Abstract
14-3-3σ (also called stratifin) is specifically expressed in the stratified squamous epithelium and its function was recently shown to be linked to epidermal stratification and differentiation in the skin. In this study, we investigated its role in corneal epithelium cell proliferation and differentiation. We showed that the 14-3-3σ mutation in repeated epilation (Er) mutant mice results in a dominant negative truncated protein. Primary corneal epithelial cells expressing the dominant negative protein failed to undergo high calcium-induced cell cycle arrest and differentiation. We further demonstrated that blocking endogenous 14-3-3σ activity in corneal epithelial cells by overexpressing dominative negative 14-3-3σ led to reduced Notch activity and Notch1/2 transcription. Significantly, expression of the active Notch intracellular domain overcame the block in epithelial cell differentiation in 14-3-3σ mutant-expressing corneal epithelial cells. We conclude that 14-3-3σ is critical for regulating corneal epithelial proliferation and differentiation by regulating Notch signaling activity.
Keywords: corneal epithelial, proliferation, differentiation, 14-3-3σ, Notch
Introduction
The corneal epithelium is a stratified squamous epithelium that undergoes constant renewal with an estimated turnover time of about 7–10 days in mammals [1] The corneal epithelium is composed of a mitotically active basal monolayer and differentiating suprabasal layers. The edge of the cornea at the junction with the sclera is comprised of a limbal region which is a niche for corneal stem cells that generate new basal cells [2; 3; 4]. Recent studies have shown that some stem-like cells scattered throughout the corneal epithelium can regenerate the corneal and conjunctival epithelia [5]. The corneal epithelial progenitor cells in the basal layer of the normal corneal epithelium generate transient amplifying (TA) cells that undergo multiple divisions and gradually migrate to the upper layer. During this process, the differentiating epithelial cells switch expression of differentiation-specific cytokeratins from the universal stratified epithelial cytokeratin pair of keratin-5 (K5) and K-14 to cytokeratin-3 (K3) and K12 [6]. The terminally differentiated epithelial cells in the most superficial layer form desmosomes and tight junctions, which act as a barrier to protect the cornea from environmental insults [7; 8]. The same corneal epithelial differentiation process can be largely achieved in cell culture using culture conditions that allow the culture, expansion, and differentiation of corneal epithelial progenitor cells. The molecular machinery that controls and maintains corneal epithelial cell homeostasis is still poorly understood.
14-3-3σ belongs to a seven member family of highly conserved phosphoserine/phosphothreonine-binding proteins regulating cell signaling of numerous processes that control the cell cycle, cell growth, differentiation, apoptosis, and cell migration [9]. In contrast to other 14-3-3 proteins, 14-3-3σ is specifically expressed in the stratified squamous epithelium and forms homodimers with a target specificity different from those of dimers of other family members [10]. Because of its unique target substrates, 14-3-3σ has specific cellular functions; for example, it is the only tumor suppressor protein in the family. It is also an important G2M cell cycle checkpoint regulator and its expression is induced by DNA damage stress through a p53-dependent pathway [11]. Epigenetic silencing of the 14-3-3σ gene through promoter methylation has been reported in various tumors of epithelial origin [12].
14-3-3σ is a key regulator of stratified squamous epithelial homeostasis. In skin, after exiting from the stem cell compartment, differentiating keratinocytes show increased expression of 14-3-3σ [13], suggesting that 14-3-3σ is involved in initiation of differentiation of epithelial cells. Studies in Er mice, in which 14-3-3σ is mutated, have clearly demonstrated that 14-3-3σ is essential for skin epithelial differentiation [14; 15]. We previously showed that, in Er mice, a single nucleotide insertion in the 14-3-3σ coding region leads to a truncated protein lacking the C-terminal 40 amino acids, which form part of the ligand-binding domain [15]. Theoretically, the mutant 14-3-3σ could still dimerize with wild-type 14-3-3σ, but the resulting complex would be inactive due to its not having any ligand binding ability, suggesting that the mutant 14-3-3σ would act as a dominant negative protein.
In the present study, we showed that 14-3-3σ is a key regulator of corneal epithelial cell homeostasis. Blocking 14-3-3σ activity with the dominant negative mutant 14-3-3σ resulted in increased cell proliferation and diminished differentiation of corneal epithelial cells. These defects were associated with reduced Notch activity and Notch receptor expression. Furthermore, reintroduction of an active form of Notch restored the differentiation of the mutant cells. Our data suggest that 14-3-3σ regulates corneal epithelial cell proliferation and differentiation by maintaining Notch activity.
