Abstract
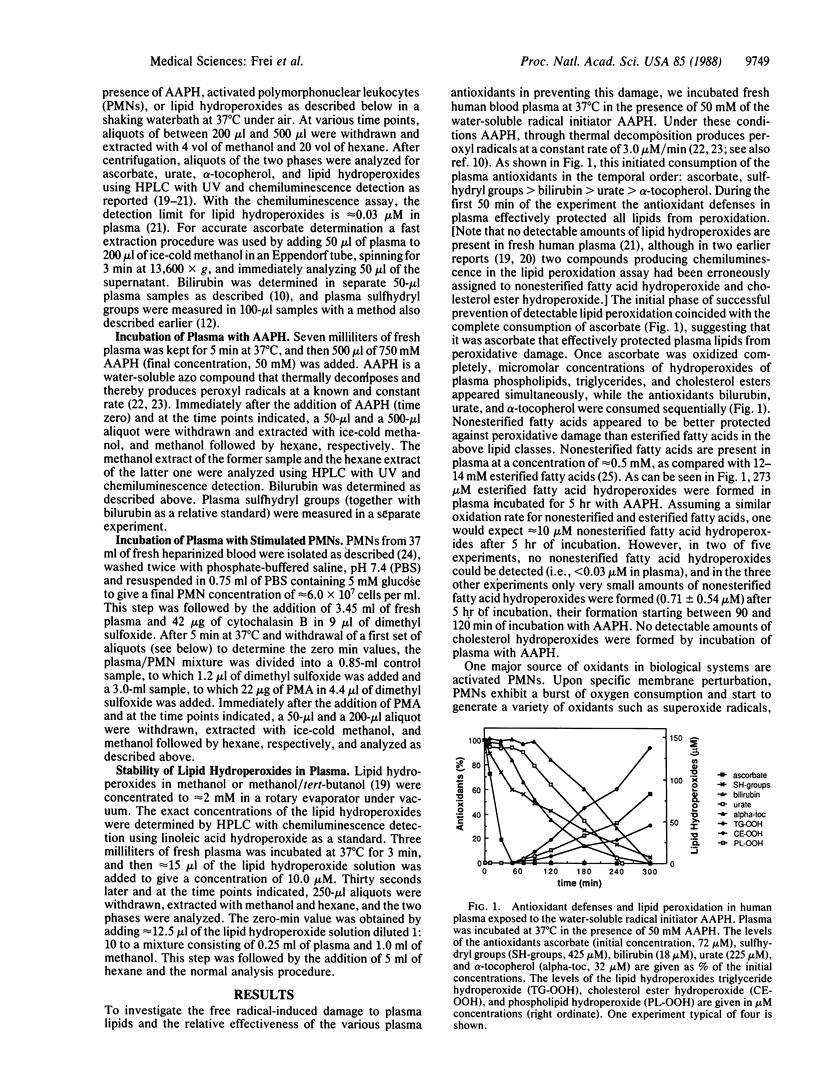
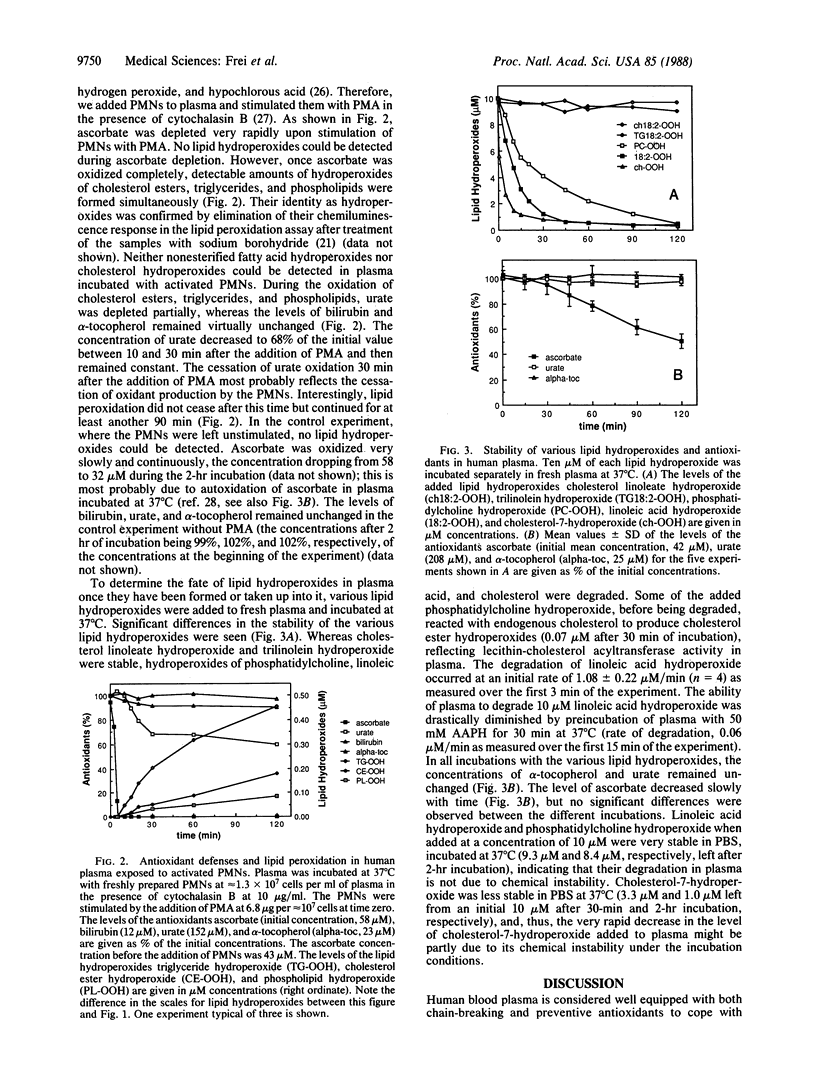
The temporal disappearance in human blood plasma of endogenous antioxidants in relation to the appearance of various classes of lipid hydroperoxides measured by HPLC postcolumn chemiluminescence detection has been investigated under two types of oxidizing conditions. Exposure of plasma to aqueous peroxyl radicals generated at a constant rate leads immediately to oxidation of endogenous ascorbate and sulfhydryl groups, followed by sequential depletion of bilirubin, urate, and alpha-tocopherol. Stimulating polymorphonuclear leukocytes in plasma initiates very rapid oxidation of ascorbate, followed by partial depletion of urate. Once ascorbate is consumed completely, micromolar concentrations of hydroperoxides of plasma phospholipids, triglycerides, and cholesterol esters appear simultaneously, even though sulfhydryl groups, bilirubin, urate, and alpha-tocopherol are still present at high concentrations. Nonesterified fatty acids, the only lipid class in plasma not transported in lipoproteins but bound to albumin, are preserved from peroxidative damage even after complete oxidation of ascorbate, most likely due to site-specific antioxidant protection by albumin-bound bilirubin and possibly by albumin itself. Thus, in plasma ascorbate and, in a site-specific manner, bilirubin appear to be much more effective in protecting lipids from peroxidative damage by aqueous oxidants than all the other endogenous antioxidants. Hydroperoxides of linoleic acid, phosphatidylcholine, and cholesterol added to plasma in the absence of added reducing substrates are degraded, in contrast to hydroperoxides of trilinolein and cholesterol linoleate. These findings indicate the presence of a selective peroxidase activity operative under physiological conditions. Our data suggest that in states of leukocyte activation and other types of acute or chronic oxidative stress such a simple regimen as controlled ascorbate supplementation could prove helpful in preventing formation of lipid hydroperoxides, some of which cannot be detoxified by endogenous plasma activities and thus might cause damage to critical targets.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. W., Joyce A., Ingold K. U. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet. 1982 Aug 7;2(8293):327–327. doi: 10.1016/s0140-6736(82)90293-8. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin G., Djursäter R. Peroxidation of phospholipids promoted by myeloperoxidase. Free Radic Res Commun. 1988;4(4):251–257. doi: 10.3109/10715768809055150. [DOI] [PubMed] [Google Scholar]
