Abstract
OBJECTIVE
High concentrations of circulating glucose are believed to contribute to defective insulin secretion and β-cell function in diabetes and at least some of this effect appears to be caused by glucose-induced β-cell apoptosis. In mammalian cells, apoptotic cell death is controlled by the interplay of proapoptotic and antiapoptotic members of the Bcl-2 family. We investigated the apoptotic pathway induced in mouse pancreatic islet cells after exposure to high concentrations of the reducing sugars ribose and glucose as a model of β-cell death due to long-term metabolic stress.
RESEARCH DESIGN AND METHODS
Islets isolated from mice lacking molecules implicated in cell death pathways were exposed to high concentrations of glucose or ribose. Apoptosis was measured by analysis of DNA fragmentation and release of mitochondrial cytochrome c.
RESULTS
Deficiency of interleukin-1 receptors or Fas did not diminish apoptosis, making involvement of inflammatory cytokine receptor or death receptor signaling in glucose-induced apoptosis unlikely. In contrast, overexpression of the prosurvival protein Bcl-2 or deficiency of the apoptosis initiating BH3-only proteins Bim or Puma, or the downstream apoptosis effector Bax, markedly reduced glucose- or ribose-induced killing of islets. Loss of other BH3-only proteins Bid or Noxa, or the Bax-related effector Bak, had no impact on glucose-induced apoptosis.
CONCLUSIONS
These results implicate the Bcl-2 regulated apoptotic pathway in glucose-induced islet cell killing and indicate points in the pathway at which interventional strategies can be designed.
Type 2 diabetes develops when insulin-resistant subjects develop pancreatic β-cell dysfunction (1–3). Progressive β-cell dysfunction results in insufficient insulin secretion to compensate for insulin resistance. The relative contribution of a decrease in β-cell mass versus a functional defect in insulin secretion toward the overall morbidity remains unclear. Using human pancreatic tissue from autopsies, Butler et al. showed that there was an ∼60% reduction in β-cell mass in type 2 diabetic patients compared with nondiabetic control subjects, and this was attributed to a 10-fold or threefold increase in β-cell apoptosis in type 2 diabetic patients who were lean or obese, respectively (4). Although the cause of this apoptosis is not yet clear, glucose, saturated fatty acids, islet amyloid polypeptides, and interleukin (IL)-1β have all been implicated, and these molecules are toxic to β-cells and β-cell lines in vitro.
High concentrations of glucose can cause β-cell apoptosis and, in addition to a potential role in β-cell dysfunction in type 2 diabetes (2), high circulating glucose concentrations may also contribute to destruction of the remaining β-cells at the time of diagnosis of type 1 diabetes or when the β-cell mass in an islet transplant is marginal. β-cell apoptosis attributed to glucose toxicity has been observed in several animal models of type 2 diabetes including the Psammomys obesus desert gerbil (5), the Zucker diabetic fatty rat (6), and the domestic cat (7). Isolated islets from P. obesus are susceptible to glucose-dependent DNA fragmentation (5).
Several mechanisms for glucose-induced islet toxicity have been proposed. In human islets, it has been suggested that glucose induces intraislet production of IL-1β, leading to nuclear factor-κB activation, Fas upregulation, and β-cell apoptosis as a consequence of engagement by Fas ligand (FasL), expressed on neighboring β-cells (8–10). However, these findings could not be reproduced in other studies (11,12), leading to alternative mechanisms being suggested. β-cells are vulnerable to endoplasmic reticulum (ER) stress due to their enormous demand to synthesize and secrete insulin, and high glucose levels may exacerbate this (reviewed in [13]). High concentrations of reducing sugars were also reported to induce intracellular peroxides that elicit β-cell death (14). The expression of intrinsic antioxidant enzymes is normally quite low in β-cells (15), and adenoviral overexpression of Gpx-1 prevented glucose-induced apoptosis (14). Glucose induced expression of the proapoptotic factor thioredoxin-interacting protein, which inhibits the redox-active protein thioredoxin and, when overexpressed, induces caspase 3–dependent β-cell apoptosis (16). Glucose also promoted degradation of cyclic AMP-responsive element binding protein (CREB) by the ubiquitin-proteasome pathway leading to β-cell apoptosis (17).
In mammalian cells, two distinct pathways control apoptosis, the “death receptor” (also called extrinsic) and the “mitochondrial” (also called intrinsic or Bcl-2 regulated) pathways. In the intrinsic pathway, the eight BH3-only proteins (Bim, Bid, Bad, Puma, Noxa, Hrk, Bik, and Bmf) initiate apoptosis signaling by binding to the Bcl-2–like prosurvival proteins (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1), thereby releasing Bax and/or Bak to promote loss of mitochondrial outer membrane potential, cytochrome c release, and activation of the caspase cascade (18). Direct activation of Bax and/or Bak by certain BH3-only proteins has also been proposed (19). Exposure of human islets to 16.5 mmol/l glucose in vitro for 5 days resulted in upregulation of Bid and Bad and downregulation of Bcl-xL, resulting in the death of β-cells (20). We have shown that Bid deficiency prevents FasL–induced β-cell apoptosis (21), whereas Bcl-2 overexpression protects β-cells from a range of apoptotic stimuli including treatment with proinflammatory cytokines, FasL, or staurosporine (21,22). Mice deficient in Bad were shown to have impaired glucose homeostasis due to defective insulin secretion in response to glucose stimulation (23), but this was not due to abnormalities in β-cell apoptosis. Bim is transcriptionally upregulated by C/EBP homologous protein (CHOP) and required for ER stress–induced apoptosis in a range of cell types (24). Puma has also been implicated in ER stress–induced apoptosis in certain cell types (25). The requirement for these molecules has not yet been examined in β-cells.
Understanding how glucose toxicity triggers islet cell apoptosis is likely to shed new light on mechanisms of β-cell loss in diabetes and may therefore pave the way to improved therapeutic intervention. We have investigated the pathways of apoptosis induced in islet cells by high concentrations of reducing sugars. We found that IL-1 receptor (IL-1R) and Fas are dispensable for this process, as was Bid, which we have shown is required for extrinsic pathway apoptosis in islet cells. In contrast, Bcl-2 overexpression, loss of the BH3-only proteins Bim or Puma, or loss of the multi-BH domain protein Bax markedly protected islets from glucose toxicity.
