Abstract
OBJECTIVE
Diabetic retinopathy is associated with progressive retinal capillary activation and proliferation, leading to vision impairment and blindness. Microparticles are submicron membrane vesicles with biological activities, released following cell activation or apoptosis. We tested the hypothesis that proangiogenic microparticles accumulate in vitreous fluid in diabetic retinopathy.
RESEARCH DESIGN AND METHODS
Levels and cellular origin of vitreous and plasma microparticles from control (n = 26) and diabetic (n = 104) patients were analyzed by flow cytometry, and their proangiogenic activity was assessed by in vitro thymidine incorporation and neovessel formation in subcutaneous Matrigel plugs in mice.
RESULTS
Microparticles of endothelial, platelet, photoreceptor, and microglial origin were identified in vitreous samples. Levels of photoreceptor and microglial microparticles were undetectable in plasmas but were comparable in diabetic and control vitreous samples. Vitreous platelet and endothelial microparticles levels were increased in diabetic patients and decreased following panretinal laser photocoagulation or intravitreal antivascular endothelial growth factor injection in proliferative diabetic retinopathy (PDR). The ratio of vitreous to plasma microparticle levels was calculated to estimate local formation versus potential plasma leakage. In PDR, the endothelial microparticles ratio—but not that for platelet—was greater than 1.0, indicating local formation of endothelial microparticles from retinal vessels and permeation of platelet microparticles from plasma. Isolated vitreous microparticles stimulated by 1.6-fold endothelial proliferation and increased new vessel formation in mice.
CONCLUSIONS
The present study demonstrates that vitreous fluid contains shed membrane microparticles of endothelial, platelet, and retinal origin. Vitreous microparticles levels are increased in patients with diabetic retinopathy, where they could contribute to disease progression.
Despite advances in medical care, diabetic retinopathy continues to be a leading cause of vision impairment and blindness in working-age adults (1). The pathogenesis of diabetic retinopathy is complex and has involved multiple pathways including accumulation of polyol compounds and advanced glycation end products, increased oxidation stress and activation of the protein kinase C pathway, production of growth factors, and inflammation (1). Although there is growing evidence for an early involvement of the neural elements of the retina (2), vision loss in diabetic retinopathy is associated with progressive alterations of the retinal vasculature, leading to the breakdown of the blood retinal barrier and pathological angiogenesis of new vessels in the vitreous cavity (1,3). The risk of vision loss results then from macular edema and bleeding of these new vessels (vitreous hemorrhage) or their contraction (retinal detachment).
Microparticles are submicron membrane vesicles shed from the cell surface of both healthy and damaged cells (4). Shedding of membrane microparticles is a physiological process that accompanies cell growth and activation and that is enhanced by cytokines, reactive oxygen species, activation of apoptotic pathways, or increases in intracellular calcium leading to cytoskeleton reorganization. Numerous studies now indicate that microparticles have biological activities and may be involved in thrombosis, cell inflammation, angiogenesis and cell-to-cell communication (5–12).
Microparticles have been identified not only in human plasma but also in other tissues with high cellular activation, inflammation, or apoptosis, such as human atherosclerotic plaques or synovial fluid in rheumatoid arthritis (13,14). Plasma microparticles from different cellular origins circulate in healthy subjects, and their levels increase in patients with cardiovascular disease (15,16). The question of changes in circulating levels of microparticles appears to be controversial in diabetic patients (17,18), but plasma levels of platelet-derived and monocyte-derived microparticles increase with the severity of diabetic retinopathy (19,20).
Diabetic retinopathy is associated with increased local activation or apoptosis of retinal, neural, and vascular endothelial cells in the eye both in humans and in animal models (21–24). These finding indicate that microparticles of different cellular origin might be locally generated in the eye of diabetic patients. Alternatively, the presence of microparticles in the eye could also result from an increased vascular permeability associated with diabetic retinopathy. Thus, we sought to investigate the presence of endothelial, platelet, and retinal-derived microparticles both in the vitreous and in the plasma of diabetic patients undergoing vitrectomy for diabetic retinopathy compared with that of nondiabetic patients. We also examined the potential biological effects of vitreous microparticles on endothelial proliferation and new vessel formation.
RESEARCH DESIGN AND METHODS
We included 130 patients who underwent vitrectomy at Lariboisiere Hospital. Baseline characteristics of diabetic and control patients are given in Table 1. Patients were eligible for inclusion when they had no vitreous surgery on the same eye previously and agreed to participate in this study, which was approved by our Institutional Ethics Committee Board and adhered to the Declaration of Helsinki. All patients signed a written informed consent. Prior to surgery, diabetic retinopathy was evaluated according to the simplified international diabetic retinopathy classification (21), made on the basis of clinical data, intraoperative assessment by the surgeon, and review of fundus photographs.
TABLE 1.
