Abstract
OBJECTIVE
Ceramide is now recognized as a negative regulator of insulin signaling by impairing protein kinase B (PKB)/Akt activation. In different cells, two distinct mechanisms have been proposed to mediate ceramide inhibition of PKB/Akt: one involving atypical protein kinase C zeta (PKCζ) and the other the protein phosphatase-2 (PP2A). We hypothesized that ceramide action through PKCζ or PP2A might depend on plasma membrane (PM) structural organization and especially on caveolin-enriched domain (CEM) abundance.
RESEARCH DESIGN AND METHODS
We have used different PKCζ mutant constructs or the PP2A inhibitor, okadaic acid (OKA), to selectively inhibit PKCζ- and PP2A-dependent pathways in cells expressing different caveolin-1 levels and evaluated the impact of insulin and ceramide on PKB/Akt activity in different PM subdomains.
RESULTS
Although the PKCζ-mediated negative effect of ceramide on insulin-stimulated PKB/Akt was dominant in adipocytes, a ceramide action through PP2A outside CEMs, prevented by OKA, was also unraveled. To test the importance of CEM to direct ceramide action through the PKCζ pathway, we treated 3T3-L1 preadipocytes devoid of CEMs with ceramide and we saw a shift of the lipid-negative action on PKB/Akt to a PP2A-mediated mechanism. In fibroblasts with low CEM abundance, the ceramide-activated PP2A pathway dominated, but could be shifted to a ceramide-activated PKCζ pathway after caveolin-1 overexpression.
CONCLUSIONS
Our results show that ceramide can switch from a PKCζ-dependent mechanism to a PP2A pathway, acting negatively on PKB/Akt, and hence revealing a critical role of CEMs of the PM in this process.
Insulin is a hormone essential for tissue development, growth, energy storage, and maintenance of glucose homeostasis. Defects in insulin secretion and action are key factors in the development of metabolic diseases such as diabetes, obesity, hypertension, atherosclerosis, and cardiovascular diseases (1).
The mechanism by which insulin resistance develops in peripheral tissue is not yet fully solved. Recent work has suggested that forcing cells to store fatty acids beyond their capacities could promote insulin resistance by inducing the accumulation of intracellular signaling molecules able to inhibit the action of insulin (2). Among these fatty acid–derived lipids, ceramides are the most active to negatively regulate intermediates of the insulin-signaling pathway and to inhibit insulin-dependent pathways such as the uptake of glucose into muscle and adipocytes (3,4).
The process of insulin signal transduction is initiated by the activated insulin receptor kinase, which tyrosine phosphorylates intracellular target substrates, in particular the family of insulin receptor substrates (IRS 1–4 proteins) (5). Although numerous proteins can dock on activated IRS, it is generally accepted that phosphoinositide 3-kinase (PI 3-kinase) and signaling effectors that lie downstream from it, in particular protein kinase B (PKB, also known as Akt) and atypical protein kinase C ζ/λ (aPKCs), play crucial roles in glucose homeostasis (6). PI 3-kinase–generated membrane phosphatidylinositol-3,4,5-triphosphates (PIP3s) recruit to the plasma membrane (PM) and activate both aPKCs and PKB/Akt (7,8). Once recruited, aPKCs are phosphorylated by a 3-phosphoinositide–dependent protein kinase-1 (PDK1) on their Thr410/403 site (9). On the other hand, binding of PIP3s to the pleckstrin homology (PH) domain of PKB/Akt induces conformational changes in the kinase that expose two regulatory sites, Thr308 and Ser473 (for PKBα/Akt1). Phosphorylation of Thr308 is mediated by PDK1 and Ser473 phosphorylation by TORC2 (mammalian target of rapamycin)-rictor (rapamycin-insensitive companion of mTOR) complex (10). The importance of the activation of aPKCs and PKB/Akt by insulin in mediating glucose metabolism is now well documented in insulin-sensitive tissues. Mice lacking PKBβ (Akt2) become insulin resistant and develop severe diabetes (11), and recently, Farese et al. (12) have demonstrated the importance of PKCλ in skeletal muscle by selectively ablating this kinase in a mouse model. They showed that these mice developed insulin resistance, reduced glucose tolerance, and dyslipidemia, all common features of the metabolic syndrome.
A consensus now exists that PKB/Akt is the primary target of ceramide. Indeed, defects in activation of this kinase induced by ceramide have been observed in cell types, such as white and brown adipocytes, skeletal and smooth muscles, mammary cells, and nerve cells (13). In some cells, ceramide acts on PKB/Akt through the direct activation of phosphatases such as the protein phosphatase-2A (PP2A) (14), a cytosolic serine/threonine phosphatase responsible for dephosphorylating PKB/Akt (15). Treatment of several cell types such as C2C12 muscle cells, PC12 nerve cells, and brown adipocytes with the PP2A inhibitor okadaic acid (OKA) (16) prevents the negative effects of ceramide on PKB/Akt (13). However, in L6 muscle cells and white adipose tissue, we and others have shown that ceramide inhibited insulin-stimulated glucose transport through a mechanism that does not involve a phosphatase (17,18). Ceramide activates PKCζ (19,20), which interacts and phosphorylates the PH domain of PKB/Akt on a Thr34 residue, preventing PKB/Akt to be recruited and activated at the PM in response to insulin (19).
Thus, two mechanisms by which ceramide can inhibit PKB/Akt are described in different cell types. We hypothesized that a PKCζ- or PP2A-mediated action of ceramides might be dependent on the structure and compartmentalization of the PM that differs among cell types. It is now well recognized that the PM is not uniform but composed of subdomains with unique lipid compositions. In particular, specialized domains called caveolin-enriched domains (CEMs) have been shown to be important in mediating insulin action (21) and are enriched in ceramide (22,23). Moreover, we, as well as others, have shown that ceramide induced the recruitment of both PKCζ and PKB/Akt in CEMs (22,23). Thus, PKCζ- and PP2A-mediated mechanisms are likely to occur in different compartments. As a unifying hypothesis to explain why two different mechanisms by which ceramide inhibits PKB/Akt exist, we propose that the structure of the PM in different cell types, particularly the relative abundance of CEMs, might be a determining factor to direct the action of ceramide toward either the PKCζ or the PP2A mechanism. To test this hypothesis, we have used different PKCζ mutant constructs or OKA, to inhibit PKCζ- and PP2A-dependent pathways and evaluated the impact of both insulin and ceramide on PKB/Akt activity in CEMs and non-CEMs. Using cells with different levels of expression of caveolin, we demonstrated that ceramide switches from one mechanism to the other to inhibit the insulin activation of PKB/Akt.
RESEARCH DESIGN AND METHODS
Materials.
