Abstract
This review focuses on the role of nano-structure and nano-scale materials for tissue engineering applications. We detail a scaffold production method (electrospinning) for the production of nanofiber-based scaffolds that can approximate many critical features of the normal cellular microenvironment, and so foster and direct tissue formation. Further, we describe new and emerging methods to increase the applicability of these scaffolds for in vitro and in vivo application. This discussion includes a focus on methods to further functionalize scaffolds to promote cell infiltration, methods to tune scaffold mechanics to meet in vivo demands, and methods to control the release of pharmaceuticals and other biologic agents to modulate the wound environment and foster tissue regeneration. This review provides a perspective in the state-of-the-art of the production, application, and functionalization of these unique nanofibrous structures, and outlines future directions in this growing field.
Keywords: Nanofibers, Electrospinning, Mechanical Properties, Controlled Release, Micro-patterning, Nanotopography, Tissue Engineering
Introduction
As a consequence of aging, disease processes, or traumatic injury, tissues and organ systems lose their capacity to carry out their given physiologic function. While these degenerative processes are increasingly well understood, options for improving regeneration or restoration of function are all too often lacking. This is particularly true in the soft tissues of the musculoskeletal system, such as tendon, ligament, articular cartilage, the intervertebral disc, and the knee meniscus. Each of these tissues possesses unique mechanical properties that allow them to carry out vital load bearing roles over many decades of life. Notably, these tissues operate with a remarkably low incidence of failure given the challenging mechanical environment in which they are expected to perform. However, when damage or degeneration does occur, these specialized structures, so critical to normal function, show markedly limited endogenous regenerative capacity.
Given the generally limited intrinsic healing responses of these tissues, and the lack of adequate clinical interventions, the last two decades has witnessed a rapid expansion in work focused on creating tissue analogues for replacement of soft tissues. Generally termed ‘tissue engineering,’ this field seeks to replicate key features of native tissues through in vitro fabrication methods or via in vivo regeneration around or within an engineered microenvironment. A key component in most, but not all, tissue engineering approaches is the scaffold, or template, upon which cells are seeded or into which cells migrate after implantation into a defect site. In vivo, cells reside in a dense extracellular matrix (ECM) network – a scaffold – from which they receive precisely-controlled cell-cell, cell-matrix, and cell-soluble factor signals which ultimately dictate activity. With the growing interest in engineering tissue substitutes to repair or replace damaged tissues, understanding these interactions is crucial. Indeed, a further understanding of the native cellular/extracellular environment may ultimately lead to more effective bio-mimetic scaffolds and ex vivo processing methods towards obtaining desired biological activities upon implantation.
The functional roles of the native ECM scaffold are structural: to support cells and provide a substrate for cell migration and survival; biochemical: to sequester growth factors and other chemical cues that regulate cell fate [1]; and biological: to present bioactive peptide sequences that can directly bind receptors and activate intracellular signaling pathways [2]. Ultimately this natural bio-scaffold directs cell activities, including proliferation, differentiation, matrix production, and apoptosis [3]. For effective repair and restoration of normal cell function, each of these key features of the ECM should be reproduced in an engineered scaffolding material.
Recently, another key characteristic of the ECM that dictates cell behavior has been identified, namely the size and topographical features of its structural elements [4]. For example, collagen is the most abundant ECM protein in the body, and therefore a common mediator within the cellular microenvironment. Biologically, cells adhere to collagen via specialized integrin receptors on their surface. Structurally, collagen provides tensile strength to tissues via its hierarchical assembly of collagen subunits. However, in addition to biologic signaling and macroscopic mechanical properties, collagen also possesses nano-scaled features that are mediators of cell activity. Tropocollagen (the basic subunit of a collagen fibril) is a nanostructure with dimensions of ~300 nm in length and 1.5 nm in width [5]. Self-assembled tropocollagen forms larger collagen fibrils [6] with diameters of ~50 nm [7]. These fibrils consist of adhesive ridges alternating with 5 to 15-nm deep non-adhesive grooves [8]. Previous studies have shown that changes made to certain features, such as structural curvature of the collagen fibrils, can regulate the activities of adherent cells [9]. Similarly, basement membranes (BMs) are dense, amorphous, sheet-like ECMs that function to provide structural support, compartmentalize tissues, and regulate cell functions [10]. While BMs vary greatly in their biochemical composition, they all share an important structural feature: nanotopography. Basement membrane fiber and pore diameters range from 30 to 400 nm, and the mean elevation of features is 150 to 200 nm [11]. Nanotopography and nanoscale feature sizes are thus a fundamental component of the normal cell microenvironment, and together with the biologic and biochemical features of the ECM, function to control cell activity and through this, the formation, maintenance, and regeneration of tissues.
