Abstract
Traumatic brain injury is a leading killer of children and is a major public health problem around the world. Using general principles of neurocritical care, various treatment strategies have been developed to attempt to restore homeostasis to the brain and allow brain healing, including mechanical factors, cerebrospinal fluid diversion, hyperventilation, hyperosmolar therapies, barbiturates and hypothermia. Careful application of these therapies, normally in a step-wise fashion as intracranial injuries evolve, is necessary in order to attain maximal neurological outcome for these children. It is hopeful that new therapies, such as early hypothermia or others currently in preclinical trials, will ultimately improve outcome and quality of life for children after traumatic brain injury.
Keywords: barbiturate therapy, children, hyperosmolar therapy, hyperventilation, traumatic brain injury
Traumatic brain injury (TBI) is the leading killer of children in the USA after the first year of life. Based on 2005 data from the CDC, trauma was the leading cause of death in every pediatric age group except for those under 1 year of age (where it was fourth), and traumatic brain injury is thought to be responsible for more than 50% of all traumatic deaths in children [1]. Between 100,000 and 150,000 children suffer from severe TBI and approximately 10–15% result in death or severe disability [2]. The burden of childhood TBI is expected to exceed US$ 30 billion annually within the USA and this estimate is only likely to grow as survivors continue to utilize resources with improvements in rehabilitation and outpatient care [3,4]. Despite this enormous burden to society, brain-specific therapies for TBI are relatively limited.
In order to understand therapies for TBI, a basic understanding of the pathogenesis of brain injury after trauma is required. TBI consists of the primary injury followed by an evolution of injury and potential secondary insults. Primary injury is the damage inflicted at the time of the traumatic event and consists of contusions to brain parenchyma, lacerations of brain substance, which can lead to hematomas (epidural and subdural) and hemorrhages (intraparenchymal and subarachnoid), white matter damage (such as diffuse axonal injury) and other damage to bony structures or blood vessels. Except for removal of extra-axial blood collections that may be compressing the brain parenchyma, there are no current treatments for these primary injuries. The evolution of TBI involves the body’s reaction to injury and begins within hours after the injury is sustained. In this early time period, cerebral blood flow (CBF) is often lower than normal in children and may be inadequate to sustain normal neurological functioning in some brain regions. Direct injury to brain tissue can lead to edema formation as intravascular fluid traverses the damaged blood–brain barrier or intracellular pathways are activated that lead to cell swelling and cell death. Secondary factors such as systemic hypoxia, hypotension, hyperpyrexia and hyperglycemia can lead to exacerbation of injury and less favorable neurological outcome. Precise assessments and determination of thresholds of these events that may lead to adverse outcome are largely unknown for children after TBI, yet remain an intense focus of research at the current time.
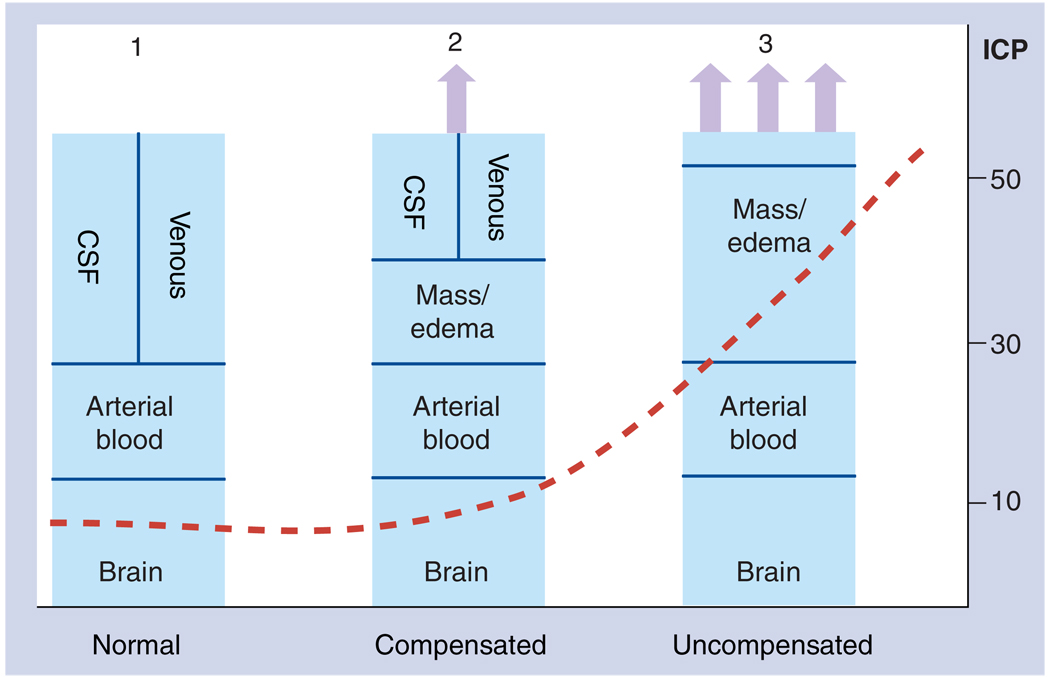
Treatment strategies for TBI in children are largely based on a few key physiological principles: the Munro–Kellie doctrine; cerebral blood pressure autoregulation; and the relationship between metabolic demands and blood flow (Figure 1). Over 100 years ago, Munro and Kellie recognized the relationship between the contents and volume of the intracranial compartment. This doctrine states that the volume of the intracranial contents (brain, cerebrospinal fluid [CSF], arterial and venous blood and any pathological mass) are related to the pressure within the relatively rigid encasement of the skull. As pathological masses accumulate in the cranial vault and after compensatory mechanisms (expansion of cranial sutures, increased CSF absorption and extrusion of venous blood into the thorax) are overcome, further increases in intracranial volume lead to critical increases in intracranial pressure (ICP). These increases in ICP, generally termed intracranial hypertension when ICP is greater than 20 mmHg, eventually lead to decreased