Abstract
Lentiviral vectors containing promoters of distinct origins, that is, strong viral promoters (cytomegalovirus [CMV] and murine stem cell virus [MSCV]), a cellular promoter (phosphoglycerate kinase [PGK]), and two composite promoters (CAG [a composite promoter sequence comprised of the CMV enhancer and portions of the chicken β-actin promoter and the rabbit β-globin gene] and SV40/CD43), were used to evaluate green fluorescent protein (GFP) reporter gene expression in human primary peripheral blood lymphocytes (PBLs) and tumor-infiltrating lymphocytes (TILs). In PBLs, vectors containing the MSCV promoter were found to be optimal for expression in both minimally stimulated and highly activated lymphocytes. The stability of gene expression was monitored for up to 7 weeks in culture and the MSCV promoter-containing vector was found to be comparable to the cellular PGK promoter-containing vector. The MSCV promoter-containing lentiviral vector was also the most active in transduced TILs and these cells retained biological activity as measured by antimelanoma antigen reactivity. Using the knowledge gained in comparing individual promoters, a series of two-gene-containing lentiviral vectors was constructed in an attempt to produce the α and β chains of antitumor antigen T cell receptors (TCRs). Dual-promoter or internal ribosome entry site (IRES)-containing vector designs were evaluated and found to be unable to produce both chains of the TCR in amounts that led to significant biological activity. In contrast, if the α and β chains were linked by a 2A ribosomal skip peptide, both proper TCR chain pairing and biologically activity were observed. This paper emphasizes the need to optimize both promoter function and protein synthesis in constructs that require stoichiometric production of multiple protein subunits.
Introduction
Adoptive immunotherapy using the transfer of highly reactive tumor-infiltrating lymphocytes (TILs) has been demonstrated to be an effective treatment for patients with metastatic melanoma (Dudley et al., 2002, 2005; Gattinoni et al., 2006; Rosenberg et al., 2008). A significant limitation to this technology lies in the generation of T cells that can recognize tumor targets. TILs have been routinely isolated from melanoma, but it has been difficult to isolate similar reactive cells from more common malignancies, and even in melanoma it is not possible to isolate TILs with antitumor functionality in about half of cases. As a possible alternative to these naturally occurring antitumor lymphocytes, T cells can be genetically engineered with antitumor antigen receptors (Sadelain et al., 2003; Stauss et al., 2007; Thomas et al., 2007). Two types of genes can be used to redirect T lymphocytes to recognize tumor-associated antigens; either naturally occurring T cell receptor (TCR) genes or chimeric antigen receptor molecules, which are hybrid proteins containing a single-chain antibody linked to T cell signaling domains.
Retroviral vectors currently used for T cell engineering do not productively transduce naive T cells and are also poor at transducing slow-growing T cells such as TILs. Lentiviral vectors have been demonstrated to effectively transduce both antitumor-reactive cytotoxic T lymphocytes (CTLs) (Zhou et al., 2003) and minimally stimulated peripheral blood lymphocytes (PBLs) (Cavalieri et al., 2003), and thus may be an attractive alternative means to genetically engineer T cells with genes designed to enhance adoptive cell transfer. The goals of this study were to (1) compare various internal promoters for use in lentiviral vectors for their ability to efficiently express genes in transduced T lymphocytes and (2) use this information to construct an effective antitumor T cell via genetic engineering.
Materials and Methods
Cell lines
The cell lines used in these experiments included 293T (CRL-11268; American Type Culture Collection [ATCC], Manassas, VA); the human lymphoid cell lines SUP-T1 (CRL-1942; ATCC) and J.RT3-T3.5 (TIB-153; ATCC); and T2, a lymphoblastoid cell line deficient in TAP (transporter associated with antigen processing) function whose HLA class I protein can be loaded with exogenous peptide. Melanoma lines developed in the Surgery Branch of the Center for Cancer Research (National Cancer Institute, National Institutes of Health, Bethesda, MD) from resected tumor lesions included HLA-A2-restricted lines (526-Mel, 624-Mel, and 2081-Mel) and two non-HLA-A2-restricted lines (888-Mel and 938-Mel). Other cell lines included H2087 (CRL-5922; ATCC) and MDA231 (HTB-26; ATCC). Cell lines were maintained in a 37°C humidified incubator with 5% CO2 supplementation. 293T cells were cultured in D10 medium consisting of Dulbecco's complete medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine, nonessential amino acids, penicillin–streptomycin, and amphotericin B (Fungizone). All other lines were cultured in R10, which consists of RPMI 1640 (Invitrogen) plus 10% fetal bovine serum (Invitrogen), l-glutamine (Invitrogen), nonessential amino acids (Invitrogen), and penicillin–streptomycin (100 U/ml) (Invitrogen).
Transduction of PBLs and TILs
Peripheral blood lymphocytes (PBLs) were from HLA-A2+ melanoma patients who had not received any prior vaccinations or immunotherapy. TILs were obtained from surgical specimens as previously described (Rosenberg et al., 1988; Topalian et al., 1989). PBLs were thawed from frozen stock stored at −180°C and placed into culture in AIM-V and interleukin-2 (IL-2; Cetus, Emeryville, CA) at 300 IU/ml. For OKT3 stimulation, the cells were either initially place in medium with anti-CD3 antibody, OKT3 (Ortho Biotech, Bridgewater, NJ) at 50 ng/ml, or were placed in OKT3 medium after transduction, at the initial changing of the culture medium. For transduction of the PBLs or TILs, 1 × 106 cells were adjusted to a final volume of 1 ml in a 24-well tissue culture-treated plate with the viral supernatant and Polybrene (final concentration, 8 μg/ml). The cells were transduced by centrifugation of the plates for 1.5 hr at 1000 × g, 32°C. The plates were placed in a 37°C, humidified 5% CO2 incubator overnight, and the medium was replaced the next day. TILs were subject to the rapid expansion protocol (REP) as previously described, using OKT3 (50 ng/ml), IL-2 (5000 IU/ml), and irradiated allogeneic peripheral blood mononuclear cells from three different donors (TIL:feeder ratio, 1:100) (Riddell and Greenberg, 1990). Six days post-REP, TILs were transduced as described and returned to culture.
