Abstract
OBJECTIVE
To characterize the relationships among long-term improvements in peripheral insulin sensitivity (glucose disposal rate [GDR]), fasting glucose, and free fatty acids (FFAs) and concomitant changes in weight and adipose tissue mass and distribution induced by lifestyle intervention in obese individuals with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We measured GDR, fasting glucose, and FFAs during a euglycemic clamp and adipose tissue mass and distribution, organ fat, and adipocyte size by dual-energy X-ray absorptiometry, CT scan, and adipose tissue biopsy in 26 men and 32 women in the Look-AHEAD trial before and after 1 year of diet and exercise aimed at weight loss.
RESULTS
Weight and fasting glucose decreased significantly (P < 0.0001) and significantly more in men than in women (−12 vs. −8% and −16 vs. −7%, respectively; P < 0.05), while FFAs during hyperinsulinemia decreased and GDR increased significantly (P < 0.00001) and similarly in both sexes (−53 vs. −41% and 63 vs. 43%; P = NS). Men achieved a more favorable fat distribution by losing more from upper compared with lower and from deeper compared with superficial adipose tissue depots (P < 0.01). Decreases in weight and adipose tissue mass predicted improvements in GDR but not in fasting glucose or fasting FFAs; however, decreases in FFAs during hyperinsulinemia significantly determined GDR improvements. Hepatic fat was the only regional fat measure whose change contributed independently to changes in metabolic variables.
CONCLUSIONS
Patients with type 2 diabetes undergoing a 1-year lifestyle intervention had significant improvements in GDR, fasting glucose, FFAs and adipose tissue distribution. However, changes in overall weight (adipose tissue mass) and hepatic fat were the most important determinants of metabolic improvements.
Most obese patients with type 2 diabetes have an unfavorable adipose tissue distribution compared with that of similarly obese men and women without type 2 diabetes (1–2). We have shown that they manifest proportionally less metabolically protective adipose tissue (gluteo-femoral) and more metabolically adverse fat depots such as abdominal adipose tissue or hepatic fat (2). Such patterns correlate with increased fasting glucose and decreased insulin sensitivity (3–5) in cross-sectional studies. From the perspective of intervention, in type 2 diabetes, both caloric restriction and relatively modest weight reduction result in fasting glucose (6–10) as well as hepatic (7,9,11–13) and peripheral insulin sensitivity (8–10,12–13) improvements. However, not all studies reporting significant weight loss or favorable fat distribution changes have observed a concomitant improvement in peripheral insulin sensitivity (11,14). Furthermore, there is a surprising paucity of data regarding the relationship between sustained lifestyle intervention–induced changes in fat mass and regional adipose tissue distribution and parallel metabolic improvements.
In several weight loss studies conducted for up to 6 months, in type 2 diabetes favorable changes in fat distribution and organ fat did not correlate with improved peripheral insulin sensitivity independent of the changes in body weight (11–14). Even fewer studies reported on longer-term (of up to 1 year) effects of weight loss on fat distribution and metabolic variables in type 2 diabetes (10,15–16). In one study, while parallel 1-year improvements were observed both in the fat distribution (measured by the waist-to-hip ratio) and in fasting glucose and fasting insulin (15), the metabolic improvements did not relate to the waist-to-hip ratio change but rather to the overall amount of weight loss (15). One interpretation is that loss of adipose tissue, regardless of depot, is the predominant factor related to the metabolic improvement in obese patients with type 2 diabetes, challenging the tenet, built mostly from cross-sectional studies, that adipose tissue distribution is a crucial and interactive determinant of the improvement. Yet, it is not clear from these studies whether the variability of the weight loss, the sometime limited number of subjects, or incomplete adipose tissue distribution measurements permitted robust evaluation of the role of specific fat depots in the improvements in metabolic control. In addition, changes in other adipose tissue characteristics, such as fat cell sizes or circulating free fatty acids (FFAs), have not been accounted for in previous studies. Larger subcutaneous abdominal fat cells predict insulin resistance and the development of type 2 diabetes (17–19), while increased circulating FFAs play an important role in the etiology of insulin resistance and hyperglycemia in type 2 diabetes (12,20–21). Whether regional fat loss contributes to improvements in FFAs during weight loss in type 2 diabetes has not previously been reported.
The current study was therefore undertaken to examine the importance of changes in adipose tissue distribution and other closely related characteristics as determinants of the improvements in metabolic fitness in response to weight loss in type 2 diabetes. We tested the hypothesis that simple measures of weight loss rather than various relative changes and permutations of adipose tissue distribution are the predominant determinant of metabolic improvement induced by a 1-year lifestyle intervention in obese patients with type 2 diabetes. Multiple aspects of adipose tissue mass and its distribution were assessed, including upper and lower adipose tissue mass (using dual-energy X-ray absorptiometry [DEXA]), adipose tissue subdivisions in the abdomen and lower extremity (using computed tomography [CT] imaging), and estimations of fat content in liver and muscle (using CT imaging). This was performed along with an adipose tissue biopsy in order to measure mean fat cell size within the abdominal subcutaneous depot both at baseline and following the 1 year of lifestyle intervention.
RESEARCH DESIGN AND METHODS
This was an ancillary study of the Look AHEAD (Action For Health in Diabetes) trial at 3 of the 16 participating sites (Pennington Biomedical Research Center, Baton Rouge, LA; the University of Pittsburgh, Pittsburgh, PA; and St. Luke's–Roosevelt Hospital Center, New York, NY). The primary goal of the Look AHEAD trial is to investigate the effects of a lifestyle intervention of weight loss and physical activity (intensive lifestyle intervention [ILI]) versus those of diabetes support and education on cardiovascular morbidity and mortality (22–23). One-year results from the Look AHEAD trial and other results of this ancillary study have previously been published (24–28).
Research volunteers.
