Abstract
OBJECTIVE
Type 2 diabetes increases breast cancer risk and mortality, and hyperinsulinemia has been identified as a major factor linking these two diseases. Thus, we hypothesized that pharmacological reduction of elevated insulin levels would attenuate type 2 diabetes–mediated mammary tumor progression.
RESEARCH DESIGN AND METHODS
We studied mammary tumor development in MKR+/+ mice, a nonobese, hyperinsulinemic mouse model of type 2 diabetes. MKR+/+ mice were either crossed with mice expressing the polyoma virus middle T oncogene specifically in the mammary gland or inoculated orthotopically with the mouse mammary tumor cell lines Met-1 and MCNeuA. MKR+/+ or control mice harboring tumors were treated with CL-316243, a specific β3-adrenergic receptor agonist, which sensitizes insulin action but has no direct effect on the mouse mammary epithelium or Met-1 and MCNeuA cells.
RESULTS
CL-316243 treatment significantly reduced the elevated insulin levels in MKR+/+ mice and, as a consequence, attenuated mammary tumor progression in the three tumor models tested. This effect was accompanied by reductions in phosphorylation of insulin and IGF-I receptors in transformed mammary tissue.
CONCLUSIONS
Insulin-sensitizing treatment is sufficient to abrogate type 2 diabetes–mediated mammary tumor progression. Therefore, early administration of insulin-sensitizing therapy may reduce breast cancer risk and mortality in patients with type 2 diabetes.
Type 2 diabetes has become a major public health problem worldwide and is associated with severe acute and chronic complications. Recently it has been shown that the disease increases breast cancer risk and mortality (1–4). In our previous studies, we have identified hyperinsulinemia as the predominant factor responsible for diabetes-mediated mammary tumor progression (5). Elevated insulin levels are observed mainly at early stages of the disease, where peripheral insulin resistance results in a compensatory increase in insulin secretion by the pancreatic β-cells to meet the higher insulin demand. Thus, before the onset of clinically overt type 2 diabetes, patients are often hyperinsulinemic but euglycemic, and hence unaware of their disease for many years. There is growing evidence that the risk for the development of breast cancer is substantially increased in patients with early stage type 2 diabetes (6,7).
Pharmacological treatment of type 2 diabetes may have an impact on cancer risk and mortality. Early stage type 2 diabetes is treated by two main approaches: insulin secretagogues (e.g., sulfonylureas) stimulate insulin secretion from the pancreatic β-cells and thus increase insulin levels. Conversely, insulin-sensitizing agents (e.g., metformin and thiazolidinediones [TZDs]) improve insulin action in peripheral tissues and, as a consequence, reduce hyperinsulinemia. There is growing evidence that antidiabetic therapy elevating insulin levels increases cancer risk as well as cancer-related mortality (8,9), whereas insulin-sensitizing drugs may reduce cancer risk, morbidity, and mortality (8–14) in patients with type 2 diabetes. However, it is as yet unclear whether the antineoplastic effects of the two mainly used insulin-sensitizing agents (metformin and TZDs) are a result of their direct action on tumor cells (15–23) or an indirect effect via a reduction of insulin levels.
Our study was aimed to explore whether lowering insulin levels in type 2 diabetes would mitigate mammary tumor progression, independent of any direct effect of the applied drug. To address this question, we used the insulin-sensitizing drug CL-316243 (24), a potent β3-adrenergic receptor (β3-AR) agonist with no known direct effects on breast cancer, in a nonobese mouse model of type 2 diabetes (MKR+/+ mice). MKR+/+ mice develop severe insulin resistance and hyperinsulinemia at an early age due to overexpression of muscle creatine kinase–driven dominant-negative IGF-I receptors (IGF-IRs), and subsequent abrogation of IGF-I and insulin signaling in skeletal muscle (25). Female MKR+/+ mice develop only mild dysglycemia but display marked insulin resistance and hyperinsulinemia, similar to early stages of type 2 diabetes in humans (5). The nonobese hyperinsulinemic phenotype of these mice makes them an ideal model to specifically study the effect of insulin reduction on mammary tumor progression, independent of numerous confounding factors originating from obesity or overt type 2 diabetes (e.g., adipokines, proinflammatory cytokines, adipose tissue–derived sex steroids, hyperglycemia) (26). To initiate mammary tumors, we used three different approaches: polyoma virus middle T (PyVmT) transgenic mice (27) served as a model for early stages of cancer development. To study solid tumor formation, PyVmT- and Neu/ErbB2-expressing tumor cells (28,29) were used in syngeneic orthotopic cell injection experiments.
Here we demonstrate that chronic CL-316243 treatment is capable of reducing insulin levels in female MKR+/+ mice, leading to an abrogation of the accelerated mammary tumor progression in all three cancer models tested. Furthermore, we show that this effect is accompanied by a reduced activation of the insulin receptor (IR) and the IGF-IR in transformed mammary tissue. Our findings indicate that insulin-sensitizing therapy is sufficient to abrogate the tumor-promoting activity of early stage type 2 diabetes. Thus, we propose that early treatment of hyperinsulinemia might contribute to lower breast cancer risk, morbidity, and mortality in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
All mice were on the FVB/N background. The generation and characterization of MKR+/+ mice (5,25) as well as mouse mammary tumor virus (MMTV)–PyVmT+/− mice (27) was described previously. The mice were housed in a clean mouse facility, had free access to a standard mouse chow (Picolab rodent diet 5053; LabDiet, St. Louis, MO) and fresh water ad libitum and were kept on a 12-h light/dark cycle. Animal care and maintenance were provided through the Mount Sinai School of Medicine Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited animal facility. All procedures were approved by the Animal Care and Use Committee of Mount Sinai School of Medicine.