RESEARCH DESIGN AND METHODS
IL-1R–deficient mice, generated on a mixed C57BL/6×129SV background (using 129SV-derived embryonic stem [ES] cells), were obtained from Dr. M. Labow (Roche) and backcrossed onto the C57BL/6 genetic background for eight generations (26). C57BL/6 mice and Fas-deficient Faslpr/lpr mice on a C57BL/6 genetic background were obtained from the Walter and Eliza Hall Institute animal breeding facility (Kew, Victoria, Australia). Mice globally deficient for bim, puma, noxa, bid, bax, or bak have been previously described (27–31). Mice deficient for puma, noxa, or bid were generated on an inbred C57BL/6 background using C57BL/6-derived ES cells. Mice deficient for bim, bax, or bak were originally generated on a mixed C57BL/6×129SV genetic background using 129SV-derived ES cells and were backcrossed for >10 generations onto the C57BL/6 background. H-2bm1 RIP-Bcl-2 transgenic mice, which express human Bcl-2 in β-cells under control of the rat insulin promoter, have previously been described (22). All animal experiments were approved by the institutional animal ethics committee.
Reagents.
d-glucose and l-glucose (used at 33.3 mmol/l) and d-ribose and l-ribose (used at 50 mmol/l) were purchased from Invitrogen (Gibco products Invitrogen, Grand Island, NY) and Sigma-Aldrich (St Louis, MO), respectively. The concentration of d-glucose or d-ribose and the time of incubation were titrated to determine optimal culture conditions. The concentrations we used were similar to those used in previously published reports (9,14,32). The pan-caspase inhibitor qVD.oph (Enzyme Systems Products, Livermore, CA) was used at 50 μmol/l. Recombinant murine γ-interferon (IFNγ) was obtained from Genentech (San Francisco, CA) and used at 100 units/ml. Human recombinant interleukin-1β from R&D Systems (Minneapolis, MN) was used at 150 units/ml.
Preparation of islets.
Islets of Langerhans were isolated by collagenase P (dissolved in Hanks' balanced salt solution containing 2 mmol/l Ca2+ and 20 mmol/l HEPES) digestion and density gradient centrifugation as described previously (33). Islets were washed, hand-picked, and cultured overnight at 37°C in 5% CO2 in CMRL medium-1066 (Gibco products Invitrogen) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mmol/l glutamine, and 10% FCS (JRH Biosciences, Lenexa, KS) (referred to below as complete CMRL). We did not observe any differences in number or size of islets isolated from mice deficient in Bcl-2 family genes, suggesting that at a global level, deficiency of these genes does not affect islet development (data not shown).
DNA fragmentation assay.
Uniformly sized islets (excluding very large or necrotic islets) were handpicked into 3.5-cm Petri dishes containing 1.1 ml of complete CMRL. Islets were then cultured with the appropriate stimuli to induce apoptosis. At the end of the culture period, nonattached cells and islets were transferred into polypropylene tubes and washed in PBS. Islets were then dispersed with trypsin (0.1 mg/ml bovine trypsin [Calbiochem] and 2 mmol/l EDTA in PBS) for 5 min at 37°C. Islets were mechanically dispersed using a pipette, washed in PBS, and allowed to recover in complete CMRL medium for 1 h at 37°C in 5% CO2. Cells were then washed in PBS and resuspended in 250 μl of hypotonic buffer containing 50 μg/ml propidium iodide (Sigma-Aldrich), 0.1% sodium citrate, and 0.1% Triton X-100, which stains nuclear DNA. The cells were then analyzed on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) using the FL3 channel. Cells undergoing apoptosis were identified by their apparent subdiploid DNA content as previously described (34).
Cytochrome c release assay.
Islets were dispersed into single cells using trypsin, and cytochrome c release was measured using the method previously described (35). Briefly, cells were permeabilized in 100 μl digitonin buffer (80 mmol/l KCl, 50 ng/ml digitonin, and 1 mmol/l EDTA in PBS) for 2 min on ice. Permeabilized cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature, washed in PBS, and incubated in blocking buffer (3% BSA and 0.05% saponin in PBS) for 1 h. Cells were then stained overnight at 4°C with mouse anti–cytochrome c antibody (clone 6H2B4; BD Pharmingen) followed by staining with phycoerythrin-conjugated sheep anti-mouse IgG antibodies (Silenus Laboratories, Hawthorn, Australia) in blocking buffer for 1 h at room temperature. Cells were analyzed on a FACSCalibur. The control samples had a typical background between 5 and 10% cytochrome c release. This is consistent with published reports using this method (35).
Real-time quantitative RT-PCR analysis.
RNA was prepared using the RNeasy Kit (QIAGEN). First-strand cDNA was prepared from 0.1–0.2 μg RNA using the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems). Real-time PCR was performed using the ABI Prism 7900 (Applied Biosystems) and the Power SYBR Green PCR Master Mix (Applied Biosystems) in 15-μl reaction volumes. Data analyses were performed with the fluorescence threshold CT method using actin as an internal control. Quantitative (q) RT-PCR was performed using the forward and reverse primers in Table 1.
TABLE 1.
PCR primers used for gene expression analysis
| Gene | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|
| bak | CTCTCATCGGAGATGATATTAACCG | AGTATGATATCAGCCAAAAAGCAGG |
| bax | GGAGATGAACTGGATAGCAATATGG | GTTTGCTAGCAAAGTAGAAGAGGGC |
| bim | GAGTTGTGACAAGTCAACACAAACC | GAAGATAAAGCGTAACAGTTGTAAGATAACC |
| puma | ATGCCTGCCTCACCTTCATCT | AGCACAGGATTCACAGTCTGGA |
| actin | TATTGGCAACGAGCGGTTC | CCATACCCAAGAAGGAAGGCT |
Western blotting.