Inclusion and exclusion criteria for Look AHEAD, which include a confirmed diagnosis of type 2 diabetes, have previously been described (22–23). This ancillary study included only participants randomized to the ILI arm of the study (24–25). To simplify the potential impact of changes in antidiabetes medications during intervention, those with fasting plasma glucose ≥180 mg/dl and those on insulin or thiazolidinedione treatment were excluded from the substudy. Fifty-eight volunteers with type 2 diabetes (43 non-Hispanic whites, 12 African Americans, and 3 Hispanics) were studied at baseline (preintervention) and after 1 year of ILI. Twenty-six men (mean ± SD age 61.6 ± 1.5 years) and 32 women (58.9 ± 1.3 years) completed baseline and 1-year measurements. The sex distribution of volunteers at the three sites was as follows: 12 female and 13 male participants at Pittsburgh, 13 female and 5 male participants at St Luke's–Roosevelt, and 7 female and 8 male at Pennington. At baseline, 6 women were pre- or perimenopausal and 26 were postmenopausal (8 on hormone-replacement therapy). All participants signed informed consent, and the project was approved by the institutional review board of each institution and by the Look-AHEAD Steering Committee.
Lifestyle intervention and study protocol.
As described elsewhere (22–25), ILI was designed to achieve weight loss through decreased caloric intake (∼500 kcal/day) and increased physical activity (≥175 min/week), with an expected 1-year weight loss of ≥7% of initial value. Before and after 1 year of ILI, our participants were admitted to clinical research facilities on the afternoon preceding the metabolic studies and underwent DEXA and CT imaging. After a standardized dinner (50% carbohydrate, 30% fat, and 20% protein), participants were fasted overnight. The next morning, a metabolic weight and a percutaneous adipose tissue biopsy were obtained; 1 h later, a hyperinsulinemic-euglycemic clamp was performed.
Addition or discontinuation of antihyperglycemic medications at the 1-year testing compared with baseline was noted. Medications added were thiazolidinedione and metformin (one man each). Medications discontinued were α-glucosidase inhibitors (one woman), meglitinides and repaglinides (two men and two women), and sulfonylureas (11 men and three women). Metformin was reintroduced for a week prior to the 1-year testing at the same dose as before the study if the patients were on it at baseline and were discontinued during the intervention (six men and two women).
Body composition.
Fat mass and fat-free mass (FFM) (including all nonfat tissue, i.e., lean body mass and bone mineral content) were measured using DEXA (Hologic QDR 4500A) according to the Manual of Procedures of the Look AHEAD trial. All DEXA scans were analyzed using QDR for Windows, version 11.1, software. Fat mass and FFM, gluteo-femoral fat, and trunk and arms fat mass (upper-body fat) were measured by the standard default analysis, in which the commercial computer-based algorithm separates the mass of gluteo-femoral and upper-body fat by two oblique lines that pass through the femoral neck (2). The coefficients of variation (CVs) for repeated measures (n = 38; unpublished data) of FFM, fat mass, and percentage of body fat were 0.6, 1.1, and 1.1%, respectively. Three cross-sectional CT scans, 1 cm in width, centered, respectively, on the T12–L1 and the L4–L5 disc space and at the mid-thigh, were obtained to assess hepatic fat as well as abdominal and thigh adipose tissue composition. All CT images were analyzed at the University of Pittsburgh using image analysis software (SliceOmatic; Tomovision, Montreal, Canada). To assess hepatic fat, CT liver and spleen attenuations (Hounsfield units) were determined, and to assess adipose tissue composition, the abdominal and thigh areas for bone, adipose tissue, and skeletal muscle were measured as previously described (29). To determine visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT) areas, a separation line was drawn manually on the abdominal CT images along abdominal wall musculature in continuity with the fascia of the paraspinal muscles. Abdominal SAT was further divided into superficial and deep SAT by manually tracing the circumferential superficial fascia as previously described (30). On thigh CT images, fascia lata was used to subdivide mid-thigh adipose tissue into SAT and subfascial adipose tissue (3).
Abdominal subcutaneous adipose tissue biopsy and adipocyte size.
A percutaneous biopsy of superficial abdominal SAT (∼500 mg) was performed ∼10 cm lateral to the umbilicus using a Bergstrom needle with suction. Adipocyte size and number were determined at the Pennington Biomedical Research Center using a Coulter Counter (Multisizer-3; Beckman Coulter, Fullerton, CA) as previously described (26,28). Cell size is presented as the geometric mean.
Hyperinsulinemic-euglycemic clamp.
A primed continuous infusion of insulin (80 mU/m2 per min) was used for at least 3 h, with the stipulation that insulin be infused for at least 1 h after reaching a plasma glucose concentration of 100 mg/dl, as previously described (27). The mean rate of exogenous glucose infusion during steady-state insulin infusion (last 30 min), glucose disposal rate (GDR), was used to assess peripheral insulin sensitivity (31). Oxygen consumption and CO2 production were measured using metabolic carts (Sensor Medics Corporation, Anaheim CA) over the 40 min just preceding and during the last 40 min of the insulin infusion; fuel oxidation (carbohydrate and fat) was calculated for the last 30 min of each period (32). Glucose storage was the difference between total GDR and glucose oxidation. Glucose utilization rates were expressed per kilogram of FFM.
Blood analyses.
Blood samples were immediately centrifuged, aliquoted, and frozen at −70°C. Plasma glucose was analyzed using a glucose oxygen electrode (Synchron CX7 Delta Systems; Beckman, Brea, CA). Plasma insulin was measured by chemiluminescent immunoassays on the Immulite 2000 analyzer (Diagnostic Product, Los Angeles, CA). The intra- and interassay CVs for insulin (at 50 μU/ml) were 1.75 and 3.6%, respectively. Plasma FFA concentrations were measured on a Beckman Synchron CX5 analyzer using a WAKO NEFA C kit (Denver, CO). All samples were analyzed in the Clinical Chemistry Laboratory at the Pennington Biomedical Research Center.