Metabolic assays.
Body weight and food intake were measured twice a week. Food intake was normalized to body weight and expressed as grams of food per grams of body weight0.75 per day. Blood glucose levels were measured weekly in the nonfasting state between 9:00 a.m. and 12:00 p.m. with an automated glucometer (Elite; Bayer, Mishawaka, IN). Plasma and serum were obtained in the nonfasting state and collected in heparinized and nonheparinized capillary tubes, respectively. Plasma insulin levels were measured by a radioimmunoassay according to the manufacturer's instructions (Linco, St. Charles, MO). Serum leptin, adiponectin, tumor necrosis factor-α (TNF-α), and interleukin (IL)-6 levels were measured by ELISA (Millipore, Billerica, MA [leptin] and R&D systems, Minneapolis, MN [adiponectin, TNF-α, and IL-6]). Serum free fatty acids (FFAs) and serum triglycerides were measured by a colorimetric assay (Roche Applied Science, Indianapolis, IN [FFAs] and BioVision, Mountain View, CA [triglycerides]). Body composition was determined in nonanesthetized mice using an EchoMRI 3-in-1 NMR system (Echo Medical Systems, Houston, TX).
Transgenic mammary tumor model.
PyVmT+/− male mice were interbred with MKR+/+ or wild-type female mice to generate cohorts of PyVmT+/− and PyVmT+/−/MKR+/+ female mice. CL-316243 (Sigma Aldrich, St. Louis, MO) was dissolved in sterile saline and administered daily intraperitoneally at a dose of 1 mg/kg body wt (30) from 3 to 6 weeks of age. Control mice received an equal amount of vehicle (sterile saline). After euthanasia, inguinal mammary glands (no. 4) were subjected to whole-mount analysis or immediately snap-frozen in liquid nitrogen for further studies.
Syngeneic orthotopic tumor models.
Met-1 and MCNeuA mouse carcinoma cells were derived from MMTV-PyVmT (FVB/N) and MMTV-Neu (FVB/N) transgenic mice, respectively (28,29). The cells were allowed to grow until confluence in Dulbecco's modified Eagle's medium supplemented with 10% FBS and were detached by a nonenzymatic cell dissociation solution, and Met-1 cells (0.5 × 106) or MCNeuA cells (106) were injected into the left inguinal mammary fat pad (no. 4) of 8-week-old female MKR+/+ and wild-type mice. One week after tumor cell inoculation, CL-316243 (1 mg · kg body wt−1 · day−1 i.p.) was administered for 21 days. Tumor growth was monitored by palpation and tumor volume was measured in a three-coordinate system using calipers. Tumor volume was calculated by the formula: 4/3 × π × r1 × r2 × r3 (r = radius).
Protein extraction and Western blot analysis.
Tissues were lysed in buffer (pH 7.4) containing 50 mmol/l Tris, 150 mmol/l NaCl, 1 mmol/l EDTA, 1.25% CHAPS (Roche Applied Science), 1 mmol/l sodium orthovanadate, 2 mmol/l sodium fluoride, 10 mmol/l sodium pyrophosphate (Sigma Aldrich), 8 mmol/l β-glycerophosphate (VWR, West Chester, PA), and Complete Protease Inhibitor Cocktail (Roche). After denaturation, the proteins were subjected to SDS-PAGE (8% Tris-glycine gel; Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was sequentially blocked and probed with primary and secondary antibodies and then analyzed by direct infrared fluorescence detection using an Odyssey Infrared Imaging System (Li-cor, Lincoln, NE). Densitometric analysis was performed using MacBAS V2.52 software (Fuji PhotoFilm, Valhalla, NY). The antibodies were purchased from the following sources: Phospho-IRβTyr1150/51/IGF-IRβTyr1135/36 (Cell Signaling Technology, Danvers, MA) and IRβ (Santa Cruz Biotechnology, Santa Cruz, CA).
Whole-mount analysis of mammary glands.
The no. 4 inguinal mammary glands were carefully excised, spread out on a glass slide, and fixed for 2–4 h in Carnoy fixative (60% ethanol, 30% chloroform, 10% glacial acetic acid). The fixed glands were hydrated in decreasing concentrations of ethanol (100, 95, 70, 50, and 30% for 15 min each), rinsed in double-distilled water, and stained overnight in carmine alum staining. After dehydration in increasing ethanol concentrations (30, 50, 70, 95, and 100% for 15 min each) and clearing in xylene overnight (Fisher Scientific, Pittsburgh, PA), the glands were covered by Mount-Quick mounting medium (Daido Sangyo, Tokyo, Japan) and photographic documentation was performed using a stereomicroscope and MicroSuite FIVE imaging software (Olympus, Center Valley, PA). Quantification of the hyperplastic mammary lesion as a ratio of the total glandular area was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Determination of β3-AR expression.
RNA was extracted from Met-1, MCNeuA, and mammary epithelial cells (MECs) derived from FVB/N mice using the NucleoSpin RNA II kit (Clontech Laboratories, Mountain View, CA). After RT, the resulting cDNA was amplified by PCR using the following primers (Operon, Huntsville, AL): β3-AR: forward: 5′ ATGGCTCCGTGGCCTCAC 3′ and reverse: 5′ CTGGCTCATGATGGGCGC 3′ (31); 18S rRNA: forward: 5′ TTGACGGAAGGGCACCACCAG 3′ and reverse: 5′ GCACCACCACCCACGGAATCG 3′.
Statistical analysis.