All reagent-grade chemicals, insulin, methyl-isobutylmethylxanthine, palmitate, dexamethasone, protein A–Sepharose 4B, and BSA were purchased from Sigma-Aldrich. C2-ceramide was obtained from Tocris and OKA from Calbiochem. Complete protein phosphatase inhibitor tablets were obtained from Boehringer-Roche Diagnostics. Antibodies against caveolin-1 were purchased from BD Biosciences; native PKB/Akt, Ser473-PKB, Thr308-PKB, Ser21/9–glycogen synthase kinase 3α/β (GSK3α/β), and Thr410/403-PKCζ/λ were from Cell Signaling (New England Biolabs); and Tyr307-PP2A, PP2A A-subunit, PKCζ, and hemagglutinin (HA) were from Santa Cruz Biotechnology. Horseradish peroxidase anti-rabbit, -mouse, and -sheep/goat IgGs were from Jackson ImmunoResearch Laboratories and the enhanced chemiluminescent substrate was from Pierce-Perbio Biotechnology. [3H]2-deoxy-d-glucose was obtained from PerkinElmer.
Preparation of recombinant adenovirus.
Adenovirus containing the cDNA of wild-type PKCζ (WT-PKCζ), myristoylated PKCζ (myr-PKCζ), or kinase-dead PKCζ (KD-PKCζ) (24) was prepared as previously described (25). All PKCζ constructs contain an HA tag for monitoring their expression. Caveolin-1 enhanced green fluorescent protein (EGFP) adenovirus was constructed according to He et al. (26). Fully differentiated adipocytes were infected with either construct at 150 multiplicity of infection.
Cell culture.
3T3-L1 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) and differentiated into mature adipocytes as described previously (22). Differentiated cells were usually harvested at days 10–12 after confluence. Human fibroblasts were cultured in DMEM/F-12 (Life Technologies) until confluence.
Preparation of whole-cell lysates.
Cells were lysed after experimental manipulation (see figure legends) in an appropriate volume of lysis buffer (27).
Preparation of detergent-resistant membranes.
Detergent-resistant membranes (DRMs) were prepared as described previously (22). Cells were homogenized into 25 mmol/l MES (2-[N-Morpholino]ethanesulfonic acid), pH 6.0, 150 mmol/l NaCl, 1% (wt/vol) Triton X-100, complete inhibitor tablet, and lysate ran on a sucrose gradient. The gradient was centrifuged at 120,000g for 20 h at 4°C. DRM fractions were then collected and frozen at −20°C until required.
Immunoblotting.
Cell lysates and membrane fractions were subjected to SDS-PAGE on polyacrylamide gels and immunoblotted as previously reported (27). Nitrocellulose membranes were probed with various antibodies as described in the figure legends. Detection of primary antibodies was performed using appropriate peroxidase-conjugated IgGs, and protein signals were visualized using enhanced chemiluminescence (Thermo Scientific Pierce) by exposure to Kodak autoradiographic film.
Plasma membrane lawns.
Adipocyte plasma membrane lawns were prepared as described (28). The PKCζ antibody was detected with cyanin 3 anti-rabbit antibody.
2-Deoxy-d-glucose uptake in 3T3-L1 adipocytes.
3T3-L1 adipocytes were incubated in serum-free DMEM 5 h before transport studies and then were exposed to 100 μmol/l C2-ceramide for 2 h, before being treated with 100 nmol/l insulin in the last 30 min. The uptake of glucose was assessed as described before (4).
HA-WT-PKCζ and PKB/Akt immunoprecipitation from 3T3-L1 adipocyte lysates.
HA-WT-PKCζ or PKB/Akt was immunoprecipitated from 500 μg of 3T3-L1 adipocyte lysates. Immunocomplexes were captured by incubation with protein A–agarose beads and solubilized in Laemmli sample buffer prior to SDS-PAGE and immunoblotting as described above.
Statistical analysis.
Statistical analysis was carried out using a Student t test. Data were considered statistically significant at P values ≤0.05.
RESULTS
Ceramide activates both PKCζ and PP2A in 3T3-L1 adipocytes.
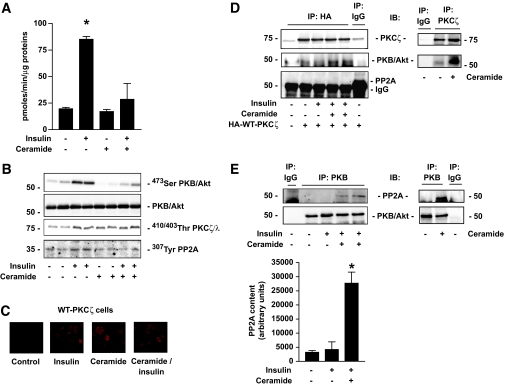
Figure 1A recapitulates insulin and ceramide effects on one of the most important end points of the insulin-signaling pathway in adipocytes. Glucose uptake in 3T3-L1 adipocytes was increased 4.5-fold after insulin treatment, an effect repressed in the presence of ceramide (Fig. 1A). It is well established that both ceramide and insulin act through PKB/Akt to mediate their actions (17,22). Indeed, 100 nmol/l insulin for 10 min induced the phosphorylation of PKB/Akt on its Ser473 site in 3T3-L1 adipocytes, and this effect is completely blunted by pretreating the cells with 100 μmol/l C2-ceramide for 2 h (Fig. 1B). To investigate the involvement of the PKCζ and PP2A in the effects of ceramide, we examined whether these proteins were activated in the presence of insulin and ceramide. 3T3-L1 adipocytes were infected with an adenovirus vector containing a WT-PKCζ construct tagged with HA. A weak membrane association in the basal state (Fig. 1C) was observed by confocal immunofluorescence microscopy of plasma membrane lawns. Both insulin and ceramide, added to the cells alone or together, induced an increase in the amount of PKCζ at the PM (Fig. 1C). As observed previously in L6 muscle cells (19), treatment of 3T3-L1 adipocytes with either 100 nmol/l insulin for 10 min or 100 μmol/l ceramide for 2 h induced the phosphorylation of PKCζ (using a PKCζ/λ antibody that recognizes the kinase phosphorylation sites Thr410/403 of both PKCζ and λ) (Fig. 1B). Insulin and ceramide added together had no additive effect on PKCζ/λ phosphorylation, indicating that these two factors targeted a similar pool of PKCζ/λ (Fig. 1B). It has been shown in fibroblasts that insulin inhibited the constitutive activity of PP2A by phosphorylating its catalytic subunit on a Tyr307 residue (29,30). In 3T3-L1 adipocytes, we observed a similar effect of the hormone (Fig. 1B). However, pretreatment of the cells with ceramide completely abolished the insulin-induced phosphorylation of PP2A (Fig. 1B), indicating that the phosphatase is fully active in the presence of ceramide. These results indicate that both PKCζ and PP2A are targets for insulin and ceramide in 3T3-L1 adipocytes.