Baseline characteristics of the patients, ophthalmology features, and performed therapies
| Diabetic patients | Control subjects | |
|---|---|---|
| Patients (n)/eyes (n) | 104/109 | 26/26 |
| Sex (male) | 63 (61) | 11 (42) |
| Age (years) | 52 ± 2 | 68 ± 2* |
| A1C (%) | 6.9 ± 2.8 | — |
| Type 1/type 2 diabetes | 72/32 | — |
| Insulin treatment | 73 (70) | — |
| Eyes with PDR (n) | 98 with/11 without | — |
| Creatinin clearance (ml/min) | 74.3 ± 3.8 | 74.8 ± 4.6 |
| Hypertension | 77 (74) | 13 (50) |
| Dyslipidemia | 53 (51%) | 5 (18) |
| Smoking | 32 (31) | 6 (22) |
| Ophthalmology features (n eyes) | ||
| PDR | 98 (90) | 0 |
| PDR with vitreous haemorrhage | 63 | 0 |
| PDR with tractional retinal detachment | 22 | 0 |
| PDR or macular edema | 14 | 0 |
| Epimacular membrane | 8 (7) | 16 (62) |
| Macular hole | 0 | 6 (23) |
| Rhegmatogenous retinal detachment | 3 (3) | 2 (8) |
| Age-related macular degenerescence | 0 | 2 (8) |
| Therapies | ||
| Intravitreal injection of Bevacizumab | 12 (11) | — |
| No panretinal laser photocoagulation | 13 (13.2) | — |
| Incomplete panretinal laser photocoagulation | 58 (59.2) | — |
| Complete panretinal laser photocoagulation | 27 (27.5) | — |
Data are n (%) unless otherwise indicated.
*Significant difference between diabetic and control patients.
Undiluted vitreous fluid samples (∼300–400 μl) were collected from patients' eyes at the start of a standard three-port pars plana vitrectomy for the treatment of retinal diseases. A central vitrectomy was performed after a 20- or 23-inch gauge sclerotomy at 4 mm from the limbus using a vitreotome (Accurus). Vitreous samples were collected at the beginning of vitrectomy before opening the balanced salt solution infusion line to maintain intraocular pressure and homogenized by gently pipetting the suspension up and down several times. Ninety-eight samples were collected from the eyes of 93 patients with proliferative diabetic retinopathy (PDR); in these cases, vitrectomy was performed for persistent vitreous hemorrhage, tractional retinal detachment, or macular edema. Among the samples, 12 eyes had received an intravitreal injection of Bevacizumab 1 week before surgery for PDR. In 11 additional diabetic patients, diabetic retinopathy was absent and vitrectomy was performed for epimacular membrane or retinal detachment (Table 1). The control group consisted of vitreous samples from 26 eyes of 26 nondiabetic patients with an idiopathic macular hole, an idiopathic epiretinal membrane, a rhegmatogenous retinal detachment, or age-related macular degeneration (Table 1).
Microparticle isolation.
Vitreous microparticles were isolated from fresh vitreous drawn at beginning of the surgery. Vitreous was separated from cells and platelets after two centrifugations (500g for 15 min and 13,500g for 5 min). Venous blood samples (10 ml) were collected on citrated tubes before the eye surgery and platelet-free plasma (PFP) from 60 patients and was immediately prepared by successive centrifugations according to the methodology of Amabile et al. (11). For each included patient, PFP and vitreous were frozen and stored at −80°C until subsequent use. Samples were frozen and thawed only once.
Materials.
Monoclonal antibodies to vascular endothelial (VE)-Cadherin (CD144) conjugated with phycoethrin, to human glycoprotein GPIIb (IIIa) (CD41) conjugated with PC5, and their corresponding isotype IgG1 were from Beckman Coulter, France. Human Annexin V solution conjugated with fluorescein isothiocyanate (FITC) was from Roche Diagnostics, France. Lectins from arachis hypogaea peanut agglutinin (PNA) and from Bandeiraea Simplicifolia Isolectin B4 (ILB4) conjugated with FITC were from Sigma Aldrich, France. Human umbilical vein endothelial cells (HUVECs) (passage 3–6) medium (Endothelium Cell Basal Medium) were obtained from Promocell (Heidelberg, Germany). Cells were cultured in endothelial growth culture medium at 37°C in a 95% air and 5% CO2 atmosphere. Matrigel basement membrane matrix was purchased from BD Bioscience (San Jose, CA), and female C57/BL6 mice (aged 7 weeks) purchased from Charles River Labs were used for the Matrigel plug assay.
Microparticle labeling and flow cytometry analysis.
Analyses were performed on a Coulter EPICS XL flow cytometer (Beckman Coulter) by two independent examiners unaware of the subject status. Human vitreous (60 μl) and PFP (20 μl) were incubated with anti-CD41-PC5 (10 μl), anti-CD144-PE (20 μl) antibodies, or their respective isotypic immunoglobulins. Microparticles expressing phosphatidylserine were labeled using FITC-conjugated Annexin V solution in the presence of CaCl2 (5 mmol/l final concentration) according to the recommendation of the supplier. Microparticles from retinal origins were labeled with lectins. FITC-conjugated lectins were diluted in PBS to reach the final concentration of 100 μg/ml. Human vitreous (60 μl) was incubated with either lectin from PNA FITC (20 μg) or lectin from ILB4 FITC (4 μg) (25,26). Their respective controls were preincubated with d-galactose (80 mmol/l for 30 min) (Sigma-Aldrich) (27–29). Microparticles were gated as events with a 0.1-to 1.0-μm diameter identified in forward-scatter and side-scatter intensity dot-plot representation using standards synthetic beads of 1 μm in diameter (Polyscience) (Fig. 1). Microparticle concentration was assessed by comparison with calibrator Flowcount beads (10 μm diameter; Beckman Coulter) with a predetermined concentration.
