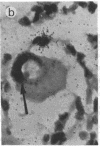
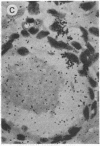
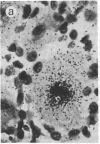
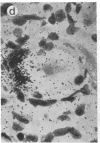
Abstract
The cellular localization and viral transcription patterns of acute and latent varicella-zoster virus (VZV) infections of human sensory nerve ganglia were studied by in situ hybridization and compared with those of latent herpes simplex virus (HSV) infection. Trigeminal and dorsal root ganglia obtained at autopsy were hybridized with 35S-labeled single-stranded RNA probes homologous to VZV or HSV fragments. We have reported that HSV persists in human sensory neurons and expresses only one family of transcripts that overlap extensively with, but are opposite in polarity to, the mRNA encoding the immediate early protein termed infected cell protein 0 (ICP0). In the present study we find that latent VZV infection involves nonneuronal cells, and multiple, but not all, VZV genes are transcribed. In contrast, during varicella both neuronal and nonneuronal cells are infected, with all regions of the VZV genome analyzed being expressed. Thus, the patterns of gene expression and cellular locations of VZV and HSV infections of human ganglia differ. The differences may underlie clinical features that distinguish these infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard S. H., Cheatham W. J., Moses H. L. Electron microscopy of zosteriform herpes simplex infection in the mouse. Lab Invest. 1972 Apr;26(4):391–402. [PubMed] [Google Scholar]
- Esiri M. M., Tomlinson A. H. Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15(1):35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- Gershon A. A., Raker R., Steinberg S., Topf-Olstein B., Drusin L. M. Antibody to Varicella-Zoster virus in parturient women and their offspring during the first year of life. Pediatrics. 1976 Nov;58(5):692–696. [PubMed] [Google Scholar]
- Gilden D. H., Rozenman Y., Murray R., Devlin M., Vafai A. Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol. 1987 Sep;22(3):377–380. doi: 10.1002/ana.410220315. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Vafai A., Shtram Y., Becker Y., Devlin M., Wellish M. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983 Dec 1;306(5942):478–480. doi: 10.1038/306478a0. [DOI] [PubMed] [Google Scholar]
- Hyman R. W., Ecker J. R., Tenser R. B. Varicella-zoster virus RNA in human trigeminal ganglia. Lancet. 1983 Oct 8;2(8354):814–816. doi: 10.1016/s0140-6736(83)90736-5. [DOI] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J Infect Dis. 1974 Jul;130(1):16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- Maples J. A. A method for the covalent attachment of cells to glass slides for use in immunohistochemical assays. Am J Clin Pathol. 1985 Mar;83(3):356–363. doi: 10.1093/ajcp/83.3.356. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Nakazawa M., Endo H. Pathology of the human spinal ganglia in varicella-zoster virus infection. Acta Neuropathol. 1975 Dec 8;33(2):105–117. doi: 10.1007/BF00687537. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Reinhold W., Fan C. M., Zorn S., Hay J., Straus S. E. Transcription mapping of the varicella-zoster virus genome. J Virol. 1985 Nov;56(2):600–606. doi: 10.1128/jvi.56.2.600-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Notkins A. L. Continued expression of a poly(A)+ transcript of herpes simplex virus type 1 in trigeminal ganglia of latently infected mice. J Virol. 1987 May;61(5):1700–1703. doi: 10.1128/jvi.61.5.1700-1703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold W. C., Straus S. E., Ostrove J. M. Directionality and further mapping of varicella zoster virus transcripts. Virus Res. 1988 Feb;9(2-3):249–261. doi: 10.1016/0168-1702(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Elizan T. S. Chronic herpes simplex virus infection. Initiation in hamsters upon implantation of infected nonpermissive glial cells. Arch Neurol. 1973 Apr;28(4):224–230. doi: 10.1001/archneur.1973.00490220032003. [DOI] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Ostrove J. M., Inchauspé G., Felser J. M., Freifeld A., Croen K. D., Sawyer M. H. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment, and prevention. Ann Intern Med. 1988 Feb;108(2):221–237. doi: 10.7326/0003-4819-108-2-221. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]