Materials and Methods
Animals
C57BL/6J mice were purchased from the Jackson Laboratory and were housed under pathogen-free conditions in accordance with institutional guidelines. Animal care and use were approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Corneal epithelial cell culture
Eyes were collected from euthanized mice and treated overnight at 4 °C for with dispase (10 mg/ml in 1640 medium) to disrupt the basement membrane, then the epithelial sheets were peeled off and digested in 0.25% trypsin-EDTA at 37 °C for 5–10 min. The cells were washed in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) [HyClone] and resuspended in keratinocyte serum-free medium (KSFM; Invitrogen) containing 0.05 mM calcium and plated on collagen-coated tissue culture plates. To induce differentiation, the cultures were exposed to 1.6 mM calcium for 48 h, then were fixed in 4% paraformaldehyde (PFA) for immunostaining or collected for Western analysis or Q-PCR analysis.
Immunohistological analysis
The PFA-fixed cell were treated for 25 min at 95 °C with Tris-EDTA buffer (10 mM Tris base, 1 mM EDTA, pH 9). All subsequent steps were at room temperature. The treated cells in 24-well plates were incubated for 1 h with 5% donkey serum, then for 90 min with primary antibodies and for 30 min with a fluorescein-conjugated secondary antibody, both in PBS containing 1% BSA, 0.2% powdered skim milk, and 0.3% Triton X-100 The primary antibodies used were mouse anti-p63 (1:200, sc-8431, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rat anti-BrdU (1:800, MAS 250c, Harlan-Sera Lab, Loughborough, UK), and rabbit anti-ZO-1 (1:600, Cat #61-7300, Zymed Laboratories Inc., San Francisco, CA). Secondary Cy3- or FITC-conjugated antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Nuclei were stained with diamidinophenylindole (DAPI).
293T culture, transfection, and co-immunoprecipitation
293T human embryonic kidney cells (Invitrogen, Carlsbad, California) in 10-cm plates were maintained in DMEM medium containing 10 % FBS and were transfected by the calcium phosphate method. Two days after transfection, the cells were lysed in cold RIPA buffer [20 mM Tris-HCl, 100 mM NaCl, 0.2% deoxycholic acid, 0.2% Triton X-100, 0.2% NP-40, and protease inhibitor cocktail (Roche)]. After clearance by incubation for 30 min with 50 μl of normal goat IgG (from Santa Cruz, both 1 μg/ml) and 100 μl of protein G-coupled agarose beads slurry (Amesham Biosciences), the lysates were incubated for 2 h at 4°C with 30 μl of protein G-coupled agarose beads slurry goat antibodies against the C-terminus of 14-3-3σ (1:200, sc-7683) or normal goat IgG (sc-2028) (both from Santa Cruz, both 1 μg/ml). The beads were then washed 5 times before the antibody-antigen complexes were released from the beads with SDS lysis buffer and loaded on 10% SDS-polyacrylamide gels and subjected to Western analysis with goat antibody against the N-terminus of 14-3-3σ (1:200, sc-7681, Santa Cruz).
Western blotting for NCID
Please see details in the supplementary materials.
Quantitative polymerase chain reaction
Total RNA was isolated from mouse primary corneal epithelial cell cultures using RNeasy Protect Mini Kit (Qiagen). Reverse transcription was performed with 2 μg of total RNA using random hexamers and a cDNA synthesis kit (SuperScript First-Strand Synthesis System, Invitrogen, Carlsbad, CA). The quantitative polymerase chain reaction (Q-PCR) was performed according to the manufacturer’s instructions (Stratagene Mx3000P QPCR System, Stratagene, San Diego, California) using a primer set for actin as the internal standard. Primers for mouse Notch1 (13177625a1), Notch2 (ID 33859592a3), Jagged1 (ID 7305197a1), Jagged2 (ID 2765404a1), and RBP1 (6755300a1) were designed based on the PrimerBank database (Harvard Medical School). The Hes1 primer sequences have been published previously [16].
Lentiviral production
The lentivector EF.hiCN1.CMV.GFP was obtained from Addgene. Details of the production of the lentiviral expression vectors CMV.GFP and CMV.14-3-3σ Mut have been published previously [15]. Lentiviruses were produced by co-transfection with lentiviral vector and the packaging vectors pMDL, pRev, and pVSVG (K4975-00, Invitrogen, Carlsbad, California) as described previously [17].