Results are expressed as means ± SEM. Statistical analyses were conducted using ANOVA followed by a Fisher test, with P ≤ 0.05 considered significant. All analyses were performed using STATVIEW version 5.0 (SAS Institute, Cary, NC).
RESULTS
Mammary epithelial cells, mammary tumor cells, and mammary gland development are not affected by CL-316243.
CL-316243 has potent antiobesity and antidiabetic effects in various rodent models (30,32–34). The action of CL-316243 is highly specific to β3-AR–expressing cells, which are predominantly the white and brown adipocytes (35,36). Chronic activation of the β3-AR increases fatty acid oxidation and energy expenditure, thereby reducing adiposity. Subsequently, chronic β3-AR stimulation leads to improved insulin sensitivity and reduced insulin levels (30,33,34).
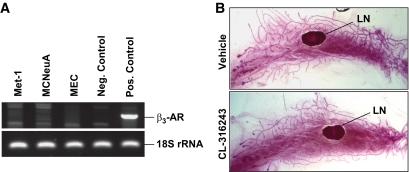
To address whether CL-316243 has direct effects on the mammary epithelium or tumor cells, we determined the expression levels of the β3-AR. As shown in Fig. 1A, RT-PCR revealed undetectable levels of β3-AR transcripts in normal mouse MECs and in the two mammary tumor cell lines: Met-1 and MCNeuA.
FIG. 1.
Normal MECs, mammary tumor cells, and mammary gland development are not affected by CL-316243. A: Determination of β3-AR expression in MECs (derived from FVB/N mice), Met-1, and MCNeuA tumor cells analyzed by PCR followed by agarose gel electrophoresis (for primers, see research design and methods section). Negative control, NIH/3T3 fibroblasts; positive control, mouse white adipose tissue. 18S rRNA was used as a loading control. B: Representative whole-mount images of the no. 4 mammary gland obtained from 7-week-old wild-type mice. The mice were treated from 4 to 7 weeks of age with CL-316243 (1 mg · kg body wt−1 · day−1 i.p.) or a vehicle control. n = 4–5 mice/group. LN, lymph node. Original magnification ×4. (A high-quality color representation of this figure is available in the online issue.)
Chronic β3-AR activation with CL-316243 leads to an increased formation of brown adipose tissue in the mammary gland (37). Thus, we assessed whether treatment with CL-316243 affects mammary gland development in vivo through alteration of the mammary fat pad. Four-week-old female wild-type mice were subjected to intraperitoneal injections of CL-316243 (1 mg · kg body wt−1 · day−1) or an equal volume of vehicle for 3 weeks. Whole-mount analyses of mammary glands obtained from these animals at the age of 7 weeks demonstrated no significant changes in mammary ductal outgrowth and side branching (Fig. 1B).
These data suggest that CL-316243 has a minimal effect on mammary gland development and that a direct effect of CL-316243 on mammary tumor cells via activation of the β3-AR is unlikely.
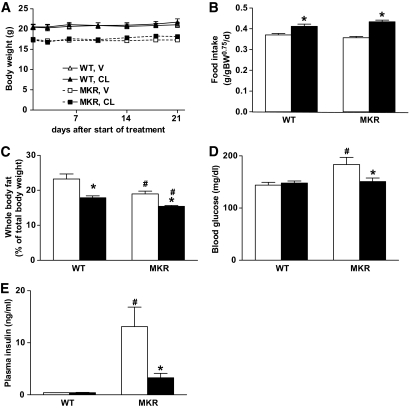
CL-316243 treatment affects food intake and body composition in female wild-type and MKR+/+ mice and reduces hyperinsulinemia in female MKR+/+ mice.
We have previously shown that chronic treatment with CL-316243 effectively reverses the diabetic phenotype in male MKR+/+ mice, which, in contrast to female MKR+/+ mice, develop overt type 2 diabetes (30). To test whether the predominantly hyperinsulinemic phenotype in female MKR+/+ mice would respond in a similar manner, we treated 8-week-old female MKR+/+ and wild-type mice chronically with CL-316243 (1 mg · kg body wt−1 · day−1) or vehicle for 3 weeks. Female MKR+/+ mice exhibit reduced body weight and body adiposity compared with wild-type mice (5). As observed in our previous study in male wild-type and MKR+/+ mice (30), we found that CL-316243 treatment did not affect body weight (Fig. 2A), but increased food intake (Fig. 2B) and decreased body adiposity (Fig. 2C), predominantly affecting the gonadal fat pads (supplemental Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1291/DC1). Additionally, we found that after 1 week of CL-316243 treatment, the moderately elevated blood glucose levels (∼20%) in MKR+/+ mice were significantly lowered (Fig. 2D). Importantly, we show that female MKR+/+ mice display severely elevated plasma insulin levels, which were significantly reduced upon CL-316243 treatment, whereas no effect of the drug on insulin levels was seen in wild-type mice (wild type, vehicle: 0.42 ± 0.01 vs. wild type, CL-316243: 0.43 ± 0.02; MKR+/+, vehicle: 13.12 ± 3.74 vs. MKR+/+, CL-316243: 3.28 ± 0.85 ng/ml) (Fig. 2E).
FIG. 2.
Chronic CL-316243 treatment abrogates the diabetic phenotype in female MKR+/+ mice. MKR+/+ and wild-type (WT) mice were treated with CL-316243 (CL, ■) or vehicle (V, □) from 9 to 12 weeks of age. Body weight (A) and food intake (B) were measured twice a week. The average food intake over 3 weeks of treatment is presented. Body adiposity (C) was measured 10 days after the onset of treatment in nonanesthetized mice using an EchoMRI 3-in-1 NMR system; glucose (D) and insulin (E) levels were measured in whole blood and plasma, respectively, 1 week after initiation of treatment in the nonfasting state. Data are expressed as means ± SEM. *P ≤ 0.05 for CL-316243 vs. vehicle group; #P ≤ 0.05 for wild-type vs. MKR+/+ from the same treatment group. n = 6–8 mice/group. These data have been reproduced in three independent experiments.