In this review, we discuss the underpinnings of tissue formation, focusing on the involvement of nanotopography. We review how certain features and sizes control cell morphology and activity, and discuss relevant man-made nano-materials in this context. We further describe an exciting scaffold production method (electrospinning) by which polymeric and natural materials can be formed into non-woven fibers with length scales of biologic relevance to the natural ECM. These nano-scale fibers, or nanofibers, hold great promise in tissue engineering and regenerative medicine applications. We describe new and emerging methods to increase the application of these scaffolds. This discussion includes considerations of enhancement of cell infiltration/placement and scaffold mechanics, while at the same time preserving the nano-scale interactions that are crucial to normal cell activity. We also describe further methods to functionalize these scaffolds, with the goal of better approximating the biochemical function (growth factor and other biomolecule release and/or sequestration) and biological features (receptor binding) of the native ECM. It is the intent of this review to describe the state-of-the-art in the production, application, and functionalization of these unique structures, as well as to describe future directions in this field.
Nanotopography Controls Cell Behavior and Tissue Formation
Since the advent of the microscope, investigators have been interested in the interaction of cells with materials. When topography was first considered, reports focused on cellular responses to structures with micron-scale features [12–15]. While these studies provided important insights into how topography can modulate cell behavior, it is now clear that more biologically favorable cell-matrix interactions in vitro require the use of nano-scale features [16–18]. Differences in cell-matrix interactions are striking when comparing micro- and nano-scale structures. For example, a cell (which has a diameter on the micron-scale) bound to a fiber of greater diameter than itself is able to spread fully atop the fiber, much like it could on a flat two-dimensional (2D) surface. In contrast, the same cell interacting with a network of fibers of much smaller diameter will adhere to multiple fibers within its microenvironment to be securely attached. Although a cell in such a scenario could theoretically spread to the same degree across multiple fibers, there would be less available surface area for the cell to bind, altering the distribution and concentration of focal adhesions (FA). Furthermore, surrounding fibers would be in close proximity to the cell in every direction, affording it a three-dimensional (3D) environment that better mimics the in vivo scenario [19]. While there are clear differences between the environments created by micro- and nano-scale materials, researchers are just beginning to understand how a cell responds detects and responds to the size disparity.
The aim of this section is to shed light on how nano-scale structures, indiscriminate of chemistry, can alter cell adhesion, morphology and cytoskeletal organization, and differentiation. A number of nanostructures, including grooves [20], ridges [21], pillars [22], and pores [23] have been used to study the responses of a wide variety of cells.
Nanotopography modulates cell adhesion
A number of studies have shown the modulation of cell adhesion in responses to nanotopgraphical features [24–26]. Although cell responses vary between cell types and nanosubstrates [27], the commonly-observed trend is that substrates with nanotopographical features enhance cell adhesion. For example, Wan et al. found that osteoblast adhesion on both textured surfaces of micro- (2.2 μm) and nano-scale (450 nm) pits was increased compared to that on the smooth surface control, and that the nano-pitted surface was superior to the micro-pitted surface [28]. Thapa et al. reported that poly(lactic-co-glycolic acid) (PLGA) nanostructures promoted greater adhesion of bladder smooth muscle cells (SMCs) compared to the micron-structured controls [29]. A similar finding using endothelial cells on PLGA nanostructured surfaces also suggested that nanotopography enhances cell adhesion [30].
Nanotopographical substrates possess increased effective surface area and so are capable of enhancing protein adsorption, which in turn impacts cell adhesion. Webster et al. showed that adsorption of select proteins onto nanophase alumina ceramics enhances the adhesion of osteoblasts [27]. Furthermore, significantly higher concentrations of vitronectin and denatured collagen were adsorbed to the nanophase alumina substrates than the microphased controls.
