinetics | Freely diffusible; metabolically inert | Metabolically trapped in myocardium | Metabolically trapped in myocardium | Complete extraction into myocardial tissue renders H215O an ideal perfusion tracer |
| Mean positron range in tissue | 1.1 mm | 0.4 mm | 2.8 mm | 82Rubidium 's high tissue penetration depth limits the spatial resolution of the perfusion imaging |
| Radiation exposure per flow measurement | ∽ 0.5 mSv | ∽ 1.0 mSv | ∽ 4.0 mSv | Actual exposure depends on administered tracer dosage adjusted for scanner hardware |
Figure 2.
A) Transaxial image at the thoracic level obtained a few minutes after injection of H215O. Note that the myocardium cannot be distinguished from the ventricular blood pool cavities as a result of free diffusion of tracer between blood and myocardial tissue, rendering the images unsuitable for diagnostic purposes. B) Through a mathematical process (i.e. factor or cluster analysis) the blood pool can be identified and subtracted from the H215O image, yielding an image of tracer activity distribution in the myocardial wall. These images can be used to qualitative grade tracer uptake. C) Parametric image of the same patient as in panel A and B. Perfusion is calculated at the voxel level through a modelling procedure based on the dynamic flux of tracer between arterial blood and myocardial tissue. Note the enhanced visual quality of the image in comparison with panel B. Moreover, for the parametric image perfusion is given in absolute terms (ml · min−1 · g−1) in the accompanying colour-coded legend.
Both 13NH3 and 82Rubidium are tracers that are actively transported across the cell membrane and effectively become metabolically trapped in myocardial tissue. As tracer is cleared from arterial blood, a high concentration gradient between blood and myocardium occurs, resulting in myocardial perfusion images of high quality. Unfortunately, extraction of both tracers is incomplete and decreases in a nonlinear fashion at increasing flow rates. This characteristic hampers accurate quantification of perfusion as correction models for incomplete extraction are mainly based on experimental animal models. 82Rubidium has the advantage of being generator produced, thereby obviating the need for an onsite cyclotron with concomitant reduction in costs. On the other hand, due to deep positron penetration depth in tissue (table 2) and relatively low count statistics of 82Rubidium, which is related to the ultra-short half-life, image quality and spatial resolution are somewhat degregated in comparison with H215O and 13NH3. These limitations make 82Rubidium the least accurate PET perfusion tracer, in terms of both qualitative and quantitative perfusion imaging, but this may well be outweighed by the fact that it is a generator produced tracer obviating the need for an (expensive) on-site cyclotron.
Diagnostic accuracy of perfusion PET
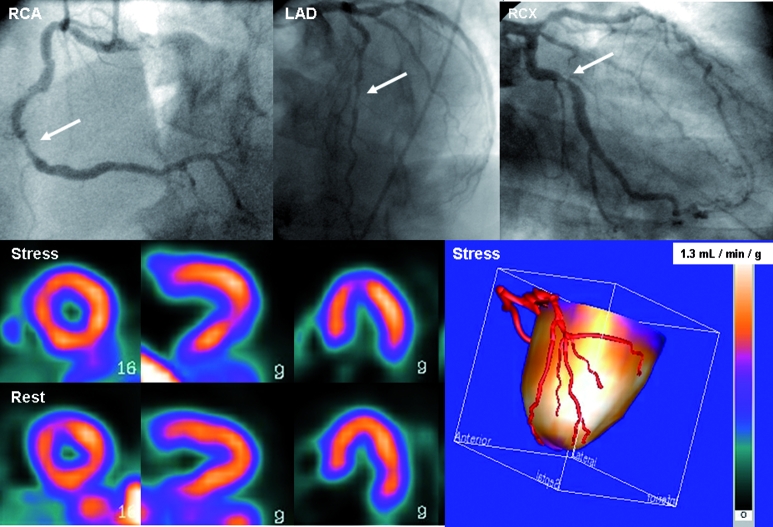
The vast majority of studies on the diagnostic accuracy of MPI with PET to detect obstructive coronary artery disease have been performed with the tracers 13NH3 and 82Rubidium. Recently, di Carli and Hachamovitch reviewed the available literature and reported weighted sensitivity and specificity values of 0.90 and 0.89, respectively, derived from analysis of nine studies including 877 patients.3 The number of studies comparing SPECT with PET head-to-head is limited, but show consistent superior diagnostic accuracy of PET. Pooled analysis of three studies comparing either 82Rubidium or 13NH3 PET with Thallium-201 SPECT demonstrated increased sensitivity (91 vs. 81%, respectively) and specificity (93 vs. 85%, respectively) in favour of PET.10 The enhanced diagnostic accuracy of 82Rubidium PET compared