Abstract
Platelet-derived growth factor (PDGF) gene therapy offers promise for tissue engineering of tooth-supporting alveolar bone defects. To date, limited information exists regarding the safety profile and systemic biodistribution of PDGF gene therapy vectors when delivered locally to periodontal osseous defects. The aim of this preclinical study was to determine the safety profile of adenovirus encoding the PDGF-B gene (AdPDGF-B) delivered in a collagen matrix to periodontal lesions. Standardized alveolar bone defects were created in rats, followed by delivery of matrix alone or containing AdPDGF-B at 5.5 × 108 or 5.5 × 109 plaque-forming units/ml. The regenerative response was confirmed histologically. Gross clinical observations, hematology, and blood chemistries were monitored to evaluate systemic involvement. Bioluminescence and quantitative polymerase chain reaction were used to assess vector biodistribution. No significant histopathological changes were noted during the investigation. Minor alterations in specific hematological and blood chemistries were seen; however, most parameters were within the normal range for all groups. Bioluminescence analysis revealed vector distribution at the axillary lymph nodes during the first 2 weeks with subsequent return to baseline levels. AdPDGF-B was well contained within the localized osseous defect area without viremia or distant organ involvement. These results indicate that AdPDGF-B delivered in a collagen matrix exhibits acceptable safety profiles for possible use in human clinical studies.
Introduction
Platelet-derived growth factor (PDGF), a member of a multifunctional polypeptide family, is composed of disulfide-bonded A, B, C, or D polypeptide chains to form a homo- or heterodimeric molecule (Andrae et al., 2008). PDGF is highly expressed in inflammatory cells, damaged bone, platelets, and mesenchymal cells (Southwood et al., 2004). PDGF mediates mitogenesis and chemotaxis of mesenchymal cells and osteoblasts through tyrosine-phosphorylated signaling pathways (Ronnstrand and Heldin, 2001; Fiedler et al., 2004). In oral tissues, PDGF also facilitates chemotaxis, matrix deposition, and attachment of periodontal ligament cells (Nishimura and Terranova, 1996; Haase et al., 1998). Delivery of PDGF-BB has also demonstrated enhancement of periodontal wound repair (Cooke et al., 2006) and regeneration preclinically (Giannobile et al., 1994, 1996; Park et al., 2000) and in humans (Howell et al., 1997; Nevins et al., 2005).
Although exogenous growth factors improve the soft and hard tissue healing response, more sophisticated delivery methods are necessary to ensure adequate protein concentration and specific cell targeting to defect sites (Ramseier et al., 2006; Cotrim and Baum, 2008). Recombinant adenoviruses (Ads) have been used as gene delivery vectors because of several unique features: (1) Ads have high transduction efficiency in both dividing and nondividing cells; (2) Ads do not induce apparent phenotypic changes in transduced cells; and (3) Ads do not integrate into the host genome and remain episomal (Gu et al., 2004). Compared with recombinant growth factors, adenovirus encoding PDGF gene sequences (AdPDGF) can successfully transduce cells, prolong growth factor expression, and induce downstream signaling pathways (Chen and Giannobile, 2002).
Adenoviral vectors administered to the head and neck for salivary gland repair have been previously studied and are now in clinical development (Cotrim et al., 2007; Voutetakis et al., 2008). Matrix-mediated delivery of DNA vectors has the potential to localize the vector and transgene products within the immediate delivery site (Chandler et al., 2000). We have previously shown that AdPDGF-B delivery in collagen significantly improves cementogenesis and osteogenesis in vivo (Jin et al., 2004). A preclinical investigation using the AdPDGF-B/collagen combination in a rabbit dermal wound model revealed robust localized wound healing responses with minimal systemic vector dissemination (Gu et al., 2004).
On the basis of our current knowledge, no existing data describe the systemic effects of adenoviral vector delivered to the osseous craniofacial complex. In this study we sought to evaluate the safety profile for the local, collagen matrix-mediated delivery of AdPDGF-B for the promotion of alveolar bone healing. Vector copy number and expression at the defect site and various organs were quantified, and systemic hematology and blood chemistry were evaluated. In combination with histological findings, the data in the present study further support the clinical development of matrix-enabled gene therapy for periodontal wound regeneration.
Materials and Methods
Adenoviral vectors
E1-,E3-deleted human adenovirus serotype 5 vectors encoding transgenes under the control of the cytomegalovirus promoter were employed in this study. Adenovirus encoding human platelet-derived growth factor-B (AdPDGF-B) and adenovirus encoding firefly luciferase (AdLuc) were used for gene transfer. Titers of viral stocks were determined on embryonic kidney 293 cells by plaque assay and expressed as plaque-forming units (PFU) per milliliter. Two different doses of adenoviral vectors were examined in this study: 5.5 × 108 and 5.5 × 109 PFU/ml in 20 μl of collagen matrix. These dose levels were equivalent to AdPDGF-B concentrations previously described (Jin et al., 2004).
Preparation of adenovirus gene-activated matrix
AdPDGF-B and AdLuc were dialyzed into GTS buffer (2.5% glycerol, 25 mM NaCl, 20 mM Tris; pH 8.0) and formulated in bovine fibrillar type I collagen matrix (Matrix Pharmaceutical, Fremont, CA) to a final concentration of 2.6%.
Periodontal alveolar bone wound model and AdPDGF-B treatment
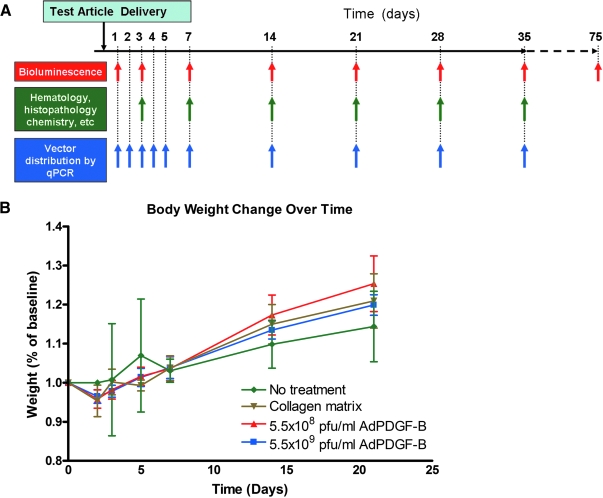
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Michigan (Ann Arbor, MI). A total of 144 (75 male, and 69 female) 10-week-old Sprague-Dawley rats (weighing 250–300 g) were used in this investigation. The general timeline, grouping criteria, and study design are shown in Fig. 1A and total gender distributions for each experiment are described separately.