arterial blood volume, decreased cerebral blood flow, brain ischemia and ultimately cerebral herniation if untreated. A second key neurocritical care principle is CBF autoregulation. Normally, CBF remains constant over a relatively wide range of mean arterial pressures (MAP), with maximal vasodilation of arteries in the lower limit of autoregulatory range (generally MAP ~50 mmHg in adults) and maximal vasoconstriction at the upper limit (MAP ~150 mmHg). Manipulations of the constriction/relaxation of cerebral arterioles can have profound effects on ICP as maximally vasodilated blood vessels within the brain will lead to increased cerebral blood volume. Lastly, cerebral metabolism, as measured by either oxygen or glucose consumption, is tightly correlated with CBF demands in normal conditions. Therefore, as metabolism is decreased by medications or decreased cerebral function, blood flow requirements will be decreased. Conversely, as metabolism is increased, CBF requirements are increased.
Figure 1. The Munro–Kellie Doctrine explains the relationship between intracranial volumes and intracranial pressure in physiological and pathophysiological conditions.
The volume of the intracranial vault (the area represented by the rectangle) is generally fixed in adults and most children. The contents of the intracranial vault in the normal condition include the brain, arterial blood, venous blood and CSF, and are maintained at a relatively low ICP (Panel 1; normal). After an injury, swelling/edema/pathological tissue can increase within the brain (Panel 2; compensated). Compensatory mechanisms, including increased CSF absorption, extrusion of CSF into the spinal canal and extrusion of venous blood into the thorax, initially limit any changes in ICP. When these compensatory changes are exhausted (Panel 3; uncompensated), any further increases in intracranial volumes are associated with concomitant increases in ICP that eventually can compromise arterial blood flow, ultimately leading to cerebral herniation. CSF: Cerebrospinal fluid; ICP: Intracranial pressure.
To a great extent, all current and future therapies for TBI in children can be understood by these principles, as we outline below. The hall-marks of the currently recommended treatment strategies for severe TBI are aimed at normalizing physiologic parameters and limiting secondary injury to the brain. In doing so, steps must be taken to optimize oxygen and glucose delivery to the brain and then brain-specific therapies need to be applied in a rational manner to maximize benefits and minimize side effects of treatment. The following sections generally outline therapies with the fewest side effects first (mechanical and CSF drainage) with increasing side effects toward the end of this section (barbiturates and hypothermia). Guidelines for the management of severe TBI in children have been formulated to synthesize the best evidence to date regarding many aspects of care [5] and they are currently under revision. This manuscript will describe the physiological basis for these therapies and summarize the evidence that support their use.
General care
Scrupulous monitoring and correction of hemodynamic, respiratory and other systemic derangements is essential to maximize outcome after TBI in children in order to maintain proper nutrient and oxygen supply to the brain during an evolving injury. Since hypoxic and ischemic injury is frequently found on autopsy of children and adults after TBI and have been linked to adverse outcome [6–9], current recommendations stress institution of mechanical ventilation with endotracheal intubation when Glasgow Coma Scale is less than 8 or when severe brain injury is suspected. Current guidelines suggest that hypoxia should be avoided by maintaining partial pressure of oxygen in arterial blood (PaO2) above 60–65 mmHg and oxygen saturation (hemoglobin [SaO2]) above 90%, but some evidence suggests targeting brain tissue oxygen tension (PbO2) of greater than 25 mmHg may be more closely linked to outcome in adults [10]. There is some preliminary evidence to support using PbO2 as a threshold for oxygenation in children, but definitive studies have not yet been reported. Finally, in supporting adequate oxygenation, positive end-expiratory pressure should be limited since increases in intrathoracic pressure may impede jugular venous drainage and diminish compensatory mechanisms based on the Munro–Kellie doctrine.