Results
The mutant 14-3-3σ has a dominant negative effect
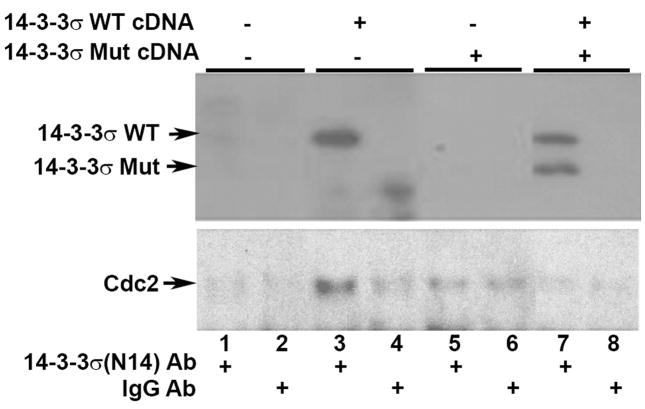
It is thought that the 14-3-3σ mutant might act as a dominant negative, as it retains the homodimerization domain and can dimerize with wild type (WT) 14-3-3σ, but lacks the region that participates in phospho-binding motif formation [15] and therefore the complex is inactive. To determine whether mutant 14-3-3σ functions as a dominant negative, we first asked whether there was an interaction between WT 14-3-3σ and mutant 14-3-3σ. 293T cells were transfected with WT 14-3-3σ cDNA and/or mutant 14-3-3σ cDNA, then the cells were solubilized and the lysate subjected to immunoprecipitation with antibodies recognizing the C-terminus of WT 14-3-3σ, but not the 14-3-3σ mutant, which lacks this region. Western blots of the immunoprecipitated protein using antibodies against the N-terminus of 14-3-3σ (recognizing both WT and mutant 14-3-3σ) showed that the mutant 14-3-3σ co-immunoprecipitated with WT 14-3-3σ (Fig. 1, top panel, lane 7), thus demonstrating that the mutant 14-3-3σ forms heterodimers with WT 14-3-3σ. In the lysates of the cell co-transfected with equal amounts of the WT and mutant cDNAs, more WT protein was immunoprecipitated than mutant protein, indicating that both WT homodimers and WT/Mut heterodimers were formed, but heterodimers were dominant. Next, we asked whether the WT/mutant 14-3-3σ dimer could bind ligands. A well-known ligand for 14-3-3σ is Cdc2, a cyclin-dependent kinase that is critical for G2M cell cycle progress [11; 18]. By Western blotting using anti-Cdc2 antibody, we showed that Cdc2 was bound to the WT 14-3-3σ homodimer (Fig. 1, lane 3 vs lane 4), but was absent in the sample containing dominantly WT/Mut heterodimers (Fig. 1, Lane 3 vs lane 7). These data demonstrate that mutant 14-3-3σ indeed functions as a dominant negative binding partner blocking the function of WT 14-3-3σ.
Fig. 1.
Mutant 14-3-3σ protein interacts with WT 14-3-3σ protein and inhibits the binding of WT 14-3-3σ protein to Cdc2. 293 T cells were transiently transfected with empty transcription vector cDNA (lanes 1–2), WT 14-3-3σ cDNA (lanes 3–4), mutant 14-3-3σ cDNA (lanes 5–6), or an equal mixture of WT and Er mutant cDNAs (lanes 7–8). Two days later, the cells were lysed and subjected to immunoprecipitation with either an anti-14-3-3σ antibody recognizing only the WT protein (lanes 1, 3, 5, and 7) or control IgG (lanes 2, 4, 6, and 8), followed by Western blotting using an anti-14-3-3σ antibody that recognizes both forms (top panel) or anti-Cdc2 antibody (bottom panel).