FIG. 1.
General study design and body weight change over time. (A) Five treatment groups (5.5 × 108 PFU/ml AdLuc/collagen, 5.5 × 109 PFU/ml AdLuc/collagen, 5.5 × 108 PFU/ml AdPDGF-B/collagen, 5.5 × 109 PFU/ml AdPDGF-B/collagen, and collagen matrix only) were investigated. The observation time points were over a period of 35 days on a weekly basis; two animals in 5.5 × 109 PFU/ml AdLuc/collagen group were observed for 75 days. Nontreated animals (neither surgical defect nor adenovirus–collagen mixture application) were also included in the experiment to evaluate systemic involvement. (B) All the surgically treated animals experienced transient body weight loss in the first few days posttreatment but thereafter gained weight continuously throughout the study period.
Two different adenovirus gene-activated matrices were prepared immediately before surgery, containing AdPDGF-B at 5.5 × 108 PFU/ml (low dose), AdPDGF-B at 5.5 × 109 PFU/ml (high dose), or collagen matrix alone. For surgical operations, the animals were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg), followed by analgesia as needed with buprenorphine (Buprenex, 0.1–0.5 mg/kg; Reckitt Benckiser Healthcare, Hull, UK). Standardized 3 × 2 × 1 mm osseous defects were created in the buccal plate overlying the mandibular first molar and second molar tooth roots as previously described (Jin et al., 2003). The exposed roots were carefully denuded of periodontal ligament, cementum, and superficial dentin. Twenty microliters of adenovirus/collagen matrix was then delivered to the defects, filling them to entirety. The wounds were closed by suturing the superficial musculature layers and approximating the skin by surgical clips. The rats received analgesics on the next day as needed for up to 7 days postsurgery. The animals also received supplemental antibiotics (ampicillin, 268 μg/liter of dextrose in distilled water) for 7 days. The surgical clips were removed 10 days after surgery. Six rats without any surgical interventions (no treatment) were also included to compare the effect on body homeostasis of the surgical procedure versus no treatment.
Body weight and clinical observations
Twenty-four male rats were distributed equally to four groups (high-dose AdPDGF-B, low-dose AdPDGF-B, collagen matrix only, and no treatment). The body weight of those animals was measured during the first 3 weeks. Clinical observation was focused on evaluation of the gross signs of swelling and lesions on days 3–35 as noted in Fig. 1A.
Tissue harvesting, and histological and histopathological observations
On sacrifice, the submandibular lymph nodes, axillary lymph nodes, brain, lung, heart, liver, spleen, kidney, and testes (from male rats), and the entire tissue within defect area as well as ovaries (from female rats), were harvested with sterile scissors for each of the specific tissues and organs. The instruments were sterilized between tissue harvests, using a glass bead sterilizer. The ipsilateral organs were chosen, and for organs with abundant DNA (heart, lung, liver, spleen, kidney, sex organs, and brain), sectioning was done at the center of each specimen. Half of the selected tissues were then preserved in a −80°C freezer for DNA extraction, and the remaining half were fixed with 10% formalin for 24 hr and transferred to 75% ethanol for subsequent histological and histopathological analysis. The defect mandibulae were decalcified with 10% acetic acid, 4% formaldehyde, and 0.85% NaCl for 3 weeks. Decalcified mandibulae and the organ specimens were then dehydrated in step gradients of ethanol and embedded in paraffin. Sections from two different regions (border and central level of defect) were made in mandibular samples and three to six slices from the central-cut sections (5–8 μm in thickness). Hematoxylin and eosin staining was performed on all histological sections, followed by pathological examination. The time points for analyses were from days 3 to 35 as described in Fig. 1A. A thorough histopathological examination was performed for all sections.
Kinetics of luciferase expression by AdLuc/GAM in vivo
Adenovirus encoding luciferase (AdLuc) was formulated at concentrations of 5.5 × 108 PFU/ml (low dose, n = 6, 3 per gender) and 5.5 × 109 PFU/ml (high dose, n = 6, 3 per gender) in 20 μl of collagen matrix. Luciferase expression within each of the animals was measured with an in vivo imaging system (Xenogen/Caliper Life Sciences, Alameda, CA). To standardize the images, the cutoff threshold was set at 5000 p/sec/cm2/sr to reduce the background signals, and the yield threshold was set at 13,000 p/sec/cm2/sr. The amplitude of luciferase expression was calculated by subtracting the intensity of luciferin signal before and 12–15 min after luciferin (Promega, Madison, WI) injection (4 mg of luciferin per 25 g of body weight). The time points for evaluation are described in Fig. 1A.
Hematology and blood chemistry
All procedures were performed by the animal health diagnostic laboratory in the Unit for Laboratory Animal Medicine (ULAM) at the University of Michigan. Twenty-four male rats were distributed equally into four groups (high-dose AdPDGF-B, low-dose AdPDGF-B, collagen alone, and no treatment), and blood was drawn from the day before surgery through 35 days postoperation (Fig. 1A). Fifty microliters of whole blood from each rat was placed into a tube containing EDTA anticoagulant for hematological specimens and a complete blood cell count (CBC) with automatic differential was performed. Serum (200 μl) was drawn from each animal and the chemical parameters examined included alkaline phosphatase, calcium, phosphorus, creatinine kinase, albumin, globulin, total protein, blood urea nitrogen (BUN), creatinine, aspartate transaminase (AST), alanine transaminase (ALT), bilirubin, total bilirubin (T. bilirubin), amylase, glucose, and cholesterol.
Quantitative polymerase chain reaction assay
Quantitative TaqMan polymerase chain reaction (PCR) was used to determine the vector copy number of AdPDGF-B in the bloodstream and organs. The primers used for quantitative real-time PCR (qPCR) bridging the vector backbone and PDGF-B prepro region were as follows: sense, 5′-GGATCTTCGAGTCGACAAGCTT-3′; antisense, 5′-ATCTCATAAAGCTCCTCGGGAAT-3′; internal fluorogenic probe, 5′-CGCCCAGCAGCGATTCATGGTGAT-3′. qPCR was performed with TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA). Briefly, a 30-μl PCR was prepared with 500 ng of DNA and a 1.5-μl mixture of gene fluorogenic probe and primers. The thermal conditions were as follows: 50°C for 2 min, 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min, and the resulting amplicon was detected with an ABI PRISM 7700 sequence detection instrument (Applied Biosystems). The standard curve was determined with a range of 101 to 105 AdPDGF-B particles (regression correlation coefficient, >95%). The possibility of cross-reactivity was evaluated by adding adenoviral vector encoding PDGF-A, PDGF-1308 (dominant-negative mutant PDGF), bone morphogenetic protein-7, noggin, bone sialoprotein, luciferase, and green fluorescent protein (GFP) for comparison. No enhancement or inhibition of signal was noted when tissues were spiked with these vectors.