FIG. 1.
Effects of insulin and ceramide on the insulin-signaling pathway in 3T3-L1 adipocytes. A: 3T3-L1 adipocytes were preincubated with 100 μmol/l C2-ceramide for 2 h and then with 100 nmol/l insulin for 30 min. 2-Deoxy-d-glucose uptake was then assessed as described in research design and methods. Values represent means ± SEM of three separate experiments. *Significant change from the control value (P < 0.05). B: 3T3-L1 adipocytes were preincubated with 100 μmol/l C2-ceramide for 2 h followed by 100 nmol/l insulin for the last 10 min. Cell lysates were immunoblotted with antibodies against either native PKB/Akt, Ser473 PKB/Akt, Thr410/403 PKCλ/ζ, or Tyr307 PP2A. C: HA-WT-PKCζ construct–infected 3T3-L1 adipocytes were treated with 100 μmol/l C2-ceramide for 2 h and then incubated with 100 nmol/l insulin for 10 min. Plasma membrane lawns were prepared as described in research design and methods and subjected to confocal fluorescent microscopy using an HA antibody. D and E: HA-WT-PKCζ–infected 3T3-L1 adipocytes were treated with 100 nmol/l insulin for 10 min, 100 μmol/l C2-ceramide for 2 h, and then with or without 100 nmol/l insulin for 10 min and lysed prior to immunoprecipitation of (D) HA or (E) PKB/Akt. HA and PKB/Akt immunoprecipitates were then immunoblotted for the presence of PKCζ, PP2A, and native PKB/Akt. Scanning densitometry was performed to quantify changes in PP2A abundance in PKB/Akt immunoprecipitates. Bars represent mean ± SEM. *Significant change P < 0.05 relative to the untreated control. Blots shown are representative of three separate experiments. IP, immunoprecipitation. (A high-quality color representation of this figure is available in the online issue.)
To find out whether both PKCζ and PP2A could associate with PKB/Akt in response to ceramide in our adipocyte culture system, coimmunoprecipitation experiments were performed. 3T3-L1 adipocytes were infected with an adenovirus expressing an HA-tagged WT-PKCζ construct. Infected cells were treated with insulin and ceramide, and WT-PKCζ was immunoprecipitated with an anti-HA antibody. Resulting immunocomplexes were analyzed by Western blotting for PKB/Akt. Figure 1D shows that, as observed before (22), ceramide, alone or combined with insulin, induced an increased association between PKCζ and PKB/Akt. As negative controls, the use of nonimmune serum or untransfected lysates did not detect any PKB/Akt in the immunoprecipitates (Fig. 1D). Interestingly, PP2A was absent from these immunocomplexes (Fig. 1D). Subsequently, we used the same cell extracts to immunoprecipitate PKB/Akt. Figure 1E shows that, whereas insulin did not induce any association between PP2A and PKB/Akt, ceramide, alone or in the presence of insulin, triggered it. Altogether, these results show that, in response to ceramide, both PKCζ and PP2A can associate with PKB/Akt, possibly targeting different pools of the kinase, because PP2A is not detected in a ceramide-induced PKCζ-PKB/Akt complex (Fig. 1D).
The inhibition of insulin-stimulated PKB/Akt by ceramide is mediated by PKCζ in 3T3-L1 adipocytes.
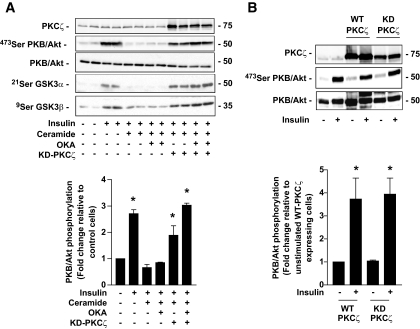
It is now well accepted that in 3T3-L1 adipocytes ceramide acts mainly, if not exclusively, on PKB/Akt through the PKCζ pathway (13,17). This is confirmed in Fig. 2, which shows that preincubation of 3T3-L1 adipocytes with ceramide, and then with insulin, led to a significant reduction in the ability of the hormone to phosphorylate PKB/Akt on its Ser473 residue. In line with previous work (17), treatment of the cells with 500 nmol/l OKA for the last 30 min of the ceramide incubation time had no effect on the action of the lipid on PKB/Akt. In contrast, infection of cells with an adenovirus-mediated transfer of an inactive dominant negative PKCζ mutant (KD-PKCζ) into these cells counteracted the action of ceramide on PKB/Akt, and on one of its physiological downstream targets, GSK3α/β (Fig. 2A). However, in contrast to data we previously obtained in muscle cells (19), overexpression of the KD-PKCζ in adipocytes did not affect the basal and insulin-stimulated PKB/Akt phosphorylation state compared with what was observed in untransfected adipocytes or in WT-PKCζ–overexpressing adipocytes (Fig. 2B). It is interesting to note, however, that the preventive effect of the KD-PKCζ on PKB/Akt was slightly potentiated in the presence of OKA (Fig. 2A). Overall, these data indicate that, whereas a PP2A activity is present in 3T3-L1 adipocytes, the involvement of the phosphatase to mediate ceramide action remains minimal.
FIG. 2.
Mechanism of ceramide action on insulin-induced phosphorylation of PKB/Akt in 3T3-L1 adipocytes. A: Control 3T3-L1 adipocytes and KD-PKCζ–infected cells were preincubated with 100 μmol/l C2-ceramide for 2 h, followed by 500 μmol/l OKA for the last 30 min. Then, 100 nmol/l insulin was added to the cells for 10 min before being lysed. Cell lysates were immunoblotted with antibodies against either native PKB/Akt, Ser473 PKB/Akt, Ser21/9 GSK3α/β, or PKCζ. Scanning densitometry was performed to quantify changes in Ser473 PKB/Akt abundance in cell lysates. Bars represent mean ± SEM. *Significant change P < 0.05 relative to the untreated control. Blots shown are representative of three separate experiments. B: Control, WT-PKCζ–, and KD-PKCζ–infected 3T3-L1 adipocytes were incubated with 100 nmol/l insulin for 10 min before being lysed. Cell lysates were immunoblotted with antibodies against either native PKB/Akt, Ser473 PKB/Akt, or PKCζ. Scanning densitometry was performed to quantify changes in Ser473 PKB/Akt abundance in cell lysates. Bars represent mean ± SEM. *Significant change P < 0.05 relative to the untreated control WT-PKCζ–expressing cells. Blots shown are representative of six separate experiments.