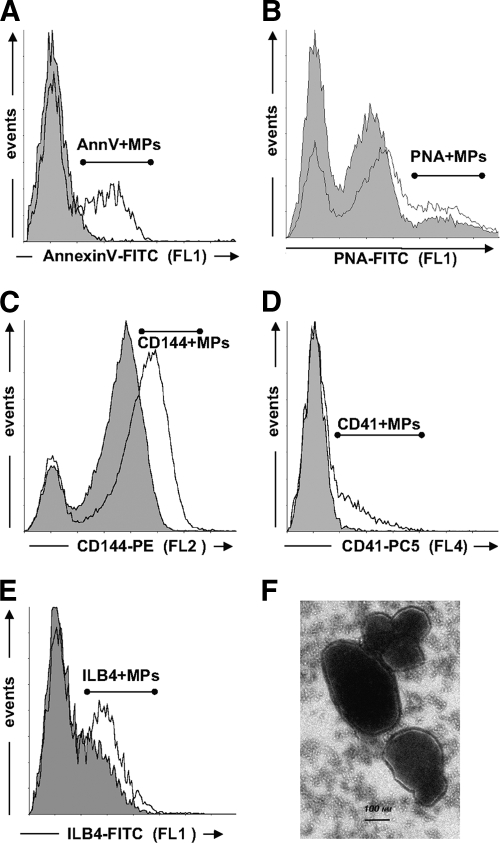
FIG. 1.
Representative traces of flow cytometry analysis of Annexin V+, platelet, endothelial photoreceptor, and microglial microparticles in human vitreous samples. A–E: Size-selected events (0.1–1.0 μm) are plotted as a function of their fluorescence for specific Annexin V–FITC binding (FL1) (A), lectin PNA-FITC labeling (B), anti-human CD144-phycoethrin (PE) labeling (C), anti-human CD41-PC5 labeling (D), or lectin ILB4-FITC labeling (E). Gray-shaded areas represent unspecific labeling determined either in the absence of calcium (A), fluorescent isotypic antibodies (C and D), or d-Galactose (B and E). F: Ultrastructural analyses of vitreous microparticles. Transmission electron microscopic picture of vitreous section showing clustering vitreous vesicles with negative staining. Following samples embedding in Epon 812, thin sections were layed on a carbon-coated grid, stained with 7.6% uranyl acetate and 0.4% lead citrate, and then examined by electron microscopy (Phillips, Eindhoven, the Netherlands; Tecnai, Fei, U.S.). Scale bar: 0.1 μm. FL1, green fluorescence; FL2, orange fluorescence; FL4, infrared fluorescence.
Isolation of vitreous microparticles for assessment of cell proliferation and angiogenesis.
To study properties of vitreous microparticles on HUVEC proliferation and angiogenesis, microparticles were isolated from vitreous samples pooled from six consecutive patients with PDR. Patients who previously had intravitreal anti-VEGF therapy injection or complete panphotocoagulation were excluded. Microparticles were pelleted and washed three times in endothelial growth culture medium medium (20,500g for 150 min). At the end of the last centrifugation, the supernatant was filtered with 0.2 μm and then 0.1 μm Acrodisc PF Syringe Filter (PALL Life Sciences) and used as vehicle. Levels of endothelial CD144+ microparticles, the most abundant vitreous microparticle subpopulation in patients with PDR, were determined in each pellet using flow cytometry, and all proliferation experiments were performed at the final concentration of 65 CD144+ microparticles/μl. Vitreous microparticles isolated using this procedure did not contain detectable levels of VEGF when determined by immunoassay (minimal detection limit of 3.3 pg/ml; n = 6).
Assessment of cell proliferation.
Endothelial cell proliferation was evaluated by 3H-thymidine (Amersham; Biosciences) incorporation in subconfluent quiescent HUVECs. At 70% confluence, endothelial cells were rendered quiescent by replacing 10% FCS with 0.1% BSA for 24 h. Endothelial cells were then incubated during 48 h with either 10% FCS (used as a positive control), vitreous microparticles from patients with PDR (corresponding to a final concentration of 65 microparticles/μl of microparticles of endothelial origin), or vehicle. The concentration of microparticles used in these experiments was the highest concentration obtained after isolation of vitreous microparticles from patients with PDR. After 48 h, 3H-thymidine was added (1 μCi/well) for 16 h at 37°C. Endothelial cell proliferation was stopped by freezing (−80°C), and free thymidine was then separated by filtration on 1.2-μm filters. The counting of the radioactivity remaining on the filters (to determine thymidine incorporated in endothelial cells) was then realized, after addition of a Microscint solution (40 μl/well), in a Microplate luminescence counter (Perkin Elmer, Boston, MA).
Matrigel angiogenesis assay.