Construction of lentiviral vector plasmids
Three lentiviral vectors were constructed from the parent pRRL-cPPT-PGK-GFP-Wsin (PGK-GFP) vector described by Lizee and colleagues (2003). The pCAG-GFP (CAG-GFP) lentiviral vector was previously described by Lizee and colleagues (2004). CMV-GFP was a gift from P. Zoltick (University of Pennsylvania, Philadelphia, PA). pBlu2SKP-CD43 vector was generated by inserting the SV40CD43 composite promoter excised with EcoRI from pDrive-SV40CD43-EF1 (Invivogen, San Diego, CA), into an EcoRI-digested pBluescript IIKS+ (pBlu2SKP) plasmid (Stratagene, La Jolla, CA). pRRL-cPPT-SV40CD43 GFP-Wsin (SV40/CD43-GFP) was constructed by removing the SV40CD43 composite promoter from pBlu2SKP-CD43 plasmid with 5′ XhoI and 3′ BamHI restriction enzymes (New England BioLabs, Ipswich, MA) and inserting the promoter into the PGK-GFP plasmid after removing the PGK promoter by 5′ XhoI and 3′ BamHI restriction digest. The pRRL-cPPT-MSCVU3-GFP-Wsin (MSCV-GFP) vector was created by amplifying the U3 promoter from the 3′ long terminal repeat (LTR) of the MSGV1 vector previously described by Hughes and colleagues (2005). A 5′ XhoI site and a 3′ BamHI site were included in the amplicon. The forward primer sequence was 5′-GATCCTCGAGGGAATGAAAGACCCCACCTGTAGG-3′. The reverse primer sequence was 5′-GACTGGATCCGGACTGGCGCGCGCCGAGTGAG-3′. Both the polymerase chain reaction (PCR) product and vector PGK-GFP were digested with XhoI and BamHI and ligated to create the pRRL-cPPT-MSCV-GFP-Wsin vector. Gammaretroviral vector MSGp53AIB, described by Cohen and colleagues (2005), was used as template for megaprimer PCRs (Ke and Madison, 1997; Tyagi et al., 2004) in combination with promoter-containing lentiviral vectors to produce p53 TCR-containing lentiviral vectors. Construction of the lentiviral vector expressing the α and β chains of an anti-gp100(154–162) TCR has been described (Yang et al., 2008). All vector constructs were confirmed by DNA sequence analysis.
Vector production
Lentiviral vectors encoding green fluorescent protein (GFP) were prepared by transient transfection of 293T cells, using a calcium phosphate-based cotransfection method, with the lentiviral gene transfer plasmid, the packaging plasmid pCMVΔ8.91, and the VSV-G envelope plasmid pMD.G2, based on a method previously described (Lizee et al., 2003). To summarize, 293T cells were seeded in a polylysine-treated 150-cm2 plate (BD Biosciences, San Jose, CA) at 11 × 106 cells per plate. The cells were transfected 12–16 hr later with 18 μg of lentiviral transfer vector, 18 μg of pCMVΔ8.91, and 8 μg of VSV-G envelope plasmid in 2 × HBSS solution, which consists of 280 mM NaCl, 100 mM HEPES, and 1.5 mM Na2HPO4 (pH = 7.12), and addition of calcium chloride to a final concentration of 124 mM. The next day the cells were washed with sterile phosphate-buffered saline (PBS) and the medium was replaced with D10 (15 ml/plate). Forty-eight to 72 hr after changing the medium on the plates, the supernatant was collected on ice, centrifuged at 1000 × g for 10 min to remove gross cellular debris, and filtered through a 0.45-μm pore size filter for ultracentrifugation. The viral supernatants were concentrated approximately 30-fold by centrifuging at 20,000 × g for 2 hr at 4°C and resuspending the viral pellets in 1 ml of cold D10 medium. The viruses were aliquoted into tubes and stored at −70°C until ready to use for titering or experiments. All lentiviruses used in the experiments were from concentrated stocks.
Determination of lentiviral titer
Titers of concentrated lentiviral vectors encoding GFP were determined by serially diluting vector preparations in D10 medium and transducing 293T cells by the Polybrene transduction method. 293T cells (100,000 per well) were seeded in a single well of a 24-well tissue culture-treated plate. The next day the medium was replaced with 1 ml of vector supernatant dilutions (1 × 103 to 1 × 106) in medium containing Polybrene at a final concentration of 8 μg/ml, and incubated overnight. The next day, the cells were treated with trypsin and transferred to 6-well plates for expansion. Six to 7 days posttransduction vector titers were determined by flow cytometry (fluorescence-activated cell sorting [FACS]), applying standard flow cytometric methods for analysis of GFP expression. The titers (transducing units [TU] = GFP-positive cells/dilution factor) of the lentiviral vectors ranged from 106 to 107 TU/ml.
Flow cytometric analysis
Cell surface expression of CD3, CD4, or CD8 was measured with phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, or allophycocyanin (APC)-conjugated antibodies and the corresponding isotype controls (BD Biosciences). PE-labeled p53264–272/HLA-A2 Pro5 pentamer was supplied by ProImmune (Oxford, UK). Immunofluorescence, analyzed as the relative log fluorescence of live cells, was measured with a FACScan flow cytometer (BD Biosciences). A combination of forward angle light scatter and propidium iodide staining was used to gate out dead cells. Approximately 1 × 105 cells were analyzed. Cells were stained in a FACS buffer made of PBS (Lonza Walkersville, Walkersville, MD) and 0.5% bovine serum albumin (BSA). Immunofluorescence was analyzed with CellQuest Pro software (BD Biosciences).
Measurement of lymphocyte antigen reactivity
To assess the recognition of tumor antigens by lentivirus-transduced PBLs or TILs, cells were cocultured with the indicated tumor cell lines or T2 cells pulsed with MART-1:27–35, gp100:209–217, or influenza virus (Flu) peptides, as described by Morgan and colleagues (2003). Target cells were prepared by using T2 cells pulsed with peptides (10 ng/ml) in cell culture medium for 2 hr at 37°C, and then washed two times in PBS. For the assay, 100,000 responder cells (PBLs or TILs) and 100,000 stimulator cells (peptide-pulsed T2 or 50,000 tumor cells) were incubated in a 0.2-ml culture volume in the wells of a 96-well culture plate. The cells were cocultured for 18 hr and the supernatant was harvested. The supernatants were analyzed for interferon (IFN)-γ secretion, using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Endogen, Cambridge, MA). The supernatants were serially diluted to be in the linear range of the ELISA.
