Abstract
AIM: To establish the prognosis and feasibility of en-bloc vascular resection of stage II pancreatic adenocarcinoma of the head and uncinate process.
METHODS: We retrospectively analyzed 87 patients with stage II pancreatic adenocarcinoma, who were subjected to pancreaticoduodenectomy (PD) and pylorus-preserving PD (PPPD) between 1996 and 2006 in Chang Gung Memorial Hospital, Taiwan. Twelve and 75 patients underwent PD/PPPD with and without resection of portal vein/superior mesenteric vein (PV/SMV), respectively.
RESULTS: The overall 1- and 3-year survival rates of patients undergoing PD/PPPD with and without vascular resection were 50.0% and 16.7%, and 44.4% and 12.2%, respectively. Morbidity and mortality rates in the PV/SMV resection vs non-resection group were 50.0% and 0.0%, and 40.0% and 2.7%, respectively. In multivariate analysis, serum bilirubin, histological differentiation and adjuvant chemotherapy were independent prognostic factors that influenced survival.
CONCLUSION: In stage II adenocarcinoma of the pancreatic head and uncinate process, serum bilirubin, histological differentiation and adjuvant chemotherapy were independent prognostic factors, and en-bloc vascular resection is a feasible option in carefully selected patients.
Keywords: Pancreatic neoplasms, Adenocarcinoma, Portal vein, Superior mesenteric vein, Pancreaticoduodenectomy, Chemotherapy
INTRODUCTION
Pancreaticoduodenectomy (PD) combined with vascular resection in locally advanced pancreatic malignancies was initially associated with high morbidity and mortality rates[1]. However, with improvement in surgical techniques and postoperative care, more aggressive en-bloc resection of pancreatic malignancies, along with portal vein/superior mesenteric vein (PV/SMV) is being carried out at present, without any increase in surgical complications[2]. Unexpected tumor invasion to the lateral or posterolateral wall of the confluence of the PV/SMV is a common finding during PD for pancreatic malignancies[3]. The 5-year survival rate following PD or pylorus-preserving PD (PPPD) is 10%-15%, and the reported survival for > 5 years is less[4-6]. Mortality associated with PD has decreased dramatically to 0%-5% over the past two decades, but morbidity remains as high as 35%-60%[7-11]. Few studies are available regarding stage II pancreatic adenocarcinoma with special attention to adenocarcinoma located in the pancreatic head and uncinate process, which is more likely to invade the PV/SMV because of its close proximity to these vessels. The aim of the present study was to establish the prognostic factors and feasibility of en-bloc vascular resection in patients with stage II adenocarcinoma of the pancreatic head and uncinate process following PD/PPPD.
MATERIALS AND METHODS
Patient population
From January 1996 to December 2006, 129 consecutive patients with stage I to III adenocarcinoma of the pancreatic head and uncinate process were subjected to surgery at Chang Gung Memorial Hospital, Taipei, Taiwan. We included only stage II adenocarcinoma of the pancreatic head and uncinate process [87 patients; stage IIA (n = 14) and IIB (n = 73)], as there is more possibility of vascular encasement in stage II pancreatic cancers located in the head and uncinate process. The tumors were staged according to the 6th edition of the American Joint Committee on Cancer Staging System (2002). Survival duration was calculated from the time of surgery to death or the last follow-up date (December 31, 2007), irrespective of the cause of death.
Surgical procedure
PD and PPPD were considered as standard procedures. Resection margins from the common bile duct, pancreatic neck, retropancreatic tissue, and from the PV or SMV (in PV/SMV resection) were sent routinely for frozen sectioning, and in cases with positive resection margins, wider resection was performed until a negative resection margin was achieved. Lymph nodes around the hepaticoduodenal ligament, common hepatic artery, celiac trunk, PV, SMV and retropancreatic area were routinely dissected and removed. Reconstruction was performed by pancreaticojejunostomy. In case of PV/SMV encasement, segmental resection and reconstruction by end-to-end anastomosis (n = 9) or a vascular graft (n = 3; one autologous and two ePTFE grafts) were performed. Ten patients had segmental resection of the PV and two had combined PV/SMV segmental resection, and the splenic vein was anastomosed to the main portal trunk in both cases. All the patients who underwent vascular reconstruction were treated with a single dose of heparin intraoperatively and postoperatively; heparin was not used routinely. These patients were monitored by Doppler study for vascular graft patency in the early postoperative period.