Statistical analyses.
Data were expressed and shown as means ± SEM unless otherwise indicated. For each variable, data were presented for completed, valid measurements both at baseline and after 1 year. Data were missing for men (out of 26) for liver, spleen, and muscle attenuations (1 man each) and for adipose tissue cell size (2 men) and for women (out of 32) for abdominal adipose tissue measurements (1 woman); thigh adipose tissue, liver, spleen, and muscle attenuations (3 women each); and for adipose tissue cell size (1 woman). Variables with significant deviation from normal distribution were log transformed before analyses (insulin, FFAs, and their changes). ANOVA with repeated measures was used to assess significant changes over the 1 year of the intervention; interactions by sex were tested for significance. General linear models were built according to a priori hypotheses. Specifically, we tested the following: 1) whether changes in any of the regional fat measures were predictors of the 1-year changes in metabolic variables independent of the change in overall weight or fat mass; 2) whether the change in clamp FFAs was a predictor of the changes in GDR or fasting glucose, independent of changes in weight, fat mass, or any of the regional fat measures; and 3) whether the change in GDR was a predictor of the change in fasting glucose, independent of changes in weight, fat mass, or any of the regional fat measures. P < 0.05 was considered significant. Statistica, version 6.0 (Statsoft, Tulsa, OK), was used for analyses.
RESULTS
One-year changes in weight.
Weight decreased significantly (P < 0.00001) in both men and women (Table 1), but there was a wide range of weight change (between −26.5 and 3.5 kg in men and −27.3 and 0.9 kg in women). Men lost a higher percentage of their initial weight than women (−12.1 ± 1.2 vs. −8.1 ± 1.1%; P < 0.05). Higher 1-year weight loss was predicted by higher weight at baseline in both sexes (β = −0.41; P < 0.01) but was not related to baseline adipose tissue mass or distribution or to fat cell size or metabolic variables.
TABLE 1.
Weight, adipose tissue mass and distribution, organ fat, and abdominal subcutaneous fat cell size before and after 1-year lifestyle intervention
| Men (n = 26)* |
Women (n = 32)† |
|||
|---|---|---|---|---|
| Baseline | 1 year | Baseline | 1 year | |
| Weight (kg)‡ | 101.2 ± 1.9 | 88.8 ± 1.8 | 91.4 ± 1.7 | 83.9 ± 1.7 |
| BMI (kg/m2)‡ | 32.4 ± 0.5 | 28.4 ± 0.5 | 34.8 ± 0.6 | 32.0 ± 0.6 |
| FFM (kg)‡ | 70.9 ± 1.1 | 66.9 ± 1.0 | 54.2 ± 1.0 | 52.1 ± 0.9 |
| Fat mass (Kg)‡ | 30.3 ± 1.2 | 22.0 ± 1.2 | 37.1 ± 1.1 | 31.8 ± 1.1 |
| Percent fat mass (of weight)‡ | 29.8 ± 0.8 | 24.5 ± 0.9 | 40.4 ± 0.7 | 37.5 ± 0.8 |
| Upper-body fat (kg)‡ | 21.4 ± 0.9 | 15.1 ± 0.9 | 24.4 ± 0.9 | 20.7 ± 0.8 |
| Gluteo-femoral fat (kg)§ | 8.2 ± 0.3 | 6.2 ± 0.3 | 12.1 ± 0.6 | 10.6 ± 0.5 |
| VAT (cm2)‡ | 311.7 ± 18.3 | 216.5 ± 18.3 | 259.5 ± 16.8 | 213.3 ± 16.7 |
| Deep abdominal SAT (cm2)‡ | 170.9 ± 11.6 | 120.4 ± 10.2 | 148.2 ± 10.6 | 130.6 ± 9.3 |
| Superficial abdominal SAT (cm2)§ | 120.9 ± 11.5 | 92.0 ± 10.6 | 237.1 ± 10.6 | 206.8 ± 9.7 |
| Subfascial thigh AT (cm2)(one leg)§ | 18.1 ± 1.5 | 12.9 ± 1.1 | 22.7 ± 1.5 | 18.1 ± 1.1 |
| Superficial thigh SAT (cm2) (one leg)§ | 84.7 ± 7.9 | 66.8 ± 7.5 | 156.5 ± 7.5 | 138.4 ± 7.1 |
| Liver attenuation (HU)§ | 51.2 ± 2.1 | 59.7 ± 1.8 | 46.5 ± 1.9 | 54.6 ± 1.7 |
| Spleen attenuation (HU)§ | 50.4 ± 0.8 | 51.3 ± 0.8 | 47.6 ± 0.8 | 48.8 ± 0.7 |
| L/S attenuation ratio§ | 1.01 ± 0.04 | 1.17 ± 0.04 | 0.99 ± 0.04 | 1.13 ± 0.04 |
| Muscle area (cm2) (both legs)‡ | 311.5 ± 6.8 | 292.7 ± 6.9 | 223.4 ± 6.3 | 215.2 ± 6.4 |
| Muscle attenuation (HU) | 46.8 ± 0.9 | 47.4 ± 0.8 | 45.0 ± 0.8 | 45.1 ± 0.7 |
| Fat cell size§ | 0.73 ± 0.05 | 0.50 ± 0.04 | 0.96 ± 0.04 | 0.76 ± 0.03 |
| Fat cell number§ | 3,756 ± 271 | 4,897 ± 370 | 2,982 ± 239 | 3,345 ± 325 |
Data are unadjusted means ± SE. FFM, FM, upper body fat, and gluteo-femoral fat measured by DEXA; VAT, SAT, abdominal and thigh SAT subcompartments, and organ (liver, spleen, muscle) attenuation measured by CT scan.
