Abstract
Carcinoembryonic antigen (CEeA) is a glycosylated cell surface antigen known to be highly overexpressed in several adenocarcinomas, including colorectal cancer, while demonstrating limited expression in normal tissues. Prior work has shown that the plasma clearance of T84.66, a monoclonal anti-CEA antibody, is enhanced by several-fold in a CEA-expressing xenograft mouse model, suggesting the presence of a target mediated elimination pathway. purpose of this study is to investigate the influence of tumor volume on the plasma clearance of and test the hypothesis that the plasma pharmacokinetics of T84.66 may be used as a sensitive and selective test for the diagnosis of CEA-positive tumors. plasma pharmacokinetics were studied following intravenous (iv) administration of a 1 mg/kg dose in animals without tumor and mice bearing low (20–75 mm3), medium (400–570 mm3), and high volume (800–1,200 mm3) LS174T xenografts.
Based on comparison of the disposition of in non-tumor bearing mice and mice bearing low-volume tumors, it was predicted that a single plasma concentration of obtained seven days after dosing, would provide a sensitive and selective means of determining the presence of tumor in mice. A blinded follow-up study was conducted using athymic mice with or without intraperitoneal LS174T xenografts. 1 mg/kg of 125I-T84.66 was administered iv, and plasma samples were collected on day 7. Comparison of the observed concentration of 125I-T84.66 to the pre-determined threshold value (7.63 nM) enabled identification of tumor bearing mice with a sensitivity of 93.3% and specificity of 100%.
Key words: carcinoembryonic antigen (CEA), target mediated disposition (TMD), T84.66, anti-CEA IgG, screening test, sensitivity, specificityin
Introduction
Colorectal cancer is the third most commonly occurring cancer in the United States, accounting for approximately 9% of all cancer related deaths.1 Colorectal cancer typically develops from noncancerous tissue growths (polyps) that emerge along the inner lining of the colon and rectum. Over time, the adenomatous polyps grow through the muscle wall and invade nearby organs and lymph nodes, eventually leading to widespread metastasis. Colorectal cancer is known to progress slowly over several years, and early detection of localized disease can greatly improve chances for a complete cure.2
Carcinoembryonic antigen (CEA) is a highly glycosylated cell surface protein that was first isolated from human colonic tissue extracts in 1965 by Gold and Freedman.3,4 Extensive research lead to its identification as an important tumor associated antigen, highly overexpressed in colon, breast, lung and pancreatic cancer and in other cancers of epithelial origin.5,6 Subsequent studies noted the presence of elevated serum CEA concentrations in colorectal cancer patients compared to normal subjects,7 suggesting the utility of serum CEA levels as a diagnostic tool and marker for monitoring treatment of colorectal cancer. However, serum CEA concentrations are often elevated in conditions such as pneumonia, hypothyroidism, chronic renal failure, ulcerative colitis, and also with increasing age.8–11 Due to high inter-individual variability, serum CEA has not shown sufficient sensitivity or specificity for use as a diagnostic test for CEA-positive cancers.
Current options for colorectal cancer screening include colonoscopy and computed tomography (virtual colonoscopy), which have acceptable levels of sensitivity and specificity in detecting tumors and pre-malignant adenomatous polyps. Other more convenient screening methods such as fecal occult blood tests, fecal immunochemical tests and stool DNA tests, have been shown to be capable of identifying cancers, but are of limited utility due to their poor sensitivity as “stand alone” diagnostic tests.12 Several studies have examined the use of imaging techniques coupled with radio-labeled anti-CEA antibodies and fragments to assist in the detection of tumors.13–16 These methods are associated with marginal sensitivity and not easily implemented due to the need for image analysis; as a consequence, there has been very little use of anti-CEA antibodies within first line detection tests.
Previously, we compared the plasma disposition of T84.66, a monoclonal anti-CEA antibody, in mice that were tumor-free or bearing LS174T human colorectal cancer xenografts, which express CEA. In animals bearing LS174T tumors, T84.66 time-averaged clearance was increased by 4–7 fold, suggesting the presence of an antigen (or target) mediated elimination pathway.17 In this work, we investigated the influence of tumor volume on the target mediated elimination of T84.66. Additionally, a randomized, blinded study was conducted to test the hypothesis that a tracer dose of T84.66, with collection of a single plasma sample, may be used to detect the presence of LS174T tumors in mice.