Islets were incubated for 4 days with cytokines and/or 33.3 mmol/l glucose or 50 mmol/l ribose, washed with PBS, and lysed in 10 μl of lysis buffer (20 mmol/l Tris/HCl [pH 7.4], 135 mmol/l NaCl, 1.5 mmol/l MgCl2, 1 mmol/l EGTA, 1% Triton X-100, 1× protease inhibitor cocktail [Sigma-Aldrich], and 1× phosphatase inhibitor cocktail [Sigma-Aldrich]). Samples were separated by SDS-PAGE and transferred to nitrocellulose using standard procedures. Western blotting was performed with anti-NOS2 antibody (Ab; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Bim Ab (clone 3C5; Alexis, Plymouth Meeting, PA), anti-Puma Ab (ProSci, Poway, CA), and anti-actin Ab (Santa Cruz Biotechnology) followed by horseradish peroxidase–conjugated secondary antibodies (Silenus Laboratories) and detection with ECL Western blotting detection system (GE Healthcare).
Nitrite determination.
Nitrite was detected in the cultures by mixing 100 μl supernatant with 100 μl Griess reagent (36). Absorbances were read at 540 nm, and nitrite concentrations were calculated using a sodium nitrite standard curve.
Statistical analysis.
Statistical analyses of data were performed using GraphPad Prism (GraphPad Software, San Diego, CA). All data shown as bar graphs are represented as means ± SEM. Data were analyzed by one-way or two-way ANOVA with Bonferroni post test for comparison of multiple columns, or by two-tailed paired t tests.
RESULTS
Abnormally high concentrations of glucose or ribose induce DNA fragmentation in islet cells.
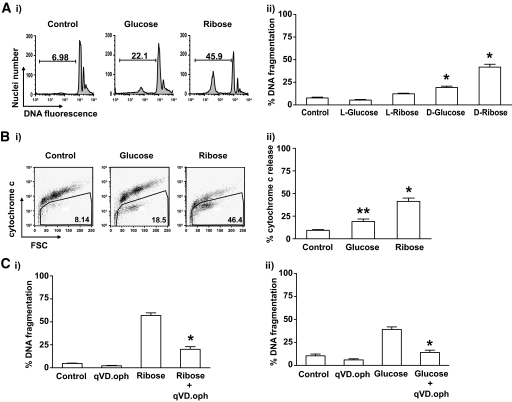
Islets from wild-type C57BL/6 mice were exposed to 33.3 mmol/l glucose or 50 mmol/l ribose in vitro, and DNA fragmentation was examined by fluorescence-activated cell sorter (FACS) analysis as a measure of apoptosis. Wild-type islets cultured in high concentrations of d-glucose for 6 days displayed a significant increase in DNA fragmentation compared with islets cultured in medium containing only 5.6 mmol/l glucose (Fig. 1A), or those cultured in medium with 33.3 mmol/l l-glucose (osmolarity control). d-Ribose mimics the effects of glucose exposure (14), and it killed islet cells more rapidly, and to a greater extent, than high glucose (Fig. 1A).
FIG. 1.
High concentrations of glucose or ribose induce DNA fragmentation, cytochrome c release, and caspase activation in islets. Islets (100–200) from wild-type mice were cultured in complete medium containing 5.5 mmol/l d-glucose (control) or were exposed to 33.3 mmol/l d-or l-glucose or 50 mmol/l d-or l-ribose. A: DNA fragmentation was measured by flow cytometry after culture for 6 days in glucose or 4 days in ribose. i: Representative FACS profiles are shown. ii: Data represent islets from a minimum of five individual mice per group. Statistical significance: *P < 0.0001 compared with control (one-way ANOVA). B: Cytochrome c release was measured by flow cytometry after 4 days of incubation in control medium or in glucose, or 3 days in ribose. i: Representative FACS profiles are shown. ii: Data represent islets from 4–8 individual mice per group. Statistical significance: *P < 0.0001 **P < 0.01 compared with control (one-way ANOVA). C: DNA fragmentation of islet cells after incubation for (i) 4 days in ribose or (ii) 6 days in glucose in the presence or absence of the caspase inhibitor qVD.oph was measured by flow cytometry. Data represent islets from 4–5 individual mice per group. Statistical significance: *P < 0.0001 compared with ribose or glucose treatment (one-way ANOVA).
To confirm that glucose toxicity triggered apoptosis in islet cells, we examined release of cytochrome c from the mitochondria, an event that occurs upstream of internucleosomal DNA fragmentation. High glucose concentrations promoted release of cytochrome c from the mitochondria (Fig. 1B). Consistent with the analysis of DNA fragmentation, high concentrations of ribose triggered mitochondrial cytochrome c release more rapidly and to a greater extent than treatment with glucose (Fig. 1B). The pan-caspase inhibitor qVD.oph significantly inhibited ribose-induced islet cell DNA fragmentation (Fig. 1C), confirming that cell killing occurred through a caspase-dependent apoptotic process.
Islets deficient in IL-1 receptors or Fas are not protected from glucose-induced DNA fragmentation.
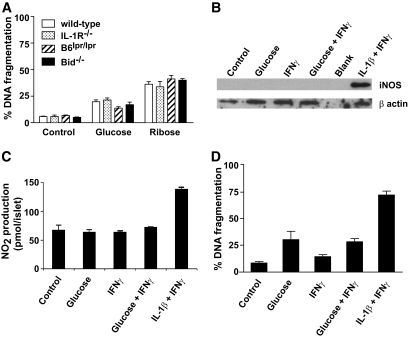
Previous studies have suggested that high glucose concentrations result in IL-1β production by β-cells, leading to Fas upregulation and autocrine or paracrine FasL-Fas–induced apoptosis signaling (9,10). To determine the contribution of this proposed cell death pathway to glucose-induced β-cell killing, DNA fragmentation was examined in islets from mice lacking IL-1 receptors (IL-1R−/−) or functional Fas (Faslpr/lpr). In response to culture in 50 mmol/l ribose or 33.3 mmol/l glucose, DNA fragmentation was comparable among islets from wild-type, IL-1R−/−, or Faslpr/lpr mice (Fig. 2A). In addition, loss of the BH3-only protein Bid, which we have shown to be essential for death receptor–mediated islet cell apoptosis (21), also failed to protect islets from ribose or glucose toxicity (Fig. 2A). These results suggest that IL-1R and Fas are not involved in glucose/ribose-induced killing of murine islets.
FIG. 2.