Nanotopography modulates cell morphology and cytoskeletal organization
Numerous studies have reported the effects of nanotopography on cell morphology [21,31,32]. Nano-scale features are able to orient cells, control cell spreading by limiting the surface area available for cell attachment, and modulate FA patterns and resultant stress fiber organization. For example, Teixeira et al. demonstrated that epithelial cell morphology was dictated by precisely controlled nanogroove and nanoridge patterns [20]. The nanotopographical surface was created with 400–4000 nm wide pitches and 150–600 nm deep grooves, and coated with silicon oxide to eliminate any effect from surface chemistry. They found that epithelial cells aligned and elongated along the nanoridges (Figure 1A), while cells on smooth surface substrates remained predominantly round. Furthermore, a greater percentage of aligned cells were observed in deeper grooves. In addition, cells extended lamellipodia and filopodia primarily along ridges and down to groove floors (Figure 1B). Lastly, the size of the FAs was dependent on the ridge width, with wider ridges allowing for larger FAs to form. Together, these data suggest that nanoscale surface features can have profound effects on cell morphology.
Figure 1. Epithelial cells cultured on nano-patterned surfaces (400 nm pitch).
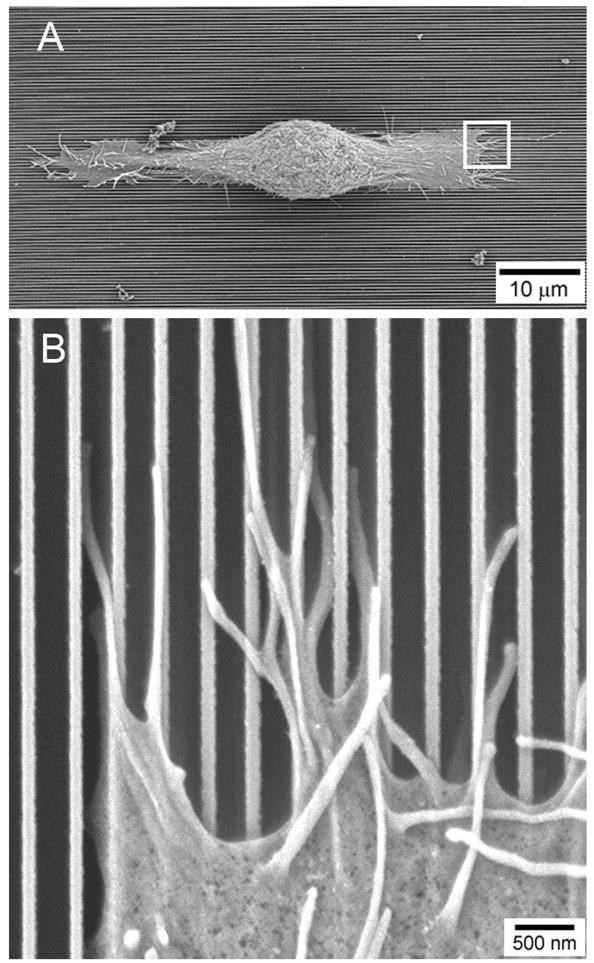
(A) Corneal epithelial cell aligned along nanostructured ridges. (B) Filopodia extend along the top of ridges and bottom of grooves, and lamellipodia protrude into the grooves along the cell edge. Reproduced from [20] with permission.
Similar cell behaviors were observed in a study using cylindrical nanocolumns to culture fibroblasts [22]. Compared to the flat surface control, fibroblasts on nanocolumns were less spread and more rounded. They also displayed fewer actin stress fibers and more filopodia, suggesting that such a substrate may better facilitate fibroblast migration.
