Abstract
OBJECTIVE
Mice with complete deletion of insulin receptor substrate 2 (IRS2) develop hyperglycemia, impaired hepatic insulin signaling, and elevated gluconeogenesis, whereas mice deficient for protein tyrosine phosphatase (PTP)1B display an opposing hepatic phenotype characterized by increased sensitivity to insulin. To define the relationship between these two signaling pathways in the regulation of liver metabolism, we used genetic and pharmacological approaches to study the effects of inhibiting PTP1B on hepatic insulin signaling and expression of gluconeogenic enzymes in IRS2−/− mice.
RESEARCH DESIGN AND METHODS
We analyzed glucose homeostasis and insulin signaling in liver and isolated hepatocytes from IRS2−/− and IRS2−/−/PTP1B−/− mice. Additionally, hepatic insulin signaling was assessed in control and IRS2−/− mice treated with resveratrol, an antioxidant present in red wine.
RESULTS
In livers of hyperglycemic IRS2−/− mice, the expression levels of PTP1B and its association with the insulin receptor (IR) were increased. The absence of PTP1B in the double-mutant mice restored hepatic IRS1-mediated phosphatidylinositol (PI) 3-kinase/Akt/Foxo1 signaling. Moreover, resveratrol treatment of hyperglycemic IRS2−/− mice decreased hepatic PTP1B mRNA and inhibited PTP1B activity, thereby restoring IRS1-mediated PI 3-kinase/Akt/Foxo1 signaling and peripheral insulin sensitivity.
CONCLUSIONS
By regulating the phosphorylation state of IR, PTB1B determines sensitivity to insulin in liver and exerts a unique role in the interplay between IRS1 and IRS2 in the modulation of hepatic insulin action.
The insulin receptor substrate (IRS) proteins are key mediators of insulin and insulin-like growth factor (IGF)-1 signaling. Of the six IRS proteins identified, IRS1 and IRS2 integrate essential signals from the insulin receptor (IR) and IGF-IR that regulate a variety of processes including metabolism and cellular growth, development, and survival (1). Signaling by IRS proteins is mediated by two main pathways: the phosphatidylinositol (PI) 3-kinase and the mitogen-activated protein kinase pathways. Although IRS1 and IRS2 share similar expression patterns, several lines of evidence suggest the tissue specificity of IRS-mediated signaling in growth and metabolism (2–5). IRS1−/− mice display reduced body size, insulin resistance, and β-cell hyperplasia (2). In contrast, complete deletion of IRS2 in mice causes defects in hepatic insulin action coincident with failed suppression of hepatic glucose production (HGP) (3,5,6) and β-cell insufficiency due to impaired IGF-1 mitogenic signaling (4). Consequently, IRS2-deficient mice develop type 2–like diabetes and most die at ∼16 week of age as a result of diabetes complications. Regarding insulin action in the liver, the transcription factor Foxo1 links IRS/PI-3 kinase-mediated signaling to the regulation of various genes involved in metabolic pathways (7). Activation of Akt/Foxo1 phosphorylation in the response to insulin treatment is disrupted in hepatocytes of IRS2−/− mice (8). However, recent studies have demonstrated that Foxo1 phosphorylation in liver may be effectively mediated by either IRS1 or IRS2 signaling (9–12).
Protein tyrosine phosphatases (PTPs) catalyze the dephosphorylation of tyrosine-phosphorylated proteins (13) and are negative regulators of tyrosine kinase receptor–mediated signaling. PTP1B directly interacts with both the IR and IGF-1R (14,15). The importance of PTP1B in hepatic metabolism has been demonstrated in vivo and in cellular models (16,17). Mice lacking the ptpn1 gene exhibit increased insulin sensitivity owing to enhanced phosphorylation of IR in liver and skeletal muscle, resistance to weight gain on a high-fat diet, and an increased basal metabolic rate (18–20). Moreover, the ability of insulin to suppress HGP is enhanced in PTP1B−/− mice. We have recently demonstrated that sensitivity to insulin in liver as a result of PTP1B deficiency is acquired during postnatal development: adult, but not neonatal, PTP1B−/− hepatocytes display increased insulin-mediated signaling via Akt/Foxo1 and a more pronounced inhibition of genes that regulate gluconeogenesis than in control hepatocytes (21).
Given that the hepatic phenotype of PTP1B−/− mice contrasts with that of IRS2−/− mice, we hypothesized that deletion of PTP1B in this model would restore sensitivity to insulin. With the present study, we demonstrate that expression of PTP1B is upregulated in the liver of IRS2−/− mice. Moreover, we have observed that the absence of this phosphatase enables activation of IRS1-mediated Akt/Foxo1/signaling, thereby restoring hepatic insulin sensitivity. Thus, genetic ablation of PTP1B or pharmacological inhibition of its expression and activity by resveratrol treatment rescues hepatic sensitivity to insulin action in IRS2−/− mice.
RESEARCH DESIGN AND METHODS
Reagents and antibodies.
Fetal serum (FS) and culture media were obtained from Invitrogen. Insulin for cell culture (I-0516), anti-mouse immunoglobulin (IgG)-agarose (A-6531), anti–β-actin antibody (A-5441), and resveratrol (R-5010) were from Sigma Aldrich. Protein A-agarose was from Roche Applied Science. (γ32P)-ATP (3,000 Ci/mmol), (α32P)-dCTP (3,000 Ci/mmol), and a cDNA labeling kit were from GE Healthcare. Anti–phospho-Foxo1 (Ser 256) (cat. no. 9461), anti–phospho-Akt (Thr 308) (cat. no. 2965), anti-Akt (cat. no. 9272), anti-phospho-AMP kinase (Thr172) (cat. no. 2531), and anti-AMP kinase (AMPK) (cat. no. 2532) antibodies were from Cell Signaling Technology. The anti–phospho-Akt (Ser 473) (sc-7985), anti-IR β-chain for Western blot (sc-711), and anti–phospho-Tyr for immunoprecipitations (Py20, sc-508) antibodies were from Santa Cruz Biotechnology. The anti-IR β-chain antibody (Ab-3; GR07) used for immunoprecipitations was from Calciochem. The anti-IRS1 (06-248), anti-p85α (06-195), anti-PTP1B (07-088), anti–acetyl-lysine (clone 4G12; 05-515), and anti–phospho-Tyr (clone 4G10, 05-321) antibodies were obtained from Upstate (Millipore). Anti-SirT1 antibody (Ab12193) was from Abcam. Human regular insulin for animal experiments (cat. no. 775502; Actrapid) was purchased from Novo Nordisk. Anti–phosphatidylinositol (PIP)3-fluorescein isothiocyanate (FITC) antibody (Z-6345) was from Echelon Research Laboratories Inc.
Animals.