IL-1R or Fas is not required for glucose- or ribose-induced islet cell apoptosis. A: Islets (200) from wild-type, IL-1R−/−, Faslpr/lpr, or bid−/− mice were cultured for 6 days in control medium or medium containing 33.3 mmol/l glucose or for 4 days in medium containing 50 mmol/l ribose. DNA fragmentation was measured by flow cytometry. No significant differences were observed among mice of the different genotypes; P = 0.1403 (one-way ANOVA). Data are representative of at least three independent experiments using a minimum of three individual mice of each genotype. B–D: Islets were cultured for 7 days in control medium or 33.3 mmol/l glucose with cytokines added in the last 4 days. The data in B–D represent islets from three independent experiments. B: Western blotting for iNOS and β-actin as a loading control. C: NO2 concentration in culture supernatant was determined. D: DNA fragmentation was measured by flow cytometry. No significant difference in NO2 production or DNA fragmentation was observed between islets cultured with glucose and those cultured with glucose + IFNγ.
Incubation of mouse islets with recombinant IL-1β and IFNγ leads to the induction of inducible nitric oxide synthase (iNOS) expression, NO2 production, and DNA fragmentation. If high concentrations of glucose induce functional concentrations of intraislet IL-1β production, then these downstream effects should be measurable. Therefore, we tested whether 33.3 mmol/l glucose together with IFNγ could induce iNOS expression, NO2 production, and DNA fragmentation. Our results show that although recombinant IL-1β and IFNγ were able to induce iNOS expression, as well as a significant increase in NO2 production and DNA fragmentation, no iNOS expression, NO2 production, or DNA fragmentation was observed in islets cultured in 33.3 mmol/l glucose in the presence of IFNγ (Fig. 2B–D). Together these data rule out a role for IL-1 in glucose toxicity of mouse islets.
Bcl-2 overexpression protects islet cells from glucose or ribose toxicity–induced cytochrome c release and DNA fragmentation.
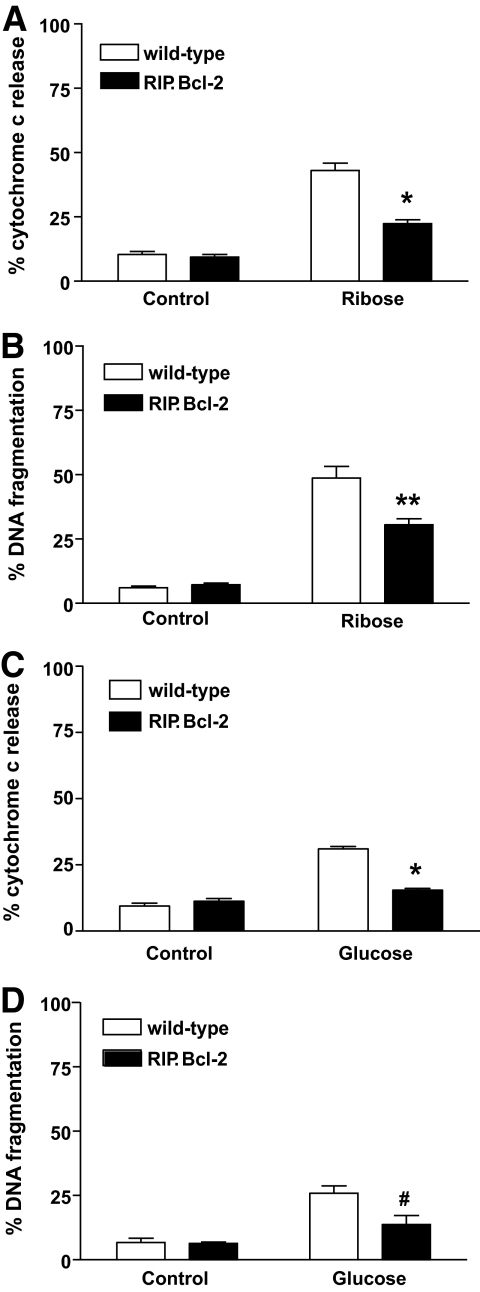
Disruption of the mitochondria and release of cytochrome c are controlled by the Bcl-2 family of proteins (37). Therefore, if cytochrome c release represents a critical point in toxicity of islets to high concentrations of ribose or glucose, overexpression of prosurvival Bcl-2 family members, such as Bcl-2 itself, should inhibit this apoptotic pathway. We therefore treated islets overexpressing Bcl-2 in β-cells and control islets from nontransgenic animals with high concentrations of ribose or glucose and compared their susceptibility to apoptosis. Indeed, overexpression of Bcl-2 reduced cytochrome c release from the mitochondria and significantly inhibited DNA fragmentation triggered by exposure to 50 mmol/l ribose (Fig. 3A and B). Similarly, overexpression of Bcl-2 substantially reduced cytochrome c release and DNA fragmentation in islets cultured in medium containing 33.3 mmol/l glucose (Fig. 3C and D).
FIG. 3.
Bcl-2 overexpression protects islets from glucose- or ribose-induced apoptosis. Islets (100) from wild-type or RIP.Bcl-2 transgenic mice were cultured in control medium or medium containing 50 mmol/l ribose or 33.3 mmol/l glucose. A: Cytochrome c release was measured by flow cytometry after 3 days of culture. Data represent islets from five individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 50 mmol/l ribose (one-way ANOVA). B: DNA fragmentation was measured by flow cytometry after culture for 4 days with 50 mmol/l ribose. Data represent islets from 5–7 individual mice per genotype. Statistical significance: **P < 0.001 compared with wild-type islets in 50 mmol/l ribose (one-way ANOVA). C: Cytochrome c release was measured by flow cytometry after 4 days of culture. Data represent islets from four individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 33.3 mmol/l glucose (one-way ANOVA). D: DNA fragmentation was measured by flow cytometry after 6-day culture in 33.3 mmol/l glucose. Data represents islets from 4–6 individual mice per genotype. Statistical significance: #P < 0.01 compared with wild-type islets in 33.3 mmol/l glucose (one-way ANOVA).
Loss of Bax but not Bak protects islets from glucose- or ribose-induced DNA fragmentation.