with more contemporary SPECT methodology (99Tc-sestamibi as a tracer and gated acquisition) was recently highlighted by Bateman and colleagues.11 Specificity was shown to be significantly higher with PET (93 vs. 73%, respectively), whereas sensitivity was only marginally increased (87 vs. 82%, respectively) in 112 subjects evaluated for CAD. These data seem to apply irrespective of the examined patient population. Nonetheless, specific clinical conditions and patient characteristics in particular stress the advantageous utility of PET over SPECT. Attenuation artefacts are common in SPECT imaging in obese patients and women with large breasts. Routine attenuation correction with PET therefore increases specificity in these patients. Furthermore, in patients with extensive CAD, balanced ischaemia may occur that can go undetected with SPECT (figure 3).12,13 Quantitative PET analysis in these cases will display a diffusely blunted flow reserve and reveal the diagnosis, despite the absence of regional stress induced perfusion defects.
Figure 3.
A 52-year-old diabetic patient with atypical chest pain. The coronary arteries were severely calcified with a calcium score of more than 3500 (Agatston), therefore no CTCA was performed given its low diagnostic value in this type of patient due to blooming artefacts. PET perfusion with oxygen-15-labelled water displayed homogeneous perfusion both during rest and pharmacologically induced vasodilation (adenosine). Quantitative stress perfusion, however, was severely blunted (1.30 ml • min–1 • g–1, population-based normal value 3.54±1.01 ml • min–1 • g–1).45 Such a uniform distribution pattern would have resulted in a false-negative SPECT examination. The patient was referred for conventional angiography which revealed three-vessel coronary artery disease, confirming the findings of the hybrid PET-CT study.
More than any other noninvasive imaging technique, the prognostic implications of MPI with SPECT have been well defined by long-term follow-up of large cohorts of patients characterised by a wide range of likelihood or already established CAD.14 In contrast, the available data on the prognostic value of perfusion PET are scarce, although extrapolation of the gained knowledge with SPECT seems reasonable. To date, three studies have explored the prognostic value of relative MPI with 82Rubidium PET. Marwick and co-workers evaluated 685 patients and reported an event-free survival during a 41-month follow-up period of 90, 87, 75, and 76% corresponding to a normal or mildly, moderately, and extensively abnormal scan, respectively.15 Yoshinaga et al. included 367 patients with a mean follow-up of 3.1±0.9 years.16 A normal scan was accompanied by a favourable prognosis with an annual event rate of 0.4% that increased to 7% in the group of patients with the most severe perfusion defects. More recently, Schenker et al. studied 695 consecutive patients who were evaluated for CAD with 82Rubidium PET. The presence of ischaemia was related to prognosis, i.e. a normal scan yielded an annual event rate of 5.9%, whereas an abnormal scan was accompanied with an annual event rate of 14.6%.17 The additive prognostic value of absolute quantitative flow in patients with ischaemic heart disease has been highlighted by Tio et al. In a cohort of 344 patients who underwent 13NH3 PET, the level of flow reserve impairment was independently related to an increased mortality.18 Moreover, it was a stronger predictor of cardiac death than LV ejection fraction. Nonetheless, it should be mentioned that all of these studies are characterised by relatively low event rates; additional and larger studies are needed to more accurately validate the prognostic value perfusion PET.