For blood DNA, the samples were collected from 6 rats per gender (total of 12 per group) in the four groups (high-dose AdPDGF-B, low-dose AdPDGF-B, collagen matrix only, and no treatment) before surgery, and throughout 35 days after gene delivery (Fig. 1A). Fifty microliters of whole blood was isolated and DNA was obtained with a QIAamp DNA blood mini kit (Qiagen, Valencia, CA). For organ and tissue DNA, total tissue in the defect area and surrounding musculature, submandibular lymph node, axillary lymph nodes, brain, lung, heart, liver, kidney, spleen, and sex organs (testes and ovaries) was excised from three rats in each of the three groups (high-dose AdPDGF-B, low-dose AdPDGF-B, and collagen matrix only) postsacrifice, and triplicate experiments were performed. The time points analyzed were from 3 to 35 days (Fig. 1A). Each PCR contained 500 ng of test DNA without spiking. Prestudy experiments demonstrated expected signal enhancement with AdPDGF-B spiking (500 copies per reaction; data not shown). The limit of detection was 30 copies per 500 ng of test DNA for all the specimens.
Statistical analysis
Analysis of variance (ANOVA) was used to evaluate the differences in body weights and hematological and chemical parameters between experimental and control groups. Test groups were evaluated for time-dependent dynamics with collagen and nonsurgical groups, using Bonferroni posttests, and the significance was assessed by repeated-measures ANOVA. Results are presented as the mean ± SD of measurements, with a p value less than 0.05 being considered statistically significant.
Results
Clinical observations and body weight
All animals survived throughout the entire experimental period and among all surgically treated animals, no significant adverse events were noted beyond local swelling at the treatment sites, presumably caused by the surgical procedures. Body weight changes were normalized, using day 0 as baseline, and the measures of weight change were evaluated as fractions relative to baseline weight. Results showed that after surgical treatment, all animals experienced slight weight loss within the first 2 days; however, they consistently gained weight over the course of the study. No significant weight changes were found among the three surgical groups at any time point (Fig. 1B).
Histology and histopathology
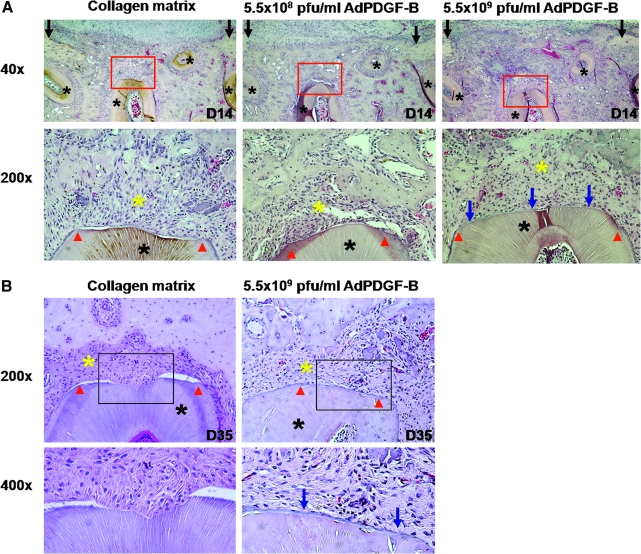
Two weeks after surgery, early bone formation could be observed within the defect area (Fig. 2A, top). Nearly complete bone bridging of the alveolar bone wounds was noted in both AdPDGF-B-treated groups, whereas there was limited bridging in the collagen-only animals. Cementogenesis could be seen in both AdPDGF-B-treated groups at 2 weeks but not in the collagen matrix group, and the defects treated with high-dose (5.5 × 109 PFU/ml) AdPDGF-B revealed more cementum formation compared with the other groups (Fig. 2A, bottom). At 35 days, the bone had completely bridged all of the defect area, and the fractions of defect fill became consistent in all animals. Animals receiving high-dose AdPDGF-B demonstrated greater evidence of cementogenesis along the tooth root (Fig. 2B).
FIG. 2.
PDGF gene delivery promotes periodontal tissue regeneration in vivo. (A) Limited bone formation and bridging had occurred by 14 days in wound treated with collagen matrix only compared with AdPDGF-B/collagen-treated defects. Top: Original magnification, × 40. Bottom: Higher power view (original magnification, × 200) of tooth/cementum/periodontal ligament (PDL)/bone interfaces outlined in red in the top row. More newly formed cementum structure (blue arrows) was observed in high-dose (5.5 × 109 PFU/ml) AdPDGF-B/collagen-treated sites. (B) At 35 days, defect treated with AdPDGF-B at 5.5 × 109 PFU/ml demonstrated a significant amount of root cementum compared with defect treated with collagen matrix only. Red arrowheads indicate the edges of exposed tooth dentin surface; blue arrows, new cementum; black asterisks, tooth roots; yellow asterisks, the area of PDL. (All images are in transverse orientation and stained with hematoxylin and eosin.)
Macroscopic evaluations of the harvested organs revealed no meaningful changes except mild enlargement of the submandibular lymph nodes in AdPDGF-B-treated (both high-dose and low-dose) and collagen matrix-only groups within the first week postsurgery. Evaluation of histological sections showed occasional but mild inflammatory infiltration in lymph nodes, spleen, and liver in all groups. However, no significant histopathological signs were noted beyond the suspected alterations associated with the surgical operation. In particular, no evidence of viral inclusions was observed for any of the evaluated tissues and organs.
Hematology and blood chemistry
Blood was analyzed from each animal before surgery and through 35 days postoperation (Fig. 1A). Also, blood from six animals in the no-treatment group was collected for comparison. All parameters for hematology and blood chemistry were consistent among groups and were generally within the normal range. Although there were some minor changes, we found no significant differences in complete blood count (CBC) and clinical chemistry parameters in any treatment group throughout the period of observation (Tables 1 and 2). There were several animals in both the high-dose and low-dose groups that revealed significant changes in amylase; however, the majority of the values were within the normal range. On day 28, animals in the low-dose group demonstrated significant elevation in serum glucose, but those levels returned to the baseline range by day 35.