Differentiation of WT corneal epithelial cells is blocked by mutant 14-3-3σ
To examine the role of 14-3-3σ in controlling corneal epithelial proliferation and differentiation, we used primary cultured mouse corneal epithelial cells isolated and cultured essentially as described in previous reports [19; 20; 21; 22]. We then studied the responses of the freshly isolated corneal epithelial cells to low and high extracellular Ca2+ and found that primary mouse corneal epithelial cells could be maintained in defined keratinocyte serum-free medium (KSFM) medium containing low extracellular Ca2+ (0.05 mM) with basal cell phenotypes, such as high proliferation, p63 expression, and low ZO1 expression. A high extracellular Ca2+ concentration (1.6 mM) rapidly induced corneal epithelial cell differentiation with suprabasal corneal epithelial cell characters, including reduced p63 expression and cell proliferation, as shown by BrdU labeling, and induction of tight junction formation, as shown by ZO-1 staining (see Fig. S1)
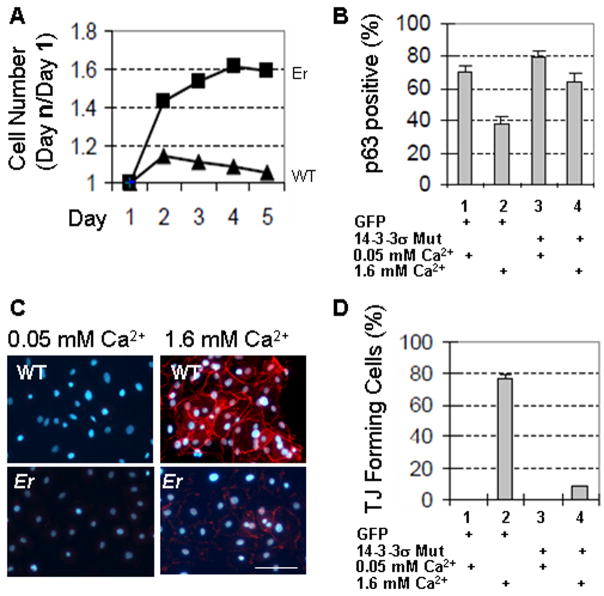
To study the function of 14-3-3σ in corneal epithelial cells, we transfected primary WT corneal epithelial cell cultures with lentiviruses expressing the dominant negative mutant 14-3-3σ to block endogenous 14-3-3σ activity, while control cells were transfected with the control protein Green Fluorescent Protein (GFP). Because high calcium-induced differentiation was accompanied by cell cycle arrest and tight junction formation, we counted cell numbers for 5 days in low or high calcium (1.6 mM) and examined whether tight junctions were formed after 48 h. The mutant 14-3-3σ significantly blocked WT corneal epithelial cell differentiation after high calcium induction, as demonstrated by continued cell growth (Fig. 2A) and expression of p63 (Fig. 2B) and by the absence of the tight junction differentiation marker ZO-1 (Fig. 2C and D). These data show that 14-3-3σ is important in promoting corneal epithelial cell differentiation and that the dominant negative mutant 14-3-3σ blocks 14-3-3σ activity, thus hampering high calcium-induced cell differentiation.
Fig. 2.
Differentiation of WT corneal epithelial cell is blocked by mutant 14-3-3σ. Primary corneal epithelial cells isolated from C57BL6J mice were transduced with GFP or Er mutant 14-3-3σ using lentiviral expression vectors. At 24 h after transduction, half of the cells were switched to high calcium medium (1.6 mM), while the others were left in low calcium medium. (A). Cell growth curve over 5 days. (B). Quantification of p63-positive cells in WT and mutant 14-3-3σ-expressing corneal epithelial cells after 48 h in low or high calcium medium. (C). Tight junction (TJ) visualized by ZO-1 staining (shown in red) in GFP- or 14-3-3σ mutant-expressing corneal epithelial cells cultures in low or high calcium medium for 48 h. Bar size=100μm (D). Quantification of TJ-forming cells in the samples shown in C.
Notch activity and Notch transcription are reduced in 14-3-3σ mutant-expressing corneal epithelial cells
The Notch pathway is a highly conserved cell signaling pathway which controls cell proliferation, differentiation, and fate [23] and is important for corneal epithelial proliferation and differentiation. It is well documented that ligand binding activates Notch to release the active form of the Notch receptor, i.e., the Notch intracellular domain (NICD), from the membrane, and the released NICD then translocates into the nucleus and regulates target gene transcription. We examined Notch activity in corneal epithelial cells expressing mutant 14-3-3σ. A high calcium concentration induced differentiation of WT corneal progenitor cells accompanied by the generation of NICD (Fig. 3A, lanes 3 and 4), whereas NICD production was completely blocked in mutant 14-3-3σ-expressing corneal epithelial cells (Fig. 3A, lanes 7 and 8). Upon ligand binding, NICD induces the transcription of multiple target genes, including Hes1. Notch1 has also been shown to be critical for vitamin A metabolism by modulating the transcription of cellular retinol binding protein 1 (RBP1), which is required to generate a pool of intracellular retinol. Hes1 and RBP1 expression was reduced or lost, respectively, in 14-3-3σ mutant-expressing corneal epithelial cells (Fig. 3B). To elucidate the cause of the reduced NICD activation, we examined the expression of the Notch receptor genes Notch1 and Notch2 and the Notch ligand genes Jagged1 and Jagged2 by Q-PCR analysis. Interestingly, we observed a significant reduction in Notch1 and Notch2 mRNA levels, but not in Jagged1 and Jagged2 mRNA levels (Fig. 3B).