*Missing data for men (out of 26) for organ attenuations (1 man each) and fat cell size (2 men).
†Missing data for women (out of 32) for abdominal adipose tissue measurements (1 woman), thigh adipose tissue and organ attenuations (3 women each), and fat cell size (1 woman).
‡Significant change in both men and women (P range <0.05 to 0.00001), with significant interaction by sex (P range <0.05 to 0.001 for the interaction term).
§Significant change in both men and women (P range <0.05 to 0.00001), with no significant interaction by sex.
One-year changes in adipose tissue mass and distribution, organ fat infiltration, and mean abdominal subcutaneous fat cell size.
Fat mass and FFM decreased significantly by 27.7 ± 2.6 and 5.5 ± 0.8% and by 14.0 ± 2.1 and 3.8 ± 0.7%, in men and women, respectively (Table 1). The change in fat mass ranged from −20.4 to 0.2 and from −19.5 to 0.8 kg, while the change in FFM ranged from −9.9 to 3.3 and from −7.8 to 2.8 kg, in men and women, respectively, resulting in a significant decrease in body fat percentage (Table 1). There were a number of significant changes in various regional adipose tissue depots (Table 1). Upper body fat, VAT, and deep abdominal SAT decreased significantly (Table 1) and significantly more in men than in women, independent of baseline values (P < 0.01). There were also significant decreases in superficial abdominal SAT and gluteo-femoral fat and in the lower extremity of the thigh, measured cross-sectionally for both the subfascial and the superficial adipose tissue depots, but without sex effect (Table 1).
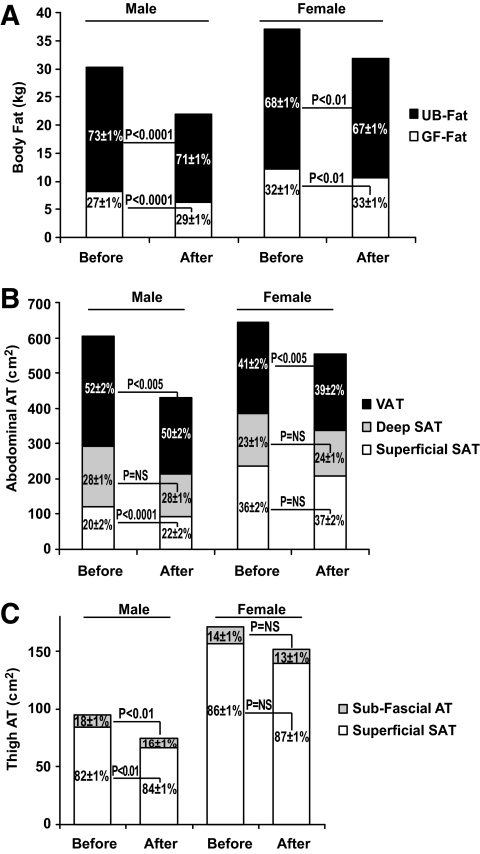
Because of the differential of greater loss from the upper compared with the lower body, the fat distribution as measured by DEXA changed significantly (Fig. 1A) in both sexes. In men, VAT decreased more than the superficial abdominal SAT; this resulted in a significant change within abdominal adipose tissue distribution (Fig. 1B). There was a similar trend evident in women, although to a lesser extent than in men (Fig. 1B). A similar pattern was noted in the lower extremity: in men, the subfascial thigh adipose tissue decreased more than the superficial thigh SAT, resulting in a significant change in the thigh adipose tissue distribution (Fig. 1C); a similar trend was observed in women (Fig. 1C).
FIG. 1.
Absolute amounts in kg (A) or cm2 (B–C) on the y-axis and the relative distribution as percent of total fat (A) or percent of total area (B–C) shown as means ± SEM of upper-body (UB-Fat) and lower-body (gluteo-femoral [GF-Fat]) fat by DEXA (A); VAT and deep (Deep SAT) and superficial (Superficial SAT) abdominal adipose tissue (AT) areas measured at L4–L5 by CT (B); and subfascial and superficial subcutaneous adipose tissue areas measured at mid-thigh (one leg) by CT (C) before and after 1 year of intervention; P values are shown for differences in the relative distribution.
The ratio of the liver-to-spleen attenuation increased, indicating a significant decrease in hepatic fat (P < 0.00001) (Table 1) in both men (−18 ± 5%) and women (−18 ± 4%). The thigh muscle attenuation, a surrogate for intramuscular fat, did not change significantly (P = 0.36) (Table 1). The thigh muscle area decreased on average by 4.6%; this decrease was similar in magnitude to the average decrease in FFM (4.6%).
Mean size of abdominal subcutaneous fat cells decreased in both men and women (Table 1). Lipid content per unit of adipose tissue together with the mean fat cell size was used to estimate the number of fat cells per unit of adipose tissue (see research design and methods). The calculated number of fat cells per unit of abdominal SAT increased in both sexes (Table 1), despite an average decrease in the overall size of the depot by ∼17%.
One-year changes in fasting glucose, FFAs, and GDR.
Fasting glucose decreased significantly in both men and women (Table 2) and significantly more in men than in women (−16.2 ± 2.8 vs. −6.8 ± 3.5%; P < 0.05). The clamped glucose levels were not different after than before the intervention (Table 2). Fasting insulin decreased significantly and equally in both sexes (Table 2), while insulin levels at steady state during the clamp were lower after intervention in women only (interaction term P = 0.05). Fasting FFAs were significantly decreased (Table 2) and were suppressed by insulin to a significantly greater extent (P < 0.00001) after (by 98 ± 0.5 and 97 ± 0.5% in men and women, respectively) than before (by 95 ± 1 and 94 ± 1%) the weight loss. Therefore, FFA levels at steady state of the clamp were significantly decreased after weight loss (Table 2) similarly in men and women (−54.9 ± 8.5 vs. −41.2 ± 15.7%). GDR increased significantly (Table 2) similarly in men and women (63.3 ± 8.1 vs. 43.1 ± 8.6%), with improvement of both glucose oxidation and storage (Table 2).