Hybrid imaging with PET-CT in patients with CAD
In recent years cardiac CT has emerged as a promising tool to noninvasively evaluate coronary artery calcium plaque burden (CAC) and, with the aid of a contrast agent, additionally acquire a coronary angiogram (CTCA). Its development has been swift and technical progress is ongoing at a rapid pace. In a relatively short period of time, much experience has been gained with this novel technique and the current status of cardiac CT has been thoroughly reviewed elsewhere.5 Although it is beyond the scope of the present review to elaborately discuss the role of cardiac CT as a stand-alone imaging tool, a few remarks are in order. Assessment of coronary plaque burden with CAC can be obtained with either electron beam CT or multidetector CT.19 Studies have unequivocally demonstrated that there is a relationship between CAC score and the likelihood of significant stenoses with high sensitivity (91%), although specificity is poor (52%) and the site of plaque frequently does not coincide with the actual coronary obstructive lesion.20 Furthermore, levels of CAC have been shown to be of prognostic value with incremental predictive information over traditional risk factors alone.21,22 Given the high negative predictive value associated with the absence of coronary calcium, i.e. CAC is zero, many have adopted the view that its role in clinical practice lies predominantly as a gatekeeper for further anatomical or functional screening.23 However, CAC does not detect non-calcified coronary plaque and its prevalence can be as high as 10%.24 Of interest, the distinction in plaque composition (i.e. non-calcified, mixed, or calcified) with CTCA is related to plaque morphology and seems to hold additional prognostic information.25,26
With the aid of multislice CT scanners, preferably 64 and higher, it is possible to noninvasively obtain high-quality images of the coronary arteries. In a recent meta-analysis of 27 CTCA studies using 64-slice scanners to detect CAD, sensitivity, specificity, positive predictive value and negative predictive value were 98, 91, 93, and 97%, respectively, on a per-patient basis.27 It is, however, important to realise that these data were obtained in cohorts of patient who were already referred for conventional angiography and displayed relatively high prevalence of CAD. Also, evaluation of CAD in these studies is generally limited to vessel size of >1.5 mm and uninterpretable images are often excluded from analysis. More recent multicentre studies, in which no segments were excluded from the analysis, have confirmed the excellent negative predictive value (97 to 99%) of 64 slice CTCA; however, positive predictive value was lower (64 to 85%) on a per-patient basis and decreased even further on a per-vessel basis (51 to 59%).28,29
The general consensus from these studies is that a negative CTCA of a good quality can be considered to have ruled out CAD, whereas the presence of significant coronary lesions at CTCA or an uninterpretable scan warrants further evaluation. Indeed, there is only a crude relation between CTCA estimated and invasively measured (with quantitative invasive coronary angiography) stenosis diameter30 and inducible ischaemia can only be observed at MPI in half of the significant coronary stenoses at CTCA (>50%).31-34
Given the profound differences in clinical information, i.e. anatomy with CT versus function of the coronary arteries with PET MPI, there is an ongoing debate as to what the best noninvasive imaging strategy is to evaluate patients with suspected CAD. Although any algorithm to optimise patient referral for diagnostic imaging heavily relies on the pre-test likelihood of disease, in general the following can be stated. An initial normal cardiac CT accurately rules out CAD, but in case of a low quality scan or presence of coronary plaques, additional functional testing is warranted. On the other hand, an initial functional test may be completely normal but certainly does not exclude subclinical atherosclerosis, which may require medical treatment. Indeed, recent studies have demonstrated that in patients with a normal perfusion study, an increased calcium score or an abnormal CTCA are accompanied by a poorer prognosis.17,35 Hence, there is an incremental value of calcium scoring and CTCA in patients with normal perfusion imaging. On the other hand, a calcium score of zero may be accompanied by reversible perfusion defects at MPI in as many as 16% of patients.17 It is clear that each single imaging approach of either CT or PET MPI will result in missed diagnoses and/or unnecessary invasive procedures due to increased rates of false-positive results. Combining perfusion PET with cardiac CT yields complementary information within a single session of approximately 45 minutes, and immediately gathers all the necessary clinical data to guide patient decision-making (figure 4). As listed in table 3, roughly four scenarios can be observed. A normal CT and PET MPI rules out CAD and justifies discharge of the patient. A high calcium score and/or coronary lesions at CTCA but a normal perfusion scan may require medical treatment, whereas signs of (extensive) ischaemia and an abnormal CT examination warrants referral to the cath-lab for potential revascularisation. In the latter scenario, the hybrid imaging approach also facilitates target vessel revascularisation in case of multivessel CAD. By pinpointing coronary lesions to areas of ischaemia by fusion of the anatomical and functional images (figure 5), the culprit coronary obstruction can be identified and selective percutaneous treatment strategies can be employed. Finally, a blunted flow reserve or regional reversible perfusion defects without signs of epicardial coronary artery lesions are compatible with coronary micovascular dysfunction.36
Figure 4.