T84.66 elimination increased with increasing tumor volume in mice, and in each study group clearance was significantly greater than that found in tumor-free animals. Using a pre-determined threshold concentration of 7.63 nM, a single plasma concentration of T84.66 allowed accurate diagnosis of 93% of animals bearing LS174T tumors with no false-positives in a 30-mouse prospective study. As such, this report provides an example for use of target-mediated disposition of an antibody for the diagnosis of cancer.
Results
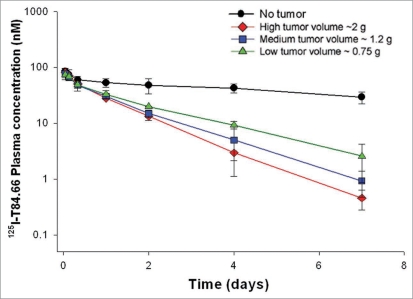
T84.66 plasma pharmacokinetics were studied following a 1 mg/kg intravenous (iv) dose to mice without tumor or with low, medium or high volume LS174T xenografts. Antibody plasma clearance was 0.905 ± 0.170, 2.31 ± 0.36, 2.83 ± 0.31 and 3.08 ± 0.31 mL/h/kg in animals with no tumor and with low, medium and high volume tumors, respectively (Fig. 1). Clearance values in xenograft bearing mice were significantly higher compared to control mice (ANOVA, p < 0.05, for the comparison of each tumor group to the control group). T84.66 clearance in tumor bearing mice increased with increasing tumor volume; however, differences in clearance values between the tumor groups were not statistically significant. The day 7 plasma concentration of T84.66 in control mice was more than 10-fold higher than the corresponding values in xenograft bearing mice (29.2 ± 7.16 nM vs. 2.56 ± 1.68, 0.92 ± 0.45 or 0.46 ± 0.18 nM for control vs. low, medium and high volume xenografts).
Figure 1.
Comparison of anti-CEeA IigG, 125I-Tt84.66 disposition in control and tumor bearing mice. Solid circles represent plasma 125I-concentration data collected from control mice (no tumor). Solid triangles represent plasma 125I-concentration data collected from low volume tumor bearing mice. Solid squares represent plasma 125I-concentration data collected from medium volume tumor bearing mice. Solid diamonds represent plasma 125I-concentration data collected from high volume tumor bearing mice.
For purposes of establishing a diagnostic test, we chose to identify a “threshold concentration” calculated as the mean day 7 plasma concentration for the low volume animals (2.56 nM) plus three times the associated standard deviation (1.68 nM). Following administration of a test dose of 1 mg/kg T84.66, we proposed to identify animals as tumor-positive where the day 7 plasma concentration was determined to be below the threshold, and to identify animals as tumor-negative where the day 7 plasma concentration was found to be above the threshold. The calculated threshold value, 7.63 nM, is expected to be greater than the day 7 concentration that would be found in 99.7% of animals bearing low volume tumors, based on the simple assumptions of a normal distribution.
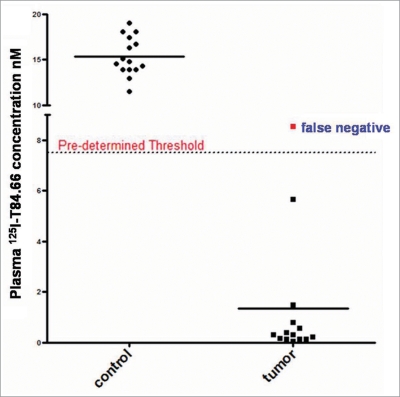
In the independent, blinded, follow up study, 1 mg/kg of 125I labeled T84.66 was administered to 30 mice, where 15 mice were inoculated with saline and the remaining 15 mice were inoculated with LS174T tumor cells in suspension. Plasma T84.66 concentrations were measured on day 7 via gamma counting. A total of 16 mice were identified as tumor negative, and 14 mice were determined to have LS174T xenografts. On comparison of these results with the known classification based on inoculation records of the independent investigator, the test allowed correct identification of 14 out of 15 mice as tumor-positive (1.35 ± 2.53 nM), and 15 out of 15 mice to be tumor-negative (15.4 ± 2.14 nM). Of note, the antibody concentrations observed were similar to those found in the initial study; specifically, the day 7 T84.66 concentrations in tumor-bearing animals were comparable to the values observed for animals bearing low and mid-sized subcutaneous tumors (Fig. 1). The sensitivity [true positive/(true positive + false negative)] of this diagnostic approach was 93.3% and the specificity [true negative/(true negative + false positive)] was 100%, as presented in Figure 2. All results were confirmed by autopsy at the end of the study. The single mouse which was incorrectly identified to be tumor negative was found to have only trace quantities of tumor cells in the peritoneum, with non-quantifiable tumor volume.