IRS2−/− mice were purchased from The Jackson Laboratories. PTP1B−/− mice were obtained from Abbot Laboratories and have previously been described (19,21,22). Male IRS2−/− and female PTP1B−/− mice, maintained on a similar mixed genetic background (C57BL/6 × 129/sv), were intercrossed to yield IRS2+/−/PTP1B+/− mice. The resulting double-heterozygous mice were then intercrossed to obtain all possible genotypes including double-null IRS2−/−/PTP1B−/− mice. Genotyping was performed by PCR as previously described (23). The various genotypes were born with the expected Mendelian frequencies. Animals were fed a standard diet ad libitum and had free access to drinking water. All animal experimentation was conducted in accordance with accepted standards of animal care. Trans-resveratrol (25 mg/ml; Sigma) was orally administrated to mice via drinking water in bottles that were protected from the light, as previously described (24). The dose of resveratrol is equivalent to ∼2.5 mg · kg−1 · day−1. During our study, we confirmed that the mice were in fact consuming the amount of water necessary to reach the desired pharmacological dose of resveratrol.
Metabolic measurements.
For measuring fed and fasted (20- to 24-h fast) glucose concentrations, blood was obtained from the tail vein and glucose was assayed using a glucometer (Accu-Check Aviva; Roche). Intraperitoneal glucose tolerance (GTT) tests were performed on mice fasted for 20–24 h and injected with 2 g d-glucose/kg body wt, and blood glucose was measured at 30, 60, 90, and 120 min after injection. Intraperitoneal insulin tolerance (ITT) tests were performed on 4 h–fasted mice injected with 0.75 units/kg human regular insulin, and blood glucose was measured at 15, 30, 45, and 60 min after injection.
Insulin signaling studies.
Fasted mice were intraperitoneally injected with PBS or 0.75 units/kg human regular insulin and killed 15 min later. Then, livers were removed and total protein extracts were prepared as previously described (21).
Primary culture of adult hepatocytes.
Hepatocytes were isolated from nonfasting male mice (8–12 weeks old) by perfusion with collagenase as previously described (25). Cells were plated in 60-mm dishes (Falcon; BD biosciences) and cultured in William's Medium E supplemented with 20 ng/ml epidermal growth factor, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FS for 48 h. Then, cells were serum starved and used for experiments.
Immunofluorescence and confocal imaging.
Cells were grown on glass coverslips, serum starved for 4–6 h, and stimulated with insulin for 5 min. Then, cells were washed twice with PBS, fixed in methanol (−20°C) for 2 min, and processed for immunofluorescence. Anti–PIP3-FIIC antibody was applied for 1 h at 37°C in PBS-1% BSA, followed by four washes of 5 min each in PBS. Immunofluorescence was examined in an MRC-1024 confocal microscope adapted to an inverted Nikon Eclipse TE 300 microscope. Immunofluorescence mounting medium was from Vector Laboratories.
PI 3-kinase activity, RNA extraction from primary hepatocytes, and Northern blot analysis.
PI 3-kinase activity was measured in the immunoprecipitates by in vitro phosphorylation of PI as previously described (8). Total RNA was isolated from primary hepatocytes with Trizol (Invitrogen) and submitted to Northern blot analysis. Blots were hybridized with cDNA probes for PEPCK and glucose-6-phosphatase (G6Pase). Membranes were subjected to autoradiography, and relative quantification of the hybridization signals was performed by densitometric scanning of the autoradiograms.
Quantitative real-time PCR analysis.
Total RNA was isolated from livers with Trizol reagent (Invitrogen). DNase I–treated RNA was reverse transcribed into cDNA before the PCR assay was performed for ptpn1 gene expression. Primer-probe sets for murine PTP1B were purchased as predesigned TaqMan gene expression assays and run per the manufacturer's instructions (Applied Biosystems). The results are given as percentage over control (wild-type mice or IRS2−/− mice treated with resveratrol) after normalization of mRNA to 18S rRNA expression.
PTP1B phosphatase activity, islet isolation, islet morphology, and β-cell mass.
Livers were homogenized in buffer containing 20 mmol/l imidazole HCl, 2 mmol/l EGTA, and 2 mmol/l EDTA (pH 7.0) supplemented with protease inhibitors (10 mg/ml leupeptin, 10 mg/ml aprotinin, and 1 mmol/l phenylmethylsulfonyl fluoride). Cell lysates were applied through a Sephadex G-25 column to remove the free phosphate. After protein content determination, PTP1B phosphatase activity was determined by measuring phosphate release using a synthetic monophosphotyrosyl-containing peptide and the malachite green assay (cat. no. 17-125; Millipore). The protocols for islet isolation, analysis of islet morphology, and β-cell mass have previously been described (26).
PTP1B immunohistochemistry in islets.
Pancreata from 8- to 12-week-old mice were fixed with 4% paraformaldehyde overnight and cryoprotected in PBS containing 30% sucrose. Cryostat sections (10 μm) were cut and kept at −80°C until used. Sections were brought to room temperature, permeabilized with methanol for 2 min (−20°C), treated with 10% normal goat serum, and then incubated overnight with the anti-PTP1B antibody (1:50 dilution, sc-14021; Santa Cruz Biotechnology) overnight at 4°C. Immunodetection was carried out with a secondary biotinylated goat anti-rabbit antiserum using 3-3′diaminobencidine (Vector). Sections were examined in a Nikon Eclipse 90i microscope, and light microphotographs were taken with an associated Leitz digital camera (Leitz DFC300 FXC).
Statistical analysis.
The data are expressed as means ± SD. The statistical significance was estimated with Student's t test for unpaired observation. The results were considered significant at P < 0.05.
RESULTS
PTP1B deficiency restores insulin-activated PI 3-kinase/Akt/Foxo1 signaling in the liver of IRS2-deficient mice.