Table 1.
Hematological Analyses for AdPDGF-B Delivery to Alveolar Bone Defectsa
| |
Before surgery |
Day 3 |
Day 7 |
Day 14 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematological parameterb | Col | L-Ad | H-Ad | Col | L-Ad | H-Ad | Col | L-Ad | H-Ad | Col | L-Ad | H-Ad |
| WBC (K/μl) | 12.53 (1.84) | 12.87 (2.21) | 13.99 (2.98) | 13.08 (1.98) | 8.491 (1.428) | 16.27 (2.29) | 12.57 (4.75) | 12.07 (3.97) | 16.90 (2.19) | 16.07 (3.15) | 13.01 (2.79) | 14.64 (0.86) |
| Neutrophil (K/μl) | 3.081 (0.887) | 4.184 (0.910) | 4.534 (1.343) | 3.493 (0.665) | 2.448 (0.559) | 4.985 (0.660) | 4.365 (2.170) | 2.781 (1.032) | 6.019 (0.678) | 6.599 (2.293) | 4.134 (1.228) | 4.811 (0.663) |
| Lymphocyte (K/μl) | 8.641 (1.481) | 7.908 (1.593) | 8.753 (1.595) | 8.784 (1.449) | 5.484 (0.949) | 10.16 (1.259) | 7.455 (2.674) | 8.491 (2.754) | 9.651 (1.673) | 8.683 (1.870) | 8.025 (1.575) | 8.974 (0.500) |
| Monocyte (K/μl) | 0.765 (0.239) | 0.745 (0.166) | 0.604 (0.180) | 0.735 (0.220) | 0.516 (0.175) | 0.841 (0.169) | 0.558 (0.299) | 0.764 (0.312) | 1.141 (0.182) | 0.687 (0.079) | 0.694 (0.174) | 0.711 (0.112) |
| Eosinophil (K/μl) | 0.033 (0.023) | 0.021 (0.015) | 0.074 (0.048) | 0.056 (0.048) | 0.043 (0.029) | 0.134 (0.075) | 0.158 (0.119) | 0.029 (0.016) | 0.05 (0.043) | 0.073 (0.079) | 0.133 (0.063) | 0.083 (0.096) |
| Basophil (K/μl) | 0.005 (0.008) | 0.009 (0.010) | 0.024 (0.035) | 0.008 (0.010) | 0.003 (0.005) | 0.026 (0.036) | 0.043 (0.041) | 0.009 (0.008) | 0.03 (0.044) | 0.027 (0.048) | 0.026 (0.031) | 0.016 (0.038) |
| RBC (M/μl) | 7.273 (0.599) | 8.164 (0.488) | 6.88 (0.646) | 7.745 (1.210) | 6.709 (0.506) | 6.223 (0.426) | 5.716 (1.068) | 7.606 (1.213) | 7.344 (0.600) | 6.763 (0.481) | 7.043 (0.344) | 7.344 (0.224) |
| Hb (g/dl) | 14.91 (0.78) | 17.35 (1.72) | 13.94 (1.42) | 15.25 (2.56) | 13.29 (1.10) | 12.58 (0.97) | 11.35 (2.29) | 15.38 (1.95) | 15.58 (1.09) | 13.51 (0.69) | 14.45 (0.60) | 15.038 (0.532) |
| Hct (%) | 44.91 (4.53) | 51.24 (2.98) | 41.76 (4.09) | 47.2 (7.67) | 40.43 (2.88) | 37.64 (2.31) | 33.5 (6.37) | 44.66 (6.58) | 44.74 (5.69) | 40.54 (3.40) | 41.75 (1.46) | 43.89 (1.18) |
| MCV (fl) | 61.7 (1.86) | 62.79 (1.20) | 60.67 (1.37) | 60.9 (1.53) | 60.31 (1.74) | 60.54 (0.73) | 58.52 (1.14) | 58.85 (1.37) | 62.85 (1.13) | 59.89 (1.80) | 59.36 (1.20) | 59.79 (1.18) |
| MCH (pg) | 20.55 (0.99) | 21.24 (1.04) | 20.27 (0.76) | 19.69 (0.81) | 19.8 (0.37) | 20.19 (0.30) | 19.77 (0.58) | 20.33 (1.27) | 21.23 (0.47) | 20.01 (0.98) | 20.54 (1.09) | 20.48 (0.34) |
| MCHC (g/dl) | 33.33 (1.75) | 33.81 (1.81) | 33.39 (0.84) | 32.28 (0.75) | 32.86 (1.06) | 33.4 (0.71) | 33.82 (1.09) | 34.55 (1.61) | 34.03 (1.03) | 33.47 (1.59) | 34.65 (1.56) | 34.288 (0.954) |
| RDW (%) | 15.44 (0.55) | 16.2 (0.52) | 15.01 (0.32) | 16.01 (0.63) | 15.76 (0.61) | 15.88 (0.46) | 15.92 (0.50) | 17.65 (0.76) | 16.89 (0.67) | 16.39 (0,98) | 16.29 (0.42) | 16.63 (0.37) |
Abbreviations: Col. collagen matrix-only group; H-Ad, high-dose (5.5 × 109 PFU/ml) AdPDGF-B-treated group; Hb, hemoglobin; Hct, hematocrit; L-Ad, low-dose (5.5 × 108 PFU/ml) AdPDGF-B-treated group; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cells; RDW, red blood cell distribution width; WBC, white blood cells.
n = 6 per group. Entries demonstrate the average value of parameters for each group; numbers in parentheses indicate standard deviations. No significant differences were noted among the AdPDGF-B and collagen matrix groups during early time points, as well as beyond 14 days (data not shown).
K/μl, thousands per microliter (entry × 103); M/μl, millions per microliter (entry × 106).
Table 2.