Figure 2.
Screening test results for detection of carcinoembryonic antigen (CEeA) overexpressing adenocarcinoma. Tthe left panel presents 125I-Tt84.66 plasma concentrations in mice identified as tumor negative. 125I-concentrations in all mice lie above the predetermined threshold value of 7.63 nM, and hence are correctly identified to be lacking tumor (specificity: 100%). right panel presents 125I-plasma concentrations in mice identified as tumor positive. 125I-concentrations in 14 out of 15 mice lie below the predetermined threshold value of 7.63 nM and, hence, are correctly identified to be lacking tumor (sensitivity: 93.3%). single concentration value above the threshold represents a false negative.
Discussion
There has been rapid, recent growth in the development and use of antibody-based therapies for the treatment and diagnosis of disease, including cancer. Many antibodies demonstrate “target mediated disposition” (TMD),19,20 where antibody binding to the target antigen influences the rate and extent of antibody distribution or elimination. Key determinants of the significance of target-mediated antibody disposition include antigen density, antigen accessibility to the antibody, the extent of differential expression (normal versus cancer tissues), the site of localization (circulating versus membrane bound), antigen turnover, antigen internalization and shedding into systemic circulation. TMD has been shown to contribute to the elimination of therapeutic antibodies directed against antigens such as epidermal growth factor receptor (EGFR),21 HER2 (EGFR2),22 CD20, CD1123 and glycoprotein IIb/IIIa,24 among others.
In previous work conducted in our lab, we reported the influence of tumor-associated CEA on the disposition of a monoclonal anti-CEA antibody, T84.66.17 The present work was conducted to study the influence of tumor volume on T84.66 TMD, and investigate the possible use of T84.66 disposition kinetics for the detection of CEA-positive tumors. Although target-mediated elimination of antibodies has been studied previously by several groups, the possible role of this mechanistic pathway in diagnosis of cancers has not, to our knowledge, been examined.
Since the isolation of CEA from human colonic tissue extracts in the 1960s, extensive work has been conducted examining its role as a tumor marker in several adenocarcinomas25 and also as a target for radioimmunodetection26 and treatment.27 Although there are literature reports describing the distribution of anti-CEA antibodies in animal models28 and clinical studies,29,30 there is a lack of clear understanding of the contribution of this antigen to the plasma clearance of anti-CEA antibodies. In our previous work, we investigated the plasma pharmacokinetics of T84.66 following iv doses of 1, 10 and 25 mg/kg, and we observed that the plasma clearance of T84.66 was enhanced by several fold in animals bearing CEA-positive LS174T tumors. Through the use of a physiologically-based model, we estimated that CEA-mediated elimination of T84.66 was responsible for over 85% of the total body clearance of the antibody, following a dose of 1 mg/kg. This prior work was performed in athymic mice bearing mid-sized tumors (1–1.5 g tumor weight).
To further explore the influence of CEA on anti-CEA IgG disposition, T84.66 pharmacokinetics were investigated in tumor volumes as small as 500–750 mg, to large tumors weighing close to 2 grams. As expected, increasing tumor volumes showed a trend towards increased T84.66 elimination, most likely caused by an increase in the number of CEA sites available for antibody binding and target mediated elimination. Perhaps due to the high variability in tumor volume and the small group sizes used in this study, no significant differences in T84.66 plasma clearance were identified between the groups of xenograft bearing mice. Other factors likely also contribute to the observed variability in T84.66 plasma clearance, potentially including heterogeneous antigen distribution, restricted antigen availability, and barriers to antibody penetration.31,32
Although the study did not identify significant differences in T84.66 plasma clearance between groups of tumor-bearing animals, significant differences were observed between clearance values observed between control mice and tumor-bearing mice. Additionally, very dramatic differences were found between the day 7 T84.66 concentrations observed for control and tumor bearing animals. Day 7 plasma concentrations in the high volume group were 63-fold lower than the corresponding values in control mice. Similarly, T84.66 concentrations in the medium and low volume tumor groups were 30-fold and 10-fold lower than control values. Using the threshold value of 7.63 nM that was derived from the day 7 T84.66 concentration in the low volume group, we were able to identify animals bearing LS174T intraperitoneal tumors with a sensitivity of 93.3% and specificity of 100%.