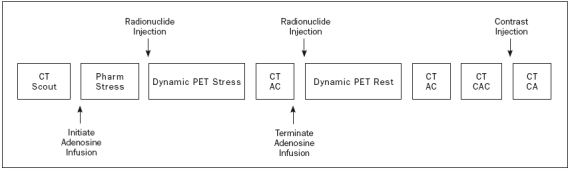
Potential cardiac PET-CT scanning protocol. After an initial CT scout to delineate the field of view, pharmacological stress is induced by infusion of adenosine intravenously. Maximum vasodilation reaches a steady state after two minutes and the radionuclide is injected followed immediately by a dynamic PET frame sequence (approximately 5 minutes, which varies slightly for different PET tracers). Directly after the PET sequence, a CT attenuation scan (CTAC) is acquired after which the infusion of adenosine is terminated. Generally, stress imaging is performed early in the protocol to allow the heart rate to return to baseline level in order to reach a low heart rate during the CT coronary angiography (CTCA). After sufficient time to allow for radiodecay of the tracer, the rest PET sequence and a separate CTAC are acquired. Subsequently, a calcium score is performed (CTCAC) followed by CTCA during infusion of a contrast agent. During CTCAC and CTCA, a low and steady heart rate of less than 65 beats/min must be ensured. If necessary, intravenous β-blockers are administered to achieve this goal. The length of the protocol depends on the PET tracer used. It can be completed in less than 45 minutes with H215O and 82Rubidium, and in less than one hour with 13NH3.
Table 3.
Scenarios in cardiac PET-CT imaging for (suspected) coronary artery disease (CAD).
| PET | CTCA | Diagnosis | Proposed Strategy |
|---|---|---|---|
| Normal | Normal | No CAD | Discharge |
| Normal | Abnormal | Non-significant CAD | Medical treatment and follow-up |
| Abnormal | Abnormal | Significant CAD | Medical treatment and consider invasive coronary angiography |
| Abnormal | Normal | Coronary microvascular dysfunction | Risk profile modification and consider antianginal medical treatment |
Abnormal PET=summed stress score >3 (17 segment model) or diffuse blunted flow reserve, abnormal CTCA=at least 1 stenosis >50%.
Figure 5.
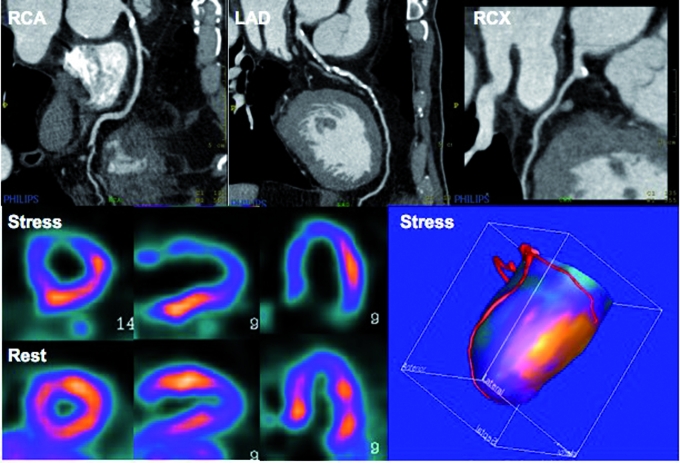
Cardiac PET-CT images of a patient who is being evaluated for coronary artery disease. CTCA images display calcified lesions in the proximal RCA and throughout the proximal course of the LAD. PET images display a large reversible perfusion defect in the anterior wall, coinciding with the LAD territory on the fusion images, whereas the RCA perfusion territory has a normal hyperaemic response. This type of imaging therefore facilitates target vessel revascularisation in case of multivessel disease. Please note that the entire acquisition protocol is completed in less than 45 minutes.