Nanotopography alters cell phenotype
Recent reports indicate that SMCs express different gene profiles when cultured on 20-nm and 200-nm pores [23]. Using cDNA microarrays, the differential regulation of 500 genes was observed between the two different sized surfaces. Importantly, groups of genes related to cell adhesion and morphology were identified. Specifically, biglycan was upregulated 15-fold in cells exposed to the larger pores. Other genes related to the ECM and cytoskeleton, including laminin, collagen type IV, myosin Ib, villin, connexin, and cofilin, were also upregulated on the larger pores, while surface proteoglycans, like glypican, perlecan, and syndecan, were downregulated. Groups of genes related to cell cycle, proliferation, and signal transduction were identified as being modulated by the nanopores, as well. Clearly, the size of nanotopographical features can elicit great changes in cellular transcriptional activity.
Further evidence indicates that nanotopography can also direct stem cells to differentiate towards specific lineages. For example, synthetic nanostructures have been used to direct neurogenesis of human mesenchymal stem cells (hMSCs) without induction medium [33]. In this study, hMSCs were cultured on 350 nm, 1 μm, and 10 μm wide gratings to compare cell morphology and gene expression. Cells cultured on the nanosized features aligned along the grating axis, exhibited elongated and parallel stress fibers, and had more extended cell protrusions. These surface-dependent cell behaviors may facilitate neurogenic lineage commitment. Notably, mature neuronal markers, such as MAP2 and β-tubulin III, and the synapse marker synaptophysin, were also highly expressed in nanosurfaced substrates. Moreover, upregulation of ECM and adhesion molecule signaling was observed, suggesting that the induction of neuronal differentiation may be associated with changes in ECM signaling and cytoskeletal arrangement.
Nanoscale Materials and Nanofibrous Scaffolds: Production and Biological Implications
While 2D surfaces are valuable tools for studying basic cellular response to nanotopography, translation of these findings towards clinical application will require 3D structures. In this section, we describe three such structures: nanotubes, nanoparticles, and nanofibers. Each of these distinct nanostructures and its effect on biological regulation will be discussed separately. Nanoparticles and nanotubes will only be discussed briefly to summarize their importance and potential uses in tissue engineering applications, with the focus centered on the production and biological effects of nanofibrous scaffolds. Of note, while not yet common, both nanoparticles and nanotubes can be complexed with nanofibers to create composite hierarchical systems, with as yet unknown functionalities.
Nanoparticles
Nanoparticles are sub-micron (10–100 nm) sized materials produced via a number of methods, including attrition or pyrolysis, and can be formed in many shapes, including spheres and regular or irregular boxes. In tissue engineering, nanoparticles have been used for scaffold synthesis [34], scaffold reinforcement [35], cell patterning [36–38], in vivo imaging [39–41], and enhancing scaffold biocompatibility [42]. The most common application of nanoparticles in tissue engineering, however, is for drug [43–46], gene [47,48], and other bioactive factor delivery [49]. Due to their ultra-small size and high surface area-to-volume ratio, nanoparticle delivery systems are an emerging tool designed to carry molecules of interest for therapeutic applications. For example, polymeric nanoparticles have the ability to target specific cells and release loaded molecules in a predetermined, spatially- and temporally-controlled manner. Moreover, the properties of nanoparticles can be tailored to enhance cell uptake. One of the major existing challenges of using nanoparticle for drug delivery is how to precisely deliver molecules of interest to target cellular compartments. Understanding the mechanism by which nanoparticles are internalized by cells and trafficked intracellularly will be critical to overcoming this challenge [50].
Nanotubes
Nanotubes are nano-scale diameter materials that can be produced from inorganic and organic elements. These materials have a very large length to diameter ratio, and have attracted a great deal of attention for potential biomedical applications. For example, nanotubes have been used to modulate cell behavior through their electrical conductivity [51–53], and mechanically to reinforce or tailor the structural properties of tissue engineered scaffolds [54–56]. In addition, nanotubes have been used to increase the surface roughness and surface area of scaffolds for cell adhesion [57,58]. Lastly, by measuring changes in electrical conductivity, nanotubes can be used as molecular sensors to quantify the amount of a particular molecule adsorbed to their surface [59,60].