It is important to note that detection of colorectal cancer in humans is likely to be much more difficult than detection of xenograft tumors within mice. The sensitivity and specificity of the proposed testing strategy will be dependent on the relative rates of target-mediated antibody elimination in tumors (TMDT), target-mediated elimination by non-cancerous tissue (TMDNC), and antibody elimination from all other processes (CLO). Sensitivity and specificity will be greatest when TMDT is much greater than the sum of TMDNC and CLO.
Main determinants of TMDT include the rate of blood flow to colorectal tumors, the lymph flow to the tumors, the efficiency of the convective transport of antibody into the tumor (which is related to the “leakiness” of the tumor vasculature), CEA expression, and the rate of internalization of antibody-CEA complexes. As with any type of “organ clearance”, the maximal possible rate of TMDT is equal to the rate of tumor blood flow. In murine models, the rate of blood flow to the xenograft has been reported to approach 2% of cardiac output,33 whereas in man, the blood flow rate to solid colorectal tumors may be as low as 0.72 ml/min per gram34 of tumor, which equates to ∼0.02% of cardiac output for tumors at the limit of detection by colonoscopy (∼0.5 g). This rate of blood flow, while quite low (∼60 ml/h), compares favorably with the expected rate of CLO (∼8–12 ml/h) for an IgG antibody in man;35,36 however, it is quite possible that the rate of TMDT will be far lower than the rate of blood flow due to limitations associated with the determinants of antibody transport into tumor tissue (convective efficiency, lymph flow) and limitations associated with determinants of antibody elimination from tumor tissue (rates of internalization of CEA-antibody complexes). As such, the ratio of TMTT to CLO may be significantly higher in mice than man.
Additionally, in the xenograft tumor model, human CEA is expressed only on the LS174T tumor surface and, consequently, non-cancerous tissues are unable to contribute to the observed target-mediated elimination of T84.66 (i.e., TMDNC = 0). In humans, low level expression is also found in healthy tissues, including the gastrointestinal tract, prostate cells, esophageal tissue and sweat glands.37–40 It is difficult to predict, a priori, the rate of non-tumor target-mediated clearance of anti-CEA antibody in man; however, it is possible that TMDNC will approximate or exceed TMDT, which will significantly reduce the sensitivity and selectivity of the proposed diagnostic strategy. The magnitude of TMDNC may be addressed, at least to some extent, through the use of a transgenic CEA mouse model.41
The present work demonstrates the application of a simple pharmacokinetic test, based on the concept of target-mediated drug disposition, to detect colorectal tumor xenografts in mice with high sensitivity and selectivity. It is expected that this strategy will be most useful for detection of tumors in cases where the target-mediated clearance pathway is highly specific to tumor tissue, and where this pathway represents the predominant route of antibody elimination. Future work will utilize a physiologically- based model and computer simulation to predict the sensitivity and selectivity of the proposed test for detection of human colorectal cancers.
Materials and Methods
Production and purification of T84.66, anti-CEA IgG.
T84.66 hybridoma cells producing monoclonal anti-CEA IgG were purchased from the American Type Culture Collection (ATCC # HB-8747, Manassas, VA, USA) and grown in T-flasks with complete growth media (DMEM + 10% FBS). Antibody production was scaled up by transferring the cells from T-flasks to 1L spinner flasks containing serum free media (Hybridoma SFM, Invitrogen, Grand Island, NY, USA). T84.66 was purified from culture supernatant by protein-G chromatography (Amersham Biosciences, Uppsala Sweden) using a Bio-Rad medium-pressure chromatography system (Bio-Rad Laboratories, CA, USA). 20 mM Na2HPO4 (pH 7.0, Sigma Chemical) was used as the loading buffer, and the elution buffer was 100 mM glycine (pH 2.8, Sigma Chemical). Eluted antibody was collected in glass tubes containing 1 M Tris buffer (pH 9.0) to neutralize the solution and minimize antibody aggregation.
Radiolabeling of T84.66 with 125I.
