Abstract
OBJECTIVE
Intestinal lipoprotein production has recently been shown to be increased in insulin resistance, but it is not known whether it is regulated by insulin in humans. Here, we investigated the effect of acute hyperinsulinemia on intestinal (and hepatic) lipoprotein production in six healthy men in the presence and absence of concomitant suppression of plasma free fatty acids (FFAs).
RESEARCH DESIGN AND METHODS
Each subject underwent the following three lipoprotein turnover studies, in random order, 4–6 weeks apart: 1) insulin and glucose infusion (euglycemic-hyperinsulinemic clamp) to induce hyperinsulinemia, 2) insulin and glucose infusion plus Intralipid and heparin infusion to prevent the insulin-induced suppression of plasma FFAs, and 3) saline control.
RESULTS
VLDL1 and VLDL2-apoB48 and -apoB100 production rates were suppressed by 47–62% by insulin, with no change in clearance. When the decline in FFAs was prevented by concomitant infusion of Intralipid and heparin, the production rates of VLDL1 and VLDL2-apoB48 and -apoB100 were intermediate between insulin and glucose infusion and saline control.
CONCLUSIONS
This is the first demonstration in humans that intestinal apoB48-containing lipoprotein production is acutely suppressed by insulin, which may involve insulin's direct effects and insulin-mediated suppression of circulating FFAs.
Dyslipidemia is a well-recognized feature of insulin resistance and type 2 diabetes and is a common risk factor for atherosclerotic cardiovascular disease. Hypertriglyceridemia, low plasma concentrations of HDL, and qualitative changes in LDL comprise the typical dyslipidemia, which is felt to play an important but not exclusive role in accelerated atherosclerosis of affected individuals (1,2). Overproduction of large, triglyceride-rich, apolipoprotein (apo)B100-containing, hepatic VLDL1 particles has been well documented in animal models and in humans with insulin resistance and type 2 diabetes and contributes to dyslipidemia (3,4). In addition, postprandial hyperlipidemia and elevated plasma concentrations of intestinal apoB48-containing particles have been demonstrated in insulin-resistant states (5–9). We and others have recently shown that insulin-resistant animal models and humans have overproduction of intestinal, apoB48-containing lipoproteins (10–12). Whereas numerous factors are known to regulate hepatic lipoprotein particle overproduction, less is known about factors that regulate intestinal lipoprotein production in insulin-resistant conditions.
We have recently shown that acute elevation of plasma free fatty acids (FFAs) stimulates not only hepatic (13) but also intestinally derived apoB48-containing lipoprotein particles in fed humans (14), demonstrating that at least one of the factors involved in the regulation of hepatic lipoprotein production also regulates intestinal lipoprotein particle production. Insulin has been shown both in vitro (9,15,16) and in vivo (13,17–22) in animals and humans to acutely suppress hepatic apoB100-containing lipoprotein particle production. This acute suppressive effect on VLDL in fasting individuals has been shown to be in part dependent on the FFA suppression induced by acute hyperinsulinemia in vivo (13,21) and is due predominantly to suppression of the VLDL1 fraction with no (22) or an opposite (17) effect on VLDL2. Insulin-resistant hyperinsulinemic, obese humans and those with type 2 diabetes are resistant to the acute inhibitory effect of insulin on VLDL production (18,20), as are primary cultured hepatocytes derived from insulin-resistant rats (23). At least part of the effect of insulin is direct, occurring by co- and posttranslational mechanisms through increasing posttranslational protein degradation (24). Incubation of human fetal small intestinal cells with insulin has also been shown to reduce chylomicron secretion (25). Recent studies have shown that intestinal lipoprotein production in chow-fed hamsters is responsive to the acute inhibitory effect of insulin, whereas enterocytes derived from insulin-resistant, fructose-fed hamsters are resistant to this acute suppressive effect of insulin through an aberrant intestinal insulin-signaling cascade (26). The responsiveness of intestinal lipoprotein secretion to acute hyperinsulinemia has not previously been examined in humans.
In the present study, we used the euglycemic-hyperinsulinemic clamp technique to examine the effects of acute hyperinsulinemia on VLDL1 and VLDL2 intestinal (apoB48) and hepatic (apoB100) lipoprotein production in six healthy men in a constant fed state. We found that insulin infusion suppresses both VLDL1 and 2 apoB48 and apoB100 concentrations as a result of suppression of VLDL1 lipoprotein secretion, with consequently less VLDL2 formed from VLDL1. When Intralipid and heparin were coinfused to prevent insulin-induced suppression of plasma FFAs, production rates of these lipoproteins were still suppressed, although to a lesser extent. These results indicate that insulin directly suppresses both intestinal and hepatic lipoprotein production in humans.
RESEARCH DESIGN AND METHODS
The demographic characteristics and fasting biochemical profiles of the six healthy, normolipidemic male participants in this study are outlined in Table 1. None of the participants had any previous history of cardiovascular disease, gastrointestinal or systemic illness, or surgical intervention within 6 months prior to the studies. No subjects were taking medications, and all had normal oral glucose tolerance tests performed immediately prior to enrollment in the study. The Research Ethics Board of the University Health Network, University of Toronto, approved the study, and all subjects gave written informed consent prior to their participation.
TABLE 1.
Demographic characteristics and fasting biochemical parameters of subjects (n = 6)
| Mean ± SE | Range | |
|---|---|---|
| Age (years) | 44.7 ± 4.9 | 21–54 |
| Weight (kg) | 78.3 ± 2.6 | 65.3–81.8 |
| BMI (kg/m2) | 24.0 ± 0.8 | 21.7–25.9 |
| Glucose (mmol/l) | 4.98 ± 0.13 | 4.4–5.3 |
| Insulin (pmol/l) | 39.7 ± 1.7 | 27.0–76.2 |
| Plasma FFA (mmol/l) | 0.40 ± 0.05 | 0.3–0.6 |
| Plasma TG (mmol/l) | 0.77 ± 0.07 | 0.5–1.00 |
| Plasma total cholesterol (mmol/l) | 3.73 ± 0.22 | 3.0–4.2 |
| VLDL1 apoB48 (mg/l) | 0.35 ± 0.06 | 0.13–0.48 |
| VLDL1 apoB100 (mg/dl) | 1.14 ± 0.20 | 0.8–1.96 |
| VLDL2 apoB48 (mg/l) | 0.66 ± 0.07 | 0.38–0.82 |
| VLDL2 apoB100 (mg/dl) | 1.22 ± 0.23 | 0.61–1.92 |
Experimental protocol for lipoprotein kinetic studies.