Results
Comparison of vector expression in transduced PBLs
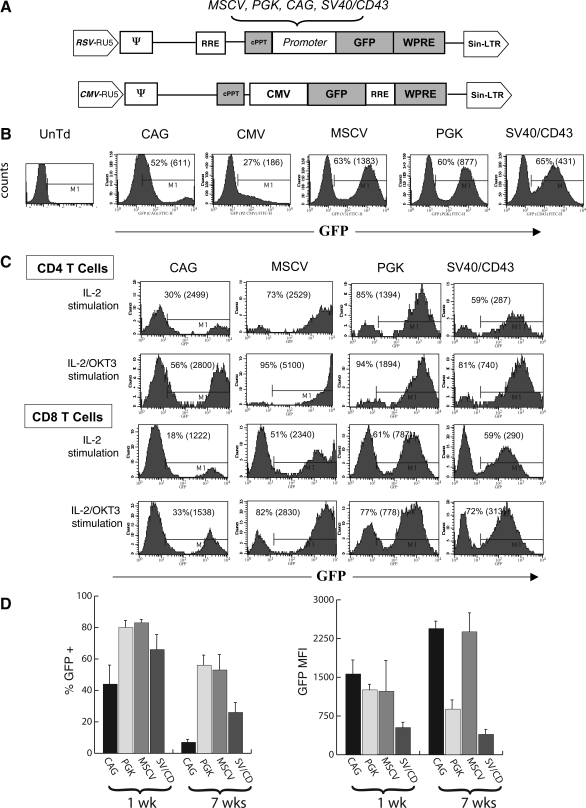
Lentiviral vectors were constructed to use a variety of different promoter elements, with the intent of optimizing gene expression in primary human T cells (Fig. 1A). PBLs were incubated for 24 hr in medium plus the cytokine IL-2, and then exposed to each vector at a multiplicity of infection (MOI) of 10. To expand PBLs after transduction, the cells were stimulated the next day with the anti-CD3 antibody OKT3. Six days posttransduction T cells were analyzed for GFP expression by FACS (Fig. 1B). We observed that all the lentiviral vectors were able to transduce T cells, with the MSCV U3, PGK, and SV40/CD43 promoter-containing vectors yielding 60–65% GFP+ T cells. The MSCV U3 promoter vector had the highest GFP expression as evidenced by a mean fluorescence intensity (MFI) of 1383. Both the PGK and SV40/CD43 lentiviral vectors were able to transduce with similar efficiency, but the MFI was less than that of cells transduced with the MSCV U3 promoter-containing vector (877 and 431, respectively). The CAG and CMV promoter-containing vectors were not particularly effective in driving GFP expression in human T cells. Because of the generally poor GFP expression from the CMV-promoted vector, it was not used in subsequent experiments.
FIG. 1.
Lentiviral vector comparison in peripheral blood lymphocytes (PBLs). (A) Diagrams of lentiviral vectors encoding the green fluorescent protein (GFP) reporter gene. LTR, long terminal repeat; RRE, Rev-responsive element; cPPT, central polypurine tract; WPRE, woodchuck posttranscriptional regulatory element. The following promoters were compared in the transduction of T cells: PGK (phosphoglycerate kinase), CAG (a composite promoter sequence composed of the CMV enhancer and portions of the chicken β-actin promoter and the rabbit β-globin gene), SV40/CD43 (a composite cellular promoter composed of the SV40 enhancer, the human CD43 promoter, and the mouse EF1 5′ untranslated region [UTR]), MSCV (the U3 promoter from murine stem cell virus [MSCV]), and CMV (cytomegalovirus immediate-early region promoter/enhancer). (B) Comparison of lentiviral vectors containing the CMV, PGK, CAG, SV40/CD43, and MSCV promoters in transduced PBLs. PBLs were grown using stimulation with IL-2 alone for 1 day, and cells were transduced with the various lentiviral vectors, using the Polybrene spinfection method at an MOI of 10. The day after transduction, cells were stimulated with OKT3 and subsequently analyzed for GFP expression 6 days after transduction. UnTd, untransduced. (C) Transduction of CD4+ and CD8+ populations of PBLs with lentiviral vectors. For stimulation, PBLs were stimulated in medium with IL-2 and OKT3, or in medium containing IL-2 alone, followed by transduction with the lentiviral vectors CAG-GFP, MSCV-GFP, PGK-GFP, and SV40/CD43-GFP at an MOI of 6. Cells initially stimulated with IL-2 alone were placed in medium containing OKT3 one day posttransduction. Seven days after transduction, the PBLs were analyzed by FACS for GFP expression (gated on general lymphocyte population by size criteria) and CD4+ or CD8+ cell surface markers. (D) PBLs collected from three different donors and stimulated with IL-2 plus OKT3 for 24 hr were transduced via the Polybrene spinfection method with the lentiviral vectors CAG-GFP, MSCV-GFP, PGK-GFP, and SV40/CD43-GFP at an MOI of 6. At 7 days posttransduction, the PBLs were analyzed by FACS for GFP expression (gated on general lymphocyte population by size criteria) and then maintained in culture with IL-2 for an additional 7 weeks before reanalysis for GFP expression as shown (percent GFP positive and MFI).
We next compared the effect of immediate anti-CD3 stimulation on T cell transduction and determined transduction efficiencies in CD4+ versus CD8+ T cells. Figure 1C shows the results of these transductions, using the PBLs of various patients (MOI of 2). Overall the MSCV U3, PGK, and SV40/CD43 lentiviral vectors showed high transduction efficiency (between 51 and 95%) under both stimulation conditions, but the transduction efficiency for all vectors was increased in the cells that received immediate anti-CD3 stimulation. While the gene transfer efficiencies (percent GFP+) were again similar on comparing the MSCV U3, PGK, and SV40/CD43 lentiviral vectors, the MSCV U3 promoter had the highest MFI in both the IL-2-stimulated and IL-2 plus OKT3-stimulated populations. In general, the vector-transduced CD4+ cells expressed more GFP per cell (based on MFI) and demonstrated a higher percent transduction than the CD8+ populations, and this was observed in both IL-2- and IL-2 plus OKT3-stimulated cell populations.