All animal experiments were performed in accordance with institutional animal care guidelines as previously described (30). Briefly, 500 μl Matrigel (free of growth factors) mixed with vitreous microparticles (reaching a level of 5,000 CD144+ microparticles of endothelial origin) or vehicle was injected subcutaneously in the back of 16 7-week-old C57/BL6 mice. After 7 days, mice were killed by intraperitoneal injection of 0.1 ml pentobarbital (Ceva Sante Animale, Libourne, France) and Matrigel plugs were excised. Specimens were fixed with 4% formaldehyde overnight at 4°C and embedded in paraffin. Serial sections (5 μm thick) were submitted to Masson Trichrome staining. Two pathologists graded the samples in a masked fashion and counted capillary structures (considering each area surrounded by few contiguous cells forming a patent lumen as a capillary structure) present on three different sections for every Matrigel plug (×10, ×20, and ×40 magnification, Olympus BH-2; Leica).
Statistical analysis.
Microparticles levels are expressed as median and range and analyzed by Kruskall-Wallis nonparametric analysis to compare levels between more than two groups, followed by Mann-Whitney analysis of two groups when appropriate. Other data are expressed as means ± SEM according to the normality of distribution and analyzed by ANOVA followed by appropriate Bonferonni tests or by Student's t test. In addition, the χ2 test was performed for frequency analysis of Matrigel angiogenesis assays. Statistical analysis was performed with SPSS 10.0 software for Windows. Differences were considered significant at P < 0.05.
RESULTS
Characterization of circulating and vitreous microparticles.
Microparticles were identified by flow cytometry analysis as events with 0.1–1 μm positively labeled by specific antibodies (Fig. 1). Specific microparticle populations were defined (Fig. 1): endothelium-derived microparticles (EMPs) (CD144+ or VE-cadherin positive) and platelet-derived microparticles (PMPs) (CD41+ or glycoprotein IIb positive) as previously described (11). Photoreceptor-derived microparticles (PNA positive) and microglial-derived microparticles (ILB4 positive) were also identified.
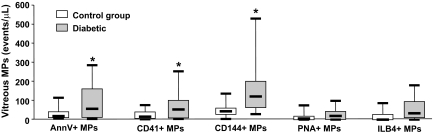
Plasma levels of Annexin V+, platelet CD41+, and endothelial CD144+ microparticles were not different in control and diabetic patients (Table 2). Microparticles from retinal origin (PNA+ or ILB4+ microparticles) were undetectable in plasma samples (Table 2). Vitreous levels of Annexin V+, platelet CD41+, and endothelial CD144+ microparticles were all markedly increased in diabetic compared with control patients (P = 0.035, 0.018, and <0.001, respectively) (Fig. 2). Microparticles of endothelial origin, identified as expressing VE-cadherin (CD144), were the most abundant microparticle subpopulation in vitreous samples from diabetic patients. Vitreous levels of Annexin V+, endothelial CD144+, and platelet CD41+ microparticles were increased in association with PDR compared with nonPDR (NPDR) (P = 0.007, 0.017, and 0.018, respectively) (Table 2). In addition, vitreous levels of Annexin V+, platelet CD41+, and endothelial CD144+ microparticles were augmented in diabetic patients with PDR compared with control subjects (P = 0.004, 0.003, and <0.0001, respectively (Fig. 2). However, there were no differences between vitreous levels of retinal microparticles (ILB4+ and PNA+) in diabetic patients and control patients. Furthermore, circulating levels of endothelial-and platelet-derived microparticles were not different between control and diabetic patients with or without PDR (Fig. 2).
TABLE 2.
Plasma levels of microparticles in control patients and in patients with or without PDR
| Plasma |
Vitreous |
|||||
|---|---|---|---|---|---|---|
| Control | NPDR | PDR | Control | NPDR | PDR | |
| N | 26 | 6 | 28 | 26 | 6 | 28 |
| Annexin V+ microparticles | 1,223 (7–5,686) | 1,436 (130–2,871) | 721 (10–6,068) | 18 (0–633) | 9 (2–43) | 92 (2–1,349)*† |
| CD41+ microparticles | 765 (25–5,018) | 718 (68–1,763) | 408 (8–2,845) | 15 (0–156) | 12 (1–58) | 64 (2–728)*† |
| CD144+ microparticles | 98 (14–901) | 190 (31–609) | 89 (21–4,402) | 44 (0–378) | 52 (18–336) | 149 (13–900)*† |
| PNA+ microparticles | 0 | 0 | 0 | 6 (0–79) | 19 (0–64) | 17 (0–791) |
| ILB4+ microparticles | 0 | 0 | 0 | 0 (0–117) | — | 33 (0–184) |
Data are expressed as median (range) of microparticles/μl. Microparticles were identified of platelet (CD41+) and endothelial (CD144+) origin or from retinal origin (PNA+ and ILB4+). Annexin V+ microparticles express phosphatidyserine and were positively labeled with Annexin V. Microparticles levels were analyzed by Kruskall-Wallis nonparametric analysis followed by Mann-Whitney analysis of two groups when appropriate.
*†Significant difference between NPDR and PDR or between nondiabetic patients and patients with PDR, respectively.
FIG. 2.
Vitreous levels of Annexin V+ (AnnV+), platelet (CD41+), endothelial (CD144+), photoreceptor (PNA+) and microglial (ILB4+) microparticles (MPs) in diabetic and control patients. Data are expressed as microparticles/μl and given as median (horizontal bar), 25th and 75th percentile (boxes), and 10th and 90th percentile (error bar). *Significantly different from control patients.