Statistical analysis
Clinical records were compared by either Fischer’s exact test or Pearson’s χ2 test, as appropriate. Age was analyzed using Student’s t test. Patient survival rate was calculated by the methods of Kaplan-Meier and log-rank test to determine univariate significance. Factors that were deemed of potential importance on the univariate analysis (P < 0.05) were included in the multivariate analysis. Cox’s regression was used for the multivariate analysis of these factors. P < 0.05 was regarded as significant. Statistical analyses were performed with SPSS for windows, version 11.5 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Patient demographics
Twelve of 87 (13.8%) patients underwent PD (n = 8) or PPPD (n = 4) with PV/SMV resection (group I) and 75/87 (86.2%) patients underwent PD (n = 57) or PPPD (n = 18) without vascular resection (group II). Tumor location was in the pancreatic head (n = 80), uncinate process (n = 1), and in both (n = 6).
Patient outcome
Analysis of clinicopathological features (Table 1) revealed predominantly male patients aged > 60 years old, and common symptoms were jaundice, abdominal pain and weight loss. Most common findings were jaundice and anemia. Preoperative biliary drainage was performed (in case of total bilirubin levels > 15 mg/dL) in six (50.0%) and 44 (58.7%) patients in group I and II, respectively. Curative resection was possible in all 12 (100.0%) patients in group I and in only 48 (64.0%) in group II. Adjuvant chemotherapy (5-fluorouracil, gemcitabine and cisplatin) was given to eight (66.7%) and 41 (54.7%) patients in group I and II, respectively. Surgical mortality rate (within 1 mo) was 0.0% and 2.7% in group I and II, respectively. The two deaths in group II were due to sepsis and multiorgan failure. The overall surgical complication rate was 41.4% and the complication rate was higher in group I (50.0% vs 40.0%) (Table 2).
Table 1.
Clinicopathological features of patients with stage II pancreatic adenocarcinoma (mean ± SD) n (%)
| Parameters | With vascular resection (n = 12) | Without vascular resection (n = 75) | P value |
| Age (yr) | 62.9 ± 11.0 | 62.9 ± 9.8 | 0.994 |
| Gender | 0.745 | ||
| Male | 7 (58.3) | 50 (66.7) | |
| Female | 5 (41.7) | 25 (33.3) | |
| Symptoms | |||
| Jaundice | 6 (50.0) | 55 (73.3) | 0.171 |
| Abdominal pain | 6 (50.0) | 35 (46.7) | 0.830 |
| Body weight loss | 6 (50.0) | 31 (41.3) | 0.573 |
| Anorexia | 3 (25.0) | 17 (22.7) | 1.000 |
| Signs | |||
| Anemic | 4 (33.3) | 38 (50.7) | 0.265 |
| Icterus | 6 (50.0) | 54 (72.0) | 0.178 |
| Abdominal tenderness | 3 (25.0) | 15 (20.0) | 0.707 |
| Albumin (g/dL) | 3.9 ± 0.6 | 3.7 ± 0.5 | 0.205 |
| Total bilirubin (mg/dL) | 6.8 ± 7.0 | 8.9 ± 7.1 | 0.333 |
| Pre-op biliary drainage | 0.573 | ||
| Yes | 6 (50.0) | 44 (58.7) | |
| No | 6 (50.0) | 31 (41.3) | |
| CEA (ng/mL) | 1.000 | ||
| ≤ 5 | 5 (62.5) | 36 (62.1) | |
| > 5 | 3 (37.5) | 22 (37.9) | |
| CA19-9 (U/mL) | 0.411 | ||
| ≤ 37 | 3 (33.3) | 13 (21.0) | |
| > 37 | 6 (66.7) | 49 (79.0) | |
| Operation time (min) | 473.9 ± 185.2 | 461.6 ± 110.4 | 0.826 |
| Blood transfusion (mL) | 396 ± 588 | 304 ± 590 | 0.618 |
| Tumor size (cm) | 3.6 ± 1.6 | 3.3 ± 1.4 | 0.526 |
| Lymph node metastases | 0.097 | ||
| Yes | 8 (66.7) | 65 (86.7) | |
| No | 4 (33.3) | 10 (13.3) | |
| Curability | 0.015 | ||
| Yes | 12 (100.0) | 48 (64.0) | |
| No | 0 (0.0) | 27 (36.0) | |
| Postoperative chemotherapy | 0.436 | ||
| Yes | 8 (66.7) | 41 (54.7) | |
| No | 4 (33.3) | 34 (45.3) | |
CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen.