PKCζ mediates the ceramide inhibitory effect on PKB/Akt within CEMs.
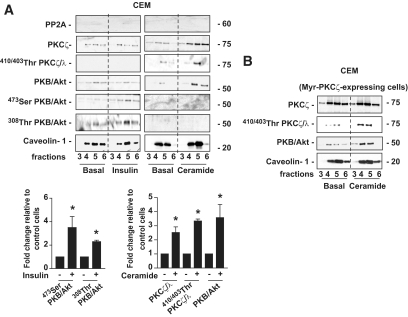
To understand why the PKCζ pathway predominates on the PP2A pathway in 3T3-L1 adipocytes, we investigated the compartmentalization of these two pathways within the cell membrane, keeping in mind that adipocytes are rich in CEM subdomains where ceramides are concentrated (23,31,32). Thus, we isolated lipid raft–containing CEMs by taking advantage of the capacity of lipid rafts to resist nonionic detergent solubilization at 4°C and purified these domains on discontinuous sucrose density gradient (22). Caveolin-1 enrichment is a characteristic of these domains that are usually recovered in the low-density fractions 3–6 of the sucrose gradient (22). In contrast to caveolin-1, PP2A was not found within CEMs at the basal state or after insulin or ceramide treatment (Fig. 3A). Insulin did not provoke any recruitment of WT-PKCζ to CEMs (Fig. 3A). Interestingly, as already described in H9c2 cardiomyoblasts (33), the total amount of PKB/Akt did not change in response to insulin in these subdomains and the hormone induced only a weak phosphorylation of the kinase on both Ser473 and Thr308 (Fig. 3A). In contrast to insulin, treatment of these cells with ceramide induced both the translocation and more than a threefold increase in the phosphorylation of WT-PKCζ on its Thr410 site, and the recruitment of PKB/Akt to these domains (Fig. 3A). These data confirmed that the activation of PKCζ by ceramide takes place in CEMs. However, it appears that PP2A is absent from these subdomains.
FIG. 3.
Differential effects of insulin and ceramide on the content of PKCζ, PKB/Akt, and PP2A in CEMs. A: 3T3-L1 adipocytes were treated with 100 μmol/l C2-ceramide for 2 h or 100 nmol/l insulin for 10 min before isolation of DRMs as described in research design and methods. Equal amounts of protein (1 μg) of the DRM-containing fractions (3–6 of the sucrose gradient) were then immunoblotted with antibodies against PKCζ, Thr410/403 PKCλ/ζ, native PKB/Akt, Ser473 PKB/Akt, Thr308 PKB/Akt, PP2A, and caveolin-1. Bands were quantified and expressed as fold increase over control. Bars represent mean ± SEM. *Significant change P < 0.05 relative to the untreated control. The blots shown are representative of at least three separate experiments. B: HA-myr-PKCζ–infected cells were treated with 100 μmol/l C2-ceramide for 2 h before isolation of DRMs as described in research design and methods. Equal amounts of protein (1 μg) of the DRM-containing fractions (3–6) were then immunoblotted with antibodies against PKCζ, Thr410/403 PKCλ/ζ, native PKB/Akt, and caveolin-1. These are representative of at least three independent experiments.
We used a membrane-associated myr-PKCζ mutant to understand whether the presence of a constitutive PKCζ into CEMs could mimic ceramide action. As expected, Fig. 3B showed that the myr-PKCζ construct was associated with the CEMs in the basal state and that ceramide could not induce a further increase in myr-PKCζ content in these domains (Fig. 3B). However, a minimal phosphorylation of the kinase was observed at the basal state and ceramide needed to be added to the cells to induce both myr-PKCζ phosphorylation and PKB/Akt recruitment to CEMs (Fig. 3B). In summary, these data show a crucial role for CEMs in the activation of PKCζ by ceramide and the subsequent sequestration of PKB/Akt.
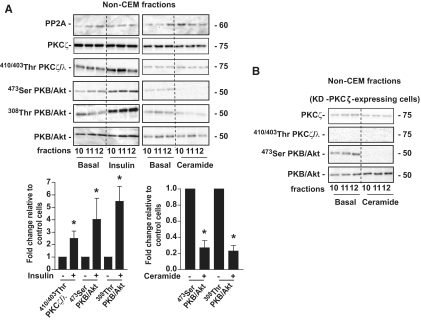
Ceramide activates PP2A outside CEMs.
The heavy non-CEM fractions (high-density fractions 10–12 of the sucrose gradient) do not contain caveolin-1 (data not shown) (22) and are a mixture of proteins contained in detergent-soluble cellular membranes and in the cytosol. In contrast to what was observed in CEMs, PP2A was detected in these fractions (Fig. 4A). In addition, both PKCζ and PKB/Akt were robustly phosphorylated in response to insulin in these fractions (Fig. 4A). The lack of phosphorylation of PKCζ by ceramide in these fractions (Fig. 4A) confirmed that this lipid is principally present in CEMs and acts on a different PKCζ intracellular pool. Surprisingly, a more than 70% decrease in PKB/Akt basal phosphorylation on its two sites was always observed in ceramide-treated fractions (Fig. 4A). This process was independent of PKCζ because the inactivation of PKB/Akt was still visible in cells overexpressing the KD-PKCζ mutant (Fig. 4B).
FIG. 4.
Differential effects of insulin and ceramide on the content of PKCζ, PKB/Akt, and PP2A in non-CEMs. A: 3T3-L1 adipocytes were treated with 100 μmol/l C2-ceramide for 2 h or 100 nmol/l insulin for 10 min before isolation of DRMs as described in research design and methods. Equal amounts of protein (1 μg) of the non–DRM-containing fractions (10–12 of the sucrose gradient) were then immunoblotted with antibodies against PKCζ, Thr410/403 PKCλ/ζ, native PKB/Akt, Ser473 PKB/Akt, Thr308 PKB/Akt, and PP2A. Bands were quantified and expressed as fold increase over control. Bars represent mean ± SEM. *Significant change P < 0.05 relative to the untreated control. The blots shown are representative of at least three separate experiments. B: KD-PKCζ–infected cells were treated with 100 μmol/l C2-ceramide for 2 h before isolation of non–CEM-containing fractions (10–12) as described in research design and methods. Equal amounts of protein (1 μg) of non–CEM-containing fractions from KD-PKCζ–infected cell lysates were immunoblotted with antibodies against PKCζ, Thr410/403 PKCλ/ζ, Ser473 PKB/Akt, native PKB/Akt, and caveolin-1. These are representative of at least three independent experiments.