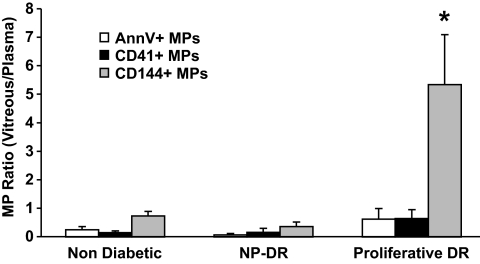
The ratio of vitreous to plasma microparticles levels indicates the importance of intraocular formation versus potential plasma leakage of microparticles from microvessels in vitreous fluid (Fig. 3). For all markers, the ratio was increased in PDR compared with control. This ratio was markedly greater than unity for only endothelial CD144+ microparticles in PDR (P = 0.02), indicating either an intraocular shedding or an abnormal clearance of endothelial microparticles.
FIG. 3.
Ratio of vitreous to plasma levels of Annexin V+ (AnnV+), platelet (CD41+), and endothelial (CD144+) microparticles (MPs) in control and diabetic patients with either PDR or NPDR. *Ratio significantly greater than unity.
Effect of therapies on vitreous microparticles levels in PDR.
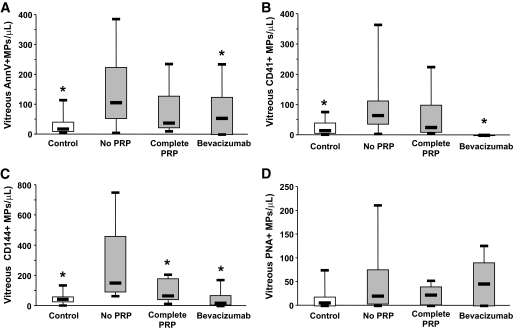
In patients with PDR, complete panretinal photocoagulation (PRP) significantly decreased vitreous endothelial microparticle levels (P = 0.037) to reach values not different from those obtained in control patients. Complete laser treatment tended to decrease vitreous levels of Annexin V+, CD41+, or PNA+ microparticles but the change did not reach statistical significance (Fig. 4).
FIG. 4.
Vitreous levels of Annexin V+ (AnnV+) (A), platelet (CD41+) (B), endothelial (CD144+) (C), and retinal (PNA+) (D) microparticles in control subjects (open bars) and diabetic patients with PDR (gray bars) who underwent either no or incomplete laser panphotocoagulation (PRP), complete PRP, or intravitreal injection of Bevacizumab prior to vitrectomy. *Significantly different when comparing patients with PDR with those with no or incomplete PRP.
One week prior to vitrectomy, 12 patients with PDR received an intravitreal injection of Bevacizumab (50 μl; 25 μg/μl), a humanized recombinant antibody binding all isoforms of VEGF-A, which causes at least short-term involution of retinal neovascularization, therefore reducing the risk of hemorrhage during surgery (Fig. 4). Bevacizumab treatment significantly decreased vitreous levels of Annexin V+ microparticles and endothelial CD144+ microparticles (P = 0.04 and P = 0.02, respectively) and led to a complete disappearance of platelet-derived CD41+ microparticles in PDR vitreous samples (Fig. 4). However, anti-VEGF treatment did not affect levels of photoreceptor microparticles (PNA+). Following Bevacizumab treatment, vitreous levels of endothelial CD144+ microparticles were no longer different from those found in control patients.
Vitreous microparticles induce endothelial cell proliferation and new vessel formation.
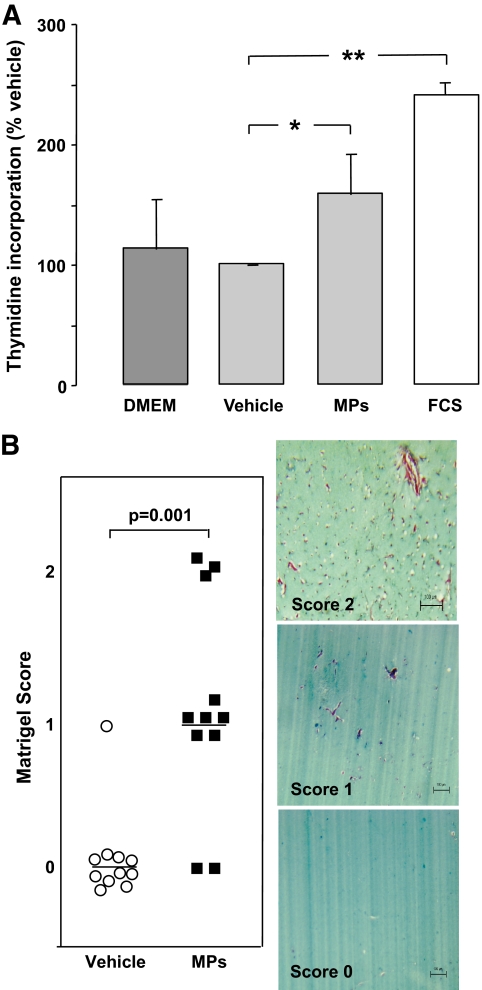
Vitreous microparticles or supernatant, as previously prepared, were incubated with subconfluent quiescent HUVECs during 48 h (n = 4). Vitreous microparticles significantly increased 3H-thymidine incorporation, by 1.6-fold, compared with microparticle vehicle (vitreous supernatant; P = 0.029). FCS (10%; used here as a positive control) increased 3H-thymidine incorporation by 2.4-fold (P = 0.003) (Fig. 5A). Vitreous microparticles did not contain detectable levels of VEGF when determined by immunoassay with a minimal detection limit of 3.3 pg/ml (n = 6). In the in vivo Matrigel plug assay, vitreous microparticles significantly induced endothelial cell migration (score 1) and new vessels formation (score 2) compared with vehicle (vitreous supernatant; score 0) (P = 0.001) (Fig. 5B).