Table 2.
Morbidity and mortality in patients with stage II pancreatic adenocarcinoma n (%)
| Parameters | With vascular resection (n = 12) | Without vascular resection (n = 75) |
| Morbidity | 6/12 (50.0) | 30/75 (40.0) |
| Pancreatic leakage | 2 (16.7) | 9 (12.0) |
| Pancreatic fistula | 0 (0.0) | 8 (10.7) |
| Gastrointestinal bleeding | 1 (8.3) | 6 (8.0) |
| Pleural effusion | 2 (8.3) | 4 (5.3) |
| Delayed gastric emptying | 1 (8.3) | 3 (4.0) |
| Wound infection | 0 (0.0) | 4 (4.0) |
| Intra-abdominal abscess | 0 (0.0) | 4 (5.3) |
| Bile leakage | 1 (8.3) | 3 (4.0) |
| Sepsis | 0 (0.0) | 3 (4.0) |
| Intra-abdominal bleeding | 1 (8.3) | 2 (2.7) |
| Mortality | 0 (0.0) | 2 (2.7) |
Survival period
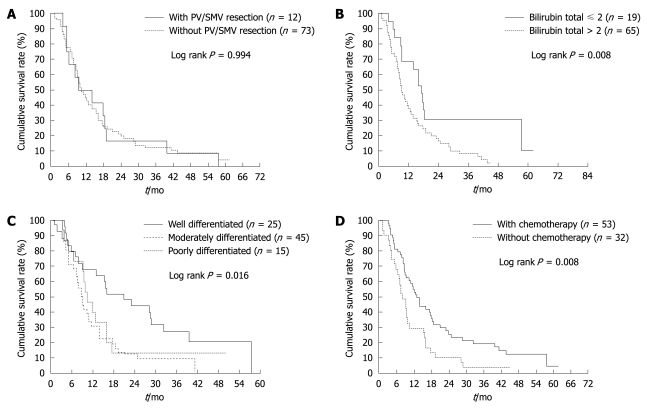
The median period of follow-up was 10.36 mo (range: 1.18-61.68 mo), and the last follow up date was December 31, 2007. The overall survival rate at 1 year and 3 years for stage II adenocarcinoma of the pancreatic head and uncinate process, with PV/SMV resection, was 50.0% and 16.7%, respectively in group I and 44.4% and 12.2% in group II (Figure 1A). Two patients in group I survived for > 3 years; one patient died after 4 years and the other survived for 4.5 years and is still under follow-up. Univariate (Table 3) and multivariate (Table 4) analyses revealed that serum bilirubin, histological differentiation and adjuvant chemotherapy were significant prognostic factors (P < 0.05).
Figure 1.
Overall survival in stage II adenocarcinoma of pancreatic head and uncinate process after pancreaticoduodenectomy (PD)/pylorus-preserving PD (PPPD). A: With and without PV/SMV resection; B: With preoperative total bilirubin level ≤ or > 2 mg/dL; C: In terms of histological differentiation; D: Treated with and without adjuvant chemotherapy.
Table 3.