Each subject underwent three separate lipoprotein kinetic studies as described below, in random order, 4–6 weeks apart (Fig. 1A). In each study, following an overnight fast (no food ingested after 5:00 p.m. the night before the kinetic study), an intravenous catheter was inserted into a superficial vein in each forearm: one for infusion and one for blood sampling. Kinetic studies were performed in the constant fed state because apoB48 levels are too low in the fasted state to accurately assess isotopic enrichments for calculation of kinetic parameters. To achieve a constant fed state, the subjects ingested aliquots of a liquid food supplement (Hormel Great Shake Plus from Hormel Health Labs: total fat 10% by weight, saturated fat 1.5%, trans fat 0%, monounsaturated fat 2.6%, polyunsaturated fat 5.6%, and cholesterol 0%; 49% calories from fat, 38% from carbohydrates, and 13% from proteins) every hour for the first 3 h starting at 4:00 a.m., with each hourly aliquot equivalent to one-sixteenth of their total daily caloric needs. After the first 3 h (after 7:00 a.m.), the subjects ingested the same formula every half hour for the remainder of the study, with each aliquot equivalent to 1/34th of their daily caloric intake. The Harris-Benedict Equation was used to estimate the total daily energy requirement for each subject.
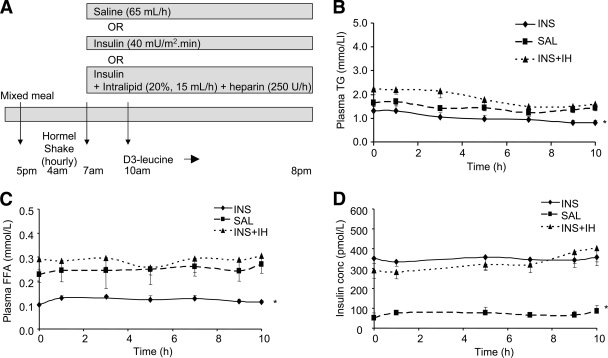
FIG. 1.
Study protocol (A) and plasma TG (B), FFA (C), and insulin (D) levels over the time course of the kinetic study. At 4:00 a.m., subjects started to ingest identical hourly and then half-hourly volumes of a liquid food supplement. Three hours after starting to ingest the formula, an intravenous infusion of INS, SAL, or INS + IH was started. Three hours after starting the intravenous infusions, a primed, continuous intravenous infusion of l-[5,5,5-2H3]-leucine was administered for an additional 10 h to enrich apoB48 and -B100 (A). Plasma TG (B), FFA (C), and insulin (D) levels were measured after an overnight fast and then throughout the 10-h lipoprotein turnover study (0–10 h) in subjects receiving INS, SAL, or INS + IH (n = 6). Values are means ± SEM for each group. Over the time course of the kinetic study, the overall significance between the three studies was *P < 0.0001 for both plasma TG and FFA concentrations, with TG and FFA concentrations significantly lower in INS vs. SAL and INS + IH but no significant difference between INS + IH and SAL. The overall significance of insulin concentrations between the three studies was *P < 0.0001, with insulin concentrations significantly higher in INS and INS + IH vs. SAL but no significant difference between INS and INS + IH.
In the insulin (INS) study, circulating insulin levels were increased through intravenous infusion of insulin (40 mU/m2 per min, Humulin R; Eli Lilly Canada, Toronto, ON, Canada), starting at 7:00 a.m. Blood glucose was assessed at the bedside using a Beckman Glucose Analyser (Beckman Coulter Canada, Mississauga, Canada) every 5–10 min, and a 20% dextrose solution was infused at a varied rate to maintain the blood glucose in the 5–6 mmol/l range. In the saline (SAL) study, saline was infused at 65 ml/h. Because insulin suppresses circulating FFAs, a second control study (insulin plus Intralipid and heparin [INS+IH]) was performed, where Intralipid (20% solution at 15 ml/h) and heparin (250 units/h) were coinfused with insulin and dextrose to prevent a decrease in circulating FFAs. The Intralipid plus heparin infusion protocol has been routinely used to elevate circulating FFAs (14,27).
Six hours after starting the liquid formula ingestion and 3 h after starting the saline, insulin, or insulin plus Intralipid and heparin infusions (i.e., 10:00 a.m.), all subjects received a primed, constant infusion (10 μmol/kg bolus followed by 10 μmol · kg−1 · h−1 for 10 h) of l-[5,5,5-2H3]-leucine (D3-leucine, 98%; Cambridge Isotope Laboratories, Andover, MA) for assessment of production and clearance rates of the lipoprotein particles as previously described (28). After the start of the D3-leucine infusion, blood samples were collected at 1, 2, 3, 5, 7, 9 and 10 h for isolation of lipoproteins. Blood samples for insulin, FFA, and triglyceride (TG) analysis were collected as previously described (14).
Laboratory methods.
Plasma was separated from blood samples within 2 h and subjected to cumulative flotation gradient ultracentrifugation to isolate VLDL1 and VLDL2 fractions (29,30). Briefly, plasma was adjusted to d = 1.10 g/ml with NaCl. A discontinuous density gradient consisting of 4 ml of d = 1.10 g/ml of plasma, 3 ml of d = 1.063 g/ml, 3 ml of d = 1.019 g/ml, and 2.8 ml of d = 1.006 g/ml NaCl solution was created. The Ti40 SW rotor (Beckman, Palo Alto, CA) was subjected to centrifugation at 40,000 rpm at 4°C. Consecutive runs were preformed to separate fractions that correspond to chylomicron (Sf >400 for 38 min), VLDL1 (Sf 60–400 for 3 h and 28 min), and VLDL2 (Sf 20–60 for 17 h). After each step, 1 ml of the gradient containing specific lipoprotein fraction was aspirated and 1 ml of d = 1.006 g/ml was used to refill the tubes before the next run.