Critical considerations
Radiation burden is of concern with PET- and/or CT-based strategies. The PET study is performed during rest and pharmacologically induced vasodilation (usually adenosine). Depending on the tracer used (table 2), radiation exposure of the flow tracer ranges between 1.0 and 8.0 mSv (N.B. average annual background radiation level ranges from 2.0 to 2.5 mSv and legislated allowed radiation exposure for professional heath care workers in the Netherlands may not exceed 20 mSv). In addition, a low-dose-CT derived attenuation map is acquired for each condition, as the anatomical position of the heart within the thorax varies between resting and stress conditions. Such an attenuation map yields an additional radiation exposure of 0.5 mSv for each run.37 It must be kept in mind, however, that MPI with 99mTechnetium SPECT is generally accompanied by a higher radiation burden (10 to 15 mSv),38 although clinical experience with lower dosage of SPECT tracers is promising and may significantly reduce the radiation burden of SPECT imaging.39
With respect to cardiac CT imaging, the calcium scoring adds approximately 2 mSv to the protocol, whereas CTCA may convey as much as 10 to 20 mSv radiation exposure. In comparison, 2 to 6 mSv is generated during a conventional invasive coronary angiogram.38 Particularly the radiation exposure of CTCA demands scrutiny and warrants further study of techniques to decrease the level of radiation. Some simple measures to reduce radiation should always be considered, such as keeping the length of the scan volume as short and tube current as low as possible. Furthermore, reducing tube voltage to 100 kV instead of the commonly used 120 kV results in a substantial further reduction of radiation exposure and should be considered in patients with a low-tomoderate body mass.40 New developments such as tube modulation (in which full tube current is limited to a short-time period in diastole, resulting in the reduction of radiation dose by 30 to 40%) and prospective ECG triggering (radiation is only applied at a predefined time point of the cardiac cycle, rather than during the entire cardiac cycle as with retrospective ECG gating) have made it possible to limit radiation well below 10 mSv.41-43 In fact, it is anticipated that ultimately the entire imaging protocol (PET MPI, CAC, and CTCA) could be completed with a radiation exposure of less than 10 mSv, as recently demonstrated by Kajander et al.44 Another cause of concern is the use of nephrotoxic contrast agents that prohibits CTCA in patients with kidney failure. Furthermore, in contrast to the PET MPI, cardiac CT requires a slow regular heart beat (preferably <65 beats/min) to obtain images of good quality, excluding this type of imaging in patients with (relative) tachycardia or arrhythmia.5 Finally, given the novelty of hybrid imaging with cardiac PET/CT, it is currently unclear where it fits in the clinical strategy in diagnosing CAD. Particularly cost-effectiveness data will be pivotal to justify its implementation into routine clinical practice. Current data support that cardiac PET MPI alone may already be used in clinical practice, particularly in patients in whom SPECT imaging is anticipated to yield false-positive results (i.e. obese patients and women with large breasts) or when there is doubt about the negative results of conventional tests. Given the low positive predictive value of cardiac CT, the hybrid imaging approach should particularly prove to be useful in patients with high likelihood for CAD in whom the significance of potential coronary lesions warrants evaluation with simultaneous PET MPI.3
Conclusions
Cardiac PET-CT is a new hybrid imaging technique in the evaluation of patients suspected of CAD. In a single scanning session, complementary information on coronary anatomy and its function is acquired. This imaging modality is therefore also popularly referred to as the one-stop-shop. Although its role in clinical practice warrants further study, it is clear that it holds great potential for the diagnostic evaluation of these patients.