In potential clinical applications, it would be desirable that transgene expression be maintained over time. Whereas there are few data concerning the duration of expression from human clinical trials using lentiviral vectors, in murine models the MSCV promoter was demonstrated to mediate high levels of gene expression, and was shown to be more resistant than similar retrovirus-derived promoters to gene silencing (Gao et al., 2001; Swindle et al., 2004). In Fig. 1D, a comparison was made between the four different promoter-containing vectors for their ability to maintain GFP expression in lentiviral vector-transduced PBLs from three donors over 7 weeks in culture (cells were stimulated once with OKT3 and IL-2 on initiation of culture and were then maintained in medium containing IL-2 alone). Transduction efficiencies were approximately 40–80% on average at week 1 posttransduction (CAG < SV40/CD43 < PGK = MSCV). The percentage of transduced cells declined in all cultures at 7 weeks posttransduction, with the CAG promoter-transduced T cells displaying the greatest decrease (average, 8% in the three donors). Analysis of GFP expression per cell (MFI) demonstrated an increase over time in the CAG and MSCV promoter-containing vector cultures and a slight decrease in the PGK and SV40/CD43 promoter cultures.
Comparison of transgene expression and evaluation of antigen reactivity in tumor-infiltrating lymphocytes
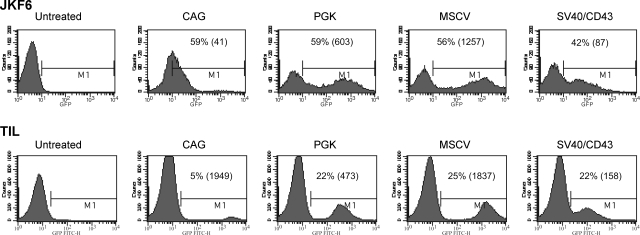
Analysis of the biological properties of TILs suggests that genetic engineering to enhance cell persistence and perhaps augmentation of costimulatory molecule expression might serve to increase TIL effectiveness in vivo. Two TIL cultures were chosen for transduction with lentiviral vectors demonstrated to effectively transduce PBLs (Fig. 2). As target cells for lentiviral vector transduction we chose an established TIL clone, JKF6, as an example of a rapidly growing TIL clone and additionally used a slow-growing primary polyclonal TIL population that was newly established in culture. Both cell types were specific for the melanoma tumor antigen MART-1 (melanoma antigen recognized by T cells-1). Using the same MOI (3) and similar transduction conditions, between 42 and 59% of the JKF6 clone was transduced with the various lentiviral vector constructs. Similar to our PBL data, the MSCV U3 and PGK vectors were the most efficient at expressing the GFP reporter gene in TILs. MSCV U3-transduced cells demonstrated higher MFIs than the other vector-transduced cells. Results from the transduction of the less rapidly dividing polyclonal TIL line demonstrated a lower number of transduced cells (between 5 and 25%). The MSCV U3 promoter-containing vector showed higher (25%) transduction efficiency and MFI (1837) than the other vectors, with the PGK vector and then the SV40/CD43 vector next in terms of efficiency and expression.
FIG. 2.
GFP expression in lentivirus-transduced tumor-infiltrating lymphocyte. Top: JKF6. FACS analysis of GFP expression in JKF6 TIL clone transduced with the following GFP-encoding lentiviral vectors: CAG-GFP, PGK-GFP, MSCV-GFP, and SV40/CD43-GFP. Cells were transduced by the Polybrene spinfection method with the lentiviral vectors at an MOI of 3. Twelve hours after transduction, the medium was replaced and cells were analyzed by FACS for GFP expression 6 days after transduction. Bottom: TIL. A polyclonal population of MART-1-reactive TILs was cultured in medium containing IL-2 and were transduced by the Polybrene spinfection method with the lentiviral vectors CAG-GFP, MSCV-GFP, PGK-GFP, and SV40/CD43-GFP at an MOI of 3. The medium was changed 12 hr after transduction, and TILs were subjected to a rapid expansion protocol (REP) with irradiated feeder PBLs, IL-2, and OKT3. On day 12 post-REP, the TILs were analyzed for GFP expression by FACS analysis.
To determine the effect of lentiviral vector transduction on the biological activity of TILs, both cultures were incubated with known melanoma antigen-expressing melanoma cell lines and T2 cells pulsed with MART-1 peptide. Both the established TIL line JKF6 and the transduced polyclonal primary TILs maintained their tumor specificity after the transduction (Table 1). These data show that both established TILs and early-passage polyclonal TILs could be transduced with lentiviral vectors and maintained their tumor antigen specificity posttransduction.
Table 1.
|
JKF6 | ||||||
|---|---|---|---|---|---|---|
| Medium | 526-Mel | 624-Mel | 888-Mel | 938-Mel | T2-MART | T2-gp100 |
| 0 | 2,132 | 4,741 | 0 | 0 | 9,401 | 0 |
| 1003 | 3,172 | 12,943 | 348 | 298 | 9,467 | 455 |
| 469 | 3,482 | 8,812 | 292 | 650 | 13,725 | 324 |
| 273 | 4,571 | 9,636 | 216 | 212 | 16,540 | 271 |
| 576 | 3,890 | 10,803 | 381 | 373 | 14,731 | 613 |
| TILs | ||||||
| T2-Flu | ||||||
| 15 | 1,109 | 3,606 | 12 | 11 | 13,506 | 69 |
| 14 | 774 | 4,488 | 12 | 12 | 15,339 | 95 |
| 22 | 861 | 2,800 | 12 | 10 | 8,890 | 250 |
| 16 | 739 | 3,569 | 11 | 11 | 10,917 | 95 |
| 22 | 868 | 3,306 | 20 | 18 | 13,154 | 83 |
Abbreviation: TILs, tumor-infiltrating lymphocytes.
As represented by IFN-γ secretion, expressed as picograms per milliliter.
TIL line JKF6 or bulk TILs were transduced with the indicated vectors and co-cultured with melanoma cell lines 526-Mel (MART-1+HLA-A2+), 624-Mel (MART-1+HLA-A2+), 888-Mel (MART-1+HLA-A2−), and 938-Mel (MART-1+HLA-A2−) and T2 cells pulsed with MART-1, gp100, or Flu peptide at 1 μg/ml.