Nanofibers
There are currently three manufacturing approaches to fabricating nanofibrous scaffolds: electrospinning [61], phase separation [62], and self-assembly [63]. Structures created by each of these approaches are quite different and thus have their own unique advantages. For example, the phase separation technique allows for control of pore architectures [64]. The most common method for fabricating nanofibers is electrospinning. In this process, nanofibers are produced from polymer solutions via the application of a high electric field and the presentation of a grounded region some distance away (Figure 2). When charge accumulation in the solution overcomes surface tension, a fine jet emits from the solution. This jet is drawn into fibers which undergo whipping and further elongation as the solvent evaporates during transit to the collecting surface. The resulting nanofibers have fiber diameters ranging in size from 50 nm to several microns. As this length scale mimics that of native collagen fibrils ex vivo, nanofibers are an ideal substrate for tissue engineering applications. Aside from the morphological similarity to collagen, scaffolds formed by electrospinning also have a high surface area-to-volume ratio, variable pore-size distribution, and high porosity [65].
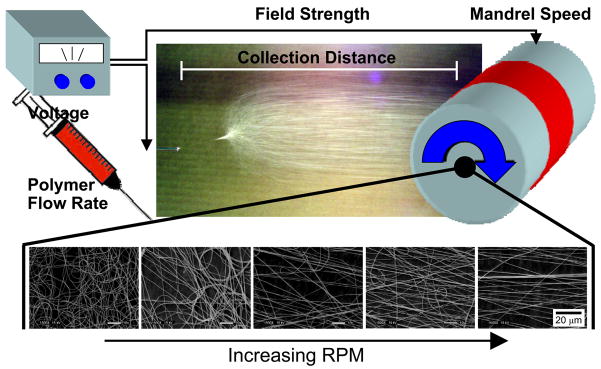
Figure 2. Production of nanofibers through electrospinning.
Nanofibers are produced from polymer solutions under a number of controlled experimental conditions, including applied voltage, flow rate, field strength, and collecting distance. When the collector is a rotating mandrel, increasing velocity of the grounded surface leads to increasing fiber alignment.
Nanofibers enhance cell adhesion
Adhesion is the first biological event that takes place when a cell is seeded onto a substrate. Once the cell is securely attached, it can then begin to migrate, proliferate, differentiate, or synthesize ECM [66]. Cells seeded on fibrous scaffolds preferentially adhere to nanofibers over microfibers of the same composition [67]. Tian et al. seeded NIH3T3 fibroblasts on composite poly(glycolic acid) (PGA)/collagen nanofibers with the PGA composition ranging from 7 to 86%, and fiber diameters ranging from 500 nm to 10 μm. They found that regardless of the PGA percentage in the composite, there were significantly more cells attached on the 500 nm fibers compared to the 3 to 5-μm and 10-μm fibers.
The mechanism by which nanofibers enhance cell adhesion is not completely understood. One possible explanation is through the enhanced and selective adsorption of adhesion molecules to the nanofibers [68]. Poly(L-lactic acid) (PLLA) scaffolds were created with nanofibrous pore walls of 50 to 500 nm, or with smooth pore walls, to compare the effect of pore wall architecture on protein adsorption. It was found that the nanofibrous scaffolds adsorbed four times more human serum proteins than the scaffolds with solid pore walls. These nanofibrous scaffolds tended to selectively adsorb fibronectin and vitronectin, two important cell adhesive proteins. Fittingly, cell adhesion was increased almost two-fold on these nanofibrous scaffolds.
Nanofibers modulate cell morphology and cytoskeletal organization
Several research teams have reported that cell morphology and cytoskeletal organization are modulated by culture on nanofibers [67,69,70]. For instance, primary mouse embryonic fibroblasts (MEFs) were seeded on 2D surfaces and 3D polyamide nanofibers to compare morphology and cytoskeletal organization. MEFs tended to adhere with a smaller projected area and a more elongated morphology on 3D nanofibrous surfaces [69]. Furthermore, cells cultured on nanofibrous surfaces displayed few or no stress fibers, with vinculin localized only to punctate structures on the dorsal membrane surface, indicative of fewer FAs and the adaptation of a more in vivo-like morphology [69].