As chronic CL-316243 treatment lowers body adiposity in both wild-type and MKR+/+ mice, serum levels of circulating lipids, adipokines, and proinflammatory cytokines were determined (Table 1). Serum levels of FFAs were unchanged in female MKR+/+ mice compared with wild-type mice, whereas there was a moderate (∼30%) increase in triglyceride levels. Upon chronic treatment with CL-316243, FFAs and triglycerides were significantly reduced to a similar level in both MKR+/+ and wild-type mice. Serum leptin levels were lower in MKR+/+ mice compared with wild-type mice, and CL-316243 treatment significantly reduced leptin levels in both MKR+/+ and wild-type mice. The levels of adiponectin and the proinflammatory cytokines TNF-α and IL-6 were not affected by CL-316243 treatment.
TABLE 1.
Effect of chronic treatment with CL-316243 on serum lipids, adipokines, and proinflammatory cytokines in female wild-type and MKR+/+ mice
| Wild-type mice |
MKR+/+ mice |
|||
|---|---|---|---|---|
| Vehicle | CL-316243 | Vehicle | CL-316243 | |
| FFAs (mmol/l) | 0.34 ± 0.04 | 0.16 ± 0.03* | 0.29 ± 0.05 | 0.17 ± 0.03* |
| Triglycerides (mmol/l) | 1.40 ± 0.09 | 1.01 ± 0.08* | 1.83 ± 0.14† | 1.13 ± 0.15* |
| Leptin (ng/ml) | 3.11 ± 0.44 | 2.04 ± 0.19* | 2.02 ± 0.28† | 1.00 ± 0.06*† |
| Adiponectin (μg/ml) | 8.39 ± 0.58 | 9.61 ± 0.34 | 9.74 ± 0.38† | 9.16 ± 0.42 |
| TNF-α (pg/ml) | 20.95 ± 1.23 | 19.54 ± 0.81 | 18.48 ± 0.47† | 18.27 ± 0.66 |
| IL-6 (pg/ml) | 4.26 ± 0.51 | 5.03 ± 1.82 | 6.75 ± 1.96 | 7.32 ± 3.27 |
Data are means ± SEM. Serum was obtained in the nonfasting state at the end of the study, 21 days after the onset of treatment with CL-316243 (1 mg · kg−1 · day−1).
*P ≤ 0.05 for CL-316243 vs. vehicle group;
†P ≤ 0.05 for wild type vs. MKR+/+ from the same treatment group. FFA and triglycerides: n = 10–16 mice/group; adiponectin, leptin, IL-6, and TNF-α: n = 5–7 mice/group.
Taken together, these data show that chronic treatment with CL-316243 effectively reduces insulin levels in female MKR+/+ mice alone while having a comparable effect on food intake, relative body adiposity, circulating lipids, and leptin levels in both wild-type and MKR+/+ mice. This allows us to study the effect of insulin reduction on mammary tumor progression in MKR+/+ mice.
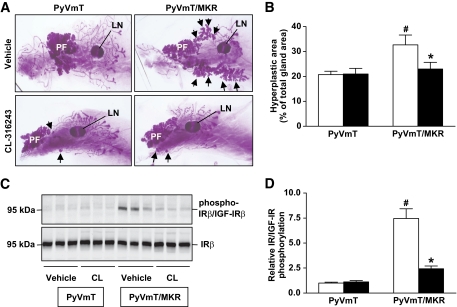
CL-316243 treatment abrogates the accelerated formation of hyperplastic mammary lesions in PyVmT+/−/MKR+/+ mice.
To investigate the effect of insulin-sensitizing therapy on early stages of mammary tumor development, we used a PyVmT-induced transgenic tumor model (27). PyVmT+/− transgenic mice express the PyVmT oncogene exclusively in the mammary epithelium under the control of the MMTV promoter. PyVmT-induced mammary tumors recapitulate many morphologic and pathophysiological processes found in human breast cancer, and their development begins with the formation of a single hyperplastic focus in the lateral part of the mammary gland at 3–4 weeks of age (38).
To assess the effect of antidiabetic therapy on mammary tumor development, we treated PyVmT+/−/MKR+/+ and PyVmT+/− mice with CL-316243 (1 mg · kg body wt−1 · day−1) or vehicle from 3 to 6 weeks of age. The metabolic effects of CL-316243 treatment were similar to those observed in adult mice (supplementary Fig. 2). The inguinal mammary glands (no. 4) were dissected after treatment at 6 weeks of age. Whole-mount analyses of vehicle-treated PyVmT+/−/MKR+/+ mice revealed a larger area of hyperplastic mammary lesions (increase of 57%) compared with the PyVmT+/− mice (Fig. 3A and B) due to accelerated formation of hyperplasia in a hyperinsulinemic milieu. In contrast, pharmacological correction of hyperinsulinemia in PyVmT+/−/MKR+/+ mice resulted in a tumor phenotype similar to nondiabetic PyVmT+/− mice (Fig. 3A and B). Note that the CL-316243–treated nondiabetic PyVmT+/− mice show no changes in mammary transformation. To determine whether the attenuation in tumor progression in the CL-316243–treated mice resulted from an inhibition of insulin signaling, we analyzed the phosphorylation of the IR/IGF-IR in the hyperplastic mammary tissue of vehicle-and CL-316243–treated mice (Fig. 3C and D and supplementary Fig. 3). We found that hyperinsulinemia led to an increased activation of the IR/IGF-IR in transformed mammary tissue of PyVmT+/−/MKR+/+ mice, whereas pharmacological correction of insulin levels by CL-316243 treatment significantly reduced this effect. Taken together, we demonstrate that lowering insulin levels in type 2 diabetic PyVmT+/−/MKR+/+ mice reduces IR/IGF-IR activation and abrogates the accelerated formation of hyperplastic mammary lesions.