Construction of TCR gene-expressing lentiviral vectors
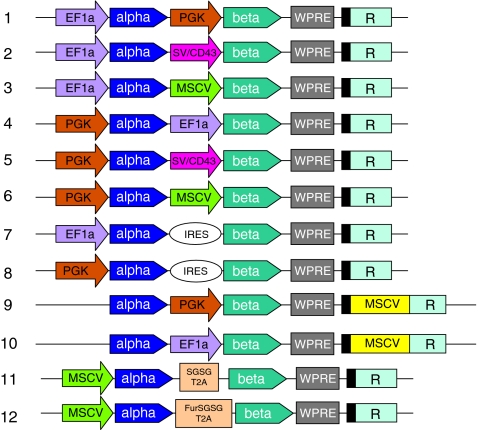
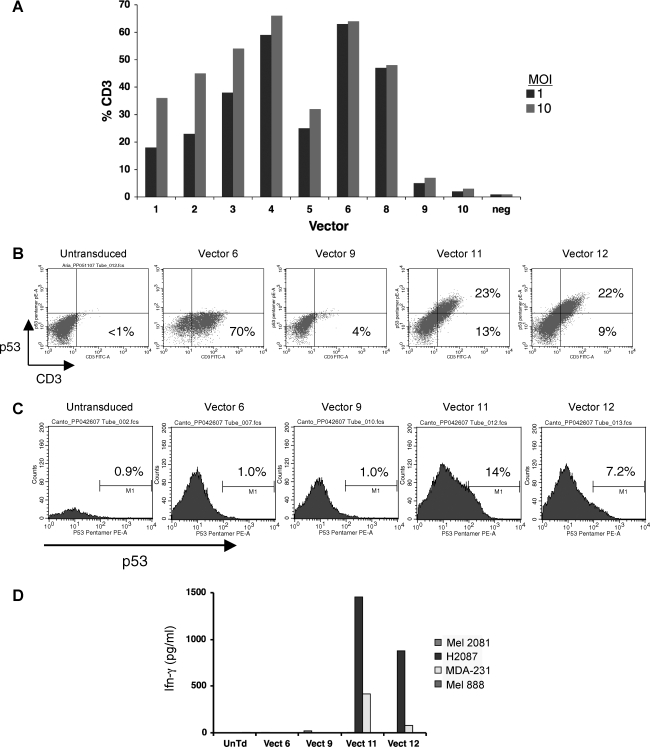
On the basis of results obtained in the previous promoter comparison experiments, we designed a series of new lentiviral vectors aimed at expressing the α and β chains of an anti-p53 tumor antigen TCR (Fig. 3) (Cohen et al., 2005). In this new series of vectors, we replaced the relatively weak CAG promoter with a promoter (EF1α) previously shown to be active in human T cells (Zhou et al., 2003; Serafini et al., 2004). These two-gene-expressing vectors were of three different designs: dual internal promoters (vectors 1–6), use of an internal ribosome entry site (IRES) sequence (vectors 7 and 8), and 3′ HIV-SIN LTR substitutions (insertions into the 3′ U3 region are copied to the 5′ LTR after reverse transcription/integration; vectors 9 and 10). Individual vector preparations were used to transduce the SUP-T1 cell line and TCR expression initially measured by CD3 surface staining. SUP-T1 is defective in endogenous TCR α chain biosynthesis and will not mobilize the CD3 protein to the cell surface unless supplied with a substitute α chain. SUP-T1 cells transduced with p53 TCR vectors 1–8 displayed CD3 staining at 30–65% (at an MOI of 10; Fig. 4A) whereas the LTR substitution vectors (vectors 9 and 10) yielded minimal CD3 staining. We next measured TCR β chain expression by staining transduced SUP-T1 cells with an anti-mouse Vβ3 antibody and in repeated experiments. We uniformly observed poor (<10%) TCR β chain expression for constructs 1–9 (vector 10 did stain for Vβ3 but did not produce the α chain; data not shown). The lack of concomitant TCR α and β chain expression necessitated two additional vector constructs.
FIG. 3.
TCR-expressing lentiviral vectors. Shown in diagram form are the lentiviral vectors designed to express the α and β chains of the anti-p53 tumor antigen TCR. Dual internal promoters were vectors 1–6. IRES-containing constructs were vectors 7 and 8. Vectors 9 and 10 used 3′ HIV-SIN LTR substitutions (insertions into the 3′ U3 region are copied to the 5′ LTR after reverse transcription/integration). 2A peptide-fusion protein vectors (constructs 11 and 12) use ribosomal skipping to produced the individual α and β chains.
FIG. 4.
Expression and function of p53 TCR lentiviral vectors. (A) p53 TCR-expressing lentiviral vector constructs 1–10 were used to transduce SUP-T1 cells at MOIs of 1 and 10 (vector 7, not shown). Six days posttransduction, cells were stained for CD3 mobilization as a measure of TCR α chain expression. (B) SUP-T1 cells were transduced with constructs 6, 9, 11, and 12 (MOI of 10) and 6 days posttransduction cells were analyzed by FACS after staining for p53 pentamer and CD3. The number in each quadrant is the percentage of positive cells for that region. (C) PBLs were stimulated with IL-2 and OKT3 for 1 day and then transduced with constructs 6, 9, 11, and 12 (MOI of 10) and 6 days posttransduction cells were analyzed by FACS after staining for p53 pentamer. (D) Human PBLs expressing the p53 TCR shown in (C) were cocultured for 16 hr with the indicated tumor cell lines (H2087 and MDA-231, HLA-A*0201+p53+; 2081-Mel, p53−; 888-Mel, HLA-A2−). The concentration of IFN-γ secreted into the medium was measured in an ELISA. Color images available online at www.liebertonline.com/hum.
The lack of effective TCR gene expression necessitated a final vector design in which the α and β chains were linked with a 2A ribosomal skip peptide (see constructs 11 and 12; Fig. 3). The 2A peptide linker had been previously demonstrated to be effective in retroviral vectors for the expression of multiple genes (Szymczak et al., 2004; Quintarelli et al., 2007). Before transducing PBLs, we screened the 2A fusion vectors in the SUP-T1 cell line. Transduced cells were costained with CD3 and p53 pentamer, which detects specific p53 TCR α/β chain pairing. Data in Fig. 4B demonstrate that only the 2A fusion vector designs mobilized CD3 to the cell surface and coexpressed both p53 TCR α and β chains (as demonstrated by pentamer staining). When the same vector preparations were used to transduce PBLs, again only constructs 11 and 12 demonstrated significant p53 pentamer staining, indicating proper α and β chain coexpression and pairing (Fig. 4C). Biological activity of the 2A fusion vectors was further demonstrated by transduction of PBLs and coculture with p53-expressing tumor cell lines (Fig. 4D). We observed that only PBLs transduced with the 2A fusion vectors (vectors 11 and 12) released cytokine in the appropriate tumor cell line cocultures.