Purified T84.66 was labeled with I125 through use of chloramine-T, as described previously.18 Briefly, 10 µl of 125I (100 mCi/ml) was added to 40 µl of T84.66 (∼1 mg/ml in phosphate buffered saline). The reaction was initiated by adding 20 µl of 1 mg/ml chloramine-T. After 90 seconds, the reaction was stopped by addition of 25 µl sodium metabisulfite (2 mg/ml-in phosphate buffer) followed by 40 µl of 10 mg/ml potassium iodide in double distilled water. The reaction mixture was lightly vortexed and immediately loaded on a Sephadex G-25M pre-packed column (GE Healthcare, Uppsala, Sweden). Radiochemical purity of 125I-T84.66 was determined by instant thin-layer chromatography (ITLC, Pall Corporation, NY, USA) and the labeled T84.66 was stored at 4°C until used.
Xenograft development
LS174T human colon cancer cells (ATCC# CL-188, Manassas, VA, USA) known to express human CEA were used to establish xenografts in male athymic Foxnu mice (20–25 g, 5–6 weeks old, Harlan, Indianapolis, IN, USA). Mice were housed in a sterile room, handled under aseptic conditions and fed autoclaved chow and water. Mice were divided into three groups comprising four mice each. 50 µl of LS174T cells (∼1 × 106 cells) were injected subcutaneously into right flank of mice in group I, 100 µl of LS174T cells (∼2 × 106 cells) for group II and 200 µl of LS174T cells (∼4 × 106 cells) in group III. Mice were examined regularly to assess tumor growth and body weight. The tumor size was measured by vernier calipers, and tumor volume was defined by the standard formula: l × w2/2, where ‘l’ represents the length of the longest diameter (mm) and ‘w’ represents the length of the axis perpendicular to l. On day 14, xenograft bearing mice were categorized into three groups based on their tumor volumes. The low tumor volume group comprised mice with tumor volumes in the range of 20–75 mm3, the medium tumor volume group included mice with tumor volumes ranging between 400–570 mm3, and the high tumor volume group consisted of mice with tumor volumes ranging from 800–1,200 mm3. All animal procedures were performed in accordance with the protocols approved by the Institutional Animal Use and Care Committee of the University at Buffalo.
Influence of xenograft volume (LS174T) on T84.66 pharmacokinetics
125I-labelled T84.66 was administered via penile vein injection to control athymic nude mice (without tumor xenografts) and mice bearing low, medium and high volume tumors (n = 4/group). The antibody was injected at a dose of 1 mg/kg (125I activity ∼10 µCi/mouse). Two days prior to administration of 125I-T84.66, all animals were given potassium iodide (0.2 g/L) in their autoclaved drinking water to block the thyroid uptake of 125I. Blood samples were collected from the retro-orbital plexus using calibrated capillary pipettes (Drummond Scientific Company, Cat # 2-000-020) at 1, 3 and 8 hours, and then at 1, 2, 4 and 7 days. Plasma concentrations of T84.66 were determined from radioactive counts obtained on a gamma counter and corrected for background counts and decay.
Use of pharmacokinetics to detect LS174T tumor xenografts
A blinded study was performed to test the hypothesis that a single plasma concentration of T84.66 would allow accurate diagnosis of animals bearing LS174T colorectal cancer xenografts. Thirty male athymic nude mice were used in this study. 200 µl of LS174T adenocarcinoma cells (5 × 106 cells) were injected into the peritoneum of 15 nude mice and 200 µl of sterile saline were injected into the peritoneal cavity of the remaining mice. Use of 5 × 106 cells was based on results of a pilot study that demonstrated generation of low volume tumors and a “take” rate of 100%, with assessment made 10 days after inoculation. Intra-peritoneal administration of cells precludes the growth of externally visible xenografts. Animals were then randomized and labeled by an independent investigator, and their body weights were routinely monitored.
One mg/kg of 125I-T84.66 was administered via penile vein injection to all mice on day 10 following inoculation with LS174T adenocarcinoma cells. A single blood sample (40 µl) was collected by retro-orbital sampling on day 7 following T84.66 administration. Blood was centrifuged at 13,000 rpm (Eppendorf Centrifuge 5415D, 16,000 xg) for 3 min, and the plasma fraction was separated. Radioactive counts were determined by gamma counting, corrected for background counts and decay, and plasma T84.66 concentrations were determined.