Univariate analyses of predictive factors for survival of patients with stage II adenocarcinoma of the pancreas after PD or PPPD, with or without vascular resection
| Parameters | Median (mo) | 95% CI of median | 3-yr survival (%) | P value |
| Age (yr) | 0.852 | |||
| ≤ 70 (n = 62) | 11.51 | 7.78-15.25 | 12.0 | |
| > 70 (n = 23) | 9.40 | 9.15-9.65 | 16.3 | |
| Sex | 0.191 | |||
| Male (n = 55) | 9.76 | 7.61-11.91 | 9.6 | |
| Female (n = 30) | 14.01 | 3.20-24.82 | 19.4 | |
| Albumin (g/dL) | 0.107 | |||
| ≤ 3.5 (n = 21) | 7.40 | 3.86-10.94 | 12.7 | |
| > 3.5 (n = 47) | 11.67 | 5.96-17.38 | 12.8 | |
| Total bilirubin (mg/dL) | 0.008 | |||
| ≤ 2 (n = 19) | 17.39 | 15.23-19.55 | 30.7 | |
| > 2 (n = 65) | 9.40 | 7.66-11.14 | 3.5 | |
| Serum CA 19-9 (U/L) | 0.167 | |||
| ≤ 37 (n = 16) | 16.11 | 1.47-30.75 | 25.0 | |
| > 37 (n = 54) | 11.51 | 8.17-14.85 | 12.3 | |
| Serum CEA (ng/mL) | 0.455 | |||
| ≤ 5 (n = 40) | 16.08 | 10.78-21.38 | 13.4 | |
| > 5 (n = 24) | 7.82 | 5.18-10.46. | 16.7 | |
| Blood transfusion | 0.491 | |||
| Yes (n = 47) | 10.88 | 7.17-14.59 | 14.9 | |
| No (n = 38) | 9.24 | 5.59-12.89 | 9.8 | |
| Tumor size (cm) | ||||
| ≤ 3 (n = 43) | 13.97 | 10.77-17.18 | 13.3 | 0.508 |
| > 3 (n = 41) | 9.27 | 8.82-9.72 | 12.5 | |
| Nodal metastases | 0.557 | |||
| Yes (n = 71) | 9.76 | 7.69-11.83 | 13.6 | |
| No (n = 14) | 12.16 | 0.00-28.36 | 8.3 | |
| Pre-op biliary drainage | 0.262 | |||
| Yes (n = 49) | 9.24 | 7.80-10.68 | 12.0 | |
| No (n = 36) | 13.97 | 9.44-18.50 | 14.3 | |
| PV/SMV resection | 0.994 | |||
| Yes (n = 12) | 9.27 | 0.00-19.54 | 16.7 | |
| No (n = 73) | 10.36 | 7.87-12.86 | 12.2 | |
| Resection margin1 | 0.071 | |||
| Negative (n = 58) | 11.51 | 6.46-16.56 | 16.1 | |
| Positive (n = 27) | 9.27 | 7.46-11.08 | 5.1 | |
| Differentiation | 0.016 | |||
| Well (n = 25) | 20.98 | 9.02-32.94 | 24.0 | |
| Moderately (n = 45) | 8.81 | 7.81-9.81 | 8.8 | |
| Poorly (n = 15) | 10.49 | 7.01-13.97 | 6.7 | |
| Adjuvant chemotherapy | 0.008 | |||
| Yes (n = 53) | 12.99 | 10.44-12.74 | 18.8 | |
| No (n = 32) | 7.96 | 5.74-10.19 | 3.2 | |
Two cases of mortality were not included in survival analysis.
Resection margin was negative in all 12 patients who underwent vascular resection.
Table 4.
Multivariate analysis in stage II pancreatic adenocarcinoma of the head and uncinate process
| Parameters | Hazard ratio (95% CI) | P value |
| Bilirubin (mg/dL) | ||
| ≤ 2/> 2 | 2.024 (1.613-3.774) | 0.026 |
| Differentiation | 0.005 | |
| Moderately/well | 2.412 (1.379-4.217) | 0.002 |
| Poorly/well | 2.091 (1.034-4.225) | 0.040 |
| Adjuvant chemotherapy | ||
| No/yes | 2.068 (1.270-3.366) | 0.003 |
| PV/SMV resection | ||
| Yes/no | 0.591 |
The 1- and 3-year survival rate in patients with and without elevation of serum bilirubin (> or ≤ 2 mg/dL) was 39.1% and 8.2%, and 68.4% and 30.7%, respectively (Figure 1B). In analysis of tumor factors, that is, histological differentiation, the 1-year survival rates in well, moderately well and poorly differentiated groups were 68.0%, 31.8% and 46.7%, respectively. The 3-year survival rates in these three categories were 24.0%, 8.8% and 6.7%, respectively. Better survival was found in the well-differentiated group (Figure 1C). The 1- and 3-year survival in patients treated with or without adjuvant chemotherapy was 54.7% and 18.8%, and 29.0% and 3.2%, respectively (Figure 1D).