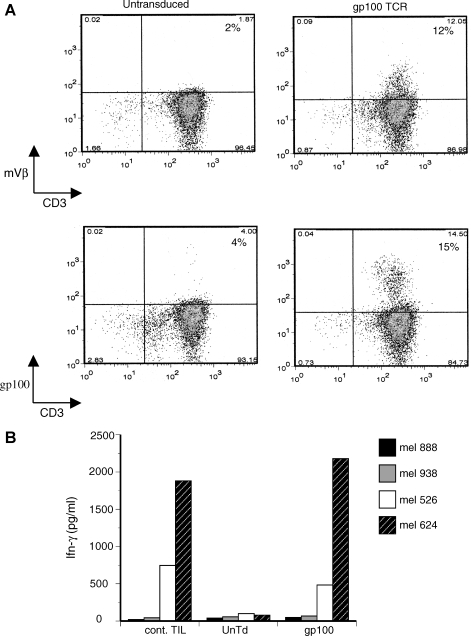
To determine the reproducibility of this approach, another TCR vector was constructed on the basis of a TCR known to be specific for the melanoma tumor antigen gp100(154–162) (Yang et al., 2008). This vector contained the same ribosomal skip peptide used to express the p53 TCR, but contained an added furin cleavage site and peptide spacer sequence. On the basis of the ability of our lentiviral vector to transduce TILs (Fig. 4), we selected a non-melanoma-reactive TIL culture for transduction with the anti-gp100 TCR vector. Lentiviral vector transduction of this nonreactive TIL culture, demonstrated both Vβ staining and specific tetramer staining on FACS analysis (12 to 15%, respectively; Fig. 5A). When these nonreactive TILs were cocultured with melanoma cell lines, IFN-γ production was demonstrated at levels comparable to that of a TIL culture with known melanoma reactive (Fig. 5B).
FIG. 5.
Lentiviral vector transduction of nonreactive TILs. (A) A nonreactive TIL culture was transduced with a gp100-reactive TCR vector, using a 2A peptide to link the α and β chains (MOI of 3). Six days posttransduction cells were analyzed for Vβ expression and gp100 tetramer staining by FACS (performed by costaining for CD3 and gating on viable lymphocytes). (B) Coculture with melanoma cell lines was performed with a TIL culture with known antimelanoma reactivity (cont. TIL), untransduced and nonreactive TILs (UnTd), and nonreactive TILs transduced with the gp100 TCR lentiviral vector (gp100). Melanoma cell lines used were two HLA-A*0201+gp100+ lines (526-Mel and 624-Mel) and two HLA-A*0201−gp100+ cell lines (888-Mel and 938-Mel). Shown is the resulting IFN-γ release after overnight coculture as determined by ELISA.
Discussion
The first approved human gene therapy clinical trials used T cell-directed gene transfer approaches, and these approaches have remained an active area of investigation for applications involving the correction of inborn errors of metabolism (Blaese et al., 1995; Aiuti et al., 2002), as a potential therapy of HIV-1 infection (Ranga et al., 1998; Morgan et al., 2005), and as a treatment for a variety of malignancies (Rossig and Brenner, 2004). The largest number of protocols using genetically engineered T cells has been for applications involving novel cancer treatments. The transgenes used in these applications were designed to provide direct therapeutic benefit (such as cytokines or TCR) or were indirectly used to monitor cell fate and/or as a potential safety switch (e.g., using HSV-Tk). A number of clinical trials using T cells engineered with antitumor-reactive TCRs or chimeric antigen receptors have been undertaken (Kershaw et al., 2006; Lamers et al., 2006, 2007; Morgan et al., 2006b; Park et al., 2007; Till et al., 2008). We demonstrated the successful use of TCR-based T cell gene therapy for the treatment of melanoma using gammaretroviral vectors (Morgan et al., 2006a). Although the response rate reported in this trial was lower than that observed for naturally occurring TILs (12% vs. >50%), the two responses observed were durable (>20 months) and additional patients have responded to the same TCR gene therapy (R.A. Morgan, unpublished observations).
The extensive analysis of promoter function in lentiviral vector-transduced T cells, performed herein, was undertaken in an attempt to develop an effective lentivirus-based TCR expression vector. Lentiviral vectors have a number of potential advantages in comparison with gammaretroviral vectors, including the ability to transduce minimally stimulated PBLs, and a potentially safer integration site preference (Cavalieri et al., 2003; Montini et al., 2006). Our previous observations using gammaretroviral vectors demonstrated that both internal promoters and IRES elements mediated coordinated TCR α and β chain expression leading to functional TCR chain pairing and biological activity (Morgan et al., 2003; Hughes et al., 2005). Although it was previously reported that the use of IRES elements in bicistronic lentiviral vectors led to biased expression of the transgenes (Yu et al., 2003; Amendola et al., 2005; Chinnasamy et al., 2006; Osti et al., 2006), the complete lack of functional TCR gene expression in IRES vectors 7 and 8 (Fig. 4) was not expected. It was also reported that lentiviral vectors containing two independent internal promoters transferred high-level expression of multiple transgenes in human hematopoietic stem progenitor cells (Yu et al., 2003). Using highly active T cell promoters, we tried serial combinations of dual promoters in eight different lentiviral vectors, but consistently failed to achieve a high percentage of TCR expression in transduced PBLs. Expression of the first gene (the α chain) was observed by CD3 mobilization studies in SUP-T1 cells, yet these cells expressed significantly less of the second gene (the β chain), as functional pairing was not observed by tetramer staining (Fig. 4). Naldini and coworkers developed lentiviral vectors coordinately expressing dual genes driven by synthetic bidirectional promoters (Amendola et al., 2005). Although they observed coordinated gene expression in various cells and tissues, these synthetic promoters exhibited lower activities in activated PBLs (10%) and naive PBLs (5%). Why internal promoter and IRES vector designs were functional in gammaretroviral vectors but not in these lentiviral vectors is unknown.