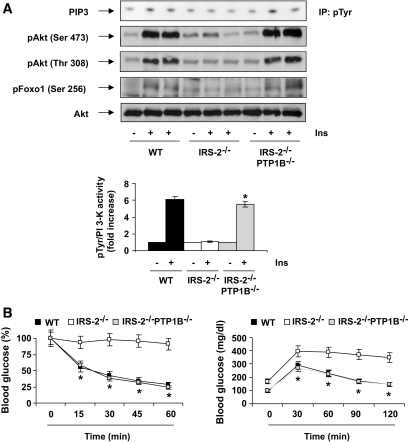
IRS2-deficient mice display hepatic insulin resistance and β-cell failure (3,5). Previous results published by Withers et al. (3) have demonstrated that insulin-stimulated IRS1-associated PI 3-kinase activity is significantly reduced in liver of IRS2−/− mice. Conversely, PTP1B-deficient mice are characterized by hypersensitivity to insulin in the activation of hepatic PI 3-kinase associated with IRS1/2 (18,20). Based on these observations, we postulated that enhanced insulin signaling in the liver as promoted by PTP1B deficiency would compensate for the lack of IRS2 and thereby restore insulin action in hepatocytes. As shown in Fig. 1A, total (anti–pTyr associated) PI-3 kinase activity was activated by insulin in liver from wild-type mice, whereas this response to insulin was absent in 12- to 16-week-old hyperglycemic (fasting blood glucose >300 mg/dl) IRS2−/− mice. However, insulin-stimulated PI-3 kinase activity was completely restored in the liver of IRS2−/−/PTP1B−/− mice of the same age. Downstream of PI 3-kinase, the phosphorylation of Akt at the catalytic (Thr 308) and hydrophobic (Ser 473) sites was reduced in the liver of hyperglycemic IRS2−/− mice but was also rescued by the absence of PTB1B in double-mutant mice (Fig. 1A). Consistent with this, insulin failed to induce the phosphorylation of Foxo1 in the absence of IRS2, but this response was normal in liver of IRS2−/−/PTP1B−/− mice. Collectively, these results indicate that the defective PI 3-kinase/Akt/Foxo1 signaling in the liver of hyperglycemic IRS2−/− mice can be completely reversed by the absence of PTP1B expression. In agreement with the data reported by Kushner et al. (23), PTP1B deficiency normalized fasting blood glucose (wild type 63 ± 2.35 mg/100 ml, IRS2−/− 169 ± 19.15 mg/100 ml, and IRS2−/−/PTP1B−/− 111 ± 4.37 mg/100 ml), fed blood glucose (wild type 138.81 ± 2.6 mg/100 ml, IRS2−/− 426.8 ± 35.7 mg/100 ml, and IRS2−/−/PTP1B−/− 129.6 ± 3.5 mg/100 ml), peripheral insulin sensitivity, and glucose tolerance in IRS2−/−/PTP1B−/− mice (Fig. 1B).
FIG. 1.
PTP1B deficiency recovers PI 3-kinase/Akt/Foxo1 insulin signaling in the liver of IRS2-deficient mice. A: Wild-type (WT), IRS2−/−, and IRS2−/−/PTP1B−/− mice (n = 9 animals per genotype) were fasted for 4 h, injected intraperitoneally with PBS or human regular insulin (0.75 units/kg), and killed 15 min later. Livers were removed and total protein extracts were prepared. Then, 600 μg total protein were immunoprecipitated with anti-pTyr antibody and used for an in vitro PI 3-kinase assay. The conversion of PI to PIP3 in the presence of [γ32-P] ATP was analyzed by thin-layer chromatography. Total protein (50 μg) was analyzed by Western blot with the antibodies against phospho-Akt (Ser 473), phospho-Akt (Thr 308), total Akt, and phospho-Foxo (Ser 256). A representative experiment is shown from four independent experiments performed. The autoradiograms showing PI 3-kinase activity were quantitated by scanning densitometry. Results are expressed as fold increase of PI 3-kinase activity and are means ± SEM. *P < 0.05, IRS2−/−/PTP1B−/− vs. IRS2−/−. B: ITT (intraperitoneal injection of 0.75 units/kg human regular insulin) and GTT (intraperitoneal injection of 2 g d-glucose/kg) tests in wild-type, IRS2−/−, and IRS2−/−/PTP1B−/− mice [n = 6–8 per genotype]). Animals were fasted for 4 and 20 h prior to insulin and glucose tolerance tests, respectively. For ITTs, results are expressed as mean ± SEM of percentage of initial blood glucose value. For GTTs, results are expressed as means ± SEM of blood glucose value (mg/dl). *P < 0.05, IRS2−/−/PTP1B−/− vs. IRS2−/−.
Restoration of insulin-mediated inhibition of PEPCK and G6Pase in primary hepatocytes from IRS2−/−/PTP1B−/− mice.
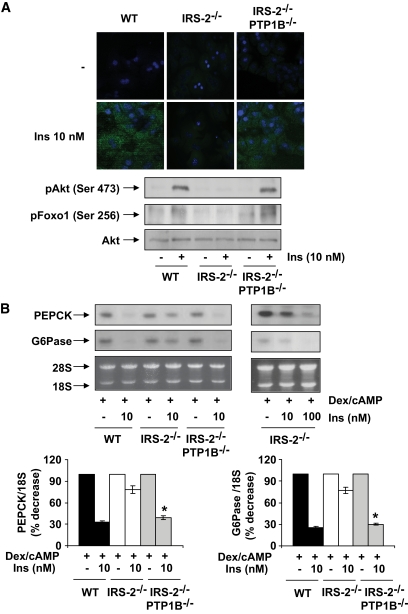
We have previously shown that in primary hepatocytes lacking IRS2, insulin failed to activate PI 3-kinase–mediated signaling, which inhibits expression of the gluconeogenic enzymes PEPCK and G6Pase (8). Thus, we investigated the effect of PTP1B deficiency on insulin-stimulated signaling in IRS2−/− hepatocytes. Consistent with observations in vivo, insulin failed to increase PIP3 levels or to induce the phosphorylation of Akt/Foxo1 in hepatocytes prepared from 12- to 16-week-old hyperglycemic IRS2−/− mice compared with wild-type controls. However, in primary hepatocytes lacking both IRS2 and PTP1B, insulin promoted PIP3 formation and Akt/Foxo1 phosphorylation at levels comparable with those in wild-type hepatocytes (Fig. 2A).
FIG. 2.
Recovery of insulin signaling and the inhibition of PEPCK and G6Pase in primary hepatocytes from IRS2−/−/PTP1B−/− mice. Primary hepatocytes obtained from mice of each genotype (wild type [WT], IRS2−/−, and IRS2−/PTP1B−) were cultured as described in research design and methods. A: Cells were serum starved for 4–6 h and further stimulated with 10 nmol/l insulin for 5 min. Upper panel: Cells were fixed in methanol (−20°C), and the generation of PIP3 was measured by confocal immunofluorescence with the anti–PIP3-FITC antibody. Representative images corresponding to primary hepatocytes isolated from one mouse are shown. Lower panel: Cell lysates were prepared, and total protein (50 μg) was used for Western blot analysis with the corresponding antibodies against phospho-Akt (Ser 473), phospho-Foxo (Ser 256), and total Akt. A representative experiment corresponding to primary hepatocytes isolated from one animal is shown from three independent experiments performed in triplicate. B: Cells were cultured in serum-free medium for 4–6 h and further stimulated with dex/cAMP (0.5 mmol/l dibutyril cAMP plus 1 μmol/l dexamethasone) in the absence or presence of insulin (10 or 100 nmol/l) for 6 h. At the end of the culture time, RNA was isolated and submitted to Northern blot analysis. A representative experiment corresponding to primary hepatocytes isolated from one mouse is shown. The autoradiograms corresponding to three independent experiments performed in hepatocytes were quantitated by scanning densitometry. The value of dex/cAMP-treated cells was set to 100%. Results are expressed as percentage of decrease by insulin of PEPCK and G6Pase mRNAs and are means ± SEM. *P < 0.05, IRS2−/−/PTP1B−/− vs. IRS2−/−. (A high-quality digital representation of this figure is available in the online issue.)