TABLE 2.
Metabolic parameters during the euglycemic-hyperinsulinemic clamp before and after 1-year lifestyle intervention
| Men (n = 26) |
Women (n = 32) |
|||
|---|---|---|---|---|
| Baseline | 1 year | Baseline | 1 year | |
| Postabsorptive state | ||||
| Glucose (μmol/l)* | 8.2 ± 0.4 | 6.7 ± 0.3 | 7.8 ± 0.3 | 7.3 ± 0.3 |
| Insulin (pmol/l)* | 71.1 ± 7.2 | 52.5 ± 8.9 | 91.5 ± 6.5 | 80.0 ± 8.0 |
| FFAs (mmol/l)* | 0.56 ± 0.02 | 0.45 ± 0.03 | 0.79 ± 0.03 | 0.63 ± 0.02 |
| Steady state during clamp | ||||
| Glucose (μmol/l) | 5.7 ± 0.1 | 5.7 ± 0.1 | 5.8 ± 0.1 | 5.8 ± 0.1 |
| Insulin (pmol/l)† | 834.3 ± 49.1 | 820.1 ± 39.2 | 982.9 ± 44.2 | 859.5 ± 35.3 |
| FFAs (mmol/l)* | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.05 ± 0.06 | 0.02 ± 0.003 |
| GDR (mg · kg FFM−1 · min−1)* | 5.7 ± 0.4 | 8.9 ± 0.5 | 6.2 ± 0.4 | 8.4 ± 0.5 |
| Glucose oxidation (mg · kg FFM−1 · min−1)*‡ | 2.7 ± 0.1 | 3.4 ± 0.2 | 3.1 ± 0.2 | 3.8 ± 0.2 |
| Glucose storage (mg · kg FFM−1 · min−1)*‡ | 3.0 ± 0.3 | 5.5 ± 0.4 | 2.9 ± 0.4 | 4.4 ± 0.5 |
Values are unadjusted means ± SE.
*Significant change in both men and women (P range <0.05 to 0.00001), with no significant interaction by sex.
†Significant change in women only (P < 0.05).
‡Men, n = 26; women, n = 30.
Determinants of 1-year changes in metabolic variables (GDR, fasting glucose, and clamp FFAs).
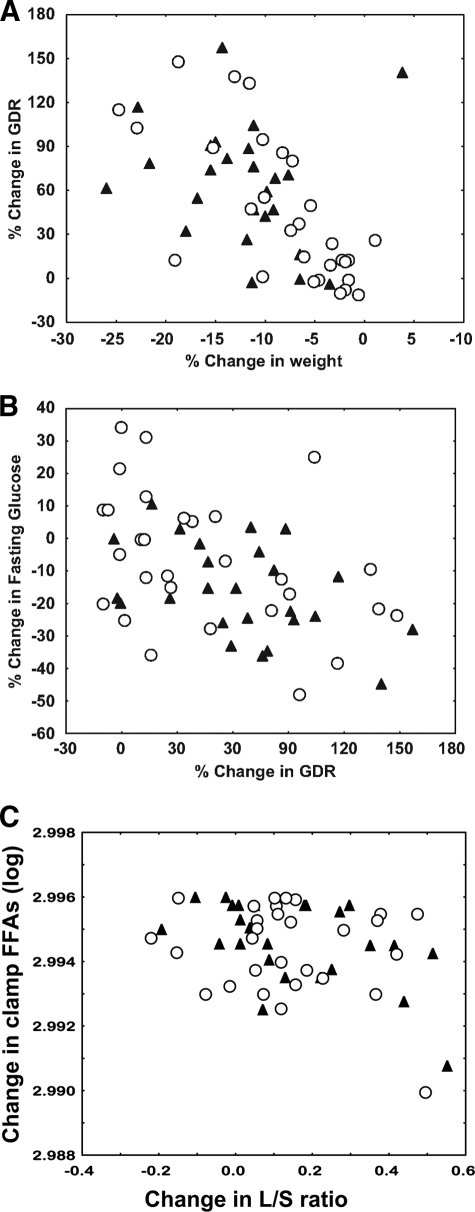
The 1-year improvement in insulin sensitivity (ΔGDR or as a percentage of baseline value [%ΔGDR]) did not relate to any baseline variables but was significantly related to the decrease in weight (%Δweight; r = −0.65; P = 0.000002 [Fig. 2A]) and fat mass (%ΔFM; r = −0.71; P = 0.00004). It was also significantly related to the decreases in all regional fat depots (r range −0.65 to −0.50; P < 0.01 for all) and in mean abdominal fat cell size (r = −0.27; P < 0.05) as well as to the increase in the relative proportion of the superficial abdominal SAT (r = 0.34; P < 0.01). However, none of these relationships were independent of the changes in weight or fat mass. In multiple regression analyses, the best predictive model for ΔGDR included %Δfat mass and %Δclamp FFA (overall R2 = 0.48; P = 0.000049) and for %ΔGDR included independent contributions from the %Δweight, %ΔL/S ratio (ratio of the CT attenuation value of the liver to that of the spleen), and %Δclamp FFA (overall R2 = 0.52; P = 0.000027).
FIG. 2.
A: The relationships between the change in weight and the change in GDR (mg · kg−1 · FFM−1), both expressed as a percent of the baseline values (r = −0.50; P = 0.0006). B: The change in GDR (mg · kg FFM−1 · min−1) and the change in fasting glucose, both expressed as percent of the baseline values (r = −0.37; P = 0.005). C: The change in the liver-to-spleen attenuation ratio measured by CT and the change in plasma FFAs at steady state during the clamp (clamp FFAs [logged values]) (r = −0.33; P = 0.014); correlation coefficients and P values shown are from models where sex and site were added as factors. ▴, men; ○, women.