We have demonstrated similar morphological and cytoskeletal alterations with primary chondrocytes seeded on PLLA electrospun nanofibers when compared to chemically-identical microfibers [70]. Chondrocytes seeded on nanofibers were found to have a rounded morphology with a disorganized actin cytoskeletal structure. In contrast, chondrocytes cultured on PLLA microfibers displayed a well-spread morphology and defined cytoskeleton (Figure 3). Such a flat, well-spread morphology is generally found in dedifferentiated chondrocytes on 2D culture surfaces [71], which might suggest that the regulatory signals that modulate cell morphology and cytoskeletal organization in a 3D microfibrous environment may more closely resemble those found in a 2D environment.
Figure 3. Morphology of chondrocytes seeded on microfibrous (MFS) and nanofibrous scaffolds (NFS).
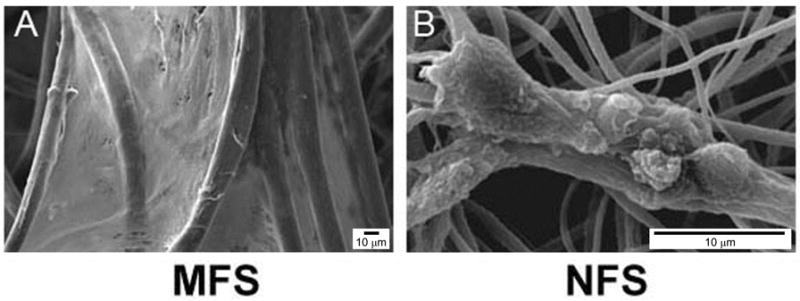
(A) Well-spread fibroblast-like cells spanned between microfibers after 28 days of culture, whereas (B) cellular aggregates composed of globular, chondrocyte-like cells grew on nanofibers. Bar, 10 μm. Reproduced from [70] with permission from Mary-Ann Leibert.
Nanofibers alter cell phenotype
Several recent findings suggest that cell shape [72] and cytoskeletal organization [73] might play a significant role in regulating cell phenotype. In the study described above, cartilage-specific gene and protein levels, such as collagens type II and IX, were upregulated in nanofibrous cultures compared to microfibrous cultures [70]. This suggests that nanofibers are capable of maintaining the chondrogenic phenotype, and provides futher evidence for a correlation between the morphological/cytoskeletal modulation and phenotypic control.
While it’s apparent that cells respond favorably to a 3D nanofibrous environment through adopting a more in vivo-like phenotype, the underlying mechanisms have yet to be elucidated. It appears, however, that such environments may promote Rac activation [74], a GTPase important in cell adhesion and signal transduction [75], and F-actin assembly [76]. The sustained activation of Rac leads to increased cell proliferation and deposition of fibrillar fibronectin by NIH 3T3 fibroblasts and normal rat kidney cells, suggesting that Rac is an important signaling molecule in directing cell activities in 3D nanofibrous culture [74].
Xie et al. recently demonstrated that nanofibers can enhance the differentiation of mouse embryonic stem cells into neural lineages [77]. Furthermore, aligned nanofibers guided neurite outgrowth along the length of the fibers. We recently showed that aligned nanofibrous scaffolds promoted ordered cytoskeleton formation by hMSCs [78] and ordered matrix deposition by cells isolated from the annulus fibrosus [79,80] and meniscus [81,82], as well as by MSCs [81]. Taken together, nanofibers provide a suitable substrate for control over stem cell differentiation and organization of deposited matrix, important features for generating functional tissue constructs.
Structural and Mechanical Features of Nanofibrous Scaffolds
From the above text, it is clear that nanofibrous scaffolds are a powerful tool for controlling cell biology and directing tissue formation. Since first described for tissue engineering applications, numerous advances in controlling the diameter and organization of these materials have been made [83]. Several recent reviews describe these advances in detail (see [84–88]). Here, we build on our understanding of nanofiber production, and point to several new concepts in the formation of these structures with the goal of improving their utility for tissue engineering applications. Specifically, we describe how the nanofiber palette has expanded tremendously over the last decade, unique fabrication strategies for producing composite structures that better replicate structural features of native tissues, and new methods for enhancing cellular colonization of these matrices, both at the time of production and after in vivo implantation. The methods described are evidence of the growing sophistication of nanofibrous arrays for tissue engineering, and are, indeed, just the tip of the iceberg reflecting ongoing modifications that will be required to access the full potential of these unique scaffolds for regenerative applications.