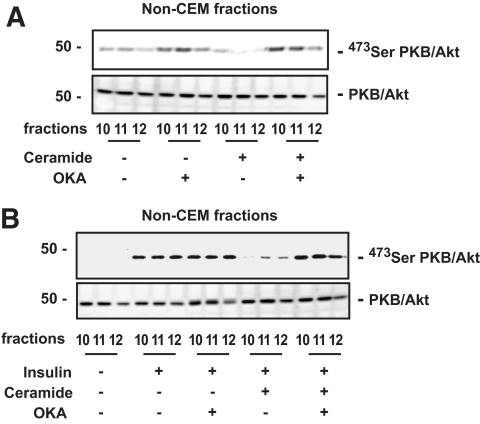
Given the presence of PP2A in these non-CEM fractions, we then decided to investigate whether a ceramide-activated PP2A mechanism could be responsible here for the inhibitory effect of ceramide on PKB/Akt by using OKA. OKA has previously been shown to inhibit PP2A activity and therefore increase PKB/Akt basal phosphorylation in adipocytes (15). We treated 3T3-L1 adipocytes with ceramide for 2 h and added 500 nmol/l OKA during the last 30 min of the incubation before assessing the basal phosphorylation state of PKB/Akt in non-CEM fractions. Figure 5A showed that incubation of 3T3-L1 adipocytes with OKA was able to reverse the inhibitory effect of ceramide on PKB/Akt basal phosphorylation and to induce the phosphorylation of the kinase to a level observed after OKA treatment alone, suggesting that the whole bulk of PP2A was inhibited in basal and ceramide-treated cells. Furthermore, OKA was also able to prevent an inhibitory effect of ceramide on insulin-induced PKB/Akt phosphorylation observed in non-CEM fractions (Fig. 5B). These results show that in non-CEMs, an active PP2A pathway for PKB/Akt inhibition exists in 3T3-L1 adipocytes. The fact that the PP2A pathway is only marginally involved in 3T3-L1 adipocytes when considering the global effect of ceramide in cells (Fig. 2) may be explained by the overabundance of CEMs in these cells.
FIG. 5.
Effect of OKA on PKB/Akt phosphorylation in non-CEMs. A: 3T3-L1 adipocytes were treated with 100 μmol/l ceramide for 2 h and/or with 500 nmol/l OKA for the last 30 min. Equal amounts of protein (1 μg) of the non–DRM-containing fractions (10–12) were then immunoblotted with antibodies against native PKB/Akt and Ser473 PKB/Akt. B: 3T3-L1 adipocytes were treated with 100 μmol/l ceramide for 2 h and with 500 nmol/l OKA for the last 30 min, followed by insulin for the last 10 min. Equal amounts of protein (1 μg) of the non–DRM-containing fractions (10–12) were then immunoblotted with antibodies against native PKB/Akt and Ser473 PKB/Akt. These are representative of at least three independent experiments.
The PP2A mechanism mediates ceramide action in cells lacking CEMs.
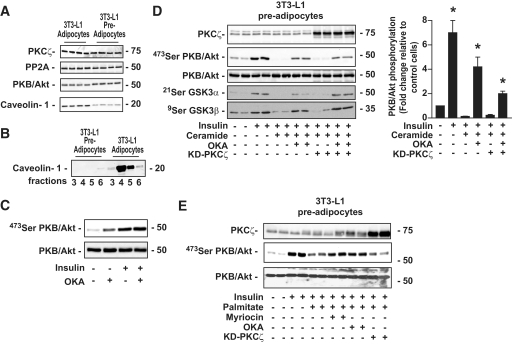
We have shown that both ceramide-activated PKCζ and PP2A pathways coexist in 3T3-L1 adipocytes, although in different membrane subdomains and only to a small extent for the latter. Our hypothesis is that the relative abundance of CEMs versus non-CEMs might determine the route by which ceramide acts on PKB/Akt. To test this hypothesis, we modulated the relative abundance of CEMs by manipulating caveolin-1 expression because this protein is a key structural component required for CEM formation (34). First, to test the effect of caveolin-1 downregulation, we used undifferentiated 3T3-L1 preadipocytes that expressed much lower quantities of caveolin-1 than fully differentiated 3T3-L1 adipocytes (35) (Fig. 6A). Total expressions of PKCζ, PP2A, and PKB/Akt did not differ significantly in differentiated and undifferentiated 3T3-L1 (Fig. 6A). As expected, Fig. 6B shows a near-complete absence of CEMs in 3T3-L1 preadipocytes compared with differentiated 3T3-L1 adipocytes. Interestingly, in these cells, OKA did not potentiate the insulin effect on PKB/Akt, suggesting that both insulin and OKA were targeting the same pool of PKB/Akt into the cell (Fig. 6C). In both adipocytes (Fig. 2) and preadipocytes (Fig. 6D), ceramide completely inhibited the Ser473 residue phosphorylation of PKB/Akt by insulin. However, unlike in adipocytes, treatment of the preadipocytes with OKA prevented the ceramide effect on PKB/Akt by 60%, whereas overexpression of the KD-PKCζ mutant did not change the ability of ceramide to suppress the hormonal activation of PKB/Akt (Fig. 6D). Identical results were observed on both GSK3 isoforms (Fig. 6D). Thus, decreasing the relative abundance of CEMs in adipocytes favors the ceramide-activated PP2A pathway over the ceramide-activated PKCζ pathway.
FIG. 6.
Effect of ceramide and palmitate on insulin-induced phosphorylation of PKB/Akt in 3T3-L1 preadipocytes. A: 3T3-L1 preadipocyte lysates were immunoblotted with antibodies against either PKCζ, PP2A, native PKB/Akt, or caveolin-1. B: CEMs prepared from 3T3-L1 preadipocytes were isolated as described in research design and methods. Equal amounts of protein (1 μg) were then immunoblotted with the antibody against caveolin-1. C: Preadipocytes were treated with 500 μmol/l OKA for 30 min and 100 nmol/l insulin for the last 10 min before being lysed. Cell lysates were immunoblotted with antibodies against either native PKB/Akt or Ser473 PKB/Akt. D: Control 3T3-L1 preadipocytes and KD-PKCζ–infected preadipocytes were preincubated with 100 μmol/l C2-ceramide for 2 h, followed by 500 μmol/l OKA for the last 30 min. Then, 100 nmol/l insulin was added to the cells for 10 min before being lysed. Cell lysates were immunoblotted with antibodies against either native PKB/Akt, Ser473 PKB/Akt, Ser21/9 GSK3α/β, and PKCζ. Scanning densitometry was performed to quantify changes in Ser473 PKB/Akt abundance in cell lysates. Bars represent mean ± SEM. *Significant change P < 0.05 relative to the untreated control. Blots shown represent three separate experiments. E: Control 3T3-L1 preadipocytes and KD-PKCζ–infected preadipocytes were preincubated with 0.75 mmol/l palmitate (conjugated with 0.2% [wt/vol] BSA) for 20 h. In some experiments, fatty acid incubation was also performed in the presence of 10 μmol/l myriocin. OKA (500 μmol/l) was added for the last 30 min and 100 nmol/l insulin for 10 min before being lysed. Cell lysates were immunoblotted with antibodies against either native PKB/Akt, Ser473 PKB/Akt, or PKCζ.