FIG. 3.
Treatment with CL-316243 prevents the accelerated formation of hyperplastic mammary lesions in diabetic mice and attenuates IR/IGF-IR activation. A: Whole-mount analysis of the no. 4 mammary gland of 6-week-old virgin PyVmT+/−/MKR+/+ mice and PyVmT+/− mice. Both groups were treated with CL-316243 or vehicle from 3 to 6 weeks of age. LN, lymph node; PF, primary focus. Arrows indicate secondary foci. Original magnification ×10. Representative whole mounts are presented. B: Quantification of the area of hyperplastic lesions is presented as percentage of the total gland area (n = 6–8 mice/group). CL-316243, ■; vehicle, □. C: Proteins extracted from hyperplastic mammary tissue of 6-week-old PyVmT+/−/MKR+/+ and PyVmT+/− mice treated with either CL-316243 (CL) or vehicle were size fractioned by SDS-PAGE and immunoblotted against phospho-IRβY1150/51/IGF-IRβY1135/36 and the IRβ. Representative Western blot analysis is shown. D: Densitometric analysis of the relative IR/IGF-IR phosphorylation (normalized to the IR) is presented as a fold change compared with the vehicle-treated control group (PyVmT+/−, vehicle) (n = 6–8 mice/group). CL-316243, ■; vehicle, □. Data are expressed as means ± SEM. *P ≤ 0.05 for CL-316243 vs. vehicle group; #P ≤ 0.05 for PyVmT+/− vs. PyVmT+/−/MKR+/+ from the same treatment group. (A high-quality color representation of this figure is available in the online issue.)
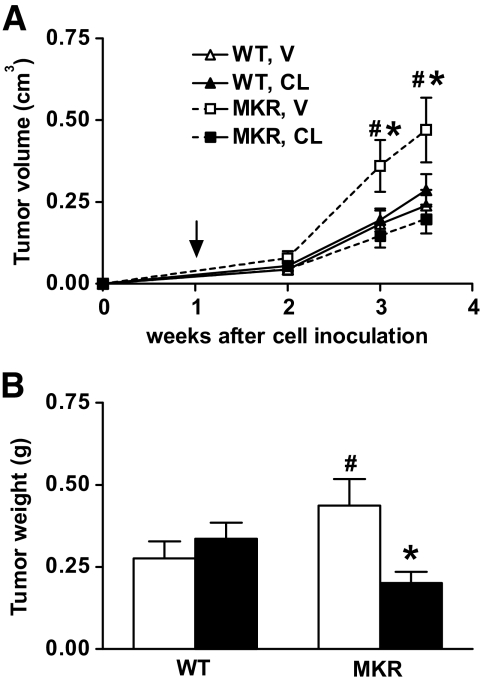
Treatment with CL-316243 abrogates the accelerated growth of advanced mammary tumors in MKR+/+ mice.
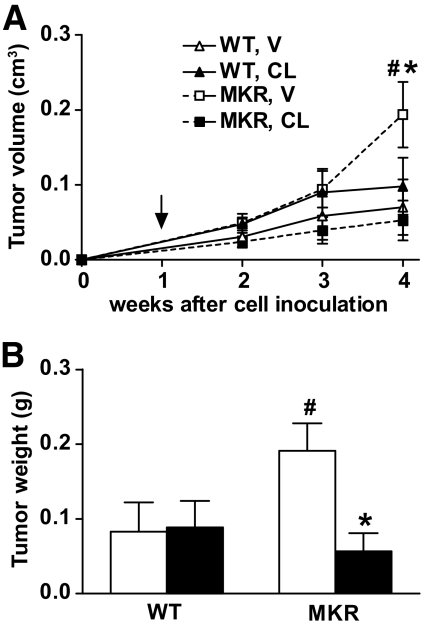
To investigate whether CL-316243 treatment would also affect growth of fully transformed tumor cells, we injected estrogen receptor–negative PyVmT-transformed mammary tumor cells (Met-1 cells [28]) orthotopically into the no. 4 mammary fat pad (0.5 × 106 cells) of 8-week-old female MKR+/+ or wild-type mice. Starting 7 days after tumor cell inoculation, when tumors were palpable, the animals were treated with CL-316243 (1 mg · kg body wt−1 · day−1) for 3 weeks. As shown in Fig. 4A, tumor volume increased in vehicle-treated MKR+/+ mice, whereas MKR+/+ mice treated with CL-316243 displayed a significant reduction in tumor growth and were similar in size to wild-type controls. Importantly, CL-316243 treatment had no effect on tumor growth in the wild-type mice. These findings were confirmed by measuring wet tumor weight at the end of the study (Fig. 4B).
FIG. 4.
Treatment with CL-316243 reverses the accelerated growth of advanced PyVmT-induced mammary tumors. Met-1 cells (0.5 × 106) were orthotopically injected into the no. 4 mammary fat pad of 8-week-old virgin wild-type or MKR+/+ mice. Seven days after cell injection, treatment with either CL-316243 (CL) or vehicle (V) was initiated. A: Tumor growth was followed by measuring tumor volume weekly and was calculated as indicated in the research design and methods section. Arrow indicates the beginning of treatment. B: Wet tumor weight was determined at the end of the study. CL-316243, ■; vehicle, □. Data are expressed as means ± SEM. *P ≤ 0.05 for CL-316243 vs. vehicle group; #P ≤ 0.05 for wild-type vs. MKR+/+ from the same treatment group. n = 7–8 mice/group.