Acknowledgements
This work was supported by grants CA114612 and CA118213 from the National Institutes of Health
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/10781
References
- 1.American Cancer Society, authors. Cancer facts & figures 2008. http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
- 2.American Cancer Society, authors. Colorectal cancer facts & figures 2008–2010. http://www.cancer.org/downloads/STT/F861708_finalforweb.pdf.
- 3.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shively JE, Beatty JD. CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol. 1985;2:355–399. doi: 10.1016/s1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- 6.Hefta LJ, Neumaier M, Shively JE. Kinetic and affinity constants of epitope specific anti-carcinoembryonic antigen (CEA) monoclonal antibodies for CEA and engineered CEA domain constructs. Immunotechnology. 1998;4:49–57. doi: 10.1016/s1380-2933(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 7.Thomson DM, Krupey J, Freedman SO, Gold P. The radioimmunoassay of circulating carcinoembryonic antigen of the human digestive system. Proc Natl Acad Sci USA. 1969;64:161–167. doi: 10.1073/pnas.64.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Diego A, Compte L, Sanchis J, Enguidanos MJ, Marco V. Usefulness of carcinoembryonic antigen determination in bronchoalveolar lavage fluid. A comparative study among patients with peripheral lung cancer, pneumonia and healthy individuals. Chest. 1991;100:1060–1063. doi: 10.1378/chest.100.4.1060. [DOI] [PubMed] [Google Scholar]
- 9.Amino N, Kuro R, Yabu Y, Takai SI, Kawashima M, Morimoto S, et al. Elevated levels of circulating carcinoembryonic antigen in hypothyroidism. J Clin Endocrinol Metab. 1981;52:457–462. doi: 10.1210/jcem-52-3-457. [DOI] [PubMed] [Google Scholar]
- 10.Stevens DP, Mackay IR. Increased carcinoembryonic antigen in heavy cigarette smokers. Lancet. 1973;2:1238–1239. doi: 10.1016/s0140-6736(73)90975-6. [DOI] [PubMed] [Google Scholar]
- 11.Witherspoon LR, Shuler SE, Alyea K, Husserl FE. Carcinoembryonic antigen: assay following heat compared with perchloric acid extraction in patients with colon cancer, non-neoplastic gastrointestinal diseases, or chronic renal failure. J Nucl Med. 1983;24:916–921. [PubMed] [Google Scholar]
- 12.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Yazaki PJ, Wu AM, Tsai SW, Williams LE, Ikler DN, Wong JY, et al. Tumor targeting of radiometal labeled anti-CEA recombinant T84.66 diabody and t84.66 minibody: comparison to radioiodinated fragments. Bioconjug Chem. 2001;12:220–228. doi: 10.1021/bc000092h. [DOI] [PubMed] [Google Scholar]
- 14.Sundaresan G, Yazaki PJ, Shively JE, Finn RD, Larson SM, Raubitschek AA, et al. 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med. 2003;44:1962–1969. [PMC free article] [PubMed] [Google Scholar]
- 15.Olafsen T, Cheung CW, Yazaki PJ, Li L, Sundaresan G, Gambhir SS, et al. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng Des Sel. 2004;17:21–27. doi: 10.1093/protein/gzh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride WJ, Zanzonico P, Sharkey RM, Noren C, Karacay H, Rossi EA, et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med. 2006;47:1678–1688. [PubMed] [Google Scholar]
- 17.Urva SR, Yang VC, Balthasar JP. Physiologically based pharmacokinetic model for T84.66: A monoclonal anti-CEA antibody. J Pharm Sci. 2010;99:1582–1600. doi: 10.1002/jps.21918. [DOI] [PubMed] [Google Scholar]
- 18.Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34:687–709. doi: 10.1007/s10928-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 19.Levy G. Pharmacologic target-mediated drug disposition. Clinical pharmacology and therapeutics. 1994;56:248–252. doi: 10.1038/clpt.1994.134. [DOI] [PubMed] [Google Scholar]
- 20.Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–532. doi: 10.1023/a:1014414520282. [DOI] [PubMed] [Google Scholar]