FIG. 5.
A: HUVECs proliferation assay (n = 4). 3H-thymidine incorporation with the vehicle (vitreous supernatant) was used as the baseline (100%). Levels of 3H-thymidine incorporation are represented with endothelium cell basal medium (Dulbecco's modified Eagle's medium [DMEM]), FCS (2%), and vitreous microparticles (MPs). Vitreous microparticles (estimated as 65 CD144+ microparticles/μl for the endothelial subpopulation) increased 3H-thymidine incorporation by 1.6-fold compared with vehicle (vitreous supernatant without CD144+ microparticles) (*P = 0.029). FCS (10%) increased it by 2.4-fold (**P = 0.003). B: Matrigel scoring and angiogenesis assay. Serial sections of Matrigel were quantified by two independent pathologists. The presence of capillary structures with massive cells invasion was noticed (2). In some plugs, angiogenesis response was limited to cell invasion (1). Low concentration of vitreous microparticles (10 EMP/μl; 5,000 EMPs added to 500 μl Matrigel) induced endothelial cell migration (score 1; n = 6) and new vessel formation (score 2; n = 3) compared with vitreous supernatant (score 0; n = 12) (P = 0.001). (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
This study reveals, for the first time, the presence of submicron membrane vesicles shed from platelet, endothelial, and retinal cells in human vitreous samples of patients undergoing vitrectomy and demonstrates the specific vitreous accumulation of endothelial microparticles in patients with PDR. In addition, we show that vitreous microparticles isolated from patients with diabetic retinopathy stimulate endothelial cell proliferation and formation of new vessels, suggesting that they could contribute to retinal angiogenesis.
Presence of shed-membrane microparticles has been reported in several human body fluids, such as plasma or synovial fluid (13,15,16). Plasma levels of microparticles of different cellular origins are increased in patients with high atherothrombotic risk or inflammatory diseases (15,16,31). In this study, we observed no significant differences in circulating microparticle levels between diabetic patients and the control group. However, our diabetic group included mostly patients with type 2 diabetes, a disease for which conflicting results on changes in circulating microparticles have been reported (17–20). Another potential confounding factor is that significant numbers of patients from the control group have cardiovascular risk factors such as hypertension or dislipidemia, which are known to be associated with increased circulating levels of microparticles (32,33).
PDR is associated with ocular increases in oxidative stress, protein glycation, growth factors, inflammatory cytokines, and cell apoptosis—all of which stimulate the shedding of membrane microparticles from retinal or vascular cells (1,4,15,16,21,22,24,34,35). We observed significant increases in the overall pool of vitreous Annexin V+ microparticles in PDR compared with control patients or patients with nonproliferative diabetic retinopathy. In addition, we demonstrate the presence of microparticles positively labeled with either PNA or ILB4 in vitreous samples but not in plasma, indicating their photoreceptor or microglial origin, respectively (25–29). If anything, levels of microparticles derived from photoreceptors or microglia tend to increase in patients with PDR, but the difference did not reach statistical significance. These microparticles originate from cells localized in deeper retinal layers and may be released in vitreous fluid following the tear of the retinal internal limiting membrane. Microfracture of the internal limiting membrane is a likely hypothesis in our control patients, who were included for epimacular membrane or macular hole, and even more so in patients with PDR, where proliferating microvessels need to perforate the internal limiting membrane (36,37).
The present study also demonstrates increased vitreous levels of microparticles originating from vascular or circulating cells such as platelets. Diabetic retinopathy is characterized by an augmented vascular permeability, and microparticles present in the blood could permeate through the leaky vascular wall into the vitreous fluid. By the same token, vitreous microparticles expressing endothelial VE-cadherin may originate from the circulating pool of microparticles, but they may be also locally generated from the retinal new vessel wall. To appreciate the relative importance of local formation versus potential leakage of microparticle from microvessels into the vitreous fluid, we determined the ratio of vitreous to plasma microparticles levels. This ratio was greater than unity only for endothelial CD144+ microparticles in diabetic patients with PDR, suggesting that significant numbers of endothelial microparticles found in the vitreous fluid could be generated from local microvascular endothelial cells in PDR or that clearance of endothelial microparticles was abnormal. On the contrary, the ratio for platelet CD41+ microparticles was lower than unity, favoring the interpretation that platelet microparticles present in PDR vitreous fluid likely originate from the plasma.