Clinical Chemistry Analyses For Ad-PDGF-B Deliverya
| |
Before surgery |
Day 3 |
Day 7 |
Day 14 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical chemistry parameter | Col | L-Ad | H-Ad | Col | L-Ad | H-Ad | Col | L-Ad | H-Ad | Col | L-Ad | H-Ad |
| Albumin (g/dl) | 2.814 (0.135) | 2.763 (0.130) | 2.657 (0.181) | 2.65 (0.648) | 2.657 (0.172) | 2.825 (0.116) | 2.543 (0.113) | 2.95 (0.141) | 2.788 (0.181) | 2.786 (0.177) | 2.913 (0.125) | 3.133 (0.234) |
| ALPase (U/liter) | 260.43 (23.52) | 255 (53.18) | 239 (31.09) | 185.5 (59.34) | 238.43 (35.45) | 166.63 (29.61) | 200.86 (37.66) | 205.75 (58.94) | 153.38 (27.69) | 232.29 (29.19) | 232.63 (42.45) | 251.67 (43.48) |
| ALT (U/liter) | 67.14 (8.30) | 89 (8.45) | 87.25 (7.50) | 72.88 (8.92) | 79.86 (4.06) | 79.88 (8.15) | 100.57 (10.47) | 79.38 (9.96) | 81 (5.04) | 84.86 (5.05) | 86.5 (7.58) | 79.5 (19.99) |
| Amylase (U/liter) | 1881.14 (186.95) | 1831 (188.36) | 1554.17 (267.61) | 1905.38 (388.61) | 1857.43 (544.49) | 1770 (251.95) | 2494.86b (844.40) | 1705.75 (310.88) | 1785.13 (328.22) | 1990.71 (525.58) | 1879.38 (195.60) | 2085.33 (44.004) |
| AST (U/liter) | 71.86 (9.91) | 79.38 (9.32) | 79.88 (8.97) | 69.5 (20.76) | 83.5 (16.55) | 69.88 (5.19) | 126.14 (111.67) | 77.57 (13.23) | 70.5 (11.43) | 66.43 (11.77) | 90.5 (26.46) | 93.25 (16.34) |
| Bilirubin (mg/dl) | 20.14 (3.34) | 19.5 (3.70) | 19 (2.16) | 21.25 (2.49) | 20 (2) | 22.13 (2.59) | 24.86 (1.86) | 21.38 (2.39) | 19.13 (1.64) | 22.57 (1.62) | 23.75 (3.45) | 24.17 (1.33) |
| Calcium (mg/dl) | 10.11 (0.25) | 10.29 (0.19) | 10.16 (0.28) | 10.54 (1.30) | 10.23 (0.28) | 10.49 (0.15) | 10.41 (0.25) | 10.35 (0.23) | 10.35 (0.12) | 10.86 (0.24) | 10.28 (0.18) | 10.83 (0.23) |
| Cholesterol (mg/dl) | 83.29 (7.13) | 78 (9.20) | 63.86 (11.81) | 78.71 (29.37) | 80.29 (8.16) | 90 (7.01) | 80 (6.90) | 80.38 (7.03) | 81.13 (2.80) | 84.43 (10.88) | 77.38 (10.32) | 82.33 (5.86) |
| Creatine kinase (U/liter) | 166.67 (28.25) | 190.63 (51.90) | 176.86 (47.36) | 178.5 (70.13) | 259.83 (133.13) | 142.63 (33.18) | 156.20 (38.46) | 244.57 (106.69) | 219.38 (64.16) | 121.71 (28.96) | 186.88 (61.42) | 197.75 (61.41) |
| Creatinine (mg/dl) | 0.4 (0.058) | 0.35 (0.053) | 0.329 (0.049) | 0.363 (0.052) | 0.386 (0.038) | 0.4 (0) | 0.386 (0.038) | 0.388 (0.035) | 0.3 (0.053) | 0.4 (0) | 0.388 (0.035) | 0.629 (0.399) |
| Glucose (mg/dl) | 230.43 (18.39) | 227.88 (27.22) | 229 (35.77) | 217.65 (24.22) | 221.57 (19.15) | 191 (33.815) | 245.29 (53.94) | 232 (29.99) | 229.88 (30.19) | 192.14 (7.67) | 212.5 (39.75) | 200.4 (10.69) |
| Phosphorus (mg/dl) | 7.657 (0.660) | 7.4 (0.490) | 6.743 (0.395) | 7.225 (0.585) | 6.214 (0.157) | 6.663 (0.434) | 6.671 (0.340) | 7.65 (0.537) | 7.45 (0.407) | 5.9 (2.62) | 7.325 (0.486) | 6.833 (0.115) |
| T. bilirubin (mg/dl) | 0.1 (0) | 0.1 (0) | 0.1 (0) | 0.275 (0.456) | 0.114 (0.038) | 0.113 (0.035) | 0.1 (0) | 0.175 (0.139) | 0.213 (0.210) | 0.1 (0) | 0.113 (0.035) | 0.1 (0) |
| Total protein (g/dl) | 5.629 (0.325) | 5.625 (0.205) | 5.557 (0.276) | 5.95 (0.680) | 5.529 (0.250) | 5.925 (0.128) | 5.586 (0.219) | 5.863 (0.250) | 5.8 (0.278) | 5.943 (0.190) | 5.938 (0.262) | 6.443 (0.351) |
| Globulin (g/dl) | 2.814 (0.227) | 2.838 (0.106) | 2.886 (0.107) | 3.288 (1.272) | 2.957 (0.162) | 3.1 (0.093) | 3.043 (0.151) | 2.925 (0.128) | 3.025 (0.128) | 3.157 (0.140) | 3.013 (0.146) | 3.25 (0.152) |
Abbreviations: ALPase, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; Col, collagen matrix-only group; H-Ad, high-dose (5.5 × 109 PFU/ml) AdPDGF-B-treated group; L-Ad, low-dose (5.5 × 108 PFU/ml) AdPDGF-B-treated group; T. bilirubin, total bilirubin.
All comparisons are made with reference to the collagen matrix group. Entries demonstrate the mean value of parameters for each group; numbers in parentheses indicates standard deviations. Serum amylase for both AdPDGF-B-treated groups revealed significant differences with respect to the collagen matrix group, and was within the normal range for time points beyound 14 days.
Significant difference from collagen matrix group (p < 0.05; n = 6 per group).