VLDL fractions with ∼1,000 μg protein were delipidated and separated by preparative 3.3% SDS-PAGE. Gel bands corresponding with apoB48 and apoB100 were excised. ApoB48 and apoB100 gel slices were hydrolyzed and derivatized to allow for the determination of plasma leucine isotopic enrichment as previously described (11). Briefly, samples were heated at 110°C with 6N HCl and norleucine as the internal standard for 24 h and dried under vacuum before being derivatized with 100 μl mixture (1:1) of acetonitrile:N-tert-butyldimethyl-N-methyltrifluoracetamide (Sigma-Aldrich). Plasma free amino acids were recovered from 0.25 ml plasma after precipitation of proteins with 1.8 ml acetone and extraction of the aqueous phase with hexane (31). The aqueous phase was dried under vacuum and amino acids derivatized and enrichments determined as described above. Derivatized samples were analyzed by electron impact ionization gas chromatography–mass spectrometry (Agilent 5975/6890N; Agilent Technologies Canada, Mississauga, ON, Canada) using helium as the carrier gas (32). Selective ion monitoring at m/z = 200 and 203 was performed, and tracer-to-tracee ratios were calculated from isotopic ratios for each sample according to the formula derived by Cobelli et al. (33).
Triglycerides were measured using an enzymatic colorimetric kit (Roche Diagnostics, Mannheim, Germany). This assay eliminates free glycerol in a preliminary reaction prior to enzymatic hydrolysis of TG and determination of the liberated glycerol; therefore, the presence of free glycerol in Intralipid does not affect the TG assay results. Cholesterol was determined using the CHOD-PAP enzymatic colorimetric kit (Roche Diagnostics). FFAs were determined with the nonesterified fatty acid (NEFA) colorimetric method (Wako Industrials, Osaka, Japan). Plasma insulin concentrations were assayed by radioimmunoassay using a human-specific insulin kit (Linco Research, St. Louis, MO). ApoB100 was separated by 3–8% SDS-PAGE and quantified using an LDL apoB100 standard as previously described (34). ApoB48 mass was determined using a human apoB48 ELISA kit with an intra-assay coefficient of variation (CV) of 3.5% and interassay CV of 5.7% (Shibayagi, Japan).
Calculation of lipoprotein production and clearance rates by compartmental modeling.
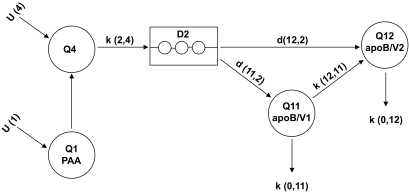
Stable isotope enrichment curves for apoB48 and apoB100 were fitted to a multicompartmental model using SAAM II software (version 1.2; University of Washington, Seattle, WA). Both apoB48 and apoB100 were modeled using the same structural model (Fig. 2). Incorporation of leucine into VLDL1 and VLDL2 occurred via the delay compartment 2. Individual enrichment (tracer-to-tracee ratios) and apoB masses were used to derive kinetic rate constants that were independent for the two subsystems. Plasma leucine enrichment was used as a forcing function. The fractional catabolic rate (FCR) of VLDL1 was the sum of the conversion from VLDL1 to VLDL2 and the direct loss from the VLDL1 compartment. Adjustment was allowed for all of the parameters except the delay, which was set to 0.5 h—as used by others (22). Production rates were calculated using the FCR of VLDL1 and VLDL2 apoB48 or VLDL1 and VLDL2 apoB100 multiplied by pool size measured over the 10 h of the kinetic study, where pool size = average plasma concentration (mg/l) between 1 and 10 h of the kinetic study × plasma volume/kg body wt (estimated as 0.045 l/kg).
FIG. 2.
Compartmental model of VLDL1 and VLDL2 apoB metabolism. The same structural model was used for both apoB48 and apoB100. D2, intracellular delay compartment that accounts for synthesis, assembly, and secretion of apolipoproteins; Q1, plasma deuterated-leucine enrichment; Q4, isotopic dilution; Q11, VLDL1 lipoprotein output for apoB48 or apoB100; Q12, VLDL2 lipoprotein output for apoB48 or apoB100.
Statistics.
Results are presented as means ± SEM. Mean values of the parameters of interest and statistical comparison between studies were calculated during the 10-h kinetic study (i.e., from ∼10:00 a.m. to 8:00 p.m., which is the time period during which deuterated leucine was infused). Repeated-measures ANOVA was used to compare the kinetic experiments and other results between the three groups, followed by a pairwise comparison post hoc (Tukey's and Tamhane's) to examine the differences between the groups. All analyses were performed with SPSS (version 15). For all of the analyses, a P value <0.05 was considered significant.
RESULTS
Plasma insulin, FFA, and TG concentrations.
By design, INS infusion increased plasma insulin levels by more than fourfold compared with SAL (INS 348 ± 34 and INS + IH 333 ± 32 vs. SAL 64.8 ± 19.9 pmol/l; P < 0.0001), while insulin concentrations were similar in INS and INS + IH (P = ns). As a result of the hyperinsulinemia, plasma FFA concentrations in INS were suppressed by more than twofold compared with SAL, which was prevented by INS + IH infusion (INS 0.12 ± 0.01 and INS+IH 0.29 ± 0.03 vs. SAL 0.25 ± 0.05 mmol/l; P = 0.0001) (Fig. 1C; Table 2). Plasma TG in all treatments increased in response to feeding and remained constant throughout the 10-h kinetics study. INS infusion resulted in decreased levels of circulating TG compared with SAL infusion (P < 0.0001, INS vs. SAL), while INS + IH coinfusion prevented the insulin-induced decrease in plasma TG and caused an additional rise in plasma TG concentration compared with SAL infusion (P < 0.0001, INS + IH vs. SAL) (Fig. 1B; Table 2).
TABLE 2.