Fig. 3.

Reduced Notch activity in 14-3-3σ mutant-expressing corneal epithelial cells. (A) Western blots showing the level of Notch1 NICD in corneal epithelial cells transduced with the control GFP-lentivirus (WT, 1–4) or with lentivirus expressing the 14-3-3σ mutant cDNA (Mut, 5–8) in low calcium (lanes 1, 2, 5, and 6) or high calcium (lanes 3, 4, 7, and 8). (B) Q-PCR analysis of Hes1, RBP1, Notch1, Notch2, Jagged1, and Jagged2 mRNAs in corneal epithelial cells transduced with lentivirus expressing either GFP or mutant 14-3-3σ.
Overexpression of NICD rescues the Er corneal cell differentiation defect
The functional role of Notch1 in controlling corneal epithelial cell fate and homeostasis has been demonstrated by studies on cornea conditional knockout mice [24]. To test whether reduced Notch activity was responsible for the defective Er mutant corneal epithelial cell phenotypes, we introduced active NICD into Er mutant corneal epithelial cells to evaluate if it would bypass the defect in 14-3-3σ and restore cell differentiation. Both wild-type and Er mutant corneal epithelial cells were transfected with NICD-expressing lentivirus and subjected to high calcium-induced cell differentiation. As anticipated, ectopically expressed NICD indeed induced expression of the Notch1 target gene Hes1 in both WT cells (Fig. 4A, lane 2) and mutant cells (Fig. 4A, lane 4). The almost complete loss of high calcium-induced differentiation in Er mutant corneal epithelial cells, as judged by lack of tight junction formation (Fig. 2D), was alleviated by forced expression of active NICD, as demonstrated by the increased number of cells that formed tight junctions (Fig. 4B and C). We conclude that ectopic overexpression of NICD enabled the Er mutant corneal epithelial cells to undergo calcium-induced differentiation and that 14-3-3σ controls corneal epithelial cell differentiation through a downstream Notch signaling pathway.
Fig. 4.
Defective mutant 14-3-3σ-expressing corneal epithelial cell differentiation can be rescued by expression of Notch1 NICD. (A). Wild-type (columns 1 and 2) or mutant 14-3-3σ-expressing (columns 3 and 4) corneal epithelial cells were transduced with NICD-expressing (columns 2 and 4) or GFP--expressing (columns 1 and 3) lentivirus (One day after transduction, the cells were switched to high calcium medium for 48 h, then Hes1 mRNA levels were assessed by Q-PCR. (B) 14-3-3σ mutant-expressing corneal epithelial cells were transduced by lentiviral GFP-expressing (top) or NICD/GFP expressing (bottom) vectors in high calcium medium for 48 h, then tight junction (TJ) formation was visualized by staining with anti-ZO-1 antibody. GFP or NICD-GFP lentiviral expression were showed in green (left). Bar size=100μm. (C) TJ-positive cells were quantified and the fold increase in TJ-positive cells in the NICD-expressing cells was calculated by comparison with the GFP-expressing controls.