DISCUSSION
In our study, the overall 1- and 3-year survival was comparable to that in the study by Fukuda et al[12], which had a survival rate of 47% and 26.8%, respectively, in group I, and 63.4% and 28.4% in group II patients. However, van Geenen et al[13] have reported 55% and 6%, respectively, Ye et al[14] have reported 37.7% and 5.6%, respectively, and Launois et al[15] have reported 42.4% and 11.9% in group I, which is slightly less than the survival rates in our study. The 3-year survival in the studies of Aramaki et al[16] and Carrère et al[17] was 21.3% and 20.0%, and 22.0% and 25.0%, in group I and II, respectively. All the above studies included all stages of pancreatic cancer, irrespective of location in the pancreas. This differs from our study, in which we focused only on stage II adenocarcinoma localized in the pancreatic head and uncinate process, where there is more probability of vascular encasement.
In our study, the mean survival time in patients undergoing curative PV/SMV resection was 16.28 mo. There was not much difference in the mortality rate in PD/PPPD with or without vascular resection, but the associated morbidity was higher in the vascular resection group. This is in contrast to earlier studies that have found that PV/SMV resection does not greatly influence morbidity and mortality in PD[12,18]. In our study, all 12 patients in group I had negative resection margins. Previous studies have reported that the resectability rate is high in PD with vascular resection[14]. PD/PPD with en-bloc vascular resection potentially provides an opportunity to achieve negative resection margins, and thus might be beneficial in achieving better survival rates in carefully selected patients with pancreatic adenocarcinoma[19,20]. Hence, in patients who were subjected to palliative treatment alone, based on their preoperative evaluation that showed PV/SMV encasement, some carefully selected patients, as determined by preoperative CT [length and severity (complete vs partial circumference) of vascular involvement], may be suitable candidates for en-bloc resection of PV/SMV, thus achieving better survival rates. Earlier studies have suggested that encasement of PV or SMV is a function of tumor location rather than more aggressive behavior, and almost equal or even better survival rates can be achieved by en-bloc resection of PV/SMV[12,19]. Our study shows that en-bloc vascular resection in stage II pancreatic adenocarcinoma is a feasible option in carefully selected patients. Hence, vascular encasement should not be considered as a contraindication for surgery; risk must be balanced against the benefit by case to case evaluation.
Serum bilirubin, histological differentiation and adjuvant chemotherapy were significant prognostic factors in our series. Previous studies have focused on the significance of depth of PV invasion[18], lymph node metastasis[21], tumor size[22], negative resection margin[23], and adjuvant chemotherapy[24] in pancreatic adenocarcinoma. These preoperative and intraoperative factors help in deciding the extent of resection, proper planning of adjuvant therapy, and predicting the survival outcome in these patients. In our study, preoperative biliary drainage has no statistical significance in the outcome of stage II pancreatic adenocarcinoma, similar to an earlier study[25]. Few studies have reported the potential advantages of preoperative biliary drainage, which include improved nutritional, metabolic and immune function, and the possibility of reduced postoperative morbidity and mortality[26,27]. In contrast, one study has reported that the biliary stents induce bacterial contamination and enhance the risk of cholangitis because of clogging. Biliary stenting also generates a severe inflammatory response adjacent to the wall of the bile duct, which is probably a factor that is responsible for increased risk of bile leakage and infection after biliodigestive reconstruction[28]. An experimental study has indicated that a period of 4-6 wk is necessary to recover metabolic and immune functions so that some benefit may be achieved by preoperative biliary drainage[29].
Histological differentiation was found to be significant in our study in determining survival outcome. Patients with well-differentiated adenocarcinoma had better survival than those with moderately well and poorly differentiated adenocarcinoma. Earlier studies by Sohn et al[22], Riediger et al[23] and Yamaguchi et al[30] have highlighted the significance of histological differentiation as a prognostic factor in pancreatic adenocarcinoma.
Adjuvant chemotherapy was also found to be statistically significant. In our study, patients who received adjuvant chemotherapy had better survival than those without chemotherapy. Adjuvant chemotherapy in pancreatic cancer substantially improved the disease-free survival and overall increase in survival rate, as shown by our study and an earlier one[31]. The drawback of our study is that it was a retrospective analysis. However, it still gives information about the prognostic factors and feasibility of en-bloc vascular resection in stage II adenocarcinoma of the pancreatic head and uncinate process.