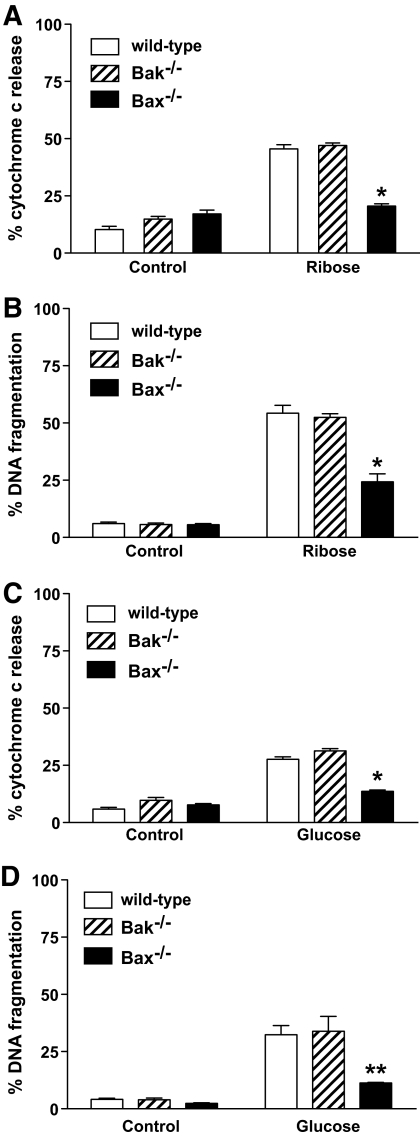
The multi-BH domain proapoptotic Bcl-2 family members Bax and Bak are essential for mitochondrial outer membrane permeabilization during apoptosis signaling and play essential and in many cell types overlapping roles in the activation of the execution phase of apoptosis (30). We tested whether loss of either of these proteins would result in protection from high concentrations of ribose or glucose. Bax-deficient islets were resistant to ribose- or glucose-induced cytochrome c release and DNA fragmentation, whereas islets from Bak-deficient mice were normally sensitive (Fig. 4). This result demonstrates that Bax plays a critical role in glucose- or ribose-induced islet killing.
FIG. 4.
Loss of Bax but not loss of Bak protects islet cells from ribose- or glucose-induced cytochrome c release and DNA fragmentation. Islets (100) from wild-type, bak−/−, or bax−/− mice were cultured in control medium or medium containing 50 mmol/l ribose or 33.3 mmol/l glucose. A: Cytochrome c release was measured by flow cytometry after 3 days of culture in ribose. Data represent islets from three individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 50 mmol/l ribose (one-way ANOVA). B: DNA fragmentation was measured by flow cytometry after 4 days of culture in ribose. Data represent islets from six individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 50 mmol/l ribose (one-way ANOVA). C: Cytochrome c release was measured by flow cytometry after 4 days of culture in glucose. Data represent islets from four individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 33.3 mmol/l glucose (one-way ANOVA). D: DNA fragmentation was measured by flow cytometry after 6-day culture in glucose. Data represent islets from three individual mice per genotype. Statistical significance: **P < 0.001 compared with wild-type islets in 33.3 mmol/l glucose (one-way ANOVA).
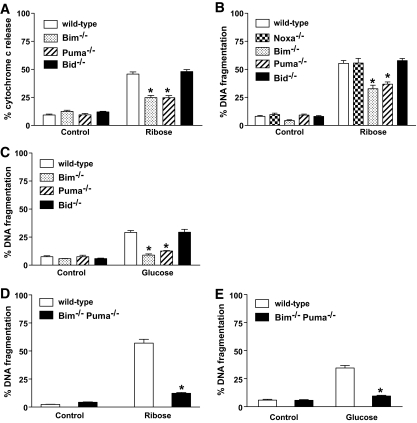
Individual loss of the BH3-only proteins Bim or Puma inhibits glucose or ribose toxicity–induced mitochondrial cytochrome c release and DNA fragmentation.
The observation that Bcl-2 overexpression or loss of Bax protects islet cells from glucose toxicity demonstrated that the mitochondrial pathway of apoptosis is involved, and indicated that it is initiated by members of the BH3-only subgroup of the Bcl-2 family. We therefore isolated islets from a panel of BH3-only–deficient mice (Bim−/−, Noxa−/−, Puma−/−, or Bid−/−) to examine their sensitivity to glucose or ribose toxicity. Deficiency of either Bim or Puma reduced cytochrome c release (Fig. 5A) and DNA fragmentation (Fig. 5B) from the mitochondria to a similar extent as overexpression of Bcl-2. In contrast, loss of the BH3-only proteins Noxa or Bid had no impact (Fig. 5A–C).
FIG. 5.
Loss of Bim or Puma protects islets from glucose- or ribose-induced mitochondrial cytochrome c release and DNA fragmentation. Islets (100) from wild-type, bim−/−, noxa−/−, puma−/−, bid−/−, or bim−/−puma−/− mice were cultured in control medium or medium containing 50 mmol/l ribose or 33.3 mmol/l glucose. A: Cytochrome c release was measured by flow cytometry after 3 days of culture in ribose. Data represent islets from a minimum of three individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 50 mmol/l ribose (one-way ANOVA). B: DNA fragmentation was measured by flow cytometry after 4 days of culture in ribose. Data represent islets from a minimum of three individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 50 mmol/l ribose (one-way ANOVA). C: DNA fragmentation was measured by flow cytometry after 6 days of culture in glucose. Data represent islets from a minimum of three individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 33.3 mmol/l glucose (one-way ANOVA). D: DNA fragmentation was measured by flow cytometry after 4 days of culture in ribose. Data represent islets from 3–4 individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 50 mmol/l ribose (one-way ANOVA). E: DNA fragmentation was measured after 6 days of culture in glucose. Data represent 4–5 individual mice per genotype. Statistical significance: *P < 0.0001 compared with wild-type islets in 33.3 mmol/l glucose (one-way ANOVA).