Expanding the Nanofiber Palette
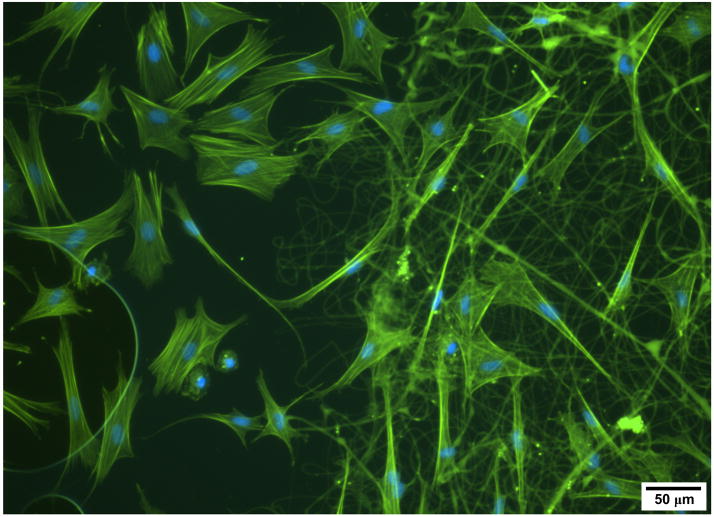
Electrospinning is an incredibly adaptable fabrication method, with dozens of input parameters all impacting the morphological, biological, and mechanical characteristics of the resultant scaffold. The effects of processing variables such as applied voltage, electric field strength, collection distances, solution viscosities, and flow rates (amongst others) have been widely investigated [83,86,89]. However, the most direct way to change the output is through polymer selection. Successful electrospinning has been achieved in enumerable biologic and synthetic polymers, and daily, new materials are being added to the repertoire (for review, see [90]). Of note, electrospinning has been carried out with synthetics such as polyurethanes [91], biodegradable polyesters (e.g., polycaprolactone (PCL) [61,92–94], PGA [95], poly(lactic acid) (PLA) [96–98], and polydiaxanone [99]), as well as natural biopolymers including collagen [93,100–103], elastin [102,103], silk fibroin [104–107], chitosan [108,109], dextran [110], and wheat gluten [111]. Natural materials in particular, such as collagen, enhance the rate with which cells initially adhere to fibers (Figure 4).
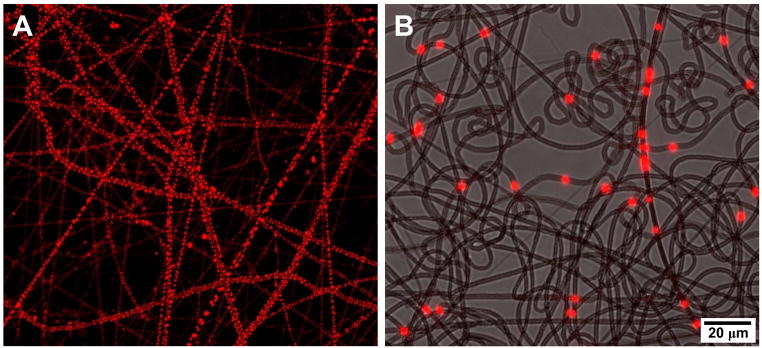
Figure 4. Biologic nanofibers control cell adhesion.
Acin (green) and nuclear staining (blue) of ovine mesenchymal stem cells on glass (left side) and collagen nanofibers (right side) for 24 hours. Note the larger areas of cell spreading and prominent stress fibers on the glass surfaces, and the well attached and well spread cells on collagen nanofibers.
Generally, a minimum molecular weight or chain length is required for a polymer to be successfully drawn into fibers. When this proves impossible to achieve, blended solutions can be utilized to “carry” the desired polymer. We have recently reported on the electrospinning of several low molecular weight elements of a library of poly(β-aminoester)s that were blended with poly(ethylene oxide) (PEO) to facilitate fiber formation [112], as well as novel photocrosslinkable and hydrolytically degradable elastomers carried by gelatin [113]. In these cases, the use of a water soluble carrier allows for the removal of this component after crosslinking, resulting in a pure fibrous mesh of the desired polymer.