Similar analyses were performed for the 1-year improvement in fasting glucose and decreases in clamp FFAs. Neither was related to the changes in weight or fat mass. Among regional fat measures, Δfasting glucose was only significantly related to the ΔL/S ratio (r = −0.37; P = 0.006) and %Δfasting glucose to the %ΔVAT (r = 0.31; P = 0.03). While Δfasting glucose was also related to the decrease in clamp FFAs (P < 0.05), in multiple regression analysis the best predictive model for Δfasting glucose included independent contributions from the ΔL/S ratio and ΔGDR (overall R2 = 0.32; P = 0.009) and for %Δfasting glucose included independent contributions from the %ΔGDR (R2 = 0.31; P = 0.005) (Fig. 2B). The 1-year decrease in clamp FFA was only related to ΔL/S ratio or %ΔL/S ratio (r = −0.33, P = 0.014, or r = −0.39, P = 0.01, respectively) (Fig. 2C), independently of changes in weight or fat mass.
DISCUSSION
After 1 year of Look AHEAD ILI (22), participants with type 2 diabetes had greatly improved levels of peripheral insulin sensitivity, fasting glucose, and FFAs in parallel to significant weight and fat loss, improvement in adipose tissue distribution, and decrease in hepatic fat. The changes in the peripheral insulin sensitivity were best predicted by the overall change in weight and fat mass; the only regional fat measure independently predicting metabolic improvements was the decrease in hepatic fat.
Several studies have examined the effect of weight loss on adipose tissue distribution and organ fat infiltration in type 2 diabetes (11–14,33). These studies varied in duration from a few weeks to up to 6 months and reported variable changes in adipose tissue distribution and organ fat depending on the measurements done and the nature of the intervention leading to the weight loss. Our study is unique in that we have studied the subjects after a 1-year intervention, have measured all aspects of adipose tissue distribution and organ fat infiltration, and enrolled a number of subjects sufficient for reporting results separately for both men and women. In general, significant loss of visceral and hepatic fat has consistently been reported, whereas a decrease in muscle fat has not been consistently observed (11–14, 33). In our study, muscle fat infiltration did not change; this could have been due to the CT measurement technique, which is less sensitive than intramyocellular lipid measurement by nuclear magnetic resonance (NMR) spectroscopy (magnetic resonance spectroscopy [MRS]), as well as to the duration and nature of the intervention. Previous studies have suggested that exercise may prevent the loss of intramyocellular lipid during weight loss induced by caloric restriction (13, 34). Thus, the exercise component of our intervention could have had a similar effect over the 1-year period.
We also found that men in our study, and to a lesser degree women, had favorable changes in adipose tissue distribution from the upper to the lower and from the deeper to the more superficial depots. Such changes have not previously been reported during weight loss by dieting in type 2 diabetes; the exercise component of the ILI could have played a role (35–36). Changes in adipose tissue distribution could accompany significant improvements in the components of the metabolic syndrome in individuals with type 2 diabetes (35–36); however, we did not find that the relationships specifically between improvements in the adipose tissue distribution and improvements in the peripheral insulin sensitivity were independent of the change in overall weight and adipose tissue mass. We therefore confirmed our original hypothesis that, with the exception of the decrease in hepatic fat, weight loss and overall adipose tissue mass reduction better predicted the improvement in peripheral insulin sensitivity than improvements in adipose tissue distribution. This finding is also in agreement with previous reports, of shorter duration, with smaller sample sizes and more homogenous weight loss (12–13).
The decrease in insulin-suppressed FFA levels and the decrease in hepatic fat were also independent determinants of improved peripheral insulin sensitivity. The latter finding is new for type 2 diabetes to the best of our knowledge, although cross-sectional independent associations between hepatic fat and insulin sensitivity have previously been described (2,37). The causative direction and the underlying pathophysiology of this association could not be determined from the present study. Changes in insulin, glucose, and FFA levels could all be potential mediators. The association between the decrease in the insulin-suppressed FFA levels and the improvement in peripheral insulin sensitivity has previously been described (12), and the role of FFAs in the etiology of insulin resistance in type 2 diabetes has been stressed in both cross-sectional (4) and weight loss (12) studies. Both glucose phosphorylation and glucose transport in skeletal muscle are known to be affected by circulating FFA levels (21,38) and, in turn, improve with weight loss in type 2 diabetes (39). We also found that the change in peripheral insulin sensitivity was related to the relative improvement in superficial adipose tissue distribution and the decrease in this depot's mean fat cell size. These relationships were not independent of the change in body weight but are significant in that they point to the importance of the subcutaneous fat characteristics in the etiology of insulin resistance in type 2 diabetes (18–19).
With regard to fasting glucose, our results are similar to those previously published (12) in that the best predictor for the improvement in fasting glucose was the improvement in insulin sensitivity (GDR). The changes in VAT and hepatic fat were associated with the improvement in fasting glucose independent of changes in overall adipose tissue mass, but only the change in hepatic fat was related to the change in fasting glucose, independent of the change in GDR. We also report for the first time that the changes in insulin-suppressed FFAs were related to the change in hepatic fat. The importance of hepatic fat as a determinant of metabolic parameters in type 2 diabetes has been underscored by cross-sectional associations with hepatic insulin resistance (12) and by associations with insulin requirements during insulin therapy in type 2 diabetes, independent of measured insulin action and FFA levels (40). In our study, a decrease in hepatic fat was associated with improvements in all three key metabolic variables studied. The exact mechanism is not known; among other possibilities is improved insulin clearance after weight loss (8), which in addition to the improved β-cell function, could result in a more physiologic insulin pattern and lower both plasma glucose and FFAs (7,41). Thus, we conclude that changes in hepatic fat play a key role in the improvement of metabolic parameters with weight loss in type 2 diabetes.