BH3-only proteins often cooperate to induce apoptosis; for example, it has been shown that combined loss of Bim and Puma protects lymphoid and myeloid cells more potently against a range of apoptotic stimuli, such as cytokine deprivation, than loss of either BH3-only protein alone (38). Because apoptosis induced by ribose or glucose toxicity was only partially inhibited by loss of either Bim or Puma, we examined the possibility that these proteins cooperate in glucose-or ribose-mediated apoptosis. Remarkably, the extent of apoptosis induced by 50 mmol/l ribose (Fig. 5D) or 33.3 mmol/l glucose (Fig. 5E) in islets deficient in both Bim and Puma was not significantly above the background level observed in islets cultured in control medium, and significantly lower than apoptosis seen in islets lacking only Bim or Puma or those overexpressing Bcl-2 (compare Fig. 5B and D). These data demonstrate that Bim and Puma are both activated and cause glucose or ribose toxicity–induced apoptosis in islet cells.
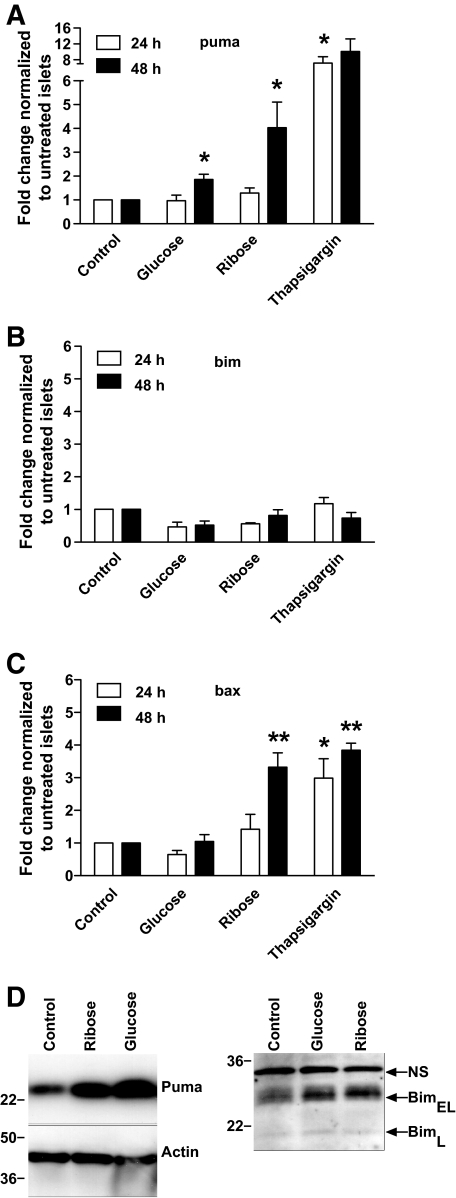
Glucose and ribose toxicity cause upregulation of Puma and Bax mRNA in islet cells.
The ability of ribose or glucose to induce expression of Bim, Puma, Bax, and Bak was measured by quantitative RT-PCR. After 48-h exposure to 50 mmol/l ribose, expression of puma and bax increased fourfold (Fig. 6A–C). Very low levels of bak were detectable, and these did not change after exposure to glucose or ribose (data not shown). Incubation with 5 μmol/l thapsigargin, which induces apoptosis of islets (39), induced a twofold to sevenfold increase in expression of puma, bax, (Fig. 6A–C) and bak (data not shown), but not bim. Bim expression remained unchanged, even though this basal level of bim was clearly sufficient to cause apoptosis in response to ribose incubation. We used PCR primers that detect BimL and BimEL, but not BimS, so we cannot exclude the possibility that BimS is playing a role. We also determined protein expression by Western blotting. Consistent with the qRT-PCR data, we observed an increase in Puma expression after incubation with 33.3 mmol/l glucose or 50 mmol/l ribose, but no change in Bim expression or phosphorylation (Fig. 6D). We therefore surmise that the basal level of Bim, or a modification that is not detectable by Western blotting, is critical for glucose toxicity–induced killing of β-cells.
FIG. 6.
Ribose or glucose induces expression of Puma and Bax in islets. Quantitative RT-PCR of wild-type islets cultured in control medium or medium containing 50 mmol/l ribose, 33.3 mmol/l glucose, or 5 μmol/l thapsigargin for 24 or 48 h. Relative RNA expression levels for (A) Puma, (B) Bim, and (C) Bax were calculated by normalizing to the signal for β-actin in each sample and comparison with islets cultured in control medium. Means ± SEM of 3–4 independent experiments is shown. Statistical significance: *P < 0.05, **P < 0.01 compared with control (two-tailed paired t test). D: Western blotting was performed with antibodies to Bim, Puma, and β-actin. Arrows indicate bands corresponding to BimEL and BimL. NS is a nonspecific band that serves as a loading control for the Bim immunoblot. Phosphorylation of Bim results in a shift of the BimEL and BimL bands to slightly greater molecular weight. Results are representative of three independent experiments.
DISCUSSION
We have exposed pancreatic islets to high concentrations of glucose or ribose in vitro as a model of the long-term metabolic stress on β-cells observed in insulin resistance and diabetes. We found conclusive evidence that excessive glucose can cause apoptosis in islet cells by the mitochondrial (also called intrinsic or Bcl-2-regulated) pathway. Glucose or ribose caused release of cytochrome c and DNA fragmentation that are both hallmarks of apoptosis mediated by the mitochondrial pathway of cell death (40). The ability of overexpression of the antiapoptotic protein Bcl-2 to partially inhibit β-cell death confirms the involvement of this pathway. Altered expression of Bcl-2 family genes has also been observed after exposure of human islets to high concentrations of glucose, also implicating the intrinsic pathway in glucose toxicity (17,20).
Mitochondrial outer membrane disruption and effector caspase activation are initiated by activation of the proapoptotic BH3-only Bcl-2 family members (37,41). BH3-only family members are activated in a death stimulus–and cell type–specific manner, and the ability to group stimuli that trigger apoptosis by the same pathway may help us to understand the mechanisms of cellular responses (42). We found that glucose and ribose toxicity–induced apoptosis requires the BH3-only proteins Bim and Puma and the multi-BH domain protein Bax, whereas deficiency of other proapoptotic proteins, including Bid and Bak, had no impact. In previous studies that examined processes that have been implicated in β-cell apoptosis in type 1 diabetes, including those triggered by perforin/granzyme B, FasL, and tumor necrosis factor, the BH3-only protein Bid was required (21,43). Therefore, glucose and ribose toxicity trigger a distinct apoptotic cascade in islet cells from that triggered by either perforin plus granzyme B or death receptor ligation. This makes it unlikely that high concentrations of glucose kill murine islet cells by the death receptor pathway or a process involving IL-1R signaling. Moreover, Bcl-2 overexpression or caspase inhibition provide minimal protection from cytokine-mediated islet cell death (44,45). This adds further weight to the notion that this pathway and glucose toxicity–induced apoptosis are distinct.