References
- 1.Knaapen P, Lubberink M. Cardiac positron emission tomography: myocardial perfusion and metabolism in clinical practice. Clin Res Cardiol. 2008;97:791–6. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005;46:75–88. [PubMed] [Google Scholar]
- 3.Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–80. [DOI] [PubMed] [Google Scholar]
- 4.Le Guludec D, Lautamaki R, Knuuti J, Bax JJ, Bengel FM. Present and future of clinical cardiovascular PET imaging in Europe a position statement by the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging. 2008;35:1709–24. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29:531–56. [DOI] [PubMed] [Google Scholar]
- 6.Camici PG. Positron emission tomography and myocardial imaging. Heart. 2000;83:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iida H, Kanno I, Takahashi A, Miura S, Murakami M, Takahashi K, et al. Measurement of absolute myocardial blood flow with H215O and dynamic positron-emission tomography. Strategy for quantification in relation to the partial-volume effect. Circulation. 1988;78:104–15. [DOI] [PubMed] [Google Scholar]
- 8.Knaapen P, Boellaard R, Gotte MJ, van der Weerdt AP, Visser CA, Lammertsma AA, et al. The perfusable tissue index: a marker of myocardial viability. J Nucl Cardiol. 2003;10:684–91. [DOI] [PubMed] [Google Scholar]
- 9.Boellaard R, Knaapen P, Rijbroek A, Luurtsema GJ, Lammertsma AA. Evaluation of basis function and linear least squares methods for generating parametric blood flow images using 15O-water and Positron Emission Tomography. Mol Imaging Biol. 2005;7:273–85. [DOI] [PubMed] [Google Scholar]
- 10.Machac J. Cardiac positron emission tomography imaging. Semin Nucl Med. 2005;35:17–36. [DOI] [PubMed] [Google Scholar]
- 11.Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECGgated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. [DOI] [PubMed] [Google Scholar]
- 12.Yoshinaga K, Katoh C, Noriyasu K, Iwado Y, Furuyama H, Ito Y, et al. Reduction of coronary flow reserve in areas with and without ischemia on stress perfusion imaging in patients with coronary artery disease: a study using oxygen 15-labeled water PET. J Nucl Cardiol. 2003;10:275–83. [DOI] [PubMed] [Google Scholar]
- 13.Parkash R, deKemp RA, Ruddy TD, Kitsikis A, Hart R, Beauchesne L, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11:440–9. [DOI] [PubMed] [Google Scholar]
- 14.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;17;97:535–43. [DOI] [PubMed] [Google Scholar]
- 15.Marwick TH, Shan K, Patel S, Go RT, Lauer MS. Incremental value of rubidium-82 positron emission tomography for prognostic assessment of known or suspected coronary artery disease. Am J Cardiol. 1997;80:865–70. [DOI] [PubMed] [Google Scholar]
- 16.Yoshinaga K, Chow BJ, Williams K, Chen L, deKemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48:1029–39. [DOI] [PubMed] [Google Scholar]
- 17.Schenker MP, Dorbala S, Hong EC, Rybicki FJ, Hachamovitch R, Kwong RY, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation. 2008;117:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tio RA, Dabeshlim A, Siebelink HM, de Sutter J, Hillege HL, Zeebregts CJ, et al. Comparison Between the Prognostic Value of Left Ventricular Function and Myocardial Perfusion Reserve in Patients with Ischemic Heart Disease. J Nucl Med. 2009;50:214–9. [DOI] [PubMed] [Google Scholar]
- 19.Stanford W, Thompson BH, Burns TL, Heery SD, Burr MC. Coronary artery calcium quantification at multi-detector row helical CT versus electron-beam CT. Radiology. 2004;230:397–402. [DOI] [PubMed] [Google Scholar]
- 20.Nallamothu BK, Saint S, Bielak LF, Sonnad SS, Peyser PA, Rubenfire M, et al. Electron-beam computed tomography in the diagnosis of coronary artery disease: a meta-analysis. Arch Intern Med. 2001;161:833–8. [DOI] [PubMed] [Google Scholar]
- 21.Brown BG, Morse J, Zhao XQ, Cheung M, Marino E, Albers JJ. Electron-beam tomography coronary calcium scores are superior to Framingham risk variables for predicting the measured proximal stenosis burden. Am J Cardiol. 2001;88:23E–26E. [DOI] [PubMed] [Google Scholar]