To further substantiate the physiological importance of ceramide on PKB/Akt, we reproduced the latter experiment using palmitate (Fig. 6E). Palmitate can produce long-chain ceramide de novo by a pathway that is dependent on serine palmitoyl transferase. Generated ceramide has been shown to promote the inactivation of the insulin-induced PKB/Akt activation by the same mechanism described above with short-chain ceramides (4). Figure 6E showed that incubation of preadipocytes with 0.75 mmol/l palmitate for 20 h downregulated the phosphorylation of PKB/Akt by insulin. Pretreatment of the cells with a serine palmitoyl transferase inhibitor, myriocin, completely prevented the inhibitory effect of palmitate on PKB/Akt (Fig. 6E), indicating that the lipid was acting on PKB/Akt through the synthesis of ceramides. As observed with C2-ceramide–treated cells, if OKA prevented the action of palmitate on PKB/Akt, overexpression of the KD-PKCζ mutant was not effective (Fig. 6E).
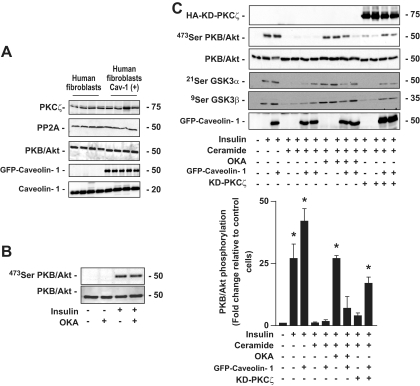
We then examined the effects of an increase of CEM relative abundance by overexpressing caveolin-1 in human fibroblasts. In contrast to adipocytes, only 3–5% of the PM of fibroblasts is of CEM origin (36), and it has been shown that overexpressing caveolin-1 could induce the formation of more of these membrane domains (34). We used a caveolin-1–GFP adenoviral construct that is known to retain the functional characteristics and intracellular distribution of the endogenous caveolin-1 (37). Caveolin-1–GFP overexpression in human fibroblasts did not change the endogenous expression of PKCζ, PP2A, and PKB/Akt (Fig. 7A). In human fibroblasts, like in preadipocytes, OKA did not increase further the phosphorylation of PKB/Akt by insulin, suggesting that both insulin and OKA act on the same pool of PKB/Akt (Fig. 7B). Caveolin-1 overexpression did not affect the ability of ceramide to inhibit insulin-induced activation of either PKB/Akt or GSK3α/β (Fig. 7C). In untransfected cells, OKA prevented ceramide from having an effect, pointing to involvement of a PP2A pathway, whereas in cells overexpressing caveolin-1, OKA was much less efficient (Fig. 7C). Conversely, although the KD-PKCζ had no effect on control fibroblasts, it was able to partially abolish ceramide effects in caveolin-1–overexpressing cells. Thus, the differential effects of both OKA and the KD-PKCζ in cells overexpressing caveolin-1 and in control cells underscore the importance of CEMs in supporting ceramide action via PKCζ. Furthermore, the results show that the lipid can switch from one mechanism to another, in order to act negatively on PKB/Akt, depending on the submembrane domain composition of the PM of the cells.
FIG. 7.
Effect of ceramide on insulin-induced phosphorylation of PKB/Akt in control human fibroblast versus caveolin-1–overexpressing human fibroblast. A: Control human fibroblast and caveolin-1–overexpressing human fibroblast lysates were immunoblotted with antibodies against either PKCζ, PP2A, native PKB/Akt, GFP, or caveolin-1. B: Human fibroblasts were treated with 500 μmol/l OKA for 30 min and 100 nmol/l insulin for the last 10 min before being lysed. Cell lysates were immunoblotted with antibodies against either native PKB/Akt or Ser473 PKB/Akt. C: Control human fibroblast and caveolin-1–overexpressing human fibroblast were preincubated with 100 μmol/l C2-ceramide for 2 h, followed by 500 μmol/l OKA for the last 30 min. Then, 100 nmol/l insulin was added to the cells for 10 min before being lysed. Cell lysates were immunoblotted with antibodies against native PKB/Akt, Ser473 PKB/Akt, Ser21/9 GSK3α/β, PKCζ, and GFP. Scanning densitometry was performed to quantify changes in Ser473 PKB/Akt abundance in cell lysates. Bars represent mean ± SEM. *Significant change, P < 0.05 relative to the untreated control. Blots shown represent three to four separate experiments.
DISCUSSION
In the present study, we investigated the mechanisms by which the sphingolipid-derived second messenger ceramide induced insulin resistance. As it is established that ceramide does not act directly on its PKB/Akt target (17,18), two distinct mechanisms have been proposed to mediate the inhibitory action of the lipid, one involving PKCζ and the other PP2A. Although not mutually exclusive, it was unclear, until now, why one mechanism was favored over the other in a given cell type. Our present data provide an explanation for the alternative use of these pathways toward PKB/Akt inhibition by ceramide. We demonstrate that compartmentalization of the PM into subdomains, and particularly the abundance of CEMs, is a crucial determinant of the pathway used by ceramide to inhibit PKB/Akt phosphorylation. We show that in CEM-enriched PM, ceramide acts through the recruitment of both PKCζ and PKB/Akt within these membrane domains, whereas in cells with low caveolin expression, hence basically devoid of these domains, ceramide inhibits PKB/Akt mainly via PP2A.
The PM has been shown to be heterogeneous in its lipid composition, and some lipid species, such as cholesterol or sphingolipids, are known to segregate into specific subdomains called lipid rafts that form more ordered and less fluid regions within the PM (38). One specific subset of lipid rafts, called CEMs, is composed of membrane regions characterized by the presence of caveolins. These CEMs are believed to act as platforms for conducting a variety of cellular functions by recruiting or excluding specific signaling molecules and also regulating the accessibility of these proteins to other regulatory or effector molecules (38). CEM abundance is largely determined by the level of caveolin expression that is detectable in most cell types but is very abundant in adipocytes and endothelial cells where caveolae invaginations can occupy nearly 30% of the cell surface (39). Ceramide has been shown to accumulate largely in these domains (40), but it is as yet unclear how they could modulate signaling. The inability of ceramide to transfer rapidly between lipid bilayers (41) implies that downstream signaling targets with which ceramide interacts could be recruited to the PM, rather than ceramide itself being translocated internally. One of the best targets of ceramide to be characterized in vitro has been PKCζ (42,43), and very recently a specific protein fragment of the kinase has been demonstrated to bind ceramide (44). Here, we have shown that whereas ceramide directs PKCζ to CEMs to be activated specifically in response to the lipid, insulin promotes the recruitment of PKCζ outside these membrane subdomains where the kinase is activated through phosphorylation by PDK1 (45). Our results suggest that the whole insulin-signaling process occurs outside these CEMs. Submembrane compartmentalization of PKCζ, depending on the stimulus, could explain the two opposite roles that this kinase plays to regulate the insulin-signaling pathway (positive with insulin and negative with ceramide). Some studies suggest that CEMs could also play a role in insulin signaling. Indeed, contradictory data have been published, some showing that insulin receptor and GLUT4 could be localized in CEMs (46), whereas others did not (47,48). More work will be necessary to solve this discrepancy.