Both the ER stress and oxidative stress pathways have been associated with the toxic effects of glucose and ribose on islets (13,46). Both of these stress pathways have also been reported to activate BH3-only proteins, particularly Bim and Puma, in other cell types. In certain epithelial cells, thymocytes and macrophages, treatment with thapsigargin or tunicamycin caused ER stress–mediated apoptosis that required the BH3-only protein Bim (24). In contrast, in neurons Puma was found to be critical for apoptosis induced by oxidative or ER stress (25,47). Our results demonstrate that Bim and Puma have critical overlapping roles in glucose toxicity–induced killing of islets. This is reminiscent of the finding that combined loss of these two BH3-only proteins protects lymphoid cells against a range of death stimuli, such as cytokine deprivation, more potently than loss of either alone (38). It is possible that glucose toxicity induces Bim in islet cells predominantly by triggering ER stress. In other cell types, ER stress–induced activation of the transcriptional activator CHOP activates Bim, which is essential for ER stress–induced apoptosis (24). Because we found no upregulation of bim mRNA in islets exposed to high concentrations of glucose or ribose, we surmise that CHOP may be critical for the basal level of bim expression. This may relate to the fact that due to the high demand for protein synthesis and secretion, β-cells are in a constant state of ER stress. Although we were unable to detect evidence of posttranslational modification of Bim by Western blotting, we cannot exclude the possibility that glucose and ribose toxicity activate Bim posttranslationally. The mechanisms by which glucose or ribose toxicity activates Puma are currently not clear, but they may also involve the ER stress pathway.
Perhaps surprisingly, we found that loss of Bax but not loss of Bak protects islet cells from glucose toxicity. This is reminiscent of another study with β-cells and one with neuronal cells, both of which found that Bax but not Bak is critical for their Myc-induced or oxidative stress–induced apoptosis, respectively, although both proteins are expressed (47,48). In the case of glucose or ribose toxicity–induced islet killing, it remains possible that Bak does cooperate with Bax in this pathway to apoptosis because loss of Bax afforded considerably less protection than combined loss of Bim and Puma.
In conclusion, we have shown that high concentrations of glucose and ribose cause apoptosis mediated by the mitochondrial pathway in islets. This occurs by specific pathways distinct from those involved in cytokine- or death receptor–mediated apoptosis. There is increasing evidence that β-cell mass and β-cell susceptibility to apoptosis are key determinants in transition from insulin resistance to diabetes (49), and in mouse models, blocking islet cell apoptosis can prevent type 2 diabetes (50,51). Identification of Bim, Puma, and Bax as critical mediators in glucose-induced β-cell killing pinpoints possible targets for therapeutic intervention in this disease.
ACKNOWLEDGMENTS
This work was supported by fellowships and grants from the National Health and Medical Research Council of Australia (NHMRC) (NHMRC program 461221; NHMRC Career Development Awards to H.E.T. and C.L.S.; NHMRC Australia fellowship to A.S.), the National Institutes of Health (CA-043540–18 and CA-80188–6), and a joint special program grant from the Juvenile Diabetes Research Foundation/NHMRC (program 466658).
No potential conflicts of interest relevant to this article were reported.
We thank J.M. Adams, S. Cory, A. Villunger, and M. Labow for gene-targeted mice; M. Narita for qRT-PCR primers; G. Siciliano, N. Iannarella, A. Gomes, and S. Fynch for animal care; and R. Ayala-Perez, B. Helbert, C. Youngadn, and C. Dobrzelak for technical assistance and genotyping.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Muoio DM, Newgard CB: Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9: 193– 205 [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Nolan CJ: Islet beta cell failure in type 2 diabetes. J Clin Invest 2006; 116: 1802– 1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes CJ: Type 2 diabetes—a matter of beta-cell life and death? Science 2005; 307: 380– 384 [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 5.Donath MY, Gross DJ, Cerasi E, Kaiser N: Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 1999; 48: 738– 744 [DOI] [PubMed] [Google Scholar]
- 6.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS: Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 1998; 47: 358– 364 [DOI] [PubMed] [Google Scholar]
- 7.Zini E, Osto M, Franchini M, Guscetti F, Donath MY, Perren A, Heller RS, Linscheid P, Bouwman M, Ackermann M, Lutz TA, Reusch CE: Hyperglycaemia but not hyperlipidaemia causes beta cell dysfunction and beta cell loss in the domestic cat. Diabetologia 2009; 52: 336– 346 [DOI] [PubMed] [Google Scholar]
- 8.Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY: Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 2007; 56: 2356– 2370 [DOI] [PubMed] [Google Scholar]
- 9.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY: Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002; 110: 851– 860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY: Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 2001; 50: 1683– 1690 [DOI] [PubMed] [Google Scholar]
- 11.Elouil H, Cardozo AK, Eizirik DL, Henquin JC, Jonas JC: High glucose and hydrogen peroxide increase c-Myc and haeme-oxygenase 1 mRNA levels in rat pancreatic islets without activating NFkappaB. Diabetologia 2005; 48: 496– 505 [DOI] [PubMed] [Google Scholar]
- 12.Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, Eizirik DL: Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 2005; 54: 3238– 3244 [DOI] [PubMed] [Google Scholar]
- 13.Eizirik DL, Cardozo AK, Cnop M: The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008; 29: 42– 61 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Tran PO, Harmon J, Robertson RP: A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A 2002; 99: 12363– 12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiedge M, Lortz S, Drinkgern J, Lenzen S: Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997; 46: 1733– 1742 [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A: Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes 2008; 57: 938– 944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costes S, Vandewalle B, Tourrel-Cuzin C, Broca C, Linck N, Bertrand G, Kerr-Conte J, Portha B, Pattou F, Bockaert J, Dalle S: Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in beta-cells and human pancreatic islets. Diabetes 2009; 58: 1105– 1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC: Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 2007; 315: 856– 859 [DOI] [PubMed] [Google Scholar]