We investigated the effects of two treatments of PDR on vitreous levels of microparticles. Complete laser photocoagulation causes regression of preretinal neovascularization by destroying the outer layers of the retina and improving the oxygen diffusion from the choroid to the inner retina (38–41), whereas intravitreal injection of the anti-VEGF antibody Bevacizumab leads to a complete resolution of angiographic leakage of neovascularization and to a rapid involution of retinal neovascularization (42–45). Both treatments decreased levels of vitreous endothelial CD144+ microparticles to values no longer different from those found in control patients. These observations further reinforce our findings that PDR is associated with a specific increase in local shedding of endothelial microparticles originating from new vessels. Furthermore, Bevacizumab decreased vitreous platelet CD41+ microparticles to undetectable levels, which is a finding consistent with the decrease vascular leakage reported for the anti-VEGF ocular therapy (42,46).
The present study shows that vitreous microparticles stimulate in vitro endothelial proliferation and in vivo new-vessel formation in a Matrigel plug model. This finding is in agreement with previous studies showing that microparticles of different cellular origins (platelet, leukocyte, or endothelial) are proangiogenic (8–10,34,47). However, the present data contrast with studies reporting the antiangiogenic effect of microparticles of endothelial or lymphocyte origin, which is associated with the stimulation of oxidative stress (48,49). The reasons for this discrepancy are unknown but could result from the different nature or composition of microparticles. Because of the paucity of microparticles in vitreous samples and their low recovery after isolation, we were unable to identify the cell origin of vitreous microparticles that affect endothelial cells. Although the proangiogenic effect of platelet-derived microparticles is mediated in part by growth factors (8), the mechanism of the proliferative effect of vitreous microparticles unlikely involves VEGF because levels of this growth factor were below detection in isolated vitreous microparticles samples. Because of the low recovery of the isolation procedure, the present data do not permit the examination of the proangiogenic effect of vitreous microparticles from nonproliferative diabetic retinopathy or from control patients. For similar reasons, we could not evaluate the biological effects of increasing concentrations of vitreous microparticles.
In conclusion, we identified the presence in vitreous fluid of membrane microparticles shed from retinal, vascular, and circulating cells and their significant increase in patients with PDR, where they could contribute to disease progression.
ACKNOWLEDGMENTS
This study was supported by INSERM, an educational grant from the Servier Research Institute, and the European Network of Excellence on Vascular Genomics (contract no. LSHM-CT-2003-503254). C.M.B. was supported by a contrat d'Interface INSERM/Assistance Publique Hopitaux de Paris.
No potential conflicts of interest relevant to this article were reported.
The authors thank K. Averous and R. Tadayoni, who contributed to the collection of vitreous samples.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Frank RN: Diabetic retinopathy. N Engl J Med 2004; 350: 48– 58 [DOI] [PubMed] [Google Scholar]
- 2.Kern TS, Barber AJ: Retinal ganglion cells in diabetes. J Physiol 2008; 586: 4401– 4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis TM, Gardiner TA, Stitt AW: Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye 2009; 23: 1496– 1508 [DOI] [PubMed] [Google Scholar]
- 4.Zwaal RF, Schroit AJ: Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 1997; 89: 1121– 1132 [PubMed] [Google Scholar]
- 5.Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z: Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 2001; 104: 2649– 2652 [DOI] [PubMed] [Google Scholar]
- 6.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, Have K, Eijsman L, Hack CE, Sturk A: Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation 1997; 96: 3534– 3541 [DOI] [PubMed] [Google Scholar]
- 7.Barry OP, Pratico D, Savani RC, FitzGerald GA: Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest 1998; 102: 136– 144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D: Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 2005; 67: 30– 38 [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Song KS, Chung JH, Lee KR, Lee SN: Platelet microparticles induce angiogenesis in vitro. Br J Haematol 2004; 124: 376– 384 [DOI] [PubMed] [Google Scholar]
- 10.Boulanger CM, Tedgui A: Dying for attention: microparticles and angiogenesis. Cardiovasc Res 2005; 67: 1– 3 [DOI] [PubMed] [Google Scholar]
- 11.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM: Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 2005; 16: 3381– 3388 [DOI] [PubMed] [Google Scholar]
- 12.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R: Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol 2008; 173: 1210– 1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berckmans RJ, Nieuwland R, Tak PP, Boing AN, Romijn FP, Kraan MC, Breedveld FC, Hack CE, Sturk A: Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum 2002; 46: 2857– 2866 [DOI] [PubMed] [Google Scholar]
- 14.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A: Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 1999; 99: 348– 353 [DOI] [PubMed] [Google Scholar]
- 15.Boulanger CM, Amabile N, Tedgui A: Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension 2006; 48: 180– 186 [DOI] [PubMed] [Google Scholar]
- 16.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM: Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005; 20: 22– 27 [DOI] [PubMed] [Google Scholar]