Mean plasma and VLDL1 and VLDL2 lipids, apoB48 and B100 concentrations, FCRs, and production rates during the kinetic study in three experimental conditions
| Variable | INS | SAL | INS + IH |
|---|---|---|---|
| Plasma FFA (mmol/l) | 0.12 ± 0.01* | 0.25 ± 0.05 | 0.29 ± 0.03 |
| Plasma TG (mmol/l) | 1.03 ± 0.16* | 1.46 ± 0.10 | 1.91 ± 0.23 |
| VLDL1 TG (mmol/l) | 0.21 ± 0.07* | 0.40 ± 0.03 | 0.50 ± 0.09 |
| VLDL1 cholesterol (mmol/l) | 0.05 ± 0.02* | 0.10 ± 0.01 | 0.12 ± 0.03 |
| VLDL1 apoB48 | |||
| Concentration (mg/l) | 0.90 ± 0.27* | 1.83 ± 0.19 | 1.63 ± 0.15 |
| FCR (pools/day) | 17.6 ± 5.6 | 12.3 ± 2.4 | 10.3 ± 1.1 |
| Production rate (mg · kg−1 · day−1) | 0.44 ± 0.14* | 0.93 ± 0.09 | 0.73 ± 0.06 |
| VLDL1 apoB100 | |||
| Concentration (mg/dl) | 0.70 ± 0.16* | 1.76 ± 0.42 | 1.97 ± 0.51 |
| FCR (pools/day) | 11.8 ± 3.0 | 11.7 ± 0.9 | 8.2 ± 1.4 |
| Production rate (mg · kg−1 · day−1) | 3.40 ± 0.93* | 8.98 ± 2.05 | 6.09 ± 1.08 |
| VLDL2 TG (mmol/l) | 0.19 ± 0.06* | 0.25 ± 0.03 | 0.36 ± 0.12 |
| VLDL2 cholesterol (mmol/l) | 0.07 ± 0.02* | 0.11 ± 0.02 | 0.14 ± 0.03 |
| VLDL2 apoB48 | |||
| Concentration (mg/l) | 1.12 ± 0.33* | 1.58 ± 0.11 | 1.56 ± 0.15 |
| FCR (pools/day) | 10.2 ± 1.99 | 12.9 ± 1.5 | 11.3 ± 1.7 |
| Production rate (mg · kg−1 · day−1) | |||
| Total | 0.44 ± 0.14* | 0.89 ± 0.08 | 0.75 ± 0.05 |
| Direct | 0.00 ± 0.01 | −0.03 ± 0.02 | 0.01 ± 0.02 |
| Via VLDL1 | 0.44 ± 0.14* | 0.93 ± 0.09 | 0.73 ± 0.06 |
| VLDL2 apoB100 | |||
| Concentration (mg/dl) | 0.94 ± 0.31* | 1.68 ± 0.58 | 1.90 ± 0.47 |
| FCR (pools/day) | 15.6 ± 4.2 | 16.7 ± 2.8 | 11.7 ± 1.7 |
| Production rate (mg · kg−1 · day−1) | |||
| Total | 5.94 ± 1.69 | 11.2 ± 2.5 | 9.07 ± 1.52 |
| Direct | 2.54 ± 1.36 | 2.21 ± 0.65 | 2.98 ± 0.98 |
| Via VLDL1 | 3.40 ± 0.93* | 8.98 ± 2.05 | 6.09 ± 1.08 |
Data are means ± SE for the duration of the 10-h kinetic study.
*P < 0.05 vs. SAL.
Effect of acute hyperinsulinemia, with and without decreased plasma FFAs, on VLDL1 and VLDL2 apoB48 and apoB100 concentrations.
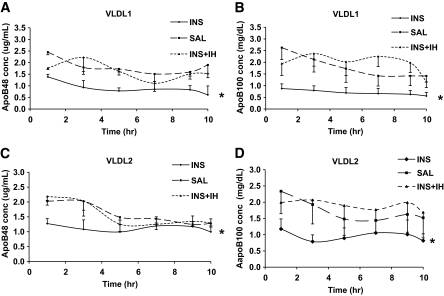
VLDL1 and VLDL2 apoB48 concentrations rose after the start of ingestion of the high-fat liquid formula (at 4:00 a.m.) and then remained elevated but constant throughout the 10-h kinetic study (from 10:00 a.m. to 8:00 p.m.). The overall difference for VLDL1 and VLDL2 apoB48 concentrations between the three studies was significant at P < 0.0001 and P = 0.036, respectively. Post hoc analysis revealed that insulin infusion in INS was associated with a significant reduction in both VLDL1 and VLDL2 apoB48 concentrations compared with SAL and INS + IH, which were not different from one another (Fig. 3A and C) (VLDL1 apoB48: INS 0.90 ± 0.27, SAL 1.83 ± 0.19, and INS + IH 1.63 ± 0.15 mg/l; VLDL2 apoB48: INS 1.12 ± 0.33, SAL 1.58 ± 0.11, and INS + IH 1.56 ± 0.15 mg/l) (Table 2). Similarly, both VLDL1 and VLDL2 apoB100 concentrations differed (P < 0.0001) between the three studies over the time course of the kinetic study. Again, with post hoc comparison, INS was shown to be significantly different from SAL and INS + IH, whereas no differences were observed between SAL and INS + IH. (Fig. 3B and D) (VLDL1 apoB100: INS 0.70 ± 0.16, SAL 1.76 ± 0.42, and INS + IH 1.97 ± 0.51 mg/dl; VLDL2 apoB100: INS 0.94 ± 0.31, SAL 1.96 ± 0.56, and INS+IH 1.90 ± 0.47 mg/dl) (Table 2).
FIG. 3.
Plasma VLDL1 and VLDL2 apoB48 and apoB100 concentrations over the time course of the kinetic study. VLDL1 (A and B) and VLDL2 (C and D) apoB48 (A and C) and -B100 (B and D) concentrations (conc) were measured after an overnight fast and then throughout the 10-h lipoprotein turnover study (0–10 h) in the three experimental conditions: INS, SAL, and INS + IH (n = 6). Subjects ingested a liquid fat formula for 16 h to achieve a constant fed state. Values are means ± SEM for each group. The overall significance for both VLDL1 and VLDL2 apoB48 concentrations between the three groups was *P < 0.0001, with VLDL1 apoB48 and apoB100 concentrations significantly lower in INS vs. SAL and IH + INS but no significant difference between INS + IH and SAL. Similarly for VLDL2, the overall significance between the three groups for apoB48 and apoB100 was *P = 0.036 for apoB48 and *P < 0.0001 for apoB100, with concentrations significantly lower in INS vs. SAL and IH + INS and no significant difference between INS + IH and SAL.
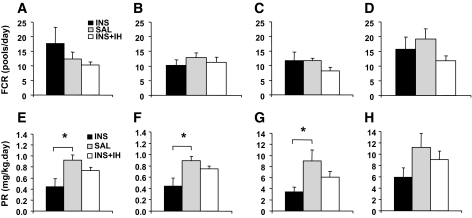
Effect of acute hyperinsulinemia, with and without decreased plasma FFAs, on VLDL1 and VLDL2 apoB48 and B100 fractional catabolic and production rates.