The failure of internal promoters or IRES to yield significant TCR gene expression led us to the construction of lentiviral vectors expressing antitumor antigen TCR by the use of 2A peptides, which were previously reported to yield functional TCR expression in gammaretroviral vectors (Szymczak et al., 2004). The main advantage of using the 2A ribosomal skip peptides in the construction of bicistronic vectors is the potential for coexpression of both genes at equal levels. Our data from two different constructs (vectors 11 and 12) demonstrated both functional α and β chain pairing and biologically activity, using this approach (Fig. 4). These results were not specific for the anti-p53 TCR, as we observed similar results with the antimelanoma gp100 TCRs (Fig. 5).
There has been one report on the clinical use of lentiviral vector-transduced T cells in humans. In this report, June and coworkers used lentiviral vectors in the setting of HIV-1 infection, in which CD4+ T cells were engineered with a vector containing an antisense gene to the HIV-1 envelope protein (Levine et al., 2006). High levels of ex vivo transduction were reported along with short-term persistence of the transferred T cells, with no adverse advents attributed to the lentiviral vector gene transfer system. On the basis of this initial report on the safety of this vector platform and the potential biological advantages in using lentiviral vectors to engineer human T cells, it is likely that lentiviral vectors expressing antitumor-reactive TCRs or similar genes may have immediate application in adoptive immunotherapy for cancer.
Acknowledgments
The authors thank the members of the FACS and TIL laboratories (Surgery Branch) for providing technical support and maintenance of tumor cells from patients. In addition, the authors thank Boro Dropulic and colleagues at Lentigen (Baltimore, MD) for assistance in the molecular cloning and production of the anti-p53 TCR lentiviral vectors. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Author Disclosure Statement
For Stephanie Jones, no competing financial interests exist; for Peter D. Peng, no competing financial interests exist; for Shicheng Yang, no competing financial interests exist; for Cary Hsu, no competing financial interests exist; for Cyrille J. Cohen, no competing financial interests exist; for Yangbing Zhao, no competing financial interests exist; for John Abad, no competing financial interests exist; for Zhili Zheng, no competing financial interests exist; for Steven A. Rosenberg, no competing financial interests exist; for Richard A. Morgan, no competing financial interests exist.
References
- Aiuti A. Vai S. Mortellaro A. Casorati G. Ficara F. Andolfi G. Ferrari G. Tabucchi A. Carlucci F. Ochs H.D. Notarangelo L.D. Roncarolo M.G. Bordignon C. Immune reconstitution in ADA-SCID after PBL gene therapy and discontinuation of enzyme replacement. Nat. Med. 2002;8:423–425. doi: 10.1038/nm0502-423. [DOI] [PubMed] [Google Scholar]
- Amendola M. Venneri M.A. Biffi A. Vigna E. Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat. Biotechnol. 2005;23:108–116. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- Blaese R.M. Culver K.W. Miller A.D. Carter C.S. Fleisher T. Clerici M. Shearer G. Chang L. Chiang Y. Tolstoshev P. Greenblatt J.J. Rosenberg S.A. Klein H. Berger M. Mullen C.A. Ramsey W.J. Muul L. Morgan R.A. Anderson W.F. T lymphocyte-directed gene therapy for ADA-SCID: Initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. Cazzaniga S. Geuna M. Magnani Z. Bordignon C. Naldini L. Bonini C. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- Chinnasamy D. Milsom M.D. Shaffer J. Neuenfeldt J. Shaaban A.F. Margison G.P. Fairbairn L.J. Chinnasamy N. Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting. MOI. Virol. J. 2006;3:14. doi: 10.1186/1743-422X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C.J. Zheng Z. Bray R. Zhao Y. Sherman L.A. Rosenberg S.A. Morgan R.A. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J. Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E. Wunderlich J.R. Robbins P.F. Yang J.C. Hwu P. Schwartzentruber D.J. Topalian S.L. Sherry R. Restifo N.P. Hubicki A.M. Robinson M.R. Raffeld M. Duray P. Seipp C.A. Rogers-Freezer L. Morton K.E. Mavroukakis S.A. White D.E. Rosenberg S.A. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E. Wunderlich J.R. Yang J.C. Sherry R.M. Topalian S.L. Restifo N.P. Royal R.E. Kammula U. White D.E. Mavroukakis S.A. Rogers L.J. Gracia G.J. Jones S.A. Mangiameli D.P. Pelletier M.M. Gea-Banacloche J. Robinson M.R. Berman D.M. Filie A.C. Abati A. Rosenberg S.A. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z. Golob J. Tanavde V.M. Civin C.I. Hawley R.G. Cheng L. High levels of transgene expression following transduction of long-term NOD/SCID-repopulating human cells with a modified lentiviral vector. Stem Cells. 2001;19:247–259. doi: 10.1634/stemcells.19-3-247. [DOI] [PubMed] [Google Scholar]
- Gattinoni L. Powell D.J., Jr. Rosenberg S.A. Restifo N.P. Adoptive immunotherapy for cancer: Building on success. Nat. Rev. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.S. Yu Y.Y. Dudley M.E. Zheng Z. Robbins P.F. Li Y. Wunderlich J. Hawley R.G. Moayeri M. Rosenberg S.A. Morgan R.A. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum. Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S.H. Madison E.L. Rapid and efficient site-directed mutagenesis by single-tube “megaprimer” PCR method. Nucleic Acids Res. 1997;25:3371–3372. doi: 10.1093/nar/25.16.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw M.H. Westwood J.A. Parker L.L. Wang G. Eshhar Z. Mavroukakis S.A. White D.E. Wunderlich J.R. Canevari S. Rogers-Freezer L. Chen C.C. Yang J.C. Rosenberg S.A. Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers C.H. Sleijfer S. Vulto A.G. Kruit W.H. Kliffen M. Debets R. Gratama J.W. Stoter G. Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- Lamers C.H. Langeveld S.C. Groot-Van Ruijven C.M. Debets R. Sleijfer S. Gratama J.W. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol. Immunother. 2007;56:1875–1883. doi: 10.1007/s00262-007-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B.L. Humeau L.M. Boyer J. Macgregor R.R. Rebello T. Lu X. Binder G.K. Slepushkin V. Lemiale F. Mascola J.R. Bushman F.D. Dropulic B. June C.H. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizee G. Aerts J.L. Gonzales M.I. Chinnasamy N. Morgan R.A. Topalian S.L. Real-time quantitative reverse transcriptase-polymerase chain reaction as a method for determining lentiviral vector titers and measuring transgene expression. Hum. Gene Ther. 2003;14:497–507. doi: 10.1089/104303403764539387. [DOI] [PubMed] [Google Scholar]