- 22.Lamont DH, Budoff MJ, Shavelle DM, Shavelle R, Brundage BH, Hagar JM. Coronary calcium scanning adds incremental value to patients with positive stress tests. Am Heart J. 2002;143:861–7. [DOI] [PubMed] [Google Scholar]
- 23.O'Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, et al. American College of Cardiology/American Heart Association Expert Consensus Document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol. 2000;36:326–40. [DOI] [PubMed] [Google Scholar]
- 24.Cheng VY, Lepor NE, Madyoon H, Eshaghian S, Naraghi AL, Shah PK. Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183–6. [DOI] [PubMed] [Google Scholar]
- 25.Pundziute G, Schuijf JD, Jukema JW, Boersma E, Scholte AJ, Kroft LJ, et al. Noninvasive assessment of plaque characteristics with multislice computed tomography coronary angiography in symptomatic diabetic patients. Diabetes Care. 2007;30:1113–9. [DOI] [PubMed] [Google Scholar]
- 26.Pundziute G, Schuijf JD, Jukema JW, Boersma E, de Roos A, van der Wall EE, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49:62–70. [DOI] [PubMed] [Google Scholar]
- 27.Abdulla J, Abildstrom SZ, Gotzsche O, Christensen E, Kober L, Torp-Pedersen C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and metaanalysis. Eur Heart J. 2007;28:3042–50. [DOI] [PubMed] [Google Scholar]
- 28.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 29.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 30.Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–54. [DOI] [PubMed] [Google Scholar]
- 31.Hacker M, Jakobs T, Matthiesen F, Vollmar C, Nikolaou K, Becker C, et al. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: first clinical experiences. J Nucl Med. 2005;46:1294–300. [PubMed] [Google Scholar]
- 32.Schuijf JD, Wijns W, Jukema JW, Atsma DE, de Roos A, Lamb HJ, et al. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48:2508–14. [DOI] [PubMed] [Google Scholar]
- 33.Rispler S, Keidar Z, Ghersin E, Roguin A, Soil A, Dragu R, et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49:1059–67. [DOI] [PubMed] [Google Scholar]
- 34.Di Carli MF, Dorbala S, Curillova Z, Kwong RJ, Goldhaber SZ, Rybicki FJ, et al. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol. 2007;14:799–809. [DOI] [PubMed] [Google Scholar]
- 35.van Werkhoven JM, Schuijf JD, Gaemperli O, Jukema JW, Boersma E, Wijns W, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol. 2009;53:623–32. [DOI] [PubMed] [Google Scholar]
- 36.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 37.Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48:783–93. [DOI] [PubMed] [Google Scholar]
- 38.Hesse B, Tagil K, Cuocolo A, Anagnostopoulos C, Bardies M, Bax J, et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging. 2005l;32:855–97. [DOI] [PubMed] [Google Scholar]
- 39.Herzog BA, Husmann L, Landmesser U, Kaufmann PA. Lowdose computed tomography coronary angiography and myocardial perfusion imaging: cardiac hybrid imaging below 3mSv. Eur Heart J. 2009;30:644. [DOI] [PubMed] [Google Scholar]
- 40.Hausleiter J, Meyer T, Hadamitzky M, Huber E, Zankl M, Martinoff S, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113:1305–10. [DOI] [PubMed] [Google Scholar]
- 41.Jakobs TF, Becker CR, Ohnesorge B, Flohr T, Suess C, Schoepf UJ, et al. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002;12:1081–6. [DOI] [PubMed] [Google Scholar]
- 42.Knuuti J. Cardiac hybrid imaging with low radiation dose. J Nucl Cardiol. 2008;15:743–4. [DOI] [PubMed] [Google Scholar]
- 43.Husmann L, Valenta I, Gaemperli O, Adda O, Treyer V, Wyss CA, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–7. [DOI] [PubMed] [Google Scholar]
- 44.Kajander S, Ukkonen H, Sipila H, Teras M, Knuuti J. Low radiation dose imaging of myocardial perfusion and coronary angiography with a hybrid PET/CT scanner. Clin Physiol Funct Imaging. 2009;29:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50:151–61. [DOI] [PubMed] [Google Scholar]