The changes in the oral hypoglycemic agents that occurred over the 1-year intervention are a potential limitation for our study. We performed separate analyses excluding the two subjects who were on insulin-sensitizing agents at the 1-year testing and not at baseline and adding discontinuation of any oral agents at 1 year compared with baseline (yes or no) as a factor. Results were essentially unchanged with a notable exception: the sex differences in the overall weight or fat loss (Table 1) were not significant anymore once the discontinuation of the oral agents was accounted for. The peripheral insulin sensitivity changes (Table 2) and the adipose tissue distribution changes presented in Fig. 1 were not affected. Therefore, we speculate that, since more men discontinued oral agents than women, this may have accounted for the sex differences in the overall weight and fat loss. The baseline menopausal status of our women and its change over time could also have potentially influenced our results. Four women changed menopausal status over the course of the study; none changed hormone-replacement therapy. Although we did not find interactions by menopausal status in our analyses (results not shown), the number of women in the different categories is too small to exclude a possible influence of baseline menopausal status on adipose tissue distribution changes over the 1 year of the study.
Finally, the Look-AHEAD trial participants had measurements of fitness at baseline and then yearly throughout the ILI, as previously described (24–25). In our cohort, fitness improved by 40 ± 8 and by 31 ± 7% in men and women, respectively (P < 0.0001). The difference in magnitude compared with the fitness improvement of the entire ILI arm (25 and 18% in men and women, respectively) (25) could be due to the special selection criteria of our study. Just for the entire ILI group (25), in our study the fitness improvement was significantly correlated with the degree of weight loss; in addition, it was significantly correlated with changes in FM, percent body fat, and GDR but not with changes in fasting glucose or FFA. The fitness improvement, however, did not predict changes in GDR independent of the overall weight or fat loss, which is consistent with results from other studies (42).
In conclusion, patients with type 2 diabetes undergoing a 1-year lifestyle intervention of diet and exercise had significant improvements in adipose tissue distribution, insulin sensitivity, fasting glucose, and circulating FFAs. Changes in overall weight, adipose mass, and hepatic fat were the most important associates of metabolic improvements.
ACKNOWLEDGMENTS
This study was funded by DK60412 (to E.R.), with additional support from U01 DK056990 (to D.E.K.), the University of Pittsburgh Obesity & Nutrition Research Center (P30 DK46204), the University of Pittsburgh General Clinical Research Center (MO1 RR000056), Pennington Biomedical Research Center Clinical Nutrition Research Unit (P30 DK072476), the Columbia University Diabetes and Endocrinology Research Center (P30 DK63608), the New York Obesity Research Center (P30 DK26687), and Columbia University Clinical Translational Service Award (UL1 RR024156).
No potential conflicts of interest relevant to this article were reported.
APPENDIX
Writing group.
Jeanine Albu, Leonie K. Heilbronn, David E. Kelley, Steven R. Smith, Evan Berk, Koichiro Azuma, F. Xavier Pi-Sunyer, and Eric Ravussin. We acknowledge the other members of the Look AHEAD adipose research group, not included in the writing group. We are grateful to the participants of the primary Look AHEAD trial for their enthusiastic willingness to participate in this ancillary study and to the nursing and nutritional staffs of the three investigational sites.
Clinical sites.
Pennington Biomedical Research Center.
George A. Bray, MD; Donna H. Ryan, MD; Donald Williamson, PhD; Frank L. Greenway, MD; Allison Strate, RN; Elizabeth Tucker; Kristi Rau; Brandi Armand, LPN; Mandy Shipp, RD; Kim Landry; and Jennifer Perault. The Pennington Biomedical Research Center is also the Coordinating Center. St. Luke's–Roosevelt Hospital Center. Jennifer Patricio, MS; Jennifer Mayer, MS; Stanley Heshka, PhD; Carmen Pal, MD; Mary Anne Holowaty, MS, CN; Diane Hirsch, RNC, MS, CDE; Linda Haselman, RN, MS, CDE; and Julia Johnson, PhD. University of Pittsburgh. Carol A. Kelley, RN; Jacqueline Wesche-Thobaben, RN, BSN, CDE; Rebecca Danchenko, BS; and Jowand Green, BS.