In vivo resistance to insulin in the livers of IRS2−/− mice is manifest by the failure of the hormone to suppress HGP (3). Thus, we investigated the ability of insulin to inhibit transcription of the genes for PEPCK and G6Pase in primary hepatocytes from wild-type, hyperglycemic IRS2−/− and IRS2−/−/PTP1B−/− mice. As shown in Fig. 2B, the mRNA levels for both PEPCK and G6Pase were upregulated by dexamethasone and cAMP in all genotypes regardless of IRS2 expression. However, insulin (10 nmol/l) failed to downregulate PEPCK and G6Pase mRNAs in IRS2-deficient hepatocytes. Of note, a higher dose of insulin (100 nmol/l) was able to suppress gluconeogenic gene expression in IRS2−/− hepatocytes, confirming the presence of severe insulin resistance. The inhibitory effect of insulin on PEPCK and G6Pase mRNAs was restored in hepatocytes from IRS2−/−/PTP1B−/− mice.
Both PTP1B expression and enzymatic activity are upregulated in the livers and islets of hyperglycemic IRS2−/− mice.
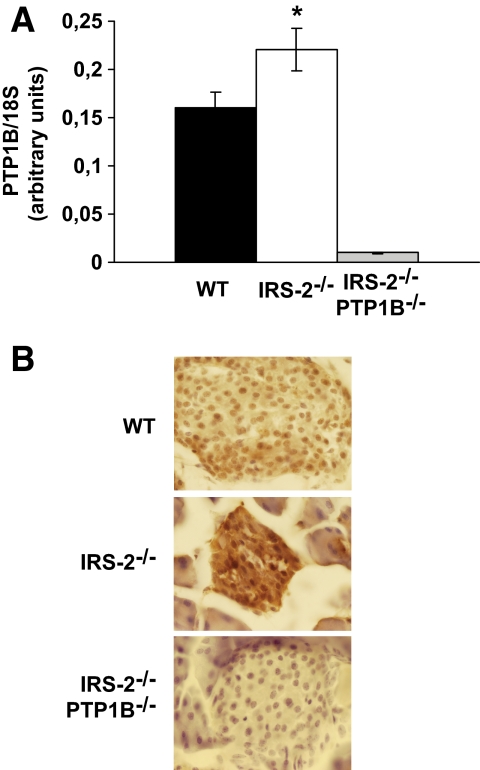
Since IR/IRS2-mediated insulin signaling is essential for maintaining glucose homeostasis, we examined the expression of key molecules that regulate the early steps of the insulin-signaling cascade in the livers of wild-type, hyperglycemic IRS2−/− and normoglycaemic IRS2−/−/PTP1B−/− mice. The expression of IR, IRS1, and p85α was equivalent between the three groups of mice (Fig. 3A). However, the levels of PTP1B protein and mRNA were significantly upregulated in the livers of hyperglycemic IRS2−/− mice compared with controls (Fig. 3A and B). PTP1B protein levels were also enhanced in primary hepatocytes prepared from hyperglycemic IRS2−/− mice (Fig. 3A). Importantly, PTP1B activity was also increased in liver extracts from hyperglycemic IRS2−/− mice compared with wild-type mice (Fig. 3C). Hepatic expression of the tyrosine phosphatase small heterdimer partner (SHP2) and the PIP3 inositol phosphatase PTEN was similar among the three genotypes of mice (Fig. 3A). As IRS2−/− mice also present abnormalities in β-cell growth that are partly reversed by PTP1B deletion (23), we investigated whether the expression of this phosphatase was also elevated in IRS2−/− pancreatic islets. Indeed, PTP1B mRNA levels were higher in islets from hyperglycemic IRS2−/− mice compared with those isolated from control mice (Fig. 4A). Moreover, immunohistochemistry analysis revealed more PTP1B staining in IRS2−/− islets than in wild-type controls (Fig. 4B).
FIG. 3.
Both PTP1B expression and its enzymatic activity were upregulated in the livers of IRS2−/− mice. A (upper panel): Liver extracts from wild-type (WT), IRS2−/−, and IRS2−/−/PTP1B−/− mice (n = 9 of each genotype) were prepared and total protein (50 μg) was used for Western blot analysis with the corresponding antibodies against insulin-signaling molecules. The anti–β-actin antibody was used as a loading control. A representative experiment is shown. The autoradiograms showing PTP1B from all animals analyzed were quantitated by scanning densitometry. Results are expressed as fold increase of PTP1B content and are means ± SEM. *P < 0.05, IRS2−/− vs. wild type. Lower panel: Primary hepatocytes obtained from mice of each genotype (wild type, IRS2−/−, and IRS2−/−/PTP1B−/−) were cultured as described in research design and methods. Cell lysates were prepared and total protein (50 μg) was used for Western blot analysis with the corresponding antibodies against PTP1B. A representative experiment corresponding to primary hepatocytes isolated from one animal is shown from three independent experiments performed in triplicate. B: Total RNA was isolated from livers of wild-type and IRS2−/− mice (n = 9 of each genotype). PTP1B mRNA levels were determined by real-time PCR. Results are expressed as arbitrary units of PTP1B/18S mRNA and are means ± SEM. *P < 0.05, IRS2−/− vs. wild type. mRNA from IRS2−/−/PTP1B−/− mice was used as a negative control. C: PTP1B activity was measured in wild-type and IRS2−/− liver extracts (n = 5 of each genotype) as described in research design and methods. Results are expressed as pmol · min−1 · μg protein−1 and are means ± SEM. PTP1B activity from IRS2−/−/PTP1B−/− liver extracts was used as a negative control. *P < 0.05, IRS2−/− vs. wild type.
FIG. 4.
Both PTP1B mRNA and protein levels were upregulated in islets of IRS2−/− mice. A: Total RNA was isolated from islets of wild-type (WT) and IRS2−/− mice (n = 8 of each genotype). PTP1B mRNA levels were determined by real-time PCR. Results are expressed as arbitrary units of PTP1B/18S mRNA and are means ± SEM. *P < 0.05, IRS2−/− vs. wild-type mice. mRNA from IRS2−/−/PTP1B−/− mice was used as a negative control. B: Immunostaining with the antibody against PTP1B was performed in pancreas sections from wild-type, IRS2−/−, and IRS2−/−/PTP1B−/− mice (n = 3 animals per genotype). Representative images are shown. (A high-quality digital representation of this figure is available in the online issue.)
The lack of PTP1B in hyperglycemic IRS2−/− mice restores hepatic IRS1-mediated PI 3-kinase signaling by releasing IR/PTP1B association.