To ascertain that our findings were not limited to PyVmT-induced mammary carcinogenesis, we also studied estrogen receptor–negative Neu-transformed mammary tumor cells (MCNeuA cells [29]) in a similar manner as the Met-1 cells. Neu is the rodent analog of the ERBB2 gene, which is amplified and/or overexpressed in 30% of human breast carcinomas (39). MCNeuA cells were inoculated orthotopically into the no. 4 mammary fat pad (106 cells) and the treatment protocol and follow-up of tumor growth were performed as described above. As shown in Fig. 5A and B, tumor volume and weight were increased in vehicle-treated MKR+/+ mice compared with wild-type controls, however, after CL-316243 treatment and subsequent decreases in insulin levels, we observed a significantly reduced tumor size in MKR+/+ mice. These data suggest that insulin-sensitizing therapy can abrogate insulin-mediated tumor progression independent of the tumor-inducing oncogene.
FIG. 5.
Treatment with CL-316243 reverses the accelerated growth of advanced Neu/ErbB2-induced mammary tumors. MCNeuA cells (106) were orthotopically injected into the no. 4 mammary fat pad of 8-week-old virgin wild-type or MKR+/+ mice. At 7 days after cell injection, treatment with either CL-316243 (CL) or vehicle (V) was initiated. A: Tumor growth was followed by measuring tumor volume weekly and was calculated as indicated in the research design and methods section. Arrow indicates the beginning of treatment. B: Wet tumor weight was determined at the end of the study. CL-316243, ■; vehicle, □. Data are expressed as means ± SEM. *P ≤ 0.05 for CL-316243 vs. vehicle group; #P ≤ 0.05 for wild-type vs. MKR+/+ from the same treatment group. n = 7–8 mice/group.
DISCUSSION
There is an increasing interest in the effect of insulin-sensitizing therapy on breast cancer risk and mortality. The current study demonstrates that treatment with the insulin-sensitizing drug CL-316243, a highly selective β3-AR agonist, reverses diabetes-induced mammary tumor progression in hyperinsulinemic, type 2 diabetic MKR+/+ mice. Our findings corroborate a major role of insulin in type 2 diabetes–mediated mammary tumor progression, which is in concert with our previous findings (5).
High concentrations of insulin are known to activate the IGF-IR (40) and our previous findings demonstrate that the IR, and to lesser extent the IGF-IR, are activated in hyperplastic mammary tumor tissue extracted from type 2 diabetic mice (5). Moreover, pharmacological blockade of the IR/IGF-IR using BMS-536924, a small-molecule tyrosine kinase inhibitor, led to abrogation of diabetes-induced mammary tumor progression in MKR+/+ mice; however, it also worsened insulin resistance and hyperinsulinemia (5). Here we demonstrate that insulin-sensitizing treatment has a comparable effect on tumor progression but simultaneously exerts antidiabetic activity. Our results imply that elevated insulin levels should be targeted pharmacologically at early stages of the disease to lower breast cancer risk and progression in patients with type 2 diabetes.
The effect of antidiabetic medications on cancer development is poorly understood and no prior experimental studies have thoroughly investigated whether pharmacological reduction of insulin levels reduces mammary tumor growth in a type 2 diabetic organism. The reason for this is that the most widely used insulin-sensitizing drugs (metformin and TZDs) are known to exert direct, predominantly antineoplastic, effects on cancer cells, both in vitro and in vivo in nondiabetic animals (15–17,19–23,41). Thus, we decided to use CL-316243, a highly selective β3-AR agonist with no known direct effect on mammary tumors, to study the consequence of lowering insulin levels on diabetes-mediated mammary tumor progression. However, it should be noted that β3-AR agonists (unlike metformin and TZDs) have not yet been developed for clinical use in humans due to various problems including a lack of selectivity and poor pharmacokinetics (42).
Algire et al. (18) have reported that metformin attenuates the effect of high-energy diet–induced insulin resistance on growth of Lewis lung LLC1 carcinoma cells. Both, the insulin-sensitizing action as well as the direct antineoplastic activity of metformin may be involved in this finding. However, in the setting of excess energy intake and obesity, other factors besides hyperinsulinemia can promote tumor growth, such as adipokines, proinflammatory cytokines, adipose tissue–derived sex steroids, or altered levels of circulating carbohydrates and lipids (43), some of which may be affected by metformin treatment (44,45).
Thus, as an experimental model for insulin resistance independent of obesity, we used a lean, hyperinsulinemic mouse model of type 2 diabetes, the female MKR+/+ mice. These mice develop severe insulin resistance and hyperinsulinemia but have only mild dysglycemia and thus recapitulate the early stages of type 2 diabetes. As these mice display reduced body adiposity and show no increase in serum FFAs, leptin, or the proinflammatory cytokines TNF-α or IL-6 and only a moderate elevation in serum triglycerides, they serve as an ideal model to study mammary tumor progression uncoupled from obesity-related tumor-promoting factors.