Footnotes
Clinical trial reg. no. NCT00017953, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mokdad A, Bowman B, Ford E, Vinicor F, Marks J, Koplan J: The continuing epidemic of obesity and diabetes in the United States. J Am Med Assoc 2001; 286: 1195– 1200 [DOI] [PubMed] [Google Scholar]
- 2.Azuma K, Heilbronn LK, Albu JB, Smith SR, Ravussin E, Kelley DE: the Look AHEAD Adipose Research Group Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2007; 293: 435– 442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Thaete FL, Kelley DE: Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000; 71: 885– 892 [DOI] [PubMed] [Google Scholar]
- 4.Kelley DE, Williams KV, Price JC, McKolanis TM, Goodpaster BH, Thaete FL: Plasma fatty acids, adiposity, and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. J Clin Endocrinol Metab 2001; 86: 5412– 5419 [DOI] [PubMed] [Google Scholar]
- 5.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC: Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003; 285: E906– E916 [DOI] [PubMed] [Google Scholar]
- 6.Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G: Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77: 7– 17 [DOI] [PubMed] [Google Scholar]
- 7.Henry RR, Wallace P, Olefsky JM: Effects of weight loss on mechanisms of hyperglycemia in obese non–insulin-dependent diabetes mellitus. Diabetes 1986; 35: 990– 998 [DOI] [PubMed] [Google Scholar]
- 8.Henry RR, Brechtel G, Griver K: Secretion and hepatic extraction of insulin after weight loss in obese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1988; 66: 979– 986 [DOI] [PubMed] [Google Scholar]
- 9.Kelley DE, Wing RR, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M: Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993; 77: 1287– 1293 [DOI] [PubMed] [Google Scholar]
- 10.Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN: Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17: 30– 36 [DOI] [PubMed] [Google Scholar]
- 11.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI: Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005; 54: 603– 608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley DE, Kuller LH, McKolanis T, Harper P, Mancino J, Kalhan S: Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care 2004; 27: 33– 40 [DOI] [PubMed] [Google Scholar]
- 13.Toledo FGS, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE: Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 2007; 56: 2142– 2147 [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R: Effects of diet and exercise on muscle and liver intracellular lipid content and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005; 90: 3191– 3196 [DOI] [PubMed] [Google Scholar]
- 15.Pascale RW, Wing RR, Blair EH, Harvey JR, Guare JC: The effect of weight loss on change in waist-to-hip ratio in patients with type II diabetes. In J Obesity 1992; 16: 59– 65 [PubMed] [Google Scholar]
- 16.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D: Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med 1987; 147: 1749– 1753 [PubMed] [Google Scholar]
- 17.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE: Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000; 43: 1498– 1506 [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW: Enhanced proportion of small adipose cells in insulin-resistant vs. insulin-sensitive individuals implicates impaired adipogenesis. Diabetologia 2007; 50: 1707– 1715 [DOI] [PubMed] [Google Scholar]
- 19.Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson J: Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia.’ Diabetologia 2007; 50: 625– 633 [DOI] [PubMed] [Google Scholar]
- 20.Williams KV, Kelley DE: Metabolic consequences of weight loss on glucose metabolism and insulin action in type 2 diabetes. Diabetes Obes and Metab 2000; 2: 121– 129 [DOI] [PubMed] [Google Scholar]
- 21.Boden G: Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997; 46: 3– 10 [PubMed] [Google Scholar]
- 22.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ: Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003; 24: 610– 628 [DOI] [PubMed] [Google Scholar]
- 23.LOOK AHEAD Research Group: The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006; 14: 737– 752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadden TA, West DS, Neiberg R, Wing RR, Ryan DH, Johnson KC, Foreyt J, Hill JO, Trence D, Vitolins M: the Look AHEAD Research Group One-year weight losses in the Look AHEAD study: factors associated with success. Obesity 2009; 17: 713– 722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakicic JM, Jaramillo SA, Balasubramanyam A, Bancroft B, Curtis JM, Mathews A, Pereira M, Regensteiner JG, Ribisl PM: Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD study. Int J Obes 2009; 33; 305– 316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois S, Heilbronn L, Smith S, Albu J, Kelley D, Ravussin E: the Look AHEAD Research Group: Adipose decreased expression of adipogenic genes in obese subjects with type 2 Diabetes. Obesity 2006; 14: 1543– 1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galgani JE, Heilbronn LK, Azuma K, Kelley DE, Albu JB, Pi-Sunyer X, Smith SR, Ravussin E: the Look AHEAD Adipose Research Group Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes 2008; 57: 841– 845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasarica M, Tchoukalova YD, Heilbronn LK, Fang X, Albu JB, Kelley DE, Smith SR, Ravussin E: the look AHEAD Adipose Research Group Differential effect of weight loss on adipocyte size subfractions in patients with type 2 diabetes. Obesity 2009; 17: 1976– 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE: Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997; 46: 1579– 1585 [DOI] [PubMed] [Google Scholar]
- 30.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH: Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000; 278: E941– E948 [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 1979; 237: E214– E223 [DOI] [PubMed] [Google Scholar]
- 32.Jequier E, Acheson K, Schutz Y: Assesment of energy expenditure and fuel utilization in man. Annu Rev Nutr 1987; 7: 187– 208 [DOI] [PubMed] [Google Scholar]
- 33.Lara-Castro C, Newcomer BR, Rowell J, Wallace P, Shaughnessy SM, Munoz AJ, Shiflett AM, Rigsby DY, Lawrence JC, Bohning DE, Buchthal S, Garvey WT: Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metabolism 2008; 57: 1– 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E: Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006; 29: 1337– 1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehman R, Vokac A, Niedermann K, Agosti K, Spinas GA: Loss of abdominal fat and improvement of the cardiovascular risk profile by regular moderate exercise training in patients with NIDDM. Diabetologia 1995; 38: 1313– 1319 [DOI] [PubMed] [Google Scholar]
- 36.Mourier A, Gautier JF, De Kerviler E, Bigard AX, Villette JM, Garnier JP, Duvallet A, Guezennec CY, Cathelineau G: Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Diabetes Care 1997; 3: 385– 391 [DOI] [PubMed] [Google Scholar]
- 37.Fabbrini E, Magkos F, Mohammed SB, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S: Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A 2009; 106: 15430– 15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felber JP, Golay A: Pathways from obesity to diabetes. Int J Obes 2002; 26( Suppl. 2): S39– S45 [DOI] [PubMed] [Google Scholar]
- 39.Williams KV, Bertoldo A, Kinahan P, Cobelli C, Kelley DE: Weight loss-induced plasticity of glucose transport and phosphorylation in the insulin resistance of obesity and type 2 diabetes. Diabetes 2003; 52: 1619– 1626 [DOI] [PubMed] [Google Scholar]
- 40.Ryysy L, Hakkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, Yki-Jarvinen H: Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 2000; 49: 749– 758 [DOI] [PubMed] [Google Scholar]
- 41.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS: Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab 2004; 89: 2704– 2710 [DOI] [PubMed] [Google Scholar]
- 42.Goodpaster BH, Katsiaras A, Kelley DE: Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 2003; 52: 2191– 2197 [DOI] [PubMed] [Google Scholar]