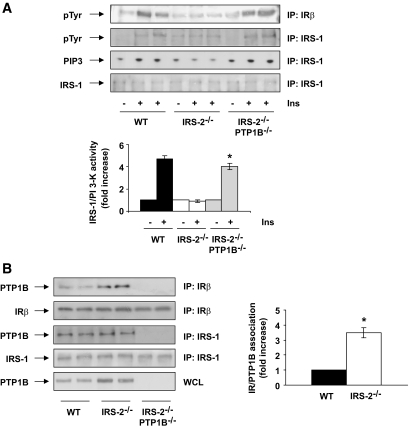
The fact that insulin-mediated PI 3-kinase/Akt/Foxo1 signaling was impaired in the livers of hyperglycemic IRS2−/− mice, along with the elevated expression and activity of PTP1B, prompted us to determine whether IRS1-mediated PI 3-kinase signaling was restored in IRS2−/−/PTP1B−/− mice. Whereas insulin-induced IRS1 tyrosine phosphorylation and PI 3-kinase activity were blunted in the liver of hyperglycemic IRS2−/− mice, these responses were completely restored in IRS2−/−/ PTP1B−/− mice (Fig. 5A). Upstream of IRS1 activation, insulin-mediated tyrosine phosphorylation of IR was completely absent in the liver of hyperglycemic IRS2−/− mice but was also rescued in IRS2−/−/PTP1B−/− mice. Consistent with this, the basal association of PTB1B with IR was significantly augmented in liver extracts from hyperglycemic IRS2−/− mice compared with the wild-type controls (Fig. 5B). However, we did not observe that IRS2 deficiency altered the PTP1B/IRS1 association. Thus, these results demonstrate that the absence of IRS2 leads to increased expression of PTP1B in the liver and enhanced association of this phosphatase with IR, thereby blocking IR/IRS1-mediated insulin signaling.
FIG. 5.
The lack of PTP1B in IRS2−/− mice restored IRS1-mediated PI 3-kinase signaling by releasing an IR/PTP1B association. A: Wild-type (WT), IRS2−/−, and IRS2−/−/PTP1B−/− mice (n = 9 animals per genotype) were fasted for 4 h, injected intraperitoneally with PBS or human regular insulin (Ins) (0.75 units/kg), and killed 15 min later. Livers were removed, and total protein extracts were prepared. Then, 600 μg total protein was immunoprecipitated with anti-IRβ and analyzed by Western blot with anti-pTyr. Total protein (600 μg) was immunoprecipitated with anti-IRS1 antibody and analyzed by Western blot with anti-pTyr and anti–total IRS1 or used for an in vitro PI 3-kinase assay. The conversion of PI to PIP3 in the presence of [γ32-P] ATP was analyzed by TLC. A representative experiment is shown. The autoradiograms showing PI 3-kinase activity were quantitated by scanning densitometry. Results are expressed as fold increase of PI 3-kinase activity and are means ± SEM. *P < 0.05, IRS2−/−/PTP1B−/− vs. IRS2−/−. B: Liver extracts from wild-type, IRS2−/−, and IRS2−/−/PTP1B−/− mice (n = 6 animals per genotype) were prepared and 600 μg total protein were immunoprecipitated with anti-IRβ or anti-IRS1 antibodies and analyzed by Western blot with the anti-PTP1B, anti-IRβ, and anti-IRS1 antibodies. Total protein (50 μg) was analyzed in whole cell lysates by Western blot with the antibody against PTP1B. The autoradiograms showing IR/PTP1B association from all animals analyzed were quantitated by scanning densitometry. Results are expressed as fold increase of IR/PTP1B association and are means ± SEM. *P < 0.05, IRS2−/− vs. wild type.
Treatment of hyperglycemic IRS2−/− mice with resveratrol downregulates PTP1B in the liver and promotes insulin-induced IRS1/Akt/Foxo1 signaling.
We next investigated whether insulin sensitivity in the liver of hyperglycemic IRS2−/− mice could be restored by pharmacologically targeting PTP1B. Resveratrol, a plant-derived polyphenol that is a potent activator of SIRT1, has been reported to repress PTP1B expression in the liver under high-fat diet–induced insulin resistance (24). Thus, hyperglycemic IRS2−/− mice were treated with resveratrol (2.5 mg · kg−1 · day−1) for 8 weeks and hepatic insulin signaling was analyzed. As shown in Fig. 6A, both insulin-induced IRS1-associated PI 3-kinase activity and Akt/Foxo1 phosphorylation were significantly increased in resveratrol-treated IRS2−/− mice compared with untreated IRS2-deficient mice. Of note, treatment with resveratrol did not alter IRS1 acetylation in the livers of IRS2−/− mice. Consistent with the restoration of insulin sensitivity, resveratrol therapy decreased PTP1B mRNA, protein levels, and phosphatase activity in the livers of IRS2−/− mice compared with untreated mice (Fig. 6A–C). Conversely, neither SirT1 levels nor the phosphorylation of its downstream effector AMPK (27) was affected by resveratrol treatment (Fig. 6A). Moreover, resveratrol improved systemic insulin sensitivity in IRS2−/− mice but did not change glucose tolerance (Fig. 6D). Blood glucose remained elevated in both the fasted (IRS2−/− 169.0 ± 19.15 mg/100 ml; IRS2−/−, resveratrol 169.77 ± 17.80 mg/100 ml) and fed (IRS2−/− 426.80 ± 35.70 mg/100 ml; IRS2−/−, resveratrol 448.9 ± 40 mg/100 ml) states compared with control untreated mice. Consistent with this, resveratrol treatment of IRS2−/− mice did not improve islet morphology or β-cell mass compared with untreated mice (results not shown).
FIG. 6.
Treatment of IRS2−/− mice with resveratrol downregulated PTP1B in the liver and restored insulin (Ins)-induced Akt/Foxo1 signaling and peripheral insulin sensitivity. A: Liver extracts from IRS2−/− mice treated (IRS-2−/−Resv) or not (IRS-2−/−) with resveratrol (n = 8 animals per condition) were fasted for 4 h, injected intraperitoneally (IP) with PBS or human regular insulin (0.75 units/kg), and killed 15 min later. Livers were removed and total protein extracts were prepared. Then, 600 μg total protein was immunoprecipitated with anti-IRS1 antibody analyzed by Western blot with anti-AcLys antibody or used for an in vitro PI 3-kinase (PI 3-K) assay. The conversion of PI to PIP3 in the presence of [γ32-P] ATP was analyzed by thin-layer chromatography. Total protein (50 μg) was analyzed by Western blot with the antibodies against phospho-Akt (pAkt) (Ser 473), phospho-Foxo (Ser 256), total Akt, and PTP1B. A representative experiment is shown from eight independent experiments performed. The autoradiograms showing PI 3-kinase activity were quantitated by scanning densitometry. Results are expressed as fold increase of PI 3-kinase activity and are means ± SEM. *P < 0.05, resveratrol treatment vs. no treatment. B: Total RNA was isolated from livers of IRS2−/− mice treated or not with resveratrol (n = 8 animals per condition). PTP1B mRNA levels were determined by real-time PCR. Results are expressed as arbitrary units of PTP1B/18S mRNA and are means ± SEM. *P < 0.05, treatment vs. no treatment. C: PTP1B activity was measured in liver extracts of IRS2−/− mice treated or not with resveratrol (n = 4 animals per condition) as described in research design and methods. Results are expressed as pmol · min−1 · μg protein−1 and are means ± SEM. *P < 0.05, treated vs. untreated-. D: ITT (injection of 0.75 units/kg human regular insulin) and GTT (injection of 2 g d-glucose/kg) tests in 8- to 12-week-old IRS2−/− mice treated or not with resveratrol (n = 6–8 per genotype). Animals were fasted for 4 and 20 h prior to insulin and glucose tolerance tests, respectively. For ITTs, results are expressed as means ± SEM of percentage of initial blood glucose value. For GTTs, results are expressed as means ± SEM of blood glucose value (mg/dl). *P < 0.05, treated vs. untreated. pAMPK, phosphorylated AMPK; Prot, protein.