We have also shown that the PP2A pathway for ceramide inhibition of PKB/Akt takes place outside the CEMs of the PM. In agreement with this, one study performed in 3T3-L1 adipocytes has shown that ceramide-activated PP2A was able to dephosphorylate a cytosolic insulin-stimulated PKB/Akt construct lacking its PH domain (49). Our present data, in which the endogenous form of PKB/Akt has been studied, demonstrate that at least in adipocytes, a ceramide-stimulated PP2A pathway coexists with the dominant ceramide-activated PKCζ pathway. The fact that the overexpression of the KD-PKCζ mutant did not completely prevent the ceramide effect on PKB/Akt in adipocytes could be explained by the fact that 1) the lack of PKCζ activity in these cells amplified the PP2A pathway activated by ceramide, and 2) the transfection efficiency of adipocytes is known to be quite poor (50). By manipulating caveolin-1 expression levels, we demonstrated that the relative abundance of CEMs within the PM represents a switch for one pathway at the expense of the other. The PP2A pathway is operating only in preadipocytes or fibroblasts with low CEM abundance and to a much smaller extent in CEM-containing cells such as adipocytes. Our previous results on mice adipocytes treated with ceramide suggested a similar shift from a PKCζ-dependent machinery in wild-type adipocytes to a mechanism involving PP2A in caveolin-1 knockout adipocytes (22). Indeed, adipocytes lacking CEMs, although partly resistant to ceramide action, still displayed a 60% reduction in insulin-induced PKB/Akt phosphorylation, suggesting that the ceramide-activated PP2A pathway substituted for the one involving PKCζ in these cells.
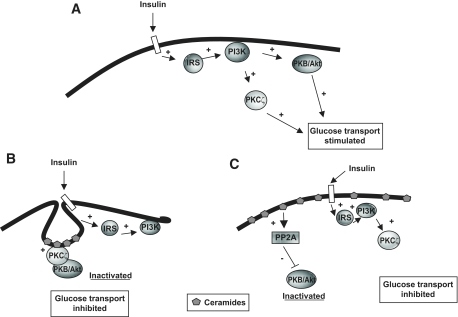
In summary, this study points to the importance of membrane composition and CEM abundance to determine the molecular mechanisms by which ceramide inhibits insulin-stimulated PKB/Akt. In adipocytes, the stimulation of glucose transport by insulin requires the activation of both PKCζ and PKB/Akt (Fig. 8A). In these cells, the preferential sublocalization of ceramide within abundant CEMs drives its negative action on PKB/Akt nearly exclusively through the mechanism involving PKCζ (Fig. 8B). However, in cells lacking CEMs, ceramide inhibits insulin-activated PKB/Akt through its dephosphorylation by PP2A (Fig. 8C). These data highlight that redundant pathways exist within cells to mediate negative actions of ceramide on insulin sensitivity. This clearly indicates that targeted approaches to a single pathway (either PKCζ or PP2A) would not be efficient strategies to fight ceramide-induced insulin resistance. Instead, efforts to prevent ceramide accumulation in insulin-sensitive tissues would be a more accurate approach.
FIG. 8.
Proposed model recapitulating mechanisms by which ceramides prevent the activation of PKB/Akt by insulin, depending on the PM composition. A: Binding of insulin to its receptor at the plasma membrane promotes the activation of both PKB/Akt and PKCζ via tyrosine phosphorylation of IRS proteins and activation of PI 3-kinase. The concomitant increase in PIP3s (not shown) facilitates PKB/Akt and PKCζ recruitment to the plasma membrane where they are activated by their upstream kinases. Both PKB/Akt and PKCζ activation is crucial for the stimulation of glucose transport in adipocytes. B: In adipocytes, an increase in CEM ceramide content induces the activation of PKCζ within CEMs and promotes the association between PKCζ and PKB/Akt in these domains. PKB/Akt is held in a repressed state within these membrane domains and is unable to support the hormonal activation of glucose transport. C: In cells lacking CEMs, ceramide activates directly the PP2A phosphatase that dephosphorylates insulin-activated PKB/Akt. In these cells, PP2A represses insulin-stimulated glucose transport by preventing PKB/Akt to be phosphorylated by its upstream kinases.
ACKNOWLEDGMENTS
This work was supported by INSERM. C.M.B. was supported by the French ministry for research.
No potential conflicts of interest relevant to this article were reported.
Part of the study was presented at the 44th annual meeting of the European Association for the Study of Diabetes, Rome, Italy, 7–11 September 2008.