Maintenance of cardiovascular performance is also essential in the treatment of TBI in children. This includes adequate fluid resuscitation and maintenance of blood pressure to ensure cerebral perfusion and resolve shock. Fluid resuscitation should be initiated in all cases of TBI as traumatically injured children often have significant fluid losses as a result of their injury, and failure to maintain adequate hemodynamic support is associated with poorer clinical outcomes [11–13]. The choice of resuscitation fluid – colloid, crystalloid, hypertonic or experimental – has been a matter of intense debate for adults after TBI. However, little is known at this time regarding the superiority of any agent, but avoidance of disturbances in serum osmolarity (particularly hyponatremia with use of hypo-osmolar solutions) is recommended. Resuscitation should have a goal of maintenance of adequate blood pressure to provide cerebral perfusion and prevent secondary organ damage. Cerebral perfusion pressure (CPP) is defined as the difference between MAP and ICP [CPP = MAP − ICP]. The range of optimal CPP is age-dependent in children, as reflected by recent recommendations (children >60 mmHg; infants and toddlers >45 mmHg), and prolonged CPP below 40 mmHg predicts mortality in a relatively large series [14]. When intravascular volume is restored (generally when central venous pressure [CVP] is at least 10mmHg), administration of vasopressor agents should be instituted to maintain CPP and cardiovascular performance. The choice of vasopressor is greatly dependent upon the systemic condition of the child, as many factors should be considered in individual children. A host of other factors, including sedation, neuromuscular paralysis, nutrition, deep vein thrombosis, prophylaxis, seizure prophylaxis, and prehospital and rehabilitation care among others, are extremely important to achieve maximal neurological recovery. This review will be limited to acute therapies for treatment of intracranial emergencies after TBI.
Mechanical therapies
Head position is an extremely important aspect in the care of children with TBI, as elevation of the child’s head can aid in draining venous blood to minimize intracranial volumes and ICP; and second, maintenance of cervical spine precautions is essential unless a critical neck injury has been excluded. This maneuver is relatively understudied, but has been a part of clinical care for years. In one telling paper, Meixensberger and colleagues found that ICP was significantly decreased in patients that had the head of their bed elevated to 30° compared with those who remained supine [15]. In this study, the authors also found that their maneuver did not appear to affect PbO2.
Surgical evacuation of hematomas and other pathological masses has been a mainstay of treatment for TBI for years, thereby treating two aspects of the Monro–Kellie doctrine: removal of the pathological mass and expansion of the intracranial cavity volume. More recently, decompressive craniectomy (removal of a portion of the skull and opening of the dura) has been advocated as a direct therapy for intractable ICP without focal mass lesion. Anecdotal reports from recent military conflicts have advocated for this practice and small case series have emerged demonstrating efficacy. Specifically, in a single-center, case-controlled study, 35 adults with TBI who underwent bifrontal craniectomy had improved neurological outcome when compared with historical controls, with young age and early operation most strongly associated with improved outcome [16]. However, in a prospective, randomized, controlled trial involving 27 children with severe TBI, early decompressive craniectomy resulted in decreased ICP for the first 48 h after surgery (average decrease in ICP of 9 mmHg), but no definitive improvement in outcome was proven [17]. While it appears very challenging, it is likely that a large, randomized, controlled trial of early decompression surgery will probably be necessary to fully define the utility of this provocative technique.
Cerebrospinal fluid drainage
Drainage of CSF is most often accomplished by placement of an externalized ventricular drain in the lateral ventricles of the brain. Occasionally, lumbar drainage of CSF is also instituted, but this must be performed with great care to prevent differential pressures within the cranial vault and potential uncal herniation. The therapeutic rationale for implementing CSF drainage for TBI is reasonably obvious – drainage of CSF from the cranial vault will decrease intracranial volumes and therefore decrease ICP. Some of the most compelling evidence linking CSF drainage to ICP, and presumably improved neurological outcome, was developed by Shapiro and Marmarou [18]. This study was developed to assess the pressure–volume index (PVI), where a given volume of fluid was instilled into the cerebral ventricle to determine the extent of ICP increase after this maneuver. Children with increased PVI (and consequent decreased cerebral compliance) were at highest risk for intracranial hypertension.
Like many of the treatment modalities of therapeutic maneuvers in TBI, definitive outcome studies are lacking, although strong suggestive evidence is present for the use of this modality. James and colleagues demonstrated that intermittent drainage of CSF from an extra-ventricular drain (EVD) resulted in immediate decreases in ICP in 32 patients ranging in age from 1 to 73 years [19]. However, this therapy alone was insufficient to control ICP secondary to severe swelling and small ventricle size after several days. Similarly, Fortune and colleagues found that CSF drainage for intracranial hypertension was the most effective therapy in 22 adults and adolescents [20]. Importantly, they also found that this therapy maintained CBF despite the large decreases in ICP, while other therapies had greater effects on cerebral hemodynamics. A more recent study by Jagannathan and colleagues demonstrated that CSF drainage via ventriculostomy improved intractable intracranial hypertension in children, and improved quality of life, despite an alarming incidence of meningitis associated with placement of the catheter (22%) [21].
As stated above, lumbar drainage is less frequently employed to treat intracranial hypertension after TBI in children. Levy and colleagues have published the largest series, where they instituted lumbar drainage of CSF in children with intractable ICP. In this series of 16 children who failed EVD-based CSF drainage, hyperventilation, furosemide, mannitol and barbiturates, lumbar drainage was safe and led to a survival rate of 88% [22]. Nevertheless, scrupulous attention to detail when utilizing this therapy should be practiced to avoid cerebral herniation.