In summary, our study concludes that serum bilirubin, histological differentiation and adjuvant chemotherapy are independent prognostic factors that influence survival in patients with stage II adenocarcinoma of the pancreatic head and uncinate process. PD/PPPD along with en-bloc vascular resection is a technically feasible option in carefully selected patients.
COMMENTS
Background
Unexpected tumor invasion to the lateral or posterolateral wall of the confluence of the portal vein/superior mesenteric vein (PV/SMV) is a common finding during pancreaticoduodenectomy for pancreatic malignancies. This study was designed to establish the prognostic factors and feasibility of en-bloc vascular resection in stage II adenocarcinoma of the pancreatic head and uncinate process.
Research frontiers
With improvement in surgical techniques and postoperative care, more aggressive en-bloc resection of pancreatic malignancies, along with PV/SMV is being carried out at present, without any increase in surgical complications.
Innovations and breakthroughs
Only a few studies have investigated stage II pancreatic adenocarcinoma, with special attention to adenocarcinoma in the head and uncinate process, which is more likely to invade the PV/SMV because of its close proximity to these vessels.
Applications
En-bloc vascular resection is a feasible option in carefully selected patients with stage II adenocarcinoma of the pancreatic head and uncinate process. Serum bilirubin, histological differentiation and adjuvant chemotherapy are the independent prognostic factors.
Peer review
This is a very well-focused paper that reports a single-center experience with en-bloc venous resection of stage II pancreatic adenocarcinoma.
Footnotes
Peer reviewers: Hiroshi Yoshida, MD, First Department of Surgery, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8603, Japan; Salvatore Gruttadauria, MD, Assistant Professor, Abdominal Transplant Surgery, ISMETT, Via E. Tricomi, 190127 Palermo, Italy
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
References
- 1.Launois B, Stasik C, Bardaxoglou E, Meunier B, Campion JP, Greco L, Sutherland F. Who benefits from portal vein resection during pancreaticoduodenectomy for pancreatic cancer? World J Surg. 1999;23:926–929. doi: 10.1007/s002689900601. [DOI] [PubMed] [Google Scholar]
- 2.Bachellier P, Nakano H, Oussoultzoglou PD, Weber JC, Boudjema K, Wolf PD, Jaeck D. Is pancreaticoduodenectomy with mesentericoportal venous resection safe and worthwhile? Am J Surg. 2001;182:120–129. doi: 10.1016/s0002-9610(01)00686-9. [DOI] [PubMed] [Google Scholar]
- 3.Cusack JC Jr, Fuhrman GM, Lee JE, Evans DB. Managing unsuspected tumor invasion of the superior mesenteric-portal venous confluence during pancreaticoduodenectomy. Am J Surg. 1994;168:352–354. doi: 10.1016/s0002-9610(05)80164-3. [DOI] [PubMed] [Google Scholar]
- 4.Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. Br J Surg. 1994;81:102–107. doi: 10.1002/bjs.1800810138. [DOI] [PubMed] [Google Scholar]
- 5.Wade TP, el-Ghazzawy AG, Virgo KS, Johnson FE. The Whipple resection for cancer in U.S. Department of Veterans Affairs Hospitals. Ann Surg. 1995;221:241–248. doi: 10.1097/00000658-199503000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosca F, Giulianotti PC, Balestracci T, Di Candio G, Pietrabissa A, Sbrana F, Rossi G. Long-term survival in pancreatic cancer: pylorus-preserving versus Whipple pancreatoduodenectomy. Surgery. 1997;122:553–566. doi: 10.1016/s0039-6060(97)90128-8. [DOI] [PubMed] [Google Scholar]
- 7.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257; discussion 257-260. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237:509–514. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540–544; discussion 544-547. doi: 10.1097/01.sla.0000184190.20289.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda S, Oussoultzoglou E, Bachellier P, Rosso E, Nakano H, Audet M, Jaeck D. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg. 2007;142:172–179; discussion 180. doi: 10.1001/archsurg.142.2.172. [DOI] [PubMed] [Google Scholar]