- Frei B., Yamamoto Y., Niclas D., Ames B. N. Evaluation of an isoluminol chemiluminescence assay for the detection of hydroperoxides in human blood plasma. Anal Biochem. 1988 Nov 15;175(1):120–130. doi: 10.1016/0003-2697(88)90369-7. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. Measurement of allantoin and uric acid in human body fluids. A potential index of free-radical reactions in vivo? Biochem J. 1987 May 1;243(3):803–808. doi: 10.1042/bj2430803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B., Moorhouse C. P. Action of uric acid, allopurinol and oxypurinol on the myeloperoxidase-derived oxidant hypochlorous acid. Free Radic Res Commun. 1987;4(2):69–76. doi: 10.3109/10715768709088090. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988 Feb 15;37(4):569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Wasil M., Grootveld M. Biologically significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by ascorbic acid. Implications for antioxidant protection in the inflamed rheumatoid joint. FEBS Lett. 1987 Mar 9;213(1):15–17. doi: 10.1016/0014-5793(87)81456-4. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Enhancement of immunologically induced granule exocytosis from neutrophils by cytochalasin B. J Immunol. 1973 Jan;110(1):290–293. [PubMed] [Google Scholar]
- Horton A. A., Fairhurst S. Lipid peroxidation and mechanisms of toxicity. Crit Rev Toxicol. 1987;18(1):27–79. doi: 10.3109/10408448709089856. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitz B., Siegel B. V. Ascorbic acid and the immune response. Adv Exp Med Biol. 1981;135:1–25. doi: 10.1007/978-1-4615-9200-6_1. [DOI] [PubMed] [Google Scholar]
- Maddipati K. R., Gasparski C., Marnett L. J. Characterization of the hydroperoxide-reducing activity of human plasma. Arch Biochem Biophys. 1987 Apr;254(1):9–17. doi: 10.1016/0003-9861(87)90075-0. [DOI] [PubMed] [Google Scholar]
- Maddipati K. R., Marnett L. J. Characterization of the major hydroperoxide-reducing activity of human plasma. Purification and properties of a selenium-dependent glutathione peroxidase. J Biol Chem. 1987 Dec 25;262(36):17398–17403. [PubMed] [Google Scholar]