- 17.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK: Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 2002; 106: 2442– 2447 [DOI] [PubMed] [Google Scholar]
- 18.Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Oliver C, Sampol J, Dignat-George F: Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 2002; 51: 2840– 2845 [DOI] [PubMed] [Google Scholar]
- 19.Ogata N, Nomura S, Shouzu A, Imaizumi M, Arichi M, Matsumura M: Elevation of monocyte-derived microparticles in patients with diabetic retinopathy. Diabetes Res Clin Pract 2006; 73: 241– 248 [DOI] [PubMed] [Google Scholar]
- 20.Ogata N, Imaizumi M, Nomura S, Shozu A, Arichi M, Matsuoka M, Matsumura M: Increased levels of platelet-derived microparticles in patients with diabetic retinopathy. Diabetes Res Clin Pract 2005; 68: 193– 201 [DOI] [PubMed] [Google Scholar]
- 21.Mizutani M, Kern TS, Lorenzi M: Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 1996; 97: 2883– 2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber AJ: A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 283– 290 [DOI] [PubMed] [Google Scholar]
- 23.Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME: TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol 1997; 115: 1031– 1035 [DOI] [PubMed] [Google Scholar]
- 24.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP: A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb J 2004; 18: 1450– 1452 [DOI] [PubMed] [Google Scholar]
- 25.Cho EY, Choi HL, Chan FL: Expression pattern of glycoconjugates in rat retina as analysed by lectin histochemistry. Histochem J 2002; 34: 589– 600 [DOI] [PubMed] [Google Scholar]
- 26.Lunemann A, Ullrich O, Diestel A, Jons T, Ninnemann O, Kovac A, Pohl EE, Hass R, Nitsch R, Hendrix S: Macrophage/microglia activation factor expression is restricted to lesion-associated microglial cells after brain trauma. Glia 2006; 53: 412– 419 [DOI] [PubMed] [Google Scholar]
- 27.Johnson LV, Hageman GS: Enzymatic characterization of peanut agglutinin-binding components in the retinal interphotoreceptor matrix. Exp Eye Res 1987; 44: 553– 565 [DOI] [PubMed] [Google Scholar]
- 28.Thornton PD, Gerke MB, Plenderleith MB: Histochemical localisation of a galactose-containing glycoconjugate expressed by sensory neurones innervating different peripheral tissues in the rat. J Peripher Nerv Syst 2005; 10: 47– 57 [DOI] [PubMed] [Google Scholar]
- 29.Blanks JC, Hageman GS, Johnson LV: Appearance of PNA-binding cells within the outer nuclear layer coinciding with photoreceptor degeneration in rd mice. Prog Clin Biol Res 1987; 247: 229– 242 [PubMed] [Google Scholar]
- 30.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR: A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 1992; 67: 519– 528 [PubMed] [Google Scholar]
- 31.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R: Microparticles in cardiovascular diseases. Cardiovasc Res 2003; 59: 277– 287 [DOI] [PubMed] [Google Scholar]
- 32.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS: Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003; 41: 211– 217 [DOI] [PubMed] [Google Scholar]
- 33.Koga H, Sugiyama S, Kugiyama K, Fukushima H, Watanabe K, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H: Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur Heart J 2006; 27: 817– 823 [DOI] [PubMed] [Google Scholar]
- 34.Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V: Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 2002; 160: 673– 680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamis AP: Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol 2002; 86: 363– 365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO: Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003; 22: 1– 29 [DOI] [PubMed] [Google Scholar]
- 37.Snead DR, Cullen N, James S, Poulson AV, Morris AH, Lukaris A, Scott JD, Richards AJ, Snead MP: Hyperconvolution of the inner limiting membrane in vitreomaculopathies. Graefes Arch Clin Exp Ophthalmol 2004; 242: 853– 862 [DOI] [PubMed] [Google Scholar]
- 38.Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978; 85: 82– 106 [DOI] [PubMed] [Google Scholar]
- 39.Stefansson E: Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol 2006; 51: 364– 380 [DOI] [PubMed] [Google Scholar]
- 40.Gariano RF, Gardner TW: Retinal angiogenesis in development and disease. Nature 2005; 438: 960– 966 [DOI] [PubMed] [Google Scholar]
- 41.Diabetic Retinopathy Study Group. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978; 85: 82– 106 [DOI] [PubMed] [Google Scholar]
- 42.Avery RL: Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006; 26: 352– 354 [DOI] [PubMed] [Google Scholar]
- 43.Spaide RF, Fisher YL: Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006; 26: 275– 278 [DOI] [PubMed] [Google Scholar]
- 44.Mason JO, 3rd, Nixon PA, White MF: Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol 2006; 142: 685– 688 [DOI] [PubMed] [Google Scholar]
- 45.Chen E, Park CH: Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006; 26: 699– 700 [DOI] [PubMed] [Google Scholar]
- 46.Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A: Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006; 113: 1695.e1– 1665.e15 [DOI] [PubMed] [Google Scholar]
- 47.Leroyer AS, Rautou PE, Silvestre JS, Castier Y, Leseche G, Devue C, Duriez M, Brandes RP, Lutgens E, Tedgui A, Boulanger CM: CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol 2008; 52: 1302– 1311 [DOI] [PubMed] [Google Scholar]
- 48.Mezentsev A, Merks RM, O'Riordan E, Chen J, Mendelev N, Goligorsky MS, Brodsky SV: Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol 2005; 289: H1106– H1114 [DOI] [PubMed] [Google Scholar]
- 49.Yang C, Mwaikambo BR, Zhu T, Gagnon C, Lafleur J, Seshadri S, Lachapelle P, Lavoie JC, Chemtob S, Hardy P: Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am J Physiol Regul Integr Comp Physiol 2008; 294: R467– R476 [DOI] [PubMed] [Google Scholar]