To elucidate the mechanism of hyperinsulinemia-associated reductions in both VLDL1 and VLDL2 apoB48 and B100 concentrations, we further examined whether the reductions were due to increased FCRs or decreased production rates. FCRs did not differ significantly between the three studies for apoB48 or apoB100 in either of the two VLDL fractions (Table 2; Fig. 4A–D) (individual kinetics parameters are shown in the supplementary Table, which is available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1297/DC1). Instead, the decreased plasma concentrations were due to reduced production, indicated by lower production rates of apoB48 and apoB100 in VLDL1 (INS vs. SAL, P = 0.009 for apoB48 and P = 0.029 for apoB100) and apoB48 in VLDL2 (P = 0.01 vs. SAL) and a trend toward a reduction in VLDL2 apoB100 (P = 0.067 vs. SAL) (Table 2; Fig. 4E–H). VLDL1 and -2 apoB48 and apoB100 production rates in INS + IH were in all cases intermediate between INS and SAL but were not significantly different from those in the other two studies. A further breakdown of the source of production indicated that the production of apoB48 in the VLDL2 fraction was entirely via VLDL1 in all treatment groups. Insulin-induced suppression of VLDL2 apoB48 concentration was therefore entirely attributable to its reduced production via VLDL1 (INS vs. SAL, P < 0.05). In contrast, a significant proportion of the production of VLDL2 apoB100 was direct (i.e., not via VLDL1), which was similar between treatments. Production of VLDL2 apoB100 via VLDL1 was significantly suppressed by insulin (INS vs. SAL, P < 0.05), whereas direct VLDL2 apoB100 production was not suppressed by insulin.
FIG. 4.
Effect of insulin infusion on VLDL1 and VLDL2 apoB48 and apoB100 FCRs and production rates. VLDL1 apoB48 (A), VLDL2 apoB48 (B), VLDL1 apoB100 (C), and VLDL2 apoB100 (D) FCRs were determined in subjects receiving either INS, SAL, or INS + IH infusion. ApoB48 and apoB100 FCR in VLDL1 and VLDL2 did not differ significantly between the three experimental protocols (A–D). VLDL1 apoB48 (E) and apoB100 (F) production rates decreased significantly in INS compared with SAL and INS + IH, with the overall significance between the three studies *P = 0.009 and *P = 0.029, respectively. VLDL2 apoB48 production rate (G) also decreased significantly with insulin infusion (*P = 0.010), while apoB100 production rate (H) did not reach significance but showed a strong trend toward reduction (P = 0.067). VLDL1 and -2 apoB48 and B100 production rates in INS + IH were in all cases intermediate between INS and SAL but were not significantly different from INS or SAL.
DISCUSSION
In this study, we investigated the effect of acute hyperinsulinemia on intestinal and hepatic lipoprotein production in humans in a constant fed state in the presence and absence of insulin-induced suppression of plasma FFAs. We have previously shown that insulin acutely suppresses TG-rich lipoprotein (TRL) total apoB (mainly apoB100) TG and particle production—at least partly as a result of hyperinsulinemia-induced suppression of plasma FFAs (13). We have also recently shown that short-term elevation of plasma FFAs stimulates hepatic apoB100-containing lipoprotein production, in both the fasted (13) and fed (14) states, as well as intestinal apoB48-containing lipoprotein production in the fed state (14), suggesting that factors similar to those involved in the regulation of hepatic lipoprotein production regulate intestinal lipoprotein production. Here, in addition to confirming our previous findings that insulin acutely suppresses hepatic apoB100-containing lipoprotein production, we also demonstrated for the first time in humans that insulin acutely suppresses intestinal apoB48-containing lipoprotein production, which could only be partly accounted for by insulin-mediated suppression of plasma FFAs and may involve mechanisms independent of suppression of plasma FFAs.
We examined apoB48 and apoB100 lipoprotein metabolism in large VLDL1 and small VLDL2 subclasses of TRL, separately. VLDL1 and VLDL2 were isolated by cumulative flotation gradient ultracentrifugation, and the chylomicron fraction was removed before the remaining two fractions were separated. The chylomicron fraction contains relatively few, very large, TRL particles with considerably less apoB48 and -B100 content compared with that of VLDL1 and VLDL2 fractions (35,36). In fact, in our study the apoB48 content in the chylomicron fraction was too low to be utilized for accurate quantification of stable isotope enrichment and kinetic modeling. We therefore limited our kinetic studies to the VLDL1 and VLDL2 fractions. A novel finding in our study, besides the demonstration that insulin acutely suppresses lipoprotein production of both the hepatic and intestinal sources, was that both VLDL1 and VLDL2 fractions responded to insulin in a qualitatively similar fashion. Several previous studies have suggested that these two fractions might possess distinct kinetic properties. Adiels et al. (17) have reported that insulin acutely suppresses VLDL1 apoB secretion while stimulating VLDL2 apoB secretion, demonstrating an inverse response between VLDL1 and VLDL2 to insulin. Malmstrom et al. (22) showed a decrease in VLDL1 apoB production with INS infusion, similar to the findings of the present study, while no net response to insulin was noted in the VLDL2 fraction. One obvious difference between our study and those of Adiels et al. (17) and Malmstrom et al. (22) is that our study was performed with subjects in the fed rather than the fasted state, with ongoing absorption of luminal fatty acids. Whether this factor accounts for the difference in insulin's effects on VLDL2 apoB100 production remains to be determined. If direct loss of VLDL1 apoB48 and B100 is minimal and the majority of VLDL1 is converted to VLDL2, as was the case in the current study, an intervention such as INS infusion that suppresses VLDL1 apoB production would subsequently lead to a reduced production of VLDL2 apoB from VLDL1. Our study therefore is in agreement with the study of Malmstrom et al. (22), with extended information on apoB48 production. The dominant effect of acute hyperinsulinemia is to suppress hepatic and intestinal production of large, VLDL1-density fraction particle production, whereas the reduction in VLDL2 apoB48 and -B100 in response to acute hyperinsulinemia was due to the reduction in production via VLDL1 apoB48 and -B100 but not their direct production. This finding would seem to suggest a similar mechanism of action in hepatocytes and intestinal enterocytes.