- 13.van Geenen RC, ten Kate FJ, de Wit LT, van Gulik TM, Obertop H, Gouma DJ. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery. 2001;129:158–163. doi: 10.1067/msy.2001.110221. [DOI] [PubMed] [Google Scholar]
- 14.Ye C, Xi PC, Hu XG. Clinical analysis of uncinate process carcinoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2003;2:605–608. [PubMed] [Google Scholar]
- 15.Launois B, Franci J, Bardaxoglou E, Ramee MP, Paul JL, Malledant Y, Campion JP. Total pancreatectomy for ductal adenocarcinoma of the pancreas with special reference to resection of the portal vein and multicentric cancer. World J Surg. 1993;17:122–126; discussion 126-127. doi: 10.1007/BF01655724. [DOI] [PubMed] [Google Scholar]
- 16.Aramaki M, Matsumoto T, Etoh T, Ishio T, Himeno Y, Sasaki A, Yada K, Kawano K, Kitano S. Clinical significance of combined pancreas and portal vein resection in surgery for pancreatic adenocarcinoma. Hepatogastroenterology. 2003;50:263–266. [PubMed] [Google Scholar]
- 17.Carrère N, Sauvanet A, Goere D, Kianmanesh R, Vullierme MP, Couvelard A, Ruszniewski P, Belghiti J. Pancreaticoduodenectomy with mesentericoportal vein resection for adenocarcinoma of the pancreatic head. World J Surg. 2006;30:1526–1535. doi: 10.1007/s00268-005-0784-4. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Sacchi M, Vrachnos P, Lygidakis NJ. Carcinoma of the pancreas with portal vein involvement--our experience with a modified technique of resection. Hepatogastroenterology. 2005;52:1596–1600. [PubMed] [Google Scholar]
- 19.Fuhrman GM, Leach SD, Staley CA, Cusack JC, Charnsangavej C, Cleary KR, El-Naggar AK, Fenoglio CJ, Lee JE, Evans DB. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154–162. doi: 10.1097/00000658-199602000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagohri T, Kinoshita T, Konishi M, Inoue K, Takahashi S. Survival benefits of portal vein resection for pancreatic cancer. Am J Surg. 2003;186:149–153. doi: 10.1016/s0002-9610(03)00173-9. [DOI] [PubMed] [Google Scholar]
- 21.Sierzega M, Popiela T, Kulig J, Nowak K. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas. 2006;33:240–245. doi: 10.1097/01.mpa.0000235306.96486.2a. [DOI] [PubMed] [Google Scholar]
- 22.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 23.Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106–1115. doi: 10.1016/j.gassur.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Boeck S, Ankerst DP, Heinemann V. The role of adjuvant chemotherapy for patients with resected pancreatic cancer: systematic review of randomized controlled trials and meta-analysis. Oncology. 2007;72:314–321. doi: 10.1159/000113054. [DOI] [PubMed] [Google Scholar]
- 25.Sewnath ME, Birjmohun RS, Rauws EA, Huibregtse K, Obertop H, Gouma DJ. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg. 2001;192:726–734. doi: 10.1016/s1072-7515(01)00819-5. [DOI] [PubMed] [Google Scholar]
- 26.Lygidakis NJ, van der Heyde MN, Lubbers MJ. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand. 1987;153:665–668. [PubMed] [Google Scholar]
- 27.Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725–731. doi: 10.1136/gut.46.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limongelli P, Pai M, Bansi D, Thiallinagram A, Tait P, Jackson J, Habib NA, Williamson RC, Jiao LR. Correlation between preoperative biliary drainage, bile duct contamination, and postoperative outcomes for pancreatic surgery. Surgery. 2007;142:313–318. doi: 10.1016/j.surg.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Krähenbühl L, Schäfer M, Krähenbühl S. Reversibility of hepatic mitochondrial damage in rats with long-term cholestasis. J Hepatol. 1998;28:1000–1007. doi: 10.1016/s0168-8278(98)80349-8. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi K, Shimizu S, Yokohata K, Noshiro H, Chijiiwa K, Tanaka M. Pancreatic carcinoma: reappraisal of surgical experiences in one Japanese university hospital. Hepatogastroenterology. 1999;46:3257–3262. [PubMed] [Google Scholar]
- 31.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]