- Lizee G. Gonzales M.I. Topalian S.L. Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum. Gene Ther. 2004;15:393–404. doi: 10.1089/104303404322959542. [DOI] [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M. Sanvito F. Ponzoni M. Bartholomae C. Sergi Sergi L. Benedicenti F. Ambrosi A. Di Serio C. Doglioni C. Von Kalle C. Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Yu Y.Y. Zheng Z. Robbins P.F. Theoret M.R. Wunderlich J.R. Hughes M.S. Restifo N.P. Rosenberg S.A. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J. Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Walker R. Carter C.S. Natarajan V. Tavel J.A. Bechtel C. Herpin B. Muul L. Zheng Z. Jagannatha S. Bunnell B.A. Fellowes V. Metcalf J.A. Stevens R. Baseler M. Leitman S.F. Read E.J. Blaese R.M. Lane H.C. Preferential survival of CD4+ T lymphocytes engineered with anti-human immunodeficiency virus (HIV) genes in HIV-infected individuals. Hum. Gene Ther. 2005;16:1065–1074. doi: 10.1089/hum.2005.16.1065. [DOI] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R. Hughes M.S. Yang J.C. Sherry R.M. Royal R.E. Topalian S.L. Kammula U.S. Restifo N.P. Zheng Z. Nahvi A. De Vries C.R. Rogers-Freezer L.J. Mavroukakis S.A. Rosenberg S.A. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006a;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R. Hughes M.S. Yang J.C. Sherry R.M. Royal R.E. Topalian S.L. Kammula U.S. Restifo N.P. Zheng Z. Nahvi A. De Vries C.R. Rogers-Freezer L.J. Mavroukakis S.A. Rosenberg S.A. Cancer regression in patients after transfer of genetically engineered lymphocytes [see comment] Science. 2006b;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osti D. Marras E. Ceriani I. Grassini G. Rubino T. Vigano D. Parolaro D. Perletti G. Comparative analysis of molecular strategies attenuating positional effects in lentiviral vectors carrying multiple genes. J. Virol. Methods. 2006;136:93–101. doi: 10.1016/j.jviromet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Park J.R. Digiusto D.L. Slovak M. Wright C. Naranjo A. Wagner J. Meechoovet H.B. Bautista C. Chang W.C. Ostberg J.R. Jensen M.C. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- Quintarelli C. Vera J.F. Savoldo B. Giordano Attianese G.M. Pule M. Foster A.E. Heslop H.E. Rooney C.M. Brenner M.K. Dotti G. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga U. Woffendin C. Verma S. Xu L. June C.H. Bishop D.K. Nabel G.J. Enhanced T cell engraftment after retroviral delivery of an antiviral gene in HIV-infected individuals. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1201–1206. doi: 10.1073/pnas.95.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S.R. Greenberg P.D. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J. Immunol. Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Packard B.S. Aebersold P.M. Solomon D. Topalian S.L. Toy S.T. Simon P. Lotze M.T. Yang J.C. Seipp C.A. Simpson C. Carter C. Bock S. Schwartzentruber D. Wei J.P. White D.E. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: A preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Restifo N.P. Yang J.C. Morgan R.A. Dudley M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossig C. Brenner M.K. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol. Ther. 2004;10:5–18. doi: 10.1016/j.ymthe.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Sadelain M. Riviere I. Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- Serafini M. Bonamino M. Golay J. Introna M. Elongation factor 1 (EF1α) promoter in a lentiviral backbone improves expression of the CD20 suicide gene in primary T lymphocytes allowing efficient rituximab-mediated lysis. Haematologica. 2004;89:86–95. [PubMed] [Google Scholar]
- Stauss H.J. Cesco-Gaspere M. Thomas S. Hart D.P. Xue S.A. Holler A. Wright G. Perro M. Little A.M. Pospori C. King J. Morris E.C. Monoclonal T-cell receptors: New reagents for cancer therapy. Mol. Ther. 2007;15:1744–1750. doi: 10.1038/sj.mt.6300216. [DOI] [PubMed] [Google Scholar]
- Swindle C.S. Kim H.G. Klug C.A. Mutation of CpGs in the murine stem cell virus retroviral vector long terminal repeat represses silencing in embryonic stem cells. J. Biol. Chem. 2004;279:34–41. doi: 10.1074/jbc.M309128200. [DOI] [PubMed] [Google Scholar]
- Szymczak A.L. Workman C.J. Wang Y. Vignali K.M. Dilioglou S. Vanin E.F. Vignali D.A. Correction of multi-gene deficiency in vivo using a single “self-cleaving” 2A peptide-based retroviral vector. Nat. Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Thomas S. Hart D.P. Xue S.A. Cesco-Gaspere M. Stauss H.J. T-cell receptor gene therapy for cancer: The progress to date and future objectives. Expert Opin. Biol. Ther. 2007;7:1207–1218. doi: 10.1517/14712598.7.8.1207. [DOI] [PubMed] [Google Scholar]
- Till B.G. Jensen M.C. Wang J. Chen E.Y. Wood B.L. Greisman H.A. Qian X. James S.E. Raubitschek A. Forman S.J. Gopal A.K. Pagel J.M. Lindgren C.G. Greenberg P.D. Riddell S.R. Press O.W. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L. Solomon D. Rosenberg S.A. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J. Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- Tyagi R. Lai R. Duggleby R.G. A new approach to “megaprimer” polymerase chain reaction mutagenesis without an intermediate gel purification step. BMC Biotechnol. 2004;4:2. doi: 10.1186/1472-6750-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Cohen C.J. Peng P.D. Zhao Y. Cassard L. Yu Z. Zheng Z. Jones S. Restifo N.P. Rosenberg S.A. Morgan R.A. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15:1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. Zhan X. D'Costa J. Tanavde V.M. Ye Z. Peng T. Malehorn M.T. Yang X. Civin C.I. Cheng L. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol. Ther. 2003;7:827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Zhou X. Cui Y. Huang X. Yu Z. Thomas A.M. Ye Z. Pardoll D.M. Jaffee E.M. Cheng L. Lentivirus-mediated gene transfer and expression in established human tumor antigen-specific cytotoxic T cells and primary unstimulated T cells. Hum. Gene Ther. 2003;14:1089–1105. doi: 10.1089/104303403322124800. [DOI] [PubMed] [Google Scholar]