Although there were no significant detectable effects of the three experimental conditions on the FCR of VLDL1 and -2 apoB48 and apoB100, we cannot definitively exclude an effect of these interventions on particle clearance. In fact, both heparin and insulin have been well described to stimulate lipase activity (37), which would be anticipated to enhance particle clearance. With the use of a constant infusion of an endogenous tracer, FCR is measured as a replacement of unlabeled VLDL with the endogenous tracer, i.e., the appearance but not the disappearance of the tracer. Although there might be a transient increase in the initial FCR immediately after INS + IH infusion, kinetics was measured in our study in a newly established steady state after 3 h of INS + IH infusion. In our previous studies that examined the effect of insulin on VLDL production in humans using a semiquantitative radiolabeling technique (13,18,19), we noted a rapid decline in VLDL pool size after the infusion of INS + IH, and this was presumably contributed to by stimulation of particle clearance (in addition to suppression of production), although the kinetics during the non–steady state initial 3-h window could not be assessed using that experimental method. However, after ∼3 h a new steady state was established, which was characterized predominantly by suppression of production. Therefore, it is possible that stimulation of TRL clearance by heparin and insulin are most marked shortly after initial administration of insulin, heparin, or both. If there was an initial increase in apoB FCR in the present study, this may not have been detectable because the kinetics study was started 3 h after INS + IH infusion began in order to allow a new steady state to occur. A second possible factor is the fact that our subjects were studied in the constant fed state—as opposed to the majority of previous studies that have used similar techniques in humans. Perhaps the constant influx of lipid in some way masks an effect of heparin and insulin on particle clearance. A third theoretical possibility that could explain the absence of a detectable effect on FCR is that accumulation of synthetic Intralipid TRL particles may have impaired the clearance of endogenously synthesized apoB-containing TRL particles (38,39), thereby neutralizing any theoretical increase in clearance by insulin or Intralipid.
The mechanism by which insulin suppresses lipoprotein production is not clear. Insulin decreases FFA flux to the liver by its antilipolytic effect in adipose and other extrahepatic tissues (40), thereby indirectly suppressing VLDL biosynthesis and hepatic lipoprotein production in previous studies (13) and intestinal lipoprotein production as shown in the present study. However, suppression of hepatic lipoprotein production was not completely abrogated by preventing the insulin-mediated suppression of FFAs; thus, when Intralipid was coinfused with insulin, production rates of intestinal and hepatic lipoprotein particles tended to be intermediate between the INS and SAL experiments—though not significantly different from either study. We speculate, therefore, that the effect of insulin on lipoprotein production is not fully attributed to its antilipolytic and FFA-mediated effects and that mechanisms independent of suppression of plasma FFAs are also involved, as we have previously demonstrated for hepatic lipoprotein production (13). Previous studies suggest that insulin regulates hepatic lipoprotein production at a co- or posttranslational level because acute hyperinsulinemia does not affect apoB mRNA levels (15,41,42), although it is also noted that direct evidence that insulin promotes apoB degradation in humans is currently not available and is still controversial in animal models. For instance, apoB secretion is increased in mice with complete deficiency of liver insulin receptor (43) but is decreased in mice with very low levels of liver insulin receptor (44). Insulin may mediate VLDL formation in the liver through regulation of the insulin-signaling cascade (45–47) and through upregulation of genes responsible for shifting TG and FFA from VLDL formation into the cytosolic storage pool and promotion of cytosolic lipid droplets formation (48,49). Microsomal TG transfer protein (MTP) is necessary for the assembly of the nascent lipoprotein particles, and insulin negatively regulates MTP expression via activation of the mitogen-activated protein kinase (50), although the time course of insulin regulation of MTP protein suggests that it is unlikely to be the key regulatory site of acute insulin action (51). More recently, in vivo and ex vivo studies in the Syrian Golden hamsters have demonstrated that intestinal lipoprotein production in the chow-fed hamsters is responsive to the inhibitory effect of insulin, whereas in insulin-resistant fructose-fed hamsters, intestinal lipoprotein production is refractory to insulin inhibition, suggesting aberrant insulin signaling as an important factor in intestinal lipoprotein particle overproduction in insulin-resistant states (26). Qin et al. (52) have recently shown that intestinal insulin resistance induced by the proinflammatory cytokine tumor necrosis factor-α (TNF-α) is associated with overproduction of intestinally derived lipoproteins in hamsters. TNF-α acts via TNF-α receptors p55 and p75, and the induction of the p38 mitogen-activated protein kinase pathway could be one of the mechanisms leading to insulin resistance and accompanying intestinal lipoprotein overproduction in those animals. LDL receptor (LDLR) can affect VLDL apoB production through modulation of intracellular apoB degradation (53,54). VLDL apoB production is increased in LDLR-null familial hypercholesterolemia patients (55). Acute administration of insulin upregulates LDLR (56); thus, acute insulin may suppress VLDL apoB production through upregulation of LDLR in our study. Future studies examining the effects of acute insulin on VLDL apoB production in LDLR-null patients would yield more direct evidence for this mechanism.
This study has extended previous findings in animal models of insulin resistance to humans and also from the liver to the intestine, demonstrating conclusively that intestinal lipoprotein particle production is inhibited in vivo by acute hyperinsuliemia. Furthermore, in the present study we have shown that insulin inhibits hepatic apoB100 production acutely in the constant fed state—not only in the fasted state, as has previously been shown (13). Postprandial lipemia and accumulation of intestinally derived apoB48-containing lipoproteins seen in diabetes is therefore due not only to delayed clearance but also to increased production of these particles. In this respect, the intestine is regulated in a fashion that is similar to the liver. Even though the absolute production rate of hepatic (apoB100) lipoprotein particles was about eightfold greater than that of intestinal (apoB48) particles under these experimental conditions, an increase in apoB48 production may contribute to postprandial lipaemia and potentially to atherosclerosis, given that those particles have been shown to be atherogenic (11,30). Future studies in humans are required to assess whether the acute suppression of intestinal lipoprotein production by insulin is blunted in the insulin-resistant state and diabetes, as has been shown recently in the fructose-fed Syrian Golden hamsters (26) and as we (18) and others (20) have previously shown to be the case for hepatic VLDL secretion. If this is indeed shown to be the case, then resistance to insulin's acute suppressive effect may play an important role in the overproduction of intestinal lipoproteins in insulin-resistant and type 2 diabetic individuals.