We thank Isabelle Hainault, Soazig Le Lay, and Xavier Le Liepvre (INSERM U872) for their expert technical support. We are grateful to Dr. Alex Toker (Harvard Medical School, Boston, MA) for providing myr-PKCζ, KD-PKCζ, and WT-PKCζ constructs and Dr. Lucas Pelkmans (Institute of Molecular Systems Biology, Zürich, Switzerland) for the caveolin-1 EGFP plasmid.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Sesti G: Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab 2006; 20: 665– 679 [DOI] [PubMed] [Google Scholar]
- 2.Schmitz-Peiffer C: Signalling aspects of insulin resistance in skeletal muscle: mechanisms induced by lipid oversupply. Cell Signal 2000; 12: 583– 594 [DOI] [PubMed] [Google Scholar]
- 3.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA: Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007; 5: 167– 179 [DOI] [PubMed] [Google Scholar]
- 4.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS: Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J 2004; 382: 619– 629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White MF: IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 2002; 283: E413– E422 [DOI] [PubMed] [Google Scholar]
- 6.Hajduch E, Litherland GJ, Hundal HS: Protein kinase B (PKB/Akt): a key regulator of glucose transport? FEBS Lett 2001; 492: 199– 203 [DOI] [PubMed] [Google Scholar]
- 7.Mora A, Komander D, van Aalten DM, Alessi DR: PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 2004; 15: 161– 170 [DOI] [PubMed] [Google Scholar]
- 8.Litherland GJ, Hajduch E, Hundal HS: Intracellular signalling mechanisms regulating glucose transport in insulin-sensitive tissues (Review). Mol Membr Biol 2001; 18: 195– 204 [DOI] [PubMed] [Google Scholar]
- 9.Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A: Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol 1998; 8: 1069– 1077 [DOI] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM: Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307: 1098– 1101 [DOI] [PubMed] [Google Scholar]
- 11.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG: Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 2003; 112: 197– 208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M: Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 2007; 117: 2289– 2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holland WL, Summers SA: Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 2008; 29: 381– 402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA: Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem 1993; 268: 15523– 15530 [PubMed] [Google Scholar]
- 15.Resjö S, Göransson O, Härndahl L, Zolnierowicz S, Manganiello V, Degerman E: Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell Signal 2002; 14: 231– 238 [DOI] [PubMed] [Google Scholar]
- 16.Haystead TA, Sim AT, Carling D, Honnor RC, Tsukitani Y, Cohen P, Hardie DG: Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature 1989; 337: 78– 81 [DOI] [PubMed] [Google Scholar]
- 17.Summers SA, Garza LA, Zhou H, Birnbaum M: Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. J Mol Cell Biol 1998; 18: 5457– 5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS: Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia 2001; 44: 173– 183 [DOI] [PubMed] [Google Scholar]
- 19.Powell DJ, Hajduch E, Kular G, Hundal HS: Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol 2003; 23: 7794– 7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourbon NA, Yun J, Kester M: Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem 2000; 275: 35617– 35623 [DOI] [PubMed] [Google Scholar]
- 21.Pilch PF, Souto RP, Liu L, Jedrychowski MP, Berg EA, Costello CE, Gygi SP: Cellular spelunking: exploring adipocyte caveolae. J Lipid Res 2007; 48: 2103– 2111 [DOI] [PubMed] [Google Scholar]
- 22.Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS: Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J 2008; 410: 369– 379 [DOI] [PubMed] [Google Scholar]
- 23.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M: Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 2007; 282: 12450– 12457 [DOI] [PubMed] [Google Scholar]
- 24.Romanelli A, Martin KA, Toker A, Blenis J: p70 S6 kinase is regulated by protein kinase Czeta and participates in a phosphoinositide 3-kinase-regulated signalling complex. Mol Cell Biol 1999; 19: 2921– 2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera JC, Vasavada RC, Stewart AF: Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem 2003; 278: 343– 351 [DOI] [PubMed] [Google Scholar]
- 26.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B: A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 1998; 95: 2509– 2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajduch E, Alessi DR, Hemmings BA, Hundal HS: Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 1998; 47: 1006– 1013 [DOI] [PubMed] [Google Scholar]
- 28.Kanzaki M, Mora S, Hwang JB, Saltiel AR, Pessin JE: Atypical protein kinase C (PKCzeta/lambda) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J Cell Biol 2004; 164: 279– 290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Parsons S, Brautigan DL: Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem 1994; 269: 7957– 7962 [PubMed] [Google Scholar]
- 30.Sontag E: Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal 2001; 13: 7– 16 [DOI] [PubMed] [Google Scholar]
- 31.Patra SK: Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta 2008; 1785: 182– 206 [DOI] [PubMed] [Google Scholar]
- 32.van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J: Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J 2003; 369: 199– 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha H, Pak Y: Modulation of the caveolin-3 and Akt status in caveolae by insulin resistance in H9c2 cardiomyoblasts. Exp Mol Med 2005; 37: 169– 178 [DOI] [PubMed] [Google Scholar]
- 34.Fra AM, Williamson E, Simons K, Parton RG: De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A 1995; 92: 8655– 8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blouin CM, Le Lay S, Lasnier F, Dugail I, Hajduch E: Regulated association of caveolins to lipid droplets during differentiation of 3T3-L1 adipocytes. Biochem Biophys Res Commun 2008; 376: 331– 335 [DOI] [PubMed] [Google Scholar]
- 36.Guillot FL, Audus KL, Raub T: Fluid-phase endocytosis by primary cultures of bovine brain microvessel endothelial cell monolayers. J Microvasc Res 1990; 39: 1– 14 [DOI] [PubMed] [Google Scholar]
- 37.Pelkmans L, Kartenbeck J, Helenius A: Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol 2001; 3: 473– 483 [DOI] [PubMed] [Google Scholar]
- 38.Simons K, Ikonen E: Functional rafts in cell membranes. Nature 1997; 387: 569– 572 [DOI] [PubMed] [Google Scholar]
- 39.Kandror KV, Stephens JM, Pilch PF: Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J Cell Biol 1995; 129: 999– 1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Megha LE: Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem 2004; 279: 9997– 10004 [DOI] [PubMed] [Google Scholar]
- 41.Simon CG, Jr, Holloway PW, Gear AR: Exchange of C(16)-ceramide between phospholipid vesicles. Biochemistry 1999; 38: 14676– 14682 [DOI] [PubMed] [Google Scholar]
- 42.Lozano J, Berra E, Municio MM, Diaz-Meco MT, Dominguez I, Sanz L, Moscat J: Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem 1994; 269: 19200– 19202 [PubMed] [Google Scholar]
- 43.Müller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K: PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J 1995; 14: 1961– 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang G, Krishnamurthy K, Umapathy NS, Verin AD, Bieberich E: The carboxyl-terminal domain of atypical protein kinase C zeta binds to ceramide and regulates junction formation in epithelial cells. J Biol Chem 2009; 284: 14469– 14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker P: Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. J Science 1998; 281: 2042– 2045 [DOI] [PubMed] [Google Scholar]
- 46.Bickel PE: Lipid rafts and insulin signaling. Am J Physiol Endocrinol Metab 2002; 282: E1– E10 [DOI] [PubMed] [Google Scholar]
- 47.Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL: The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3–L1 adipocytes. Proc Natl Acad Sci U S A 2007; 104: 1242– 1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Souto RP, Vallega G, Wharton J, Vinten J, Tranum-Jensen J, Pilch PF: Immunopurification and characterization of rat adipocyte caveolae suggest their dissociation from insulin signaling. J Biol Chem 2003; 278: 18321– 18329 [DOI] [PubMed] [Google Scholar]
- 49.Stratford S, Hoehn KL, Liu F, Summers SA: Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 2004; 279: 36608– 36615 [DOI] [PubMed] [Google Scholar]
- 50.Boizard M, Le Liepvre X, Lemarchand P, Foufelle F, Ferré P, Dugail I: Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J Biol Chem 1998; 273: 29164– 29171 [DOI] [PubMed] [Google Scholar]