Hyperventilation
As early airway support is pivotal for stabilization of a child after TBI, the control of ventilation has been the subject of controversy for many years. Fundamentally, hyperventilation leads to decreased CBF (1 mmHg change in partial pressure of carbon dioxide in the arterial blood [PaCO2] decreases CBF by 3% in regions of intact autoregulation), which ultimately leads to decreased cerebral blood volume (and decreased ICP based on the Munro–Kellie doctrine). However, the determination of the balance between adequate blood f low (and blood volume) to support neurological function while minimizing excessive flow has been very difficult over the past several decades. Bruce and colleagues demonstrated that a protocol that included aggressive hyperventilation led to good neurological outcome in children after TBI, with the rationale for this approach that excessive CBF (or hyperemia) was common. Contradicting this assumption, Adelson and colleagues reported that a significant percentage of children had cerebral hypoperfusion (CBF <20 ml/100g/min) early after injury [23], and others demonstrated that routine hyperventilation could induce brain ischemia [24–27]. As a result, brief periods of hyperventilation are now recommended to treat acute neurologic deterioration, including signs of impending brain herniation (pupillary dilation, hypertension and/or bradycardia) as other therapies are being instituted. In addition, longer periods of aggressive hyperventilation may be appropriate for refractory intracranial hypertension when CBF is known to be increased or when all other therapies have failed. However, routine, severe hyperventilation seems to represent a significant risk for brain hypoxia. In summary, it appears clear that acute hyperventilation (of duration substantial enough to allow interventions to treat a catastrophic event) is occasionally warranted, while routine hyperventilation has substantial risks and limited defined benefit.
Hyperosmolar therapies
Hyperosmolar therapies have evolved over the decades, with glycerol, mannitol and various concentrations of saline solutions predominating in clinical care. The mechanism of action of these agents on cerebrohemodynamics is more complex than other agents. Administration of these agents by intravenous infusion leads to an immediate (within seconds to minutes) decrease in blood viscosity. The net effect of this change in blood viscosity is cerebral vasoconstriction associated with unchanged CBF – thereby decreasing cerebral blood volume and causing an immediate decrease in ICP. Secondarily, administration of these agents leads to increases in serum osmolarity. In more delayed time periods (over 20 min to slightly longer), this increase in serum osmolarity leads to net fluid flux from the brain into the serum – leading to decreased edema, relative brain dehydration and decreased ICP.
Similar to other TBI therapies, definitive studies regarding hyperosmolar therapies and outcome are lacking, yet numerous studies point to the beneficial role these agents play in treating children with TBI. James and colleagues reported that a dose of mannitol of 0.5 g/kg resulted in decreased ICP in 78% of patients with a variety of CNS pathologies (e.g., trauma, brain tumor, encephalopathy and subarachnoid hemorrhage), while a larger dose of 1 g/kg was nearly universally effective (99%) [28]. Furthermore, Mendelow and colleagues demonstrated similar effects of mannitol on ICP with additional benefit also noted to CPP and CBF [29]. In the previously cited manuscript by Shapiro and Marmarou, mannitol was demonstrated to be effective in improving cerebrohemodynamics by changes in both ICP and PVI [18]. Specifically, PVI increased and ICP decreased in almost all of the 22 children studied (the two nonresponders died from uncontrolled intracranial hypertension shortly after the study). In an ambitious study to attempt to determine if mannitol can improve outcome, Kasoff and colleagues stratified 25 patients based on severity of illness and level of care required (ICP monitoring vs ICP/mannitol vs ICP/mannitol/ barbiturates) [30]. The authors found that the use of mannitol led to improved outcome as measured by expected outcomes based on trauma scores (88% observed vs 83% predicted).
Complications of mannitol include concerns for hypovolemia due to diuresis, as well as an association between mannitol use and renal failure at extremes of serum osmolarity. Hypertonic saline maintains intravascular volume and has not shown similar renal effects in studies to date. Various concentrations of hypertonic saline have been reported [31,32], but most studies in children involve administration of 3% saline. Simma and colleagues performed a prospective, randomized, controlled study of hypertonic saline versus normotonic solutions in TBI in children and found: less fluid required for resuscitation; fewer therapies needed for ICP in the first 3 days; decreased duration of mechanical ventilation; and decreased length of hospital stay [33]. More directly, Peterson and colleagues reported successful treatment of intracranial hypertension with 3% saline in a retrospective cohort of 68 children [34]. Of interest, this group demonstrated improved outcomes based on trauma scores and found no adverse events despite extreme hyperosmolarity (serum Na > 180 meq/l in some cases). Khanna and colleagues reported the use of continuous hypertonic saline solution in ten children and found that institution of this therapy resulted in decreased ICP spikes with improved CPP [35]. Finally, in a double-blind, crossover study comparing 3% saline to normal saline for intracranial hypertension, Fisher and colleagues found a 25% decrease in ICP after 3% saline and no change in the normal saline group [36].