In conclusion, the present study provides evidence that intestinal apoB48-containing lipoprotein production in humans is inhibited by acute hyperinsulinemia and that this effect is in part dependant on insulin-mediated suppression of plasma FFAs. This study extends previous observations in animal models to humans and now provides definitive proof that circulating metabolites (FFAs) and hormones (insulin) play an important regulatory role in the secretion of intestinal lipoproteins.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding from the Canadian Institutes for Health Research (MOP-43839). M.P. is the recipient of a Postdoctoral Fellowship Award from the Banting and Best Diabetes Centre, Toronto, Ontario, Canada. B.W.P. was supported by National Institutes of Health Grant P30 DK56341 (Clinical Nutrition Research Unit). G.F.L. holds a Canada Research Chair in Diabetes and is a Career Investigator of the Heart and Stroke Foundation of Canada.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
We are indebted to Patricia Harley, RN, for her assistance with subject recruitment and conducting the clinical protocol.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ginsberg HN: Insulin resistance and cardiovascular disease. J Clin Invest 2000; 106: 453– 458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis GF, Steiner G: Hypertriglyceridemia and its metabolic consequences as a risk factor for atherosclerotic cardiovascular disease in non-insulin-dependent diabetes mellitus. Diabetes Metab Rev 1996; 12: 37– 56 [DOI] [PubMed] [Google Scholar]
- 3.Adiels M, Olofsson SO, Taskinen MR, et al. : Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler ThrombVasc Biol 2008; 28: 1225– 1236 [DOI] [PubMed] [Google Scholar]
- 4.Chan DC, Barrett PH, Watts GF: Recent studies of lipoprotein kinetics in the metabolic syndrome and related disorders. Curr Opin Lipidol 2006; 17: 28– 36 [DOI] [PubMed] [Google Scholar]
- 5.Curtin A, Deegan P, Owens D, et al. : Elevated triglyceride-rich lipoproteins in diabetes: a study of apolipoprotein B-48. Acta Diabetol 1996; 33: 205– 210 [DOI] [PubMed] [Google Scholar]
- 6.Mero N, Syvanne M, Taskinen MR: Postprandial lipid metabolism in diabetes. Atherosclerosis 1998; 141( Suppl. 1): S53– S55, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi H, Saitoh S, Takagi S, et al. : Relationship between insulin-resistance and remnant-like particle cholesterol. Atherosclerosis 2002; 164: 167– 170 [DOI] [PubMed] [Google Scholar]
- 8.Schaefer EJ, McNamara JR, Shah PK, et al. : Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care 2002; 25: 989– 994 [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi A, Fukushima M, Sakai M, et al. : Remnant-like particle cholesterol, triglycerides, and insulin resistance in nonobese Japanese type 2 diabetic patients. Diabetes Care 2000; 23: 1766– 1769 [DOI] [PubMed] [Google Scholar]
- 10.Adeli K, Lewis GF: Intestinal lipoprotein overproduction in insulin-resistant states. Curr Opin Lipidol 2008; 19: 221– 228 [DOI] [PubMed] [Google Scholar]
- 11.Duez H, Lamarche B, Uffelman KD, et al. : Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler ThrombVasc Biol 2006; 26: 1357– 1363 [DOI] [PubMed] [Google Scholar]
- 12.Hogue JC, Lamarche B, Tremblay AJ, et al. : Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J Lipid Res 2007; 48: 1336– 1342 [DOI] [PubMed] [Google Scholar]
- 13.Lewis GF, Uffelman KD, Szeto LW, et al. : Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 1995; 95: 158– 166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duez H, Lamarche B, Valero R, et al. : Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation 2008; 117: 2369– 2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adeli K, Theriault A: Insulin modulation of human apolipoprotein B mRNA translation: studies in an in vitro cell-free system from HepG2 cells. Biochem Cell Biol 1992; 70: 1301– 1312 [DOI] [PubMed] [Google Scholar]
- 16.Sparks JD, Sparks CE: Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim Biophys Acta 1994; 1215: 9– 32 [DOI] [PubMed] [Google Scholar]
- 17.Adiels M, Westerbacka J, Soro-Paavonen A, et al. : Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia 2007; 50: 2356– 2365 [DOI] [PubMed] [Google Scholar]
- 18.Lewis GF, Uffelman KD, Szeto LW, et al. : Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes 1993; 42: 833– 842 [DOI] [PubMed] [Google Scholar]
- 19.Lewis GF, Zinman B, Uffelman KD, et al. : VLDL production is decreased to a similar extent by acute portal vs. peripheral venous insulin. Am J Physiol 1994; 267: E566– E572 [DOI] [PubMed] [Google Scholar]
- 20.Malmstrom R, Packard CJ, Caslake M, et al. : Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia 1997; 40: 454– 462 [DOI] [PubMed] [Google Scholar]
- 21.Malmstrom R, Packard CJ, Watson TD, et al. : Metabolic basis of hypotriglyceridemic effects of insulin in normal men. Arterioscler Thromb Vasc Biol 1997; 17: 1454– 1464 [DOI] [PubMed] [Google Scholar]
- 22.Malmstrom R, Packard CJ, Caslake M, et al. : Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes 1998; 47: 779– 787 [DOI] [PubMed] [Google Scholar]
- 23.Sparks JD, Sparks CE: Obese Zucker (fa/fa) rats are resistant to insulin's inhibitory effect on hepatic apo B secretion. Biochem Biophys Res Commun 1994; 205: 417– 422 [DOI] [PubMed] [Google Scholar]
- 24.Sparks JD, Sparks CE: Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem 1990; 265: 8854– 8862 [PubMed] [Google Scholar]
- 25.Loirdighi N, Menard D, Levy E: Insulin decreases chylomicron production in human fetal small intestine. Biochim Biophys Acta 1992; 1175: 100– 106 [DOI] [PubMed] [Google Scholar]
- 26.Federico LM, Naples M, Taylor D, et al. : Intestinal insulin resistance and aberrant production of apolipoprotein B48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes 2006; 55: 1316– 1326 [DOI] [PubMed] [Google Scholar]
- 27.Xiao C, Giacca A, Lewis GF: Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia 2008; 51: 139– 146 [DOI] [PubMed] [Google Scholar]
- 28.Batal R, Tremblay M, Barrett PH, et al. : Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J Lipid Res 2000; 41: 706– 718 [PubMed] [Google Scholar]