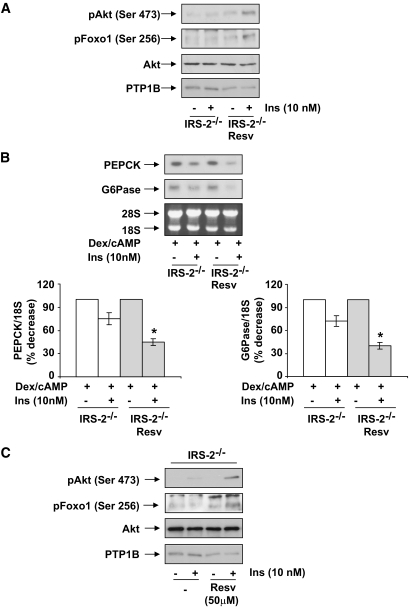
To further confirm these data, we prepared primary hepatocytes from IRS2−/− mice that had been treated with resveratrol, and PTP1B levels and insulin signaling were analyzed. Figure 7A reveals that PTP1B was downregulated and insulin strongly induced the phosphorylation of Akt/Foxo1 in hepatocytes from resveratrol-treated IRS2−/− mice compared with primary hepatocytes from untreated hyperglycemic IRS2−/− mice. Additionally, the ability of insulin to inhibit PEPCK and G6Pase mRNAs was restored in hepatocytes from resveratrol-treated IRS2−/− mice (Fig. 7B). These results demonstrate that resveratrol, by inhibiting the expression/activity of PTB1B, promotes insulin sensitivity in IRS2−/− hepatocytes. To substantiate these data, we treated primary hepatocytes from IRS2−/− mice with 50 μmol/l resveratrol for 24 h and then stimulated with insulin. As shown in Fig. 7C, resveratrol treatment downregulated PTP1B in IRS2−/− hepatocytes and, therefore, insulin-induced Akt and Foxo1 phosphorylation were restored.
FIG. 7.
Recovery of insulin (Ins) signaling and the inhibition of PEPCK and G6Pase in primary hepatocytes from resveratrol (Resv)-treated IRS2−/− mice. Primary hepatocytes obtained from mice of each condition (treated or not with resveratrol) were cultured as described in research design and methods. A: Cells were serum starved for 4–6 h and further stimulated with 10 nmol/l insulin for 5 min. After cell lysates were prepared, total protein (50 μg) was used for Western blot analysis with the corresponding antibodies against phospho-Akt (Ser 473), phospho-Foxo (Ser 256), total Akt, and PTP1B. A representative experiment corresponding to primary hepatocytes isolated from one animal is shown from three independent experiments performed in triplicate. B: Cells were cultured in serum-free medium for 4–6 h and further stimulated with dex/cAMP (0.5 mmol/l dibutyril cAMP plus 1 μmol/l dexamethasone) in the absence or presence of insulin (10 nmol/l) for 6 h. At the end of the culture time, RNA was isolated and submitted to Northern blot analysis. A representative experiment corresponding to primary hepatocytes isolated from one mouse is shown. The autoradiograms corresponding to three independent experiments performed in hepatocytes were quantitated by scanning densitometry. The value of dex/cAMP-treated cells was set to 100%. Results are expressed as percentage of decrease by insulin of PEPCK and G6Pase mRNAs and are means ± SEM. *P < 0.05, treated vs. untreated. C: Primary hepatocytes obtained from IRS2−/− mice were cultured as described in research design and methods. Cells were treated with 50 μmol/l resveratrol for 24 h, serum-starved for 2 h, and then stimulated with 10 nmol/l insulin for 5 min. After cell lysates were prepared, total protein (50 μg) was used for Western blot analysis with the corresponding antibodies against phospho-Akt (Ser 473), phospho-Foxo (Ser 256), total Akt, and PTP1B. A representative experiment corresponding to primary hepatocytes isolated from one animal is shown from three independent experiments performed in triplicate.
DISCUSSION
IRS proteins coordinate signaling pathways that regulate metabolism and cell growth and survival. Understanding the molecular regulation of IRSs is crucial for identifying new targets to treat or prevent insulin resistance and metabolic diseases. The IRS2 knockout mouse is a genetic model with defective PI 3-kinase/Akt/Foxo1–mediated insulin signaling in the liver, which causes increased HGP (3). Since inhibition of PTP1B has been demonstrated to enhance PI 3-kinase signaling in the liver (18,20), the present study has investigated whether elimination or reduction of PTP1B expression by genetic or pharmacological approaches could restore hepatic sensitivity to insulin in IRS2−/− mice that displayed fasting hyperglycemia (>300 mg/dl) at 12–16 weeks of age.
Foxo1 has been established as the molecular link between Akt phosphorylation and gluconeogenic gene expression (28,29). However, we observed that in insulin-treated IRS2−/− hepatocytes, Foxo1 remained unphosphorylated and the expression of PEPCK and G6Pase was elevated. These results are consistent with previous data (3) demonstrating that the ability of IRS1 to activate PI 3-kinase in response to insulin was severely impaired in the liver of IRS2−/− mice. Interestingly, our current results reveal that the inability of insulin to phosphorylate Foxo1 through activation of PIP3/Akt in the liver of IRS2−/− mice can be reversed by PTP1B deletion. This is a critical finding because both IRS1 and IRS2 can efficiently activate the PI 3-kinase pathway in the liver to mediate the physiological responses to insulin in regulating carbohydrate and lipid metabolism, as has been demonstrated recently in mice with liver-specific deletions of both IRS1 and IRS2 (9–12). Importantly, our results demonstrate that mRNA, protein levels, and the phosphatase activity of PTP1B were elevated in the livers of systemic IRS2−/− mice compared with wild-type controls. However, genetic deletion or pharmacological inhibition (see below) of PTP1B completely restored IRS1-mediated PI 3-kinase/Akt/Foxo1 signaling and insulin-mediated inhibition of gluconeogenic gene expression in the liver of IRS2−/− mice. Thus, our results show for the first time that the impairment of IRS1-mediated signaling in the liver of IRS2−/− mice can be fully corrected by the lack of PTP1B. Moreover, these findings suggest that the upregulation of PTP1B expression and phosphatase activity represents the molecular mechanism underlying the loss of sensitivity to insulin in liver of hyperglycemic IRS2−/− mice. IR is a major substrate for PTP1B (14), and our results further demonstrate that upregulation of PTB1B in liver of IRS2−/− mice enhances the IR/PTP1B association. Consequently, the increased dephosphorylation of IR is likely to be responsible for the inability of IRS1 to mediate insulin signaling in the liver of IRS2−/− mice. Thus, the absence of PTP1B in the double-mutant mice promotes insulin sensitivity in the liver by enabling the activation of an IR/IRS1-mediated compensatory mechanism, culminating in the inhibition of PEPCK and G6Pase in hepatocytes.