Discussion and Conclusion
14-3-3σ is specifically expressed in stratified squamous cells. The differentiated keratinocytes that exit the progenitor cell compartment show a significant increase in 14-3-3σ expression, suggesting that 14-3-3σ is linked to the initial differentiation of skin epithelial cells [13]. In epidermal basal progenitor cells, 14-3-3σ expression is repressed by p63 and, when epidermal progenitor cells are committed to differentiation [25], 14-3-3σ expression is induced due to reduced p63 expression. The identification of the 14-3-3σ mutation in Er mice clearly established the role of 14-3-3σ in controlling epidermal epithelial development and differentiation [14; 15]
Both the epidermis and corneal epithelium are derived from ectoderm during development and share a similar regulatory machinery. However, the role of 14-3-3σ in the corneal epithelium has not established. Due to paranatal lethality, it is not feasible to obtain corneal epithelial cells directly from Er/Er embryos that completely lack 14-3-3σ activity. Instead, we studied the role of 14-3-3σ in corneal epithelial cells by expressing the mutant 14-3-3σ dominant negative protein to block endogenous WT 14-3-3σ activity and showed that blocking of 14-3-3σ activity in corneal epithelial cells resulted in increased BrdU incorporation and an increased p63+ cell population. As BrdU+/p63+ are markers for the TA cell population, we conclude that 14-3-3σ is critical for controlling the proliferation capacity of TA cells. In addition, 14-3-3σ was also shown to be necessary for promoting corneal epithelial cell differentiation, as evidenced by reduced calcium-induced tight junction formation when 14-3-3σ activity was blocked. These data suggest that, as in the epidermis, 14-3-3σ is a key regulator of corneal epithelial cell homeostasis.
Notch signaling is essential for controlling both cell proliferation and differentiation. A study with cornea-specific ablation of Notch1 showed that, in response to corneal wounding, Notch1-deficient corneal progenitor cells give rise to hyperplastic, keratinized cells that continue to express markers of progenitor cells and invade the underlying stroma, producing neovascularization and ultimately a corneal plaque [24]. Vitamin A deficiency induces a similar corneal defect in humans [26]. Notch1 signaling is linked to vitamin A metabolism by regulating the expression of RBP1 [24]. In this study, we showed that Notch activity was reduced when 14-3-3σ activity was inhibited and that the reduction in Notch activity was associated with reduced transcription of Notch1 and Notch2. When Notch activity was restored in mutant cells by overexpressing NICD, both the proliferation and differentiation defects were alleviated. Our results suggest that 14-3-3σ acts upstream of Notch1 in corneal epithelial cell differentiation. Interestingly, homozygous mutation of 14-3-3σ leads to embryonic block of the differentiation of both the corneal epithelium (unpublished data) and skin keratinocytes [15]. Likewise, Notch activity is required for corneal epithelial differentiation [24]. Taken together, the results of the present study demonstrate that 14-3-3σ regulates Notch signaling after corneal stem cells are committed to terminal differentiation and that this subsequently affects corneal epithelial homeostasis.
The expression of both Notch1 and Notch2 was significantly reduced in Er mutant corneal epithelial cells. This raises the possibility that 14-3-3σ is essential for maintaining Notch1 and Notch2 transcription in the squamous epithelium. Although 14-3-3σ is not a transcription factor, there is substantial evidence that it binds to many transcription factors and regulates gene expression [9; 27]. 14-3-3σ functions as a shuttle carrier regulating protein nuclear localization, controlling transcription complex formation, and preventing transcription factors from binding to their target promoters. Alternatively, 14-3-3σ might regulate Notch signaling indirectly through other signaling pathways. Both the p63 and EGFR signaling pathways have been shown to modulate Notch1 transcription and to affect keratinocyte proliferation and differentiation [28; 29]. P63 plays an important role in the maintenance of epidermal basal cell proliferation, and, together with Notch, induces K-1 expression in suprabasal cells and the formation of the intermediate cell layers [28]. EGFR signaling negatively controls Notch1 gene expression through a mechanism involving the transcriptional downregulation of the p53 gene, and suppression of EGFR signaling activates Notch and initiates epidermal differentiation [29]. The elucidation of these signaling pathways and their relationships will facilitate our understanding of how 14-3-3σ functions in the regulation of epithelial differentiation.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Doug Dean for valuable discussion, Research to Prevent Blindness, Inc, NYC for general support, and the HistoCore funded by NIH grant EY015636. This work was supported by NIH/NCRR COBRE 5 P20 RR018733 (Q. Li), and Q.Lu were supported by NIH/NCRR 5 P20 RR017702 and NIH R01-EY018830.