- 29.Karpe F, Olivecrona T, Hamsten A, et al. : Chylomicron/chylomicron remnant turnover in humans: evidence for margination of chylomicrons and poor conversion of larger to smaller chylomicron remnants. J Lipid Res 1997; 38: 949– 961 [PubMed] [Google Scholar]
- 30.Mero N, Syvanne M, Eliasson B, et al. : Postprandial elevation of ApoB-48-containing triglyceride-rich particles and retinyl esters in normolipemic males who smoke. Arterioscler Thromb Vasc Biol 1997; 17: 2096– 2102 [DOI] [PubMed] [Google Scholar]
- 31.Patterson BW, Zhao G, Elias N, et al. : Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 1999; 40: 2118– 2124 [PubMed] [Google Scholar]
- 32.Pavlic M, Valero R, Duez H, et al. : Triglyceride-rich lipoprotein-associated apolipoprotein C-III production is stimulated by plasma free fatty acids in humans. Arterioscler ThrombVasc Biol 2008; 28: 1660– 1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobelli C, Toffolo G, Foster DM: Tracer-to-tracee ratio for analysis of stable isotope tracer data: link with radioactive kinetic formalism. Am J Physiol 1992; 262: E968– E975 [DOI] [PubMed] [Google Scholar]
- 34.Karpe F, Hamsten A, Uffelman K, et al. : Apolipoprotein B-48. Methods Enzymol 1996; 263: 95– 104 [DOI] [PubMed] [Google Scholar]
- 35.Johanson EH, Jansson PA, Gustafson B, et al. : Early alterations in the postprandial VLDL1 apoB-100 and apoB-48 metabolism in men with strong heredity for type 2 diabetes. J Intern Med 2004; 255: 273– 279 [DOI] [PubMed] [Google Scholar]
- 36.Verseyden C, Meijssen S, Castro CM: Postprandial changes of apoB-100 and apoB-48 in TG rich lipoproteins in familial combined hyperlipidemia. J Lipid Res 2002; 43: 274– 280 [PubMed] [Google Scholar]
- 37.Malmstrom R, Packard CJ, Caslake M, et al. : Effect of heparin-stimulated plasma lipolytic activity on VLDL APO B subclass metabolism in normal subjects. Atherosclerosis 1999; 146: 381– 390 [DOI] [PubMed] [Google Scholar]
- 38.Al Shayji IA, Gill JM, Cooney J, et al. : Development of a novel method to determine very low density lipoprotein kinetics. J Lipid Res 2007; 48: 2086– 2095 [DOI] [PubMed] [Google Scholar]
- 39.Bjorkegren J, Packard CJ, Hamsten A, et al. : Accumulation of large very low density lipoprotein in plasma during intravenous infusion of a chylomicron-like triglyceride emulsion reflects competition for a common lipolytic pathway. J Lipid Res 1996; 37: 76– 86 [PubMed] [Google Scholar]
- 40.Lewis GF, Carpentier A, Adeli K, et al. : Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes 2002; Endocr Rev 23: 201– 229 [DOI] [PubMed] [Google Scholar]
- 41.Dashti N, Williams DL, Alaupovic P: Effects of oleate and insulin on the production rates and cellular mRNA concentrations of apolipoproteins in HepG2 cells. J Lipid Res 1989; 30: 1365– 1373 [PubMed] [Google Scholar]
- 42.Pullinger CR, North JD, Teng BB, et al. : The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin and measurement of the mRNA half-life. J Lipid Res 1989; 30: 1065– 1077 [PubMed] [Google Scholar]
- 43.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, et al. : Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 2008; 7: 125– 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han S, Liang CP, Westerterp M, et al. : Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest 2009; 119: 1029– 1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpentier A, Taghibiglou C, Leung N, et al. : Ameliorated hepatic insulin resistance is associated with normalization of microsomal triglyceride transfer protein expression and reduction in very low density lipoprotein assembly and secretion in the fructose-fed hamster. J Biol Chem 2002; 277: 28795– 28802 [DOI] [PubMed] [Google Scholar]
- 46.Phung TL, Roncone A, Jensen KL, et al. : Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J Biol Chem 1997; 272: 30693– 30702 [DOI] [PubMed] [Google Scholar]
- 47.Sidiropoulos KG, Meshkani R, Avramoglu-Kohen R, et al. : Insulin inhibition of apolipoprotein B mRNA translation is mediated via the PI-3 kinase/mTOR signaling cascade but does not involve internal ribosomal entry site (IRES) initiation. Arch Biochem Biophys 2007; 465: 380– 388 [DOI] [PubMed] [Google Scholar]
- 48.Andersson L, Bostrom P, Ericson J, et al. : PLD1 and ERK2 regulate cytosolic lipid droplet formation. JCell Sci 2006; 119: 2246– 2257 [DOI] [PubMed] [Google Scholar]
- 49.Magnusson B, Asp L, Bostrom P, et al. : Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol 2006; 26: 1566– 1571 [DOI] [PubMed] [Google Scholar]
- 50.Au WS, Kung HF, Lin MC: Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes 2003; 52: 1073– 1080 [DOI] [PubMed] [Google Scholar]
- 51.van Greevenbroek MM, Robertus-Teunissen MG, Erkelens DW, et al. : Participation of the microsomal triglyceride transfer protein in lipoprotein assembly in Caco-2 cells: interaction with saturated and unsaturated dietary fatty acids. J Lipid Res 1998; 39: 173– 185 [PubMed] [Google Scholar]
- 52.Qin B, Qiu W, Avramoglu RK, et al. : Tumor necrosis factor-α induces intestinal insulin resistance and stimulates the overproduction of intestinal apolipoprotein B48-containing lipoproteins. Diabetes 2007; 56: 450– 461 [DOI] [PubMed] [Google Scholar]
- 53.Nassir F, Xie Y, Patterson BW, et al. : Hepatic secretion of small lipoprotein particles in apobec-1-/- mice is regulated by the LDL receptor. J Lipid Res 2004; 45: 1649– 1659 [DOI] [PubMed] [Google Scholar]
- 54.Twisk J, Gillian-Daniel DL, Tebon A, et al. : The role of the LDL receptor in apolipoprotein B secretion. J Clin Invest 2000; 105: 521– 532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millar JS, Maugeais C, Ikewaki K, et al. : Complete deficiency of the low-density lipoprotein receptor is associated with increased apolipoprotein B-100 production. Arterioscler Thromb Vasc Biol 2005; 25: 560– 565 [DOI] [PubMed] [Google Scholar]
- 56.Mazzone T, Foster D, Chait A: In vivo stimulation of low-density lipoprotein degradation by insulin. Diabetes 1984; 33: 333– 338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.