Apart from reducing insulin levels, chronic treatment with CL-316243 also decreases blood glucose levels in MKR+/+ mice. Hyperglycemia has been proposed to promote tumor growth through the increased flux of glucose, which fuels tumor cells (46). However, a major pathophysiological role of elevated blood glucose levels in the tumor-promoting action of type 2 diabetes in MKR+/+ mice is unlikely because of the following: 1) female MKR+/+ mice display only mild dysglycemia (∼20% increase in blood glucose levels compared with wild-type mice), 2) in animals with type 1 diabetes, which are insulin deficient because of immune destruction of the pancreatic β-cells, tumors regress despite severe hyperglycemia (47,48), 3) although some epidemiologic studies show a correlation between elevated blood glucose levels and cancer risk, the studies may be confounded by preexisting hyperinsulinemia (49). Chronic CL-316243 treatment reduces serum FFA, triglyceride, and leptin levels in both MKR+/+ and wild-type mice. As this decrease is observed in both genotypes and the antineoplastic effect of CL-316243 is specific to MKR+/+ mice, these factors are most likely not major players in the tumor-reducing effect of CL-316243. Nevertheless, we cannot fully exclude that changes in lipid or leptin levels may, at least in part, have an impact on breast tumor progression in MKR+/+ mice. Taken together, our experimental approach allowed us to test the effect of insulin-sensitizing therapy on tumor progression in a setting in which obesity-associated confounding factors are minimal.
Our findings demonstrate that hyperinsulinemia in mice with type 2 diabetes significantly increases IR/IGF-IR activation in transformed mammary tissue, whereas pharmacological reduction of insulin levels attenuates this effect. Law et al.(50) reported that phosphorylation of the IR/IGF-IR was present in nearly 50% of all analyzed human primary breast tumors and was predictive for a poor outcome. However, the authors did not obtain information on the insulin levels in the patients studied. It remains to be evaluated whether the extent of IR/IGF-IR activation in breast cancer specimen correlates with underlying type 2 diabetes and hyperinsulinemia in breast cancer patients. If this is the case, then insulin lowering therapy could reduce breast cancer risk and mortality.
In conclusion, we demonstrate that the administration of insulin-sensitizing therapy using a β3-AR agonist abrogates the accelerated mammary tumor progression in a nonobese mouse model of type 2 diabetes. This effect is accompanied by a reduction of insulin levels leading to a decrease in the phosphorylation of the IR/IGF-IR in transformed mammary tissue. These results demonstrate that insulin-lowering therapy is an important modifier of breast cancer progression. Our findings thus provide a rationale for early administration of insulin-sensitizing therapy in patients with hyperinsulinemia and/or type 2 diabetes, as such treatment may have a significant impact on breast cancer risk, morbidity, and mortality.
ACKNOWLEDGMENTS
This work was funded by the National Cancer Institute (1RO1CA128799-O1A1). Y.F. received grants from the Swiss National Science Foundation (PBBSB-120851 and PBBSB3-120851) as well as from the Novartis Foundation. D.L. and Y.F. received a grant from the Roche Research Foundation.
No potential conflicts of interest relevant to this article were reported.
This study was presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We thank W.J. Muller (McGill University, Montreal, Canada) for donating MMTV-PyVmT transgenic mice; S.D. Hurstings (Department of Nutritional Sciences, University of Texas, Austin, TX, and Department of Carcinogenesis, University of Texas–M.D. Anderson Cancer Center, Smithville, TX) and N.P. Nunez (Department of Nutritional Sciences, University of Texas, Austin, TX) for donating Met-1 cells; and M.J. Campbell and J.F. Youngren (University of California San Francisco, San Francisco, CA) for providing MCNeuA cells.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ: Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 2004; 159: 1160– 1167 [DOI] [PubMed] [Google Scholar]
- 2.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE: The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat 2008; 109: 389– 395 [DOI] [PubMed] [Google Scholar]
- 3.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL: Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008; 300: 2754– 2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue F, Michels KB: Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr 2007; 86: s823– s835 [DOI] [PubMed] [Google Scholar]
- 5.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, Carboni J, Gottardis MM, Pennisi PA, Molinolo AA, Kurshan N, Meija W, Santopietro S, Yakar S, Wood TL, LeRoith D: Insulin-mediated acceleration of breast cancer development and progression in a non-obese model of type 2 diabetes. Cancer Res 2010; 70: 741– 751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE: Increased prevalence of prior breast cancer in women with newly diagnosed diabetes. Breast Cancer Res Treat 2006; 98: 303– 309 [DOI] [PubMed] [Google Scholar]
- 7.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD: Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2009; 101: 48– 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowker SL, Majumdar SR, Veugelers P, Johnson JA: Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29: 254– 258 [DOI] [PubMed] [Google Scholar]
- 9.Currie CJ, Poole CD, Gale EA: The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52: 1766– 1777 [DOI] [PubMed] [Google Scholar]
- 10.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD: Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304– 1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM: Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009; 27: 3297– 3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin PJ, Ligibel JA, Stambolic V: Metformin in breast cancer: time for action. J Clin Oncol 2009; 27: 3271– 3273 [DOI] [PubMed] [Google Scholar]
- 13.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP: Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 2007; 25: 1476– 1481 [DOI] [PubMed] [Google Scholar]
- 14.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM: New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32: 1620– 1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M: Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006; 66: 10269– 10273 [DOI] [PubMed] [Google Scholar]