Abbreviation
- TA
transient amplifying
- K5
keratin-5
- K14
keratin-14
- K3
cytokeratin-3
- K12
keratin-12
- NICD
Notch intracellular domain
- RBP1
retinol binding protein 1
- WT
wild type
- Er
Repeated Epilation
- PFA
paraformaldehyde
- BrdU
5-bromo-2′-deoxyuridine
- DAPI
diamidinophenylindole
- Q-PCR
quantitative polymerase chain reaction
- GFP
Green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanna C, Bicknell DS, O’Brien JE. Cell turnover in the adult human eye. Arch Ophthalmol. 1961;65:695–8. doi: 10.1001/archopht.1961.01840020697016. [DOI] [PubMed] [Google Scholar]
- 2.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–9. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 4.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–46. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–4. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 6.Wolosin JM, Budak MT, Akinci MA. Ocular surface epithelial and stem cell development. Int J Dev Biol. 2004;48:981–91. doi: 10.1387/ijdb.041876jw. [DOI] [PubMed] [Google Scholar]
- 7.Ban Y, Dota A, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Mochida C, Kinoshita S. Tight junction-related protein expression and distribution in human corneal epithelium. Exp Eye Res. 2003;76:663–9. doi: 10.1016/s0014-4835(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 8.Sugrue SP, Zieske JD. ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp Eye Res. 1997;64:11–20. doi: 10.1006/exer.1996.0175. [DOI] [PubMed] [Google Scholar]
- 9.Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE. 2005;2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- 10.Wilker EW, Grant RA, Artim SC, Yaffe MB. A structural basis for 14-3-3sigma functional specificity. J Biol Chem. 2005;280:18891–8. doi: 10.1074/jbc.M500982200. [DOI] [PubMed] [Google Scholar]
- 11.Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–20. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 12.Lodygin D, Hermeking H. Epigenetic silencing of 14-3-3sigma in cancer. Semin Cancer Biol. 2006;16:214–24. doi: 10.1016/j.semcancer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–61. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herron BJ, Liddell RA, Parker A, Grant S, Kinne J, Fisher JK, Siracusa LD. A mutation in stratifin is responsible for the repeated epilation (Er) phenotype in mice. Nat Genet. 2005;37:1210–2. doi: 10.1038/ng1652. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Lu Q, Estepa G, Verma IM. Identification of 14-3-3sigma mutation causing cutaneous abnormality in repeated-epilation mutant mouse. Proc Natl Acad Sci U S A. 2005;102:15977–82. doi: 10.1073/pnas.0508310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, Botchkarev VA, Crabtree GR, Dotto GP. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005;8:665–76. doi: 10.1016/j.devcel.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–5. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 18.Laronga C, Yang HY, Neal C, Lee MH. Association of the cyclin-dependent kinases and 14-3-3 sigma negatively regulates cell cycle progression. J Biol Chem. 2000;275:23106–12. doi: 10.1074/jbc.M905616199. [DOI] [PubMed] [Google Scholar]
- 19.Hazlett L, Masinick S, Mezger B, Barrett R, Kurpakus M, Garrett M. Ultrastructural, immunohistological and biochemical characterization of cultured mouse corneal epithelial cells. Ophthalmic Res. 1996;28:50–6. doi: 10.1159/000267873. [DOI] [PubMed] [Google Scholar]
- 20.Ramaesh T, Ramaesh K, Leask R, Springbett A, Riley SC, Dhillon B, West JD. Increased apoptosis and abnormal wound-healing responses in the heterozygous Pax6+/− mouse cornea. Invest Ophthalmol Vis Sci. 2006;47:1911–7. doi: 10.1167/iovs.05-1028. [DOI] [PubMed] [Google Scholar]
- 21.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–72. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 22.Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3507–12. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- 23.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 24.Vauclair S, Majo F, Durham AD, Ghyselinck NB, Barrandon Y, Radtke F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13:242–53. doi: 10.1016/j.devcel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol. 2003;23:2264–76. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer A. Xerophthalmia and vitamin A status. Prog Retin Eye Res. 1998;17:9–31. doi: 10.1016/s1350-9462(97)00001-3. [DOI] [PubMed] [Google Scholar]
- 27.Benzinger A, Muster N, Koch HB, Yates JR, 3rd, Hermeking H. Targeted proteomic analysis of 14-3-3 sigma, a p53 effector commonly silenced in cancer. Mol Cell Proteomics. 2005;4:785–95. doi: 10.1074/mcp.M500021-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, Tommasi di Vignano A, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–42. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolev V, Mandinova A, Guinea-Viniegra J, Hu B, Lefort K, Lambertini C, Neel V, Dummer R, Wagner EF, Dotto GP. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–11. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.