Barbiturates & drug-induced coma
Barbiturates and other agents that induce coma have been used as therapy for TBI for decades. Such therapies are administered to decrease metabolic activity of the brain that will lead to decreased blood flow and blood volume, ultimately leading to decreases in ICP. Other effects, such as the change in cerebrovascular tone and inhibition of lipid peroxidation, may also play a role in neuroprotection [37–39]. Inducing coma by a variety of agents, including propofol, benzodiazepines and others, may lead to beneficial effects on cerebrohemodynamics. However, since the large majority of data outlines the use of pentobarbital, this review will focus entirely on this important agent.
Pentobarbital therapy has been associated with several beneficial effects in adult TBI victims, including improved ICP [40], improved brain oxygenation [41] and decreased markers of excitotoxicity in CSF [42]. However, despite its widespread use, improvements in long-term neurological outcome are lacking for both adults and children. Pittman and colleagues found that pentobarbital therapy led to decreases in ICP in more than 50% of children with TBI who were refractory to other therapies [43]. However, the study design prevented more definitive conclusions regarding pentobarbital’s effect on improved outcome. In another relatively large case series, Kasoff and colleagues found that pentobarbital use was closely linked to hemodynamic instability, with nearly all of the 25 children requiring vasoactive medications to maintain blood pressure during pentobarbital therapy [30]. As data supporting barbiturate therapy are relatively scant, the 2003 guidelines for pediatric TBI treatment contain no strategy for its use except to state that ‘high-dose barbiturate therapy may be considered in hemodynamically stable patients with salvageable severe head injury and refractory intracranial hypertension’ [5].
Experimental therapy: hypothermia
The most promising experimental therapy that may become standard care in the near term is therapeutic hypothermia. Whole-body hypothermia is categorized as moderate when the goal temperature is between 32 and 33°C, and this level of hypothermia has been demonstrated to be neuroprotective for adults with cardiac arrest [44] and neonates with perinatal asphyxia [45]. While the proposed mechanism of action of hypothermia is not entirely understood, it is assumed that hypothermia leads to decreased cerebral metabolism, ultimately leading to decreases in CBF and cerebral blood volume. It is likely that other effects of hypothermia – decreases in enzyme activity, alterations in membrane permeability, preservation of antioxidant reserve, and others – may also have significant neuroprotective effects.
The use of hypothermia for TBI is quite dichotomous at this time [46]. Almost all studies in hypothermia report a decrease in ICP with application of hypothermia, whether it is applied early after TBI as a neuroprotectant or in the delayed phase as a rescue therapy for recalcitrant intracranial hypertension. In a Phase III trial of early hypothermia including 392 adults after TBI, Clifton and colleagues found that there was an 11% difference in ICP over the hypothermia period between normothermic and hypothermic adults [47]. Similarly, Zhi and colleagues found almost a 20% decrease in ICP between hypothermic and normothermic groups in their large Phase III study [48]. In children, Adelson and colleagues report a 19.6% difference in ICP between hypothermic children and those who remained normothermic [49]. Owing to this compelling data, it appears logical to offer hypothermia as a rescue therapy when other therapies have failed.
However, the application of hypothermia as a first-line therapy to improve outcome has been less successful. After being a standard therapy decades ago, interest in hypothermia in trauma was rekindled by Marion and colleagues who demonstrated an improved neurological outcome at 6 months in adults randomized to hypothermia (32–33°C for 24 h) [50]. A multi-center trial by Clifton and colleagues sought to confirm this single-center study, but failed to demonstrate any significant beneficial effect [47]. Proponents of hypothermia point out that there were significant treatment variations within the 4-site consortium that may have played a crucial role in this negative trial.
Since the Marion study demonstrated that younger age was associated with an even greater treatment effect for hypothermia, there was enthusiasm to determine if early hypothermia could be an effective neuroprotectant in children. Adelson and colleagues performed a Phase II safety study in 48 children and found that hypothermia was safe and there was a trend toward a decrease in mortality at 3 months [49]. However, in a Phase III trial, Hutchison and colleagues have recently reported a negative study of early hypothermia that was carried out by the Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group [51]. In this study, 225 children were randomized and a detrimental effect of hypothermia was observed (31% poor outcome in the hypothermia group and 22% poor outcome in the normothermia group). Questions about the applicability of the study were raised because the outcome tested (a composite outcome score was used at 3 months by phone interview of parents) was of questionable reliability, there was a substantial amount of hypotension reported in the hypothermia group that may have affected the outcome and there was a profound degree of hyperventilation in both groups of the study that may not reflect current practice in all centers. As a result of this trial, it is possible that hypothermia treatment offers all-risk-and-no-reward for children after TBI and its use should be abandoned. Alternatively, it is possible that the particular protocol tested in this ambitious and arduous trial could be altered to provide safer neuroprotection without incurring substantial risks to the child. This hypothesis is currently being tested in an ongoing clinical trial (Pediatric Traumatic Brain Injury Consortium: Hypothermia – also known as the ‘Cool Kids Trial’).