Vector expression by bioluminescence
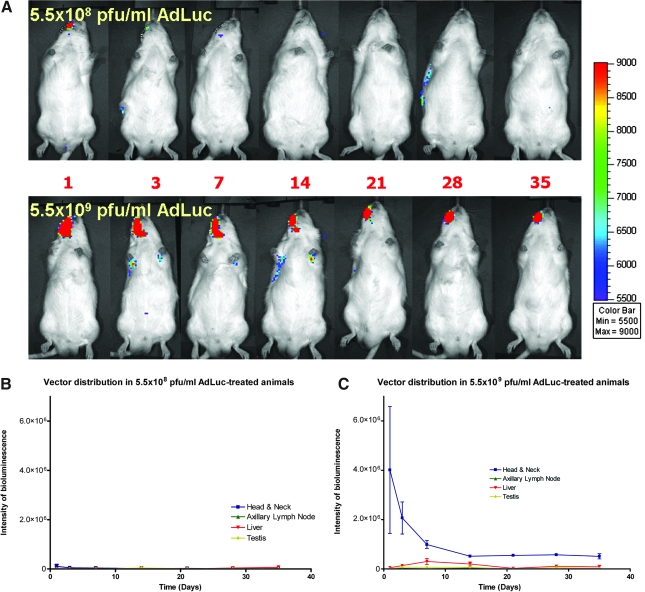
Whole body image analysis of animals treated with AdLuc/collagen matrix revealed a transduction and distribution profile from adenoviral gene delivery over the course of the experiment. Bioluminescent luciferase expression was detected in the head and neck region for all AdLuc/collagen-treated animals (n = 6 per group), with the level of expression higher in animals receiving high-dose AdLuc compared with the low-dose animals (Fig. 3A). For the low-dose AdLuc-treated group, luciferase expression gradually decreased to undetectable levels at the treated sites by 14 days without any spreading to distant organs for time points thereafter (note in Fig. 3A whole body imaging [top], some luminescence on day 28 on the animal's right side). Results also showed gradually decreasing expression of luciferase in the head and neck region within 2 weeks in high-dose AdLuc-treated animals. Further, the high-dose treated animals yielded a weak signal detected in the axillary lymph node area of three animals, and one animal showed liver expression at 1 week. However, after 2 weeks no signal was detected in any distant organs of any animal (Fig. 3B). To further investigate the persistent, low-level expression of AdLuc signal in two high-dose treated animals, bioluminescence imaging was performed until sacrifice at 75 days posttreatment. The defect mandible, surrounding musculature, axillary lymph nodes, liver, and gonadal organs were harvested and images were captured for bioluminescence quantification. Results revealed that a weak signal was restricted to only the surrounding musculature (<10 p/sec/mm2/sr), and no signal was detected in the defect site (data not shown). In addition, no significant gender differences in AdLuc expression were revealed; however, a somewhat lower signal was noted on day 1 in the head and neck region of female rats receiving high-dose AdLuc treatment (p < 0.05; data not shown).
FIG. 3.
Vector transduction efficiency and systemic distribution of bioluminescence. (A) Most of the luciferin signal is restricted to the alveolar bone defect region, with minimal systemic involvement. Signals in distant organs were absent after 14 days for both dose level groups. (B) Mild vector expression was noted during the first 3–7 days in animals treated with AdLuc at 5.5 × 108 PFU/ml. (C) Animals treated with AdLuc at 5.5 × 109 PFU/ml demonstrated significant vector expression during the first 14 days, followed by a decrease in vector expression in the head and neck region over time. The high-dose group also showed modest vector expression in liver (one of six positive on day 14) and axillary lymph nodes (one of six positive on day 3, and two of six positive on both days 7 and 10). Group size: n = 6 (three per gender). If the intensity of bioluminescence within the region of interest was less than 5000 p/sec/cm2/sr, that region was defined as “negative”.
Biodistribution by quantitative PCR
The specificity of our PCR primers and the sensitivity of the assay were determined before analysis of the study samples. We measured no primer cross-reaction with adenovirus encoding bone sialoprotein, bone morphogenetic protein-7, luciferase, noggin, PDGF-A, PDGF-1308, or GFP (data not shown). The sensitivity and detection limit of our PCR assays was 30 virus copies per 500 ng of DNA. Within the AdPDGF-B-treated area, viral vector could be detected within the first week in DNA from both high-dose and low-dose treated animals. The number of vector copies gradually decreased to undetectable levels after 2 weeks (Table 3). Vector copies measured in the blood were below the detection limit for all animals over the total period of observation. The PCR assay measured a low level of vector within spleen DNA of one animal at 3 days posttreatment, and within the lung of another animal at 2 weeks posttreatment; however, no significant vector DNA was detected in organs or tissues from the treatment groups for the remainder of the experimental time points (Table 3). These values were below the detection limit and compared similarly with vector values at the defect site, which were low to below the detection level. On examination of histological sections from the tissues (spleen and lung) positive for AdPDGF-B DNA, we found no inflammation-related phenotype or other pathological findings when compared with tissue sections from collagen matrix-treated animals.
Table 3.
AdPDGF-B PCR Results in Bloodstream and Distant Organs
| Organ/tissue | Treatment | No treatment | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 |
|---|---|---|---|---|---|---|---|---|
| Whole tissue from osseous defect | Collagen matrix | N | N | N | N | N | N | N |
| 5.5 × 108 PFU/ml AdPDGF-B | N | 3/3 (301) | 2/3 (137) | 1/3 (84) | N | N | N | |
| 5.5 × 109 PFU/ml AdPDGF-B | N | 3/3 (45,930) | 3/3 (6,097) | N | N | N | N | |
| Blood | Collagen matrix | N | N | N | N | N | N | N |
| 5.5 × 108 PFU/ml AdPDGF-B | N | N | N | N | N | N | N | |
| 5.5 × 109 PFU/ml AdPDGF-B | N | N | N | N | N | N | N | |
| Lung | Collagen matrix | N | N | N | N | N | N | N |
| 5.5 × 108 PFU/ml AdPDGF-B | N | N | N | 1/3 (38) | N | N | N | |
| 5.5 × 109 PFU/ml AdPDGF-B | N | N | N | N | N | N | N | |
| Spleen | Collagen matrix | N | N | N | N | N | N | N |
| 5.5 × 108 PFU/ml AdPDGF-B | N | 1/3 (31) | N | N | N | N | N | |
| 5.5 × 109 PFU/ml AdPDGF-B | N | N | N | N | N | N | N | |
| Brain, SLN, ALN, heart, liver, kidney, sex organs (testes or ovaries) | Collagen matrix | N | N | N | N | N | N | N |
| 5.5 × 108 PFU/ml AdPDGF-B | N | N | N | N | N | N | N | |
| 5.5 × 109 PFU/ml AdPDGF-B | N | N | N | N | N | N | N |
Abbreviations: ALN, axillary lymph nodes; N, negative; PFU, plaque-forming units; SLN, submandibular lymph nodes.
n = 3 per group (for organ analyses) and 23 per group (for blood analyses). Test sample DNAs yielding signals below the limit of detection (<30 vector particles per 500 ng of DNA) are reported as negative. Entries demonstrate “positive” animals in each group and entries in parentheses indicate the mean vector copy number per 500 ng of DNA from the positive animals.