- Orringer E. P., Roer M. E. An ascorbate-mediated transmembrane-reducing system of the human erythrocyte. J Clin Invest. 1979 Jan;63(1):53–58. doi: 10.1172/JCI109277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Steinbrecher U. P., Barnett J., Witztum J. L., Steinberg D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 May;82(9):3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O., Koller E., Jürgens G., Esterbauer H. Investigation of lipid peroxidation in human low density lipoprotein. Free Radic Res Commun. 1987;3(1-5):233–242. doi: 10.3109/10715768709069788. [DOI] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Winterhalter K. H., Richter C. Increased fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene in the phorbol myristate acetate-stimulated plasma membrane of human neutrophils. FEBS Lett. 1982 Aug 2;144(2):199–203. doi: 10.1016/0014-5793(82)80637-6. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med. 1974 Sep;47(3):223–233. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Avissar N., Whitin J., Cohen H. Purification and characterization of human plasma glutathione peroxidase: a selenoglycoprotein distinct from the known cellular enzyme. Arch Biochem Biophys. 1987 Aug 1;256(2):677–686. doi: 10.1016/0003-9861(87)90624-2. [DOI] [PubMed] [Google Scholar]
- Terao J., Shibata S. S., Matsushita S. Selective quantification of arachidonic acid hydroperoxides and their hydroxy derivatives in reverse-phase high performance liquid chromatography. Anal Biochem. 1988 Mar;169(2):415–423. doi: 10.1016/0003-2697(88)90306-5. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U., Barclay L. R., Locke S. J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987 Jun 22;924(3):408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Wendel A., Cikryt P. The level and half-life of glutathione in human plasma. FEBS Lett. 1980 Nov 3;120(2):209–211. doi: 10.1016/0014-5793(80)80299-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Ames B. N. Detection of lipid hydroperoxides and hydrogen peroxide at picomole levels by an HPLC and isoluminol chemiluminescence assay. Free Radic Biol Med. 1987;3(5):359–361. doi: 10.1016/s0891-5849(87)80048-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Brodsky M. H., Baker J. C., Ames B. N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem. 1987 Jan;160(1):7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]