Conclusion & future perspective
In summary, children with TBI have significant chances of mortality and severe disability. Current neurocritical care therapies are directed toward normalizing physiological parameters in a step-wise manner, with maintenance of normal systemic function, treatment of mechanical disturbances, application of mild hyperventilation, administration of hyperosmolar therapies and induction of coma through use of barbiturates being the currently recognized agents. Experimental use of hypothermia has great promise for periods of intracranial hypertension and may be of utility as a neuroprotectant when applied early after injury. We believe that future research to improve neurological outcome will focus on optimal neurocritical care thresholds (interstitial brain oxygen concentrations, microdialysis measurements of stress metabolites or other parameters), improved nutritional support, an understanding of targeted therapies for intracranial hypertension and a greater understanding of the role of surgery for refractory medical cases. Development of novel therapies is urgently needed to provide further breakthroughs in treatment for these unfortunate children.
Executive summary
General care
Avoidance of hypoxia by maintaining partial pressure of oxygen in arterial blood above 60–65 mmHg, oxygen saturation (hemoglobin) above 90% and brain tissue oxygen tension above 25 mmHg prevents secondary hypoxic damage.
Maintaining optimal, age-based cerebral perfusion pressure (children >60 mmHg & infants and toddlers >45 mmHg) has been demonstrated to improve outcome.
Mechanical therapies
Elevation of the child’s head by 30° to aid in draining venous blood and to minimize intracranial volumes and intracranial pressure (ICP).
Surgical evacuation of hematomas and other pathological masses allow expansion of the intracranial cavity volume.
Decompression surgery may offer benefit for recalcitrant elevations of ICP.
Cerebrospinal fluid drainage
Drainage of cerebrosprinal fluid (CSF) by externalized ventricular drain will decrease intracranial volumes and therefore decrease ICP.
Lumbar drainage of CSF can also be instituted, but this must be carried out with great care to prevent differential pressures within the cranial vault and potential uncal herniation.
Hyperventilation
Hyperventilation leads to decreased cerebral blood flow, which ultimately leads to decreased cerebral blood volume and decreased ICP.
Brief periods of hyperventilation can be used to treat acute neurologic deterioration, while longer periods of aggressive hyperventilation may be appropriate for refractory intracranial hypertension when cerebral blood flow is known to be increased or when all other therapies have failed.
Routine, severe hyperventilation appears to represent a significant risk for brain hypoxia.
Hyperosmolar therapies
Intravenous mannitol and hypertonic saline lead to an immediate decrease in blood viscosity with reflex cerebral vasoconstriction associated with no change in cerebral blood flow, thereby decreasing cerebral blood volume and exhibiting an immediate decrease in ICP.
Administration of hyperosmolar agents leads to an increase in serum osmolarity and a net fluid flux from the brain into the serum, leading to decreased edema, relative brain dehydration and decreased ICP.
Barbiturates & drug-induced coma
Pentobarbital therapy has been associated with improved ICP, improved brain oxygenation and decreased markers of excitotoxicity in CSF.
However, despite its widespread use, risks of hemodynamic instability and lack of improvements in long-term outcome studies lead to judicious use of this therapy.
Experimental therapy: hypothermia
Hypothermia is known to decrease ICP both early after traumatic brain injury as a neuroprotectant or in the delayed phase as a rescue therapy for recalcitrant intracranial hypertension.
Hypothermia has been trialled with both beneficial and detrimental effects in children, future research must be undertaken to determine efficacy.
Footnotes
Financial & competing interests disclosure
Supported in part by grants from NIH (T32HD40686 and NS052478). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
J Exo, Department of Critical Care Medicine, & The Safar Center for Resuscitation, Research, University of Pittsburgh, Pittsburgh, PA, USA.
C Smith, Department of Critical Care Medicine, & The Safar Center for Resuscitation, Research, University of Pittsburgh, Pittsburgh, PA, USA.
R Smith, Department of Critical Care Medicine, & The Safar Center for Resuscitation, Research, University of Pittsburgh, Pittsburgh, PA, USA.
MJ Bell, Department of Critical Care Medicine, & The Safar Center for Resuscitation, Research, University of Pittsburgh, Pittsburgh, PA, USA, Tel.: +1 412 692 5164, Fax: +1 412 692 6076, bellmj4@upmc.edu.