Discussion
PDGF-BB protein has demonstrated its strong potential for soft and hard tissue repair and is available for clinical use (Nevins et al., 2005; Hollinger et al., 2008). However, because of the high degradation rate and transient persistence in vivo, the treatment outcome is not entirely predictable for clinical applications (Kaigler et al., 2006). Gene delivery using an adenoviral vector provides sustained and stable transduction efficiency in vitro (Chen and Giannobile, 2002). These data confirm and extend those of Jin and colleagues (2004) demonstrating significant enhancement of tooth-supporting alveolar bone and cementum regeneration in vivo, using gene-activated matrices containing AdPDGF-B.
Although a number of studies focus on the safety profile of adenovirus-mediated gene therapy, few of them have addressed the local delivery of vectors using a gene-activated matrix and none are related to the periodontium or localized bone defects. Studies have shown that direct systemic administration of adenoviral vectors can result in acute toxicity and hepatic pathology (Nunes et al., 1999; Lenaerts et al., 2005; Ni et al., 2005). Systemic dissemination can be reduced and the efficacy-to-toxicity ratio can be improved by local gene delivery (Wang et al., 2005). With localized delivery, the vector likely enters the systemic circulation via the leaky microvessels and systemically disseminates within 10 min (Wang et al., 2005), with the inflammatory infiltrate within liver observed after 15 min in mice (Ni et al., 2005). In this study, we employed matrix (collagen)-enabled gene delivery for localized administration to alveolar bone defects. The vector dissemination in our animals beyond the alveolar bone area was limited, demonstrating well-contained localization of the gene-activated matrix.
Studies have shown that nearly 99% of systemically delivered adenoviral vectors will eventually accumulate in the liver, and are rapidly taken up by Kupffer cells and hepatocytes (Hackett et al., 2000; Manickan et al., 2006). The Kupffer cells might distribute to the lung and spleen via the circulation, but in this study we did not detect any significant vector quantities in those organs. No significant elevation of the enzymes specific to those organs further demonstrates the limited systemic influence of this approach. Although transgene luciferase expression was found in the axillary lymph nodes, spleen, and lungs of a few adenoviral vector-treated animals at 2 weeks postadministration (with no expression in these organs at later time points), the level was only slightly greater than background and no accompanying toxicological signs or histopathological changes were found. We also noted no treatment-related toxicity throughout the 35-day period. Most of the hematological and clinical chemistry parameters were within normal ranges and the only significant difference was noted for amylase (derived primarily from the pancreas and parotid gland, with some from the liver), which is one of the major enzymes to digest starch into simple sugars. Changes in serum amylase may represent a normal physiologic process, acute or chronic pancreatitis, or concomitant ongoing diseases (Garrison, 1986). However, lipase is a more sensitive and specific marker with which to diagnose pancreatitis (Tietz et al., 1986), and the lipase level in all of the animals did not change significantly. However, it is quite possible that the amylase came from the parotid salivary gland that was located in close proximity to the surgical field. The parotid gland in rats is nonencapsulated, as compared with the gland in humans. We cannot rule out this area at early time points. At later time points when we measured the luciferase signal from the harvested organs, no detectable signal was found in any of the parotid glands, but mainly in the surrounding musculature (Fig. 3). In vivo bioluminescence generated by expression of the luciferase transgene permitted quantification and localization of transgene expression and provided noninvasive, dynamic, and comprehensive monitoring of vector expression at the whole body level (Wood et al., 1999; Johnson et al., 2006). As little as 104 luciferase-expressing recombinant adenoviruses are capable of producing luminescence in the liver (Honigman et al., 2001), which is significantly higher in sensitivity than is possible with qPCR (Johnson et al., 2006), making bioluminescence a more sensitive mode of evaluation of biodistribution and subsequent vector activity. In the early time periods we detected vector in the defect area of adenovirus-treated animals, which reached undetectable levels by day 14. This result supports those reported by Jin and colleagues (2004), showing that the luciferase signal decreased to 20% by day 14 and reached an undetectable level by day 28 compared with the expression on day 1. Moreover, given that PDGF is expressed in vivo over about 10 days in periodontal wounds after injury (Green et al., 1997), this gene therapy approach demonstrates a similar expression profile that may be favorable for therapeutic application.
In summary, the results of our experiments demonstrate that local administration of AdPDGF-B with gene-activated matrix is safe when delivered to tooth-supporting alveolar bone defects. No treatment-related toxicity or systemic involvement was found. Although vector particle DNA was detectable during the first 2 weeks, primarily in the osseous defects, the titer was low and quickly attenuated at subsequent time points. These results support the further clinical development of AdPDGF-B for regeneration therapy for oral and craniofacial bone application.
Acknowledgments
The authors thank Anna Colvig for performing hematological and clinical chemical examinations, Amanda Welton for assistance with bioluminescence, Dr. John E. Wilkinson for assistance with veterinary pathology, and Christopher Strayhorn for assistance with histological processing. This study was supported in part by grants from the AO Foundation (Davos, Switzerland) and NIH/NIDCR R01-DE13397.
Author Disclosure Statement
Drs. Sosnowski and Chandler are employees of Tissue Repair Co. The University of Michigan will benefit financially by clinical development of this technology.