When the physiological consequences resulting from the complete deletion of IRS2 are compared with liver-specific conditional knockouts of IRS2, it is intriguing that IRS1 is able to compensate for the lack of IRS2 signals in the liver-specific models (9–12) but not in the total body (3) of IRS2−/− mice. Complete disruption of IRS2 causes hyperglycemia at 10–12 weeks of age (3,5), whereas hyperglycemia was not reported in liver-specific IRS2−/− mice (9–12). IRS2 deficiency impairs development and expansion of pancreatic β-cells owing to the lack of IGF-I signaling (4), thereby precluding β-cell hyperplasia as a compensatory response in the whole-body knockout model. However, this is not the case for liver-specific deletions of IRS2 where the compensatory mechanisms employed by β-cells remain intact. Interestingly, it has been demonstrated recently that high glucose enhances PTP1B transcription in human hepatocytes (30). Therefore, the presence of hyperglycemia might explain the increased levels of PTP1B mRNA and protein detected in the livers and islets of the IRS2−/− mice by the present study. Given that complete deletion of IRS2 causes multiple metabolic defects, other systemic components such as defective β-cell function, hyperleptinemia, or dyslipidemia may also modulate hepatic PTB1B expression. Mice heterozygous for both IR and IRS1 (DHet mice) display severe insulin resistance and hyperglycemia (>250 mg/dl); deletion of PTB1B in this model restored hepatic IRS1/IRS2-associated PI 3-kinase activity in response to insulin and markedly improved glucose tolerance and insulin sensitivity (31). However, no data were reported regarding PTP1B levels or activity in the liver of DHet mice.
It has recently been shown that the antioxidant resveratrol improves insulin sensitivity in insulin-resistant livers by repressing PTP1B (24). Our present findings clearly demonstrate that resveratrol exerts a protective effect against hepatic insulin resistance in IRS2−/− mice by reducing both PTP1B expression and phosphatase activity. This result is particularly interesting because AMPK phosphorylation, a downstream target of SirT1 (27), was not altered by resveratrol treatment of IRS2−/− mice. The ability of insulin to stimulate IRS1-mediated PI 3-kinase signaling was restored to normal in IRS2-deficient mice treated with resveratrol. Thus, hepatocytes from resveratrol-treated IRS2−/− mice responded to insulin by inhibiting PEPCK and G6Pase. These data are consistent with those obtained by the genetic ablation of PTB1B in the IRS2-deficient model and suggest that resveratrol might be employed to treat hepatic insulin resistance. However, the inability of resveratrol to improve glucose tolerance in IRS2−/− mice indicates that treatment with this polyphenol does not reverse the severe defects in β-cell proliferation and survival that are due to impaired IGF-1 signaling (4). The observed failure of resveratrol to improve the pancreatic phenotype of IRS2−/− mice was most likely due to the fact that this compound was administered to animals upon weaning when islet damage was already present, making it difficult to overcome the developmental reduction of β-cell mass and the loss of existing islets during the pre-diabetic and diabetic phases in mice lacking IRS2. Although the present study validates PTP1B as an attractive target for treatment of hepatic insulin–resistant states in humans, pharmacological therapies based on this phosphatase must be developed carefully given the pleiotropic effects of PTP1B on receptor tyrosine kinase signaling. In this regard, PTP1B-deficient liver cells are protected against apoptosis and liver failure (32,33). Recent studies have demonstrated that inhibition of PTP1B has beneficial effects on breast and colon tumorigenesis (34–36), suggesting that the use of PTP1B inhibitors for the treatment of hepatic insulin resistance might also protect against cancer.
In conclusion, our results demonstrate that elevated expression and activity of PTP1B in the liver of hyperglycemic IRS2−/− mice impair IR/IRS1-mediated insulin signaling by increasing the association of IR with this phosphatase. Accordingly, deletion of PTP1B promotes insulin sensitivity in the liver of IRS2−/− mice through the restoration of IRS1-mediated Akt/Foxo1 phosphorylation and the inhibition of gluconeogenic enzymes. This molecular mechanism (summarized in Fig. 8) can be mimicked by resveratrol through downregulation of PTP1B activity in liver of IRS2−/− mice. Thus, PTP1B has a unique role in modulating the signaling of IRS1 and IRS2 in the regulation of hepatic metabolic pathways and represents a rational target for the development of new pharmacological agents aimed at improving hepatic insulin sensitivity.
FIG. 8.
Schematic representation of the mechanism by which inhibition of PTP1B promotes insulin sensitivity in the liver of IRS2-deficient mice. The lack of PTP1B promotes insulin sensitivity in the liver of IRS2−/− mice through the restoration of IRS1-mediated Akt/Foxo1 phosphorylation (pAkt and pFoxo1) and the inhibition of gluconeogenic enzymes. This molecular mechanism can be mimicked by resveratrol through downregulation of PTP1B expression and activity in the liver of IRS2−/− mice. PI 3-K, PI 3-kinase.