Bibliography
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- 1.Kung HC, Hoyert DL, Xu J, et al. Deaths: final data for 2005. National Vital Statistics Reports. 2008;56(10):1–121. [PubMed]
- 2.Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient’s age. A longitudinal prospective study of adult and pediatric head injury. J. Neurosurg. 1988;68(3):409–416. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- 3.Cohadon F, Richer E, Castel JP. Head injuries: incidence and outcome. J. Neurol. Sci. 1991;103 Suppl.:S27–S31. doi: 10.1016/0022-510x(91)90005-r. [DOI] [PubMed] [Google Scholar]
- 4.Frankowski R, Annegers J, Whitman S. Epidemiology and descriptive studies. Part 1. The descriptive epidemiology of head trauma in the United States. In: Becker DP, Povlishock J, editors. Central Nervous System Trauma Status Report –1985. Bethesda, MD, USA: NIH, NINDS; 1985. pp. 33–43. [Google Scholar]
- 5. Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr. Crit. Care Med. 2003;4(3 Suppl):S1–S73. doi: 10.1097/01.CCM.0000067635.95882.24. •• The most recent version of comprehensive guidelines for the management of children after severe traumatic brain injury (TBI) – a revision of these guidelines is now ongoing and expected to be published within several months
- 6.Cooper A, DiScala C, Foltin G, et al. Prehospital endotracheal intubation for severe head injury in children: a reappraisal. Semin. Pediatr. Surg. 2001;10(1):3–6. doi: 10.1053/spsu.2001.19379. [DOI] [PubMed] [Google Scholar]
- 7.Murray JA, Demetriades D, Berne TV, et al. Prehospital intubation in patients with severe head injury. J. Trauma. 2000;49(6):1065–1070. doi: 10.1097/00005373-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J. Trauma. 1996;40(5):764–767. doi: 10.1097/00005373-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Winchell RJ, Hoyt DB. Endotracheal intubation in the field improves survival in patients with severe head injury. Trauma Research and Education Foundation of San Diego. Arch. Surg. 1997;132(6):592–597. doi: 10.1001/archsurg.1997.01430300034007. [DOI] [PubMed] [Google Scholar]
- 10. Stiefel MF, Udoetuk JD, Spiotta AM, et al. Conventional neurocritical care and cerebral oxygenation after traumatic brain injury. J. Neurosurg. 2006;105(4):568–575. doi: 10.3171/jns.2006.105.4.568. • One of the first articles to indicate that threshold values for brain tissue oxygen tension may be associated with improved outcome
- 11.Chaiwat O, Sharma D, Udomphorn Y, et al. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J. Neurotrauma. 2009;26(5):657–663. doi: 10.1089/neu.2008.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pigula FA, Wald SL, Shackford SR, et al. The effect of hypotension and hypoxia on children with severe head injuries. J. Pediatr. Surg. 2003;28(3):310–314. doi: 10.1016/0022-3468(93)90223-8. discussion 315–316. [DOI] [PubMed] [Google Scholar]
- 13.Zebrack M, Dandoy C, Hansen K, et al. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009;124(1):56–64. doi: 10.1542/peds.2008-1006. [DOI] [PubMed] [Google Scholar]
- 14.Downard C, Hulka F, Mullins RJ, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J. Trauma. 2000;49(4):654–658. doi: 10.1097/00005373-200010000-00012. discussion 658–659. [DOI] [PubMed] [Google Scholar]
- 15.Meixensberger J, Baunach S, Amschler J, et al. Influence of body position on tissue-pO2, cerebral perfusion pressure and intracranial pressure in patients with acute brain injury. Neurol. Res. 1997;19(3):249–253. doi: 10.1080/01616412.1997.11740808. [DOI] [PubMed] [Google Scholar]
- 16.Polin RS, Shaffrey ME, Bogaev CA, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41(1):84–92. doi: 10.1097/00006123-199707000-00018. discussion 92–94. [DOI] [PubMed] [Google Scholar]
- 17.Taylor A, Butt W, Rosenfeld J, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs. Nerv. Syst. 2001;17(3):154–162. doi: 10.1007/s003810000410. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro K, Marmarou A. Clinical applications of the pressure-volume index in treatment of pediatric head injuries. J. Neurosurg. 1982;56(6):819–825. doi: 10.3171/jns.1982.56.6.0819. [DOI] [PubMed] [Google Scholar]
- 19.James HE, Langfitt TW, Kumar VS. Analysis of the response to therapeutic measures to reduce intracranial pressure in head injured patients. J. Trauma. 1976;16(6):437–441. doi: 10.1097/00005373-197606000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Fortune JB, et al. Effect of hyperventilation, mannitol, and ventriculostomy drainage on cerebral blood flow after head injury. J. Trauma. 1995;39(6):1091–1097. doi: 10.1097/00005373-199512000-00014. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- 21.Jagannathan J, Feustel PJ, Graca L, et al. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J. Neurosurg. Pediatr. 2008;2(4):240–249. doi: 10.3171/PED.2008.2.10.240. [DOI] [PubMed] [Google Scholar]
- 22.Levy DI, Rekate HL, Cherny WB, et al. Controlled lumbar drainage in pediatric head injury. J. Neurosurg. 1995;83(3):453–460. doi: 10.3171/jns.1995.83.3.0453. [DOI] [PubMed] [Google Scholar]
- 23.Adelson PD, Clyde B, Kochanek PM, et al. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr. Neurosurg. 1997;26(4):200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- 24.Kiening KL, Hartl R, Unterberg AW, et al. Brain tissue pO2-monitoring in comatose patients: implications for therapy. Neurol. Res. 1997;19(3):233–240. doi: 10.1080/01616412.1997.11740805. [DOI] [PubMed] [Google Scholar]