- 19.Green DR, Chipuk JE: Apoptosis: stabbed in the BAX. Nature 2008; 455: 1047– 1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F: High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes 2001; 50: 1290– 1301 [DOI] [PubMed] [Google Scholar]
- 21.McKenzie MD, Carrington EM, Kaufmann T, Strasser A, Huang DC, Kay TW, Allison J, Thomas HE: Proapoptotic BH3-only protein Bid is essential for death receptor-induced apoptosis of pancreatic beta-cells. Diabetes 2008; 57: 1284– 1292 [DOI] [PubMed] [Google Scholar]
- 22.Allison J, Thomas H, Beck D, Brady JL, Lew AM, Elefanty A, Kosaka H, Kay TW, Huang DC, Strasser A: Transgenic overexpression of human Bcl-2 in islet beta cells inhibits apoptosis but does not prevent autoimmune destruction. Int Immunol 2000; 12: 9– 17 [DOI] [PubMed] [Google Scholar]
- 23.Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ: Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med 2008; 14: 144– 153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A: ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007; 129: 1337– 1349 [DOI] [PubMed] [Google Scholar]
- 25.Kieran D, Woods I, Villunger A, Strasser A, Prehn JH: Deletion of the BH3-only protein puma protects motoneurons from ER stress-induced apoptosis and delays motoneuron loss in ALS mice. Proc Natl Acad Sci U S A 2007; 104: 20606– 20611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW: Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol 1997; 159: 2452– 2461 [PubMed] [Google Scholar]
- 27.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A: Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735– 1738 [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A: The BH3-only protein bid is dispensable for DNA damage-and replicative stress-induced apoptosis or cell-cycle arrest. Cell 2007; 129: 423– 433 [DOI] [PubMed] [Google Scholar]
- 29.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ: Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995; 270: 96– 99 [DOI] [PubMed] [Google Scholar]
- 30.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB: The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389– 1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A: p53-and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036– 1038 [DOI] [PubMed] [Google Scholar]
- 32.Bensellam M, Van Lommel L, Overbergh L, Schuit FC, Jonas JC: Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate-and high-glucose concentrations. Diabetologia 2009; 52: 463– 476 [DOI] [PubMed] [Google Scholar]
- 33.McKenzie MD, Dudek NL, Mariana L, Chong MM, Trapani JA, Kay TW, Thomas HE: Perforin and Fas induced by IFNgamma and TNFalpha mediate beta cell death by OT-I CTL. Int Immunol 2006; 18: 837– 846 [DOI] [PubMed] [Google Scholar]
- 34.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C: A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991; 139: 271– 279 [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse NJ, Steel R, Kluck R, Trapani JA: Assaying cytochrome C translocation during apoptosis. Methods Mol Biol 2004; 284: 307– 313 [DOI] [PubMed] [Google Scholar]
- 36.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR: Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982; 126: 131– 138 [DOI] [PubMed] [Google Scholar]
- 37.Strasser A: The role of BH3-only proteins in the immune system. Nat Rev Immunol 2005; 5: 189– 200 [DOI] [PubMed] [Google Scholar]
- 38.Erlacher M, Labi V, Manzl C, Böck G, Tzankov A, Häcker G, Michalak E, Strasser A, Villunger A: Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 2006; 203: 2939– 2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrington EM, McKenzie MD, Jansen E, Myers M, Fynch S, Kos C, Strasser A, Kay TW, Scott CL, Allison J: Islet beta-cells deficient in Bcl-xL develop but are abnormally sensitive to apoptotic stimuli. Diabetes 2009; 58: 2316– 2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youle RJ, Strasser A: The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47– 59 [DOI] [PubMed] [Google Scholar]
- 41.Huang DC, Strasser A: BH3-Only proteins-essential initiators of apoptotic cell death. Cell 2000; 103: 839– 842 [DOI] [PubMed] [Google Scholar]
- 42.Puthalakath H, Strasser A: Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 2002; 9: 505– 512 [DOI] [PubMed] [Google Scholar]
- 43.Estella E, McKenzie MD, Catterall T, Sutton VR, Bird PI, Trapani JA, Kay TW, Thomas HE: Granzyme B-mediated death of pancreatic beta-cells requires the proapoptotic BH3-only molecule bid. Diabetes 2006; 55: 2212– 2219 [DOI] [PubMed] [Google Scholar]
- 44.Rabinovitch A, Suarez-Pinzon W, Strynadka K, Ju Q, Edelstein D, Brownlee M, Korbutt GS, Rajotte RV: Transfection of human islets with an anti-apoptotic gene (bcl-2) protects beta-cells from cytokine-induced destruction. Diabetes 1999; 48: 1223– 1229 [DOI] [PubMed] [Google Scholar]
- 45.Sutherland RM, Allison J, Thomas HE, Brady JL, Kay TW, Lew AM: Bcl-2 protection of islet allografts is unmasked by costimulation blockade. Transplantation 2004; 77: 1610– 1613 [DOI] [PubMed] [Google Scholar]
- 46.Robertson RP: Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 2004; 279: 42351– 42354 [DOI] [PubMed] [Google Scholar]
- 47.Steckley D, Karajgikar M, Dale LB, Fuerth B, Swan P, Drummond-Main C, Poulter MO, Ferguson SS, Strasser A, Cregan SP: Puma is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J Neurosci 2007; 27: 12989– 12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dansen TB, Whitfield J, Rostker F, Brown-Swigart L, Evan GI: Specific requirement for Bax, not Bak, in Myc-induced apoptosis and tumor suppression in vivo. J Biol Chem 2006; 281: 10890– 10895 [DOI] [PubMed] [Google Scholar]
- 49.Weir GC, Bonner-Weir S: Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004; 53( Suppl. 3): S16– S21 [DOI] [PubMed] [Google Scholar]
- 50.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M: Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 2002; 109: 525– 532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ: Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 2008; 118: 3378– 3389 [DOI] [PMC free article] [PubMed] [Google Scholar]