ACKNOWLEDGMENTS
This work was supported by Ministerio de Ciencia e Innovación Grants (Spain) BFU2008-02420, SAF2009-08114 (to A.M.V.), BFU2008-04901-C03-03 (to M.R.), BFU2005-00084, and SAF2008-00011 (to D.J.B.) and the Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) (Instituto Salud Carlos III). A.G.-R. holds a postdoctoral contract from CIBERDEM.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.White MF: Regulating insulin signaling and beta-cell function through IRS proteins. Can J Physiol Pharmacol 2006; 84: 725– 737 [DOI] [PubMed] [Google Scholar]
- 2.Araki E, Lipes MA, Patti ME, Brüning JC, Haag B, 3rd, Johnson RS, Kahn CR: Alternative pathway of insulin signaling in mice with targeted disruption of the IRS1 gene. Nature 1994; 372: 186– 190 [DOI] [PubMed] [Google Scholar]
- 3.Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF: Disruption of IRS2 causes type 2 diabetes in mice. Nature 1998; 391: 900– 904 [DOI] [PubMed] [Google Scholar]
- 4.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF: IRS2 coordinates IGF-I receptor-mediated β-cell development and peripheral insulin signaling. Nat Genet 1999; 23: 32– 40 [DOI] [PubMed] [Google Scholar]
- 5.Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T: Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes 2000; 49: 1880– 1889 [DOI] [PubMed] [Google Scholar]
- 6.Previs SF, Withers DJ, Ren JM, White MF, Shulman GI: Contrasting effects of IRS1 versus IRS2 gene disruption on carbohydrate and lipid metabolism in vivo. J Biol Chem 2000; 275: 38990– 38994 [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG: FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 2006; 281: 10105– 10117 [DOI] [PubMed] [Google Scholar]
- 8.Valverde AM, Burks D, Fabregat I, Fisher T, Carretero J, White MF, Benito M: Molecular mechanisms of insulin resistance in IRS2-deficient hepatocytes. Diabetes 2003; 52: 2239– 2248 [DOI] [PubMed] [Google Scholar]
- 9.Dong X, Park S, Lin X, Colls K, Yi X, White MF: Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest 2006; 116: 101– 114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF: Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab 2008; 8: 65– 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, Takamoto I, Mineyama T, Ogata H, Tokuyama K, Ohsugi M, Sasako T, Moroi M, Sugi K, Kakuta S, Iwakura Y, Noda T, Ohnishi S, Nagai R, Tobe K, Terauchi Y, Ueki K, Kadowaki T: Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab 2008; 8: 49– 64 [DOI] [PubMed] [Google Scholar]
- 12.Simmgen M, Knauf C, Lopez M, Choudhury AI, Charalambous M, Cantley J, Bedford DC, Claret M, Iglesias MA, Heffron H, Cani PD, Vidal-Puig A, Burcelin R, Withers DJ: Liver-specific deletion of insulin receptor substrate 2 does not impair hepatic glucose and lipid metabolism in mice. Diabetologia 2006; 49: 552– 561 [DOI] [PubMed] [Google Scholar]
- 13.Tonks NK, Neel BG: Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol 2001; 13: 182– 195 [DOI] [PubMed] [Google Scholar]
- 14.Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM: Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes 1996; 45: 1379– 1385 [DOI] [PubMed] [Google Scholar]
- 15.Buckley DA, Cheng A, Kiely PA, Tremblay ML, O′Connor R: Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol 2002; 22: 1998– 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A: Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J Biol Chem 2001; 276: 10207– 10211 [DOI] [PubMed] [Google Scholar]
- 17.Clampit JE, Meuth JL, Smith HT, Reilly RM, Jirousek MR, Trevillyan JM, Rondinone CM: Reduction of protein-tyrosine phosphatase-1B increases insulin signaling in FAO hepatoma cells. Biochem Biophys Res Commun 2003; 300: 261– 267 [DOI] [PubMed] [Google Scholar]
- 18.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP: Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999; 283: 1544– 1548 [DOI] [PubMed] [Google Scholar]
- 19.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB: Increased energy expenditure, decreased adiposity and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 2000; 20: 5479– 5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haj FG, Zabolotny JM, Kim YB, Kahn BB, Neel BJ: Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B-/- mice. J Biol Chem 2005; 280: 15038– 15046 [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rodriguez A, Clampit JE, Escribano O, Benito M, Rondinone CM, Valverde AM: Developmental switch from prolonged insulin action to increased insulin sensitivity in protein tyrosine phosphatase 1B-deficient hepatocytes. Endocrinology 2007; 148: 594– 608 [DOI] [PubMed] [Google Scholar]
- 22.Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Rondinone C, Valverde AM, Lorenzo M: Protein-tyrosine phosphatase 1B–deficient myocytes show increased insulin sensitivity and protection against tumor necrosis factor-α–induced insulin resistance. Diabetes 2007; 56: 404– 413 [DOI] [PubMed] [Google Scholar]
- 23.Kushner JA, Haj FG, Klaman LD, Dow MA, Kahn BB, Neel BG, White MF: Islet-sparing effects of protein tyrosine phosphatase-1b deficiency delay onset of diabetes in IRS2 knockout mice. Diabetes 2004; 53: 61– 66 [DOI] [PubMed] [Google Scholar]
- 24.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q: SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 2007; 6: 307– 319 [DOI] [PubMed] [Google Scholar]
- 25.Benveniste R, Danoff TM, Ilekis J, Craig HR: Epidermal growth factor receptor numbers in male and female mouse primary hepatocyte cultures. Cell Biochem Funct 1998; 4: 231– 235 [DOI] [PubMed] [Google Scholar]
- 26.Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF: Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest 2003; 112: 1521– 1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M: SirT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 2008; 283: 20015– 20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM: Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1 alpha interaction. Nature 2003; 423: 550– 555 [DOI] [PubMed] [Google Scholar]
- 29.Nakae J, Kitamura T, Silver DL, Accili D: The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 2001; 108: 1359– 1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inada S, Ikeda Y, Suehiro T, Takata H, Osaki F, Arii K, Kumon Y, Hashimoto K: Glucose enhances protein tyrosine phosphatase 1B gene transcription in hepatocytes. Mol Cell Endocrinol 2007; 271: 64– 70 [DOI] [PubMed] [Google Scholar]
- 31.Xue B, Kim YB, Lee A, Toschi E, Bonner-Weir S, Kahn CR, Neel BG, Kahn BB: Protein-tyrosine phosphatase 1B deficiency reduces insulin resistance and the diabetic phenotype in mice with polygenic insulin resistance. J Biol Chem 2007; 282: 23829– 23840 [DOI] [PubMed] [Google Scholar]
- 32.Sangwan V, Paliouras GN, Cheng A, Dubé N, Tremblay ML, Park M: Protein-tyrosine phosphatase 1B deficiency protects against Fas-induced hepatic failure. J Biol Chem 2006; 281: 221– 228 [DOI] [PubMed] [Google Scholar]
- 33.González-Rodriguez A, Escribano O, Alba J, Rondinone CM, Benito M, Valverde AM: Levels of protein tyrosine phosphatase 1B determine susceptibility to apoptosis in serum-deprived hepatocytes. J Cell Physiol 2007; 212: 76– 88 [DOI] [PubMed] [Google Scholar]
- 34.Julien SG, Dubé N, Read M, Penney J, Paquet M, Han Y, Kennedy BP, Muller WJ, Tremblay ML: Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet 2007; 39: 338– 346 [DOI] [PubMed] [Google Scholar]
- 35.Bentires-Alj M, Neel BG: Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res 2007; 67: 2420– 2424 [DOI] [PubMed] [Google Scholar]
- 36.Zhu S, Bjorge JD, Fujita DJ: PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res 2007; 67: 10129– 10137 [DOI] [PubMed] [Google Scholar]