References
- Andrae J. Gallini R. Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler L.A. Doukas J. Gonzalez A.M. Hoganson D.K. Gu D.L. Ma C. Nesbit M. Crombleholme T.M. Herlyn M. Sosnowski B.A. Pierce G.F. FGF2-targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol. Ther. 2000;2:153–160. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- Chen Q.P. Giannobile W.V. Adenoviral gene transfer of PDGF downregulates gas gene product PDGFαR and prolongs ERK and Akt/PKB activation. Am. J. Physiol. Cell Physiol. 2002;282:C538–C544. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J.W. Sarment D.P. Whitesman L.A. Miller S.E. Jin Q. Lynch S.E. Giannobile W.V. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12:1441–1450. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrim A.P. Baum B.J. Gene therapy: Some history, applications, problems, and prospects. Toxicol. Pathol. 2008;36:97–103. doi: 10.1177/0192623307309925. [DOI] [PubMed] [Google Scholar]
- Cotrim A.P. Sowers A. Mitchell J.B. Baum B.J. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol. Ther. 2007;15:2101–2106. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- Fiedler J. Etzel N. Brenner R.E. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J. Cell Biochem. 2004;93:990–998. doi: 10.1002/jcb.20219. [DOI] [PubMed] [Google Scholar]
- Garrison R. Amylase. Emerg. Med. Clin. North Am. 1986;4:315–327. [PubMed] [Google Scholar]
- Giannobile W.V. Finkelman R.D. Lynch S.E. Comparison of canine and nonhuman primate models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J. Periodontol. 1994;65:1158–1168. doi: 10.1902/jop.1994.65.12.1158. [DOI] [PubMed] [Google Scholar]
- Giannobile W.V. Hernandez R.A. Finkelman R.D. Ryan S. Kiritsy C.P. D'Andrea M.D. Lynch S.E. Comparative Effects of PDGF-BB, IGF-I singularly and in combination on periodontal regeneration in Macaca fascicularis. J. Periodont. Res. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Green R.J. Usui M.L. Hart C.E. Ammons W.F. Narayanan A.S. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-α and β receptors in human gingival wounds. J. Periodont. Res. 1997;32:209–214. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Gu D.L. Nguyen T. Gonzalez A.M. Printz M.A. Pierce G.F. Sosnowski B.A. Phillips M.L. Chandler L.A. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol. Ther. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Haase H.R. Clarkson R.W. Waters M.J. Bartold P.M. Growth factor modulation of mitogenic responses and proteoglycan synthesis by human periodontal fibroblasts. J. Cell Physiol. 1998;174:353–361. doi: 10.1002/(SICI)1097-4652(199803)174:3<353::AID-JCP9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hackett N.R. El Sawy T. Lee L.Y. Silva I. O'Leary J. Rosengart T.K. Crystal R.G. Use of quantitative TaqMan real-time PCR to track the time-dependent distribution of gene transfer vectors in vivo. Mol. Ther. 2000;2:649–656. doi: 10.1006/mthe.2000.0203. [DOI] [PubMed] [Google Scholar]
- ollinger J.O. Hart C.E. Hirsch S.N. Lynch S. Friedlaender G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Joint Surg. Am. 2008;90(Suppl. 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- Honigman A. Zeira E. Ohana P. Abramovitz R. Tavor E. Bar I. Zilberman Y. Rabinovsky R. Gazit D. Joseph A. Panet A. Shai E. Palmon A. Laster M. Galun E. Imaging transgene expression in live animals. Mol. Ther. 2001;4:239–249. doi: 10.1006/mthe.2001.0437. [DOI] [PubMed] [Google Scholar]
- Howell T.H. Fiorellini J.P. Paquette D.W. Offenbacher S. Giannobile W.V. Lynch S.E. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J. Periodontol. 1997;68:1186–1193. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- Jin Q. Anusaksathien O. Webb S.A. Rutherford R.B. Giannobile W.V. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J. Periodontol. 2003;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q. Anusaksathien O. Webb S.A. Printz M.A. Giannobile W.V. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol. Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. Huyn S. Burton J. Sato M. Wu L. Differential biodistribution of adenoviral vector in vivo as monitored by bioluminescence imaging and quantitative polymerase chain reaction. Hum. Gene Ther. 2006;17:1262–1269. doi: 10.1089/hum.2006.17.1262. [DOI] [PubMed] [Google Scholar]
- Kaigler D. Cirelli J.A. Giannobile W.V. Growth factor delivery for oral and periodontal tissue engineering. Exp. Opin. Drug Deliv. 2006;3:647–662. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts L. Verbeken E. De Clercq E. Naesens L. Mouse adenovirus type 1 infection in SCID mice: An experimental model for antiviral therapy of systemic adenovirus infections. Antimicrob. Agents Chemother. 2005;49:4689–4699. doi: 10.1128/AAC.49.11.4689-4699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickan E. Smith J.S. Tian J. Eggerman T.L. Lozier J.N. Muller J. Byrnes A.P. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Nevins M. Giannobile W.V. McGuire M.K. Kao R.T. Mellonig J.T. Hinrichs J.E. McAllister B.S. Murphy K.S. McClain P.K. Nevins M.L. Paquette D.W. Han T.J. Reddy M.S. Lavin P.T. Genco R.J. Lynch S.E. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J. Periodontol. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- Ni S. Bernt K. Gaggar A. Li Z.Y. Kiem H.P. Lieber A. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 2005;16:664–677. doi: 10.1089/hum.2005.16.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura F. Terranova V.P. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J. Dent. Res. 1996;75:986–992. doi: 10.1177/00220345960750041401. [DOI] [PubMed] [Google Scholar]
- Nunes F.A. Furth E.E. Wilson J.M. Raper S.E. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: Safety of re-administration. Hum. Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- Park Y.J. Lee Y.M. Park S.N. Sheen S.Y. Chung C.P. Lee S.J. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials. 2000;21:153–159. doi: 10.1016/s0142-9612(99)00143-x. [DOI] [PubMed] [Google Scholar]
- Ramseier C.A. Abramson Z.R. Jin Q. Giannobile W.V. Gene therapeutics for periodontal regenerative medicine. Dent. Clin. North Am. 2006;50:245–263. doi: 10.1016/j.cden.2005.12.001. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnstrand L. Heldin C.H. Mechanisms of platelet-derived growth factor-induced chemotaxis. Int. J. Cancer. 2001;91:757–762. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1136>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Southwood L.L. Frisbie D.D. Kawcak C.E. McIlwraith C.W. Delivery of growth factors using gene therapy to enhance bone healing. Vet. Surg. 2004;33:565–578. doi: 10.1111/j.1532-950x.2004.04080.x. [DOI] [PubMed] [Google Scholar]
- Tietz N.W. Huang W.Y. Rauh D.F. Shuey D.F. Laboratory tests in the differential diagnosis of hyperamylasemia. Clin. Chem. 1986;32:301–307. [PubMed] [Google Scholar]
- Voutetakis A. Zheng C. Metzger M. Cotrim A.P. Donahue R.E. Dunbar C.E. Baum B.J. Sorting of transgenic secretory proteins in rhesus macaque parotid glands following adenoviral mediated gene transfer. Hum. Gene Ther. 2008;19:1401–1405. doi: 10.1089/hum.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Yang Z. Liu S. Kon T. Krol A. Li C.Y. Yuan F. Characterisation of systemic dissemination of nonreplicating adenoviral vectors from tumours in local gene delivery. Br. J. Cancer. 2005;92:1414–1420. doi: 10.1038/sj.bjc.6602494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. Perrotte P. Onishi E. Harper M.E. Dinney C. Pagliaro L. Wilson D.R. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]