- 25.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J. Neurosurg. 1991;75(5):731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 26.Schneider GH, von Helden A, Lanksch WR, et al. Continuous monitoring of jugular bulb oxygen saturation in comatose patients – therapeutic implications. Acta Neurochir. (Wien) 1995;134(1–2):71–75. doi: 10.1007/BF01428507. [DOI] [PubMed] [Google Scholar]
- 27.von Helden A, Schneider GH, Unterberg A, et al. Monitoring of jugular venous oxygen saturation in comatose patients with subarachnoid haemorrhage and intracerebral haematomas. Acta Neurochir. Suppl. (Wien) 1993;59:102–106. doi: 10.1007/978-3-7091-9302-0_18. [DOI] [PubMed] [Google Scholar]
- 28.James HE. Methodology for the control of intracranial pressure with hypertonic mannitol. Acta Neurochir. (Wien) 1980;51(3–4):161–172. doi: 10.1007/BF01406742. [DOI] [PubMed] [Google Scholar]
- 29.Mendelow AD, Teasdale GM, Russell T, et al. Effect of mannitol on cerebral blood flow and cerebral perfusion pressure in human head injury. J. Neurosurg. 1985;63(1):43–48. doi: 10.3171/jns.1985.63.1.0043. [DOI] [PubMed] [Google Scholar]
- 30.Kasoff SS, Lansen TA, Holder D, et al. Aggressive physiologic monitoring of pediatric head trauma patients with elevated intracranial pressure. Pediatr. Neurosci. 1988;14(5):241–249. doi: 10.1159/000120397. [DOI] [PubMed] [Google Scholar]
- 31.Kerwin AJ, Schinco MA, Tepas JJ, 3rd, et al. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J. Trauma. 2009;67(2):277–282. doi: 10.1097/TA.0b013e3181acc726. [DOI] [PubMed] [Google Scholar]
- 32.Suarez JI, Qureshi AI, Bhardwaj A, et al. Treatment of refractory intracranial hypertension with 23.4% saline. Crit. Care Med. 1998;26(6):1118–1122. doi: 10.1097/00003246-199806000-00038. [DOI] [PubMed] [Google Scholar]
- 33.Simma B, Burger R, Falk M, et al. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit. Care Med. 1998;26(7):1265–1270. doi: 10.1097/00003246-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 34.Peterson B, Khanna S, Fisher B, et al. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit. Care Med. 2000;28(4):1136–1143. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- 35.Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit. Care Med. 2000;28(4):1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 36.Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J. Neurosurg. Anesthesiol. 1992;4(1):4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Demopoulos HB, Flamm ES, Pietronigro DD, et al. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol. Scand. Suppl. 1980;492:91–119. [PubMed] [Google Scholar]
- 38.Kassell NF, Hitchon PW, Gerk MK, et al. Alterations in cerebral blood flow, oxygen metabolism, and electrical activity produced by high dose sodium thiopental. Neurosurgery. 1980;7(6):598–603. doi: 10.1227/00006123-198012000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Piatt JH, Jr, Schiff SJ. High dose barbiturate therapy in neurosurgery and intensive care. Neurosurgery. 1984;15(3):427–444. doi: 10.1227/00006123-198409000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg HM, Frankowski RF, Contant CF, et al. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J. Neurosurg. 1988;69(1):15–23. doi: 10.3171/jns.1988.69.1.0015. [DOI] [PubMed] [Google Scholar]
- 41.Chen HI, Malhotra NR, Oddo M, et al. Barbiturate infusion for intractable intracranial hypertension and its effect on brain oxygenation. Neurosurgery. 2008;63(5):880–886. doi: 10.1227/01.NEU.0000327882.10629.06. discussion 886–887. [DOI] [PubMed] [Google Scholar]
- 42. Goodman JC, Valadka AB, Gopinath SP, et al. Lactate and excitatory amino acids measured by microdialysis are decreased by pentobarbital coma in head-injured patients. J. Neurotrauma. 1996;13(10):549–556. doi: 10.1089/neu.1996.13.549. • One of the first studies demonstrating biochemical effectiveness of a neurological therapy in real time
- 43.Pittman T, Bucholz R, Williams D. Efficacy of barbiturates in the treatment of resistant intracranial hypertension in severely head-injured children. Pediatr. Neurosci. 1989;15(1):13–17. doi: 10.1159/000120433. [DOI] [PubMed] [Google Scholar]
- 44.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 45.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 46.Schreckinger M, Marion DW. contemporary management of traumatic intracranial hypertension: is there a role for therapeutic hypothermia? Neurocrit. Care. 2009 doi: 10.1007/s12028-009-9256-2. [DOI] [PubMed] [Google Scholar]
- 47.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N. Engl. J. Med. 2001;344(8):556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 48.Zhi D, Zhang S, Lin X. Study on therapeutic mechanism and clinical effect of mild hypothermia in patients with severe head injury. Surg. Neurol. 2003;59(5):381–385. doi: 10.1016/s0090-3019(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 49.Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56(4):740–754. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740–754. [DOI] [PubMed] [Google Scholar]
- 50. Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N. Engl. J. Med. 1997;336(8):540–546. doi: 10.1056/NEJM199702203360803. • One of the first studies demonstrating a beneficial effect of hypothermia after TBI in adults; largely credited with starting the systematic evaluation of hypothermia as a therapy for TBI
- 51. Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 2008;358(23):2447–2456. doi: 10.1056/NEJMoa0706930. •• Large study demonstrating potential harm of hypothermia after TBI in children. It has been criticized because many children required vasopressors during rewarming, which may have led to decreased blood pressure and poorer outcomes. Remains the largest randomized, controlled trial of hypothermia for children to date