- 16.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N: Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007; 67: 10804– 10812 [DOI] [PubMed] [Google Scholar]
- 17.Blanquicett C, Roman J, Hart CM: Thiazolidinediones as anti-cancer agents. Cancer Ther 2008; 6: 25– 34 [PMC free article] [PubMed] [Google Scholar]
- 18.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M: Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer 2008; 15: 833– 839 [DOI] [PubMed] [Google Scholar]
- 19.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB: Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007; 67: 6745– 6752 [DOI] [PubMed] [Google Scholar]
- 20.Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F: The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008; 27: 3576– 3586 [DOI] [PubMed] [Google Scholar]
- 21.Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV: Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med 2005; 139: 721– 723 [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, Thor AD: Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle 2009; 8: 2031– 2040 [DOI] [PubMed] [Google Scholar]
- 23.Jarrar MH, Baranova A: PPARgamma activation by thiazolidinediones (TZDs) may modulate breast carcinoma outcome: the importance of interplay with TGFbeta signalling. J Cell Mol Med 2007; 11: 71– 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloom JD, Dutia MD, Johnson BD, Wissner A, Burns MG, Largis EE, Dolan JA, Claus TH: Disodium (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino] propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL 316,243): a potent beta-adrenergic agonist virtually specific for beta 3 receptors A promising antidiabetic and antiobesity agent J Med Chem 1992; 35: 3081– 3084 [DOI] [PubMed] [Google Scholar]
- 25.Fernández AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D: Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 2001; 15: 1926– 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vona-Davis L, Howard-McNatt M, Rose DP: Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev 2007; 8: 395– 408 [DOI] [PubMed] [Google Scholar]
- 27.Guy CT, Cardiff RD, Muller WJ: Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992; 12: 954– 961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG, McGoldrick ET, Muller WJ, Cardiff RD, Gregg JP: Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis 2005; 22: 47– 59 [DOI] [PubMed] [Google Scholar]
- 29.Campbell MJ, Wollish WS, Lobo M, Esserman LJ: Epithelial and fibroblast cell lines derived from a spontaneous mammary carcinoma in a MMTV/neu transgenic mouse. In Vitro Cell Dev Biol Anim 2002; 38: 326– 333 [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Pennisi PA, Gavrilova O, Pack S, Jou W, Setser-Portas J, East-Palmer J, Tang Y, Manganiello VC, Leroith D: Effect of adipocyte beta3-adrenergic receptor activation on the type 2 diabetic MKR mice. Am J Physiol Endocrinol Metab 2006; 290: E1227– E1236 [DOI] [PubMed] [Google Scholar]
- 31.Fève B, Elhadri K, Quignard-Boulangé A, Pairault J: Transcriptional down-regulation by insulin of the beta 3-adrenergic receptor expression in 3T3–F442A adipocytes: a mechanism for repressing the cAMP signaling pathway. Proc Natl Acad Sci U S A 1994; 91: 5677– 5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbeeny CM, Meyers DS, Hillyer DE, Bergquist KE: Metabolic alterations associated with the antidiabetic effect of beta 3-adrenergic receptor agonists in obese mice. Am J Physiol 1995; 268: E678– E684 [DOI] [PubMed] [Google Scholar]
- 33.Arch JR: beta(3)-Adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol 2002; 440: 99– 107 [DOI] [PubMed] [Google Scholar]
- 34.Weyer C, Gautier JF, Danforth E, Jr: Development of beta 3-adrenoceptor agonists for the treatment of obesity and diabetes—an update. Diabete Metab 1999; 25: 11– 21 [PubMed] [Google Scholar]
- 35.Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg AD: Molecular characterization of the human beta 3-adrenergic receptor. Science 1989; 245: 1118– 1121 [DOI] [PubMed] [Google Scholar]
- 36.Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, Fraser CM: An adipose tissue-specific beta-adrenergic receptor: molecular cloning and down-regulation in obesity J Biol Chem 1991; 266: 24053– 24058 [PubMed] [Google Scholar]
- 37.Gouon-Evans V, Pollard JW: Unexpected deposition of brown fat in mammary gland during postnatal development. Mol Endocrinol 2002; 16: 2618– 2627 [DOI] [PubMed] [Google Scholar]
- 38.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW: Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 2003; 163: 2113– 2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177– 182 [DOI] [PubMed] [Google Scholar]
- 40.Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K, Eckstein V, Nawroth PP, Bierhaus A: Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med 2008; 14: 301– 308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phoenix KN, Vumbaca F, Claffey KP: Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERalpha negative MDA-MB-435 breast cancer model. Breast Cancer Res Treat 2009; 113: 101– 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawa M, Harada H: Recent developments in the design of orally bioavailable beta3-adrenergic receptor agonists. Curr Med Chem 2006; 13: 25– 37 [PubMed] [Google Scholar]
- 43.Calle EE, Kaaks R: Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4: 579– 591 [DOI] [PubMed] [Google Scholar]
- 44.Glueck CJ, Fontaine RN, Wang P, Subbiah MT, Weber K, Illig E, Streicher P, Sieve-Smith L, Tracy TM, Lang JE, McCullough P: Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism 2001; 50: 856– 861 [DOI] [PubMed] [Google Scholar]
- 45.Kirpichnikov D, McFarlane SI, Sowers JR: Metformin: an update. Ann Intern Med 2002; 137: 25– 33 [DOI] [PubMed] [Google Scholar]
- 46.Warburg O: On the origin of cancer cells. Science 1956; 123: 309– 314 [DOI] [PubMed] [Google Scholar]
- 47.Cohen ND, Hilf R: Influence of insulin on growth and metabolism of 7,12-dimethylbenz(alpha)anthracene-induced mammary tumors. Cancer Res 1974; 34: 3245– 3252 [PubMed] [Google Scholar]
- 48.Heuson JC, Legros N: Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer Res 1972; 32: 226– 232 [PubMed] [Google Scholar]
- 49.Becker S, Dossus L, Kaaks R: Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem 2009; 115: 86– 96 [DOI] [PubMed] [Google Scholar]
- 50.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, Nicholson RI, Carboni J, Gottardis M, Pollak M, Dunn SE: Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 2008; 68: 10238– 10246 [DOI] [PubMed] [Google Scholar]