- 21.Rowinsky EK, Schwartz GH, Gollob JA, Thompson JA, Vogelzang NJ, Figlin R, et al. Safety, pharmacokinetics and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol. 2004;22:3003–3015. doi: 10.1200/JCO.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 22.McKeage K, Perry CM. Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs. 2002;62:209–243. doi: 10.2165/00003495-200262010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Coffey GP, Fox JA, Pippig S, Palmieri S, Reitz B, Gonzales M, et al. Tissue distribution and receptor-mediated clearance of anti-CD11a antibody in mice. Drug metab dispos. 2005;33:623–629. doi: 10.1124/dmd.104.002584. [DOI] [PubMed] [Google Scholar]
- 24.Mager DE, Mascelli MA, Kleiman NS, Fitzgerald DJ, Abernethy DR. Simultaneous modeling of abciximab plasma concentrations and ex vivo pharmacodynamics in patients undergoing coronary angioplasty. J Pharmacol Exp Ther. 2003;307:969–976. doi: 10.1124/jpet.103.057299. [DOI] [PubMed] [Google Scholar]
- 25.Chung JK, Jang JJ, Lee DS, Lee MC, Koh CS. Tumor concentration and distribution of carcinoembryonic antigen measured by in vitro quantitative autoradiography. J Nucl Med. 1994;35:1499–1505. [PubMed] [Google Scholar]
- 26.Goldenberg DM, Kim EE, DeLand FH, Bennett S, Primus FJ. Radioimmunodetection of cancer with radioactive antibodies to carcinoembryonic antigen. Cancer Res. 1980;40:2984–2992. [PubMed] [Google Scholar]
- 27.Blumenthal RD, Osorio L, Hayes MK, Horak ID, Hansen HJ, Goldenberg DM. Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft. Cancer Immunol Immunother. 2005;54:315–327. doi: 10.1007/s00262-004-0597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55:4611–4622. [PubMed] [Google Scholar]
- 29.Wong JY, Thomas GE, Yamauchi D, Williams LE, Odom-Maryon TL, Liu A, et al. Clinical evaluation of indium-111-labeled chimeric anti-CEA monoclonal antibody. J Nucl Med. 1997;38:1951–1959. [PubMed] [Google Scholar]
- 30.Odom-Maryon TL, Williams LE, Chai A, Lopatin G, Liu A, Wong YC, et al. Pharmacokinetic modeling and absorbed dose estimation for chimeric anti-CEA antibody in humans. J Nucl Med. 1997;38:1959–1966. [PubMed] [Google Scholar]
- 31.van Osdol W, Fujimori K, Weinstein JN. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a “binding site barrier”. Cancer Res. 1991;51:4776–4784. [PubMed] [Google Scholar]
- 32.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52:5144–5153. [PubMed] [Google Scholar]
- 33.Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54:1517–1528. [PubMed] [Google Scholar]
- 34.Goh V, Halligan S, Daley F, Wellsted DM, Guenther T, Bartram CI. Colorectal tumor vascularity: quantitative assessment with multidetector CT-do tumor perfusion measurements reflect angiogenesis? Radiology. 2008;249:510–517. doi: 10.1148/radiol.2492071365. [DOI] [PubMed] [Google Scholar]
- 35.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 37.Hammarstrom S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 38.Prall F, Nollau P, Neumaier M, Haubeck HD, Drzeniek Z, Helmchen U, et al. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium and myeloid cells in a wide range of normal human tissues. J Histochem Cytochrom. 1996;44:35–41. doi: 10.1177/44.1.8543780. [DOI] [PubMed] [Google Scholar]
- 39.Metze D, Bhardwaj R, Amann U, Eades-Perner AM, Neumaier M, Wagener C, et al. Glycoproteins of the carcinoembryonic antigen (CEA) family are expressed in sweat and sebaceous glands of human fetal and adult skin. J Invest Dermatol. 1996;106:64–69. doi: 10.1111/1523-1747.ep12327258. [DOI] [PubMed] [Google Scholar]
- 40.Nap M, Hammartrom ML, Bormer O, Hammarstrom S, Wagener C, Handt S, et al. Specificity and affinity of monoclonal antibodies against carcinoembryonic antigen. Cancer Res. 1992;52:2329–2339. [PubMed] [Google Scholar]
- 41.Wilkinson RW, Ross EL, Ellison D, Zimmermann W, Snary D, Mather SJ. Evaluation of a transgenic mouse model for anti-human CEA radioimmunotherapeutics. J Nucl Med. 2002;43:1368–1376. [PubMed] [Google Scholar]