Additionally, liquid blends of biosynthetic and natural components have been electrospun (with components thus mixed in every fiber) to create meshes with enhanced cell compatibility [114,115] or improved mechanical behavior [90]. Commonly, two dissimilar synthetic materials can be blended together to generate a fiber that has properties of both, or a natural and a synthetic fiber combined to impart biologic functionality to the fibers [116]. For example, Stankus and coworkers blended urinary bladder ECM with polyester urethane urea (PEUU), and showed enhanced cell spreading and in vivo colonization [117]. Addition of ECM proteins imparts the scaffold with biologic features of the native ECM that can control cell behavior on many levels. Additional studies have modified fiber surfaces to enhance cell binding and/or growth factor retention [118–120]. Further, methacrylate-based copolymers have been electrospun to form nanofibrous coatings that can be crosslinked after formation [121,122].
Composite Scaffolds with Properties on Demand
Despite the tremendous number of polymers that can be processed into electrospun form, scaffolds still typically fall short of design criteria based on the native ECM and tissue structural properties. Shortcomings may arise in the form of biocompatibility, degradation rates, and most frequently mechanics, including limitations in distensibility before yield, stiffness, and fatigue properties. While blended polymer solutions can sometimes address such limitations, one difficulty with this method is control over the spatial distribution of the constituent polymers in the resulting mixed fiber. Depending on the characteristics of the components, the polymers may fail to mix in solution, or disaggregate in transit as the solvent evaporates. An alternative to blending different solutions prior to electrospinning is to spin multiple polymers from separate sources, but to collect them concurrently on a common grounded collector. This generates a composite scaffold that contains several populations of distinct fibers, each with different mechanical (and potentially biological and degradation) characteristics. In the same manner that concrete and steel are combined to produce a composite material that can withstand both compressive and tensile loads, electrospun composites can amalgamate the unique and desired characteristics of the constituent polymers.
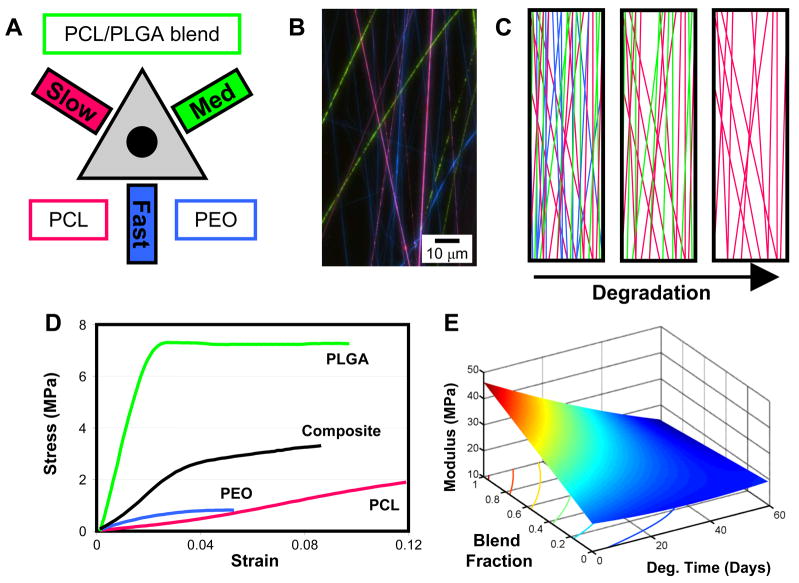
Gupta et al. established side-by-side multi-jet electrospinning as a method for generating nanofiber composites of poly(vinyl chloride), poly(vinyliediene fluoride), and segmented polyurethane [123]. Importantly, Ding and coworkers demonstrated the effects of mixing of different polymer fibers on composite mechanics. Through modulating the balance of poly(vinyl alcohol) and cellulose acetate jets, they were able to tune the modulus, yield point, and tensile strength of the composites [124]. Towards engineering temporally dynamic electrospun scaffolds with fast, medium, and slow degrading elements, we have developed a tri-jet electrospinning device to fabricate multi-polymer composites of PEO, PLGA, and PCL fibers [125–127], Figure 5. Integrating a constitutive mixture model with this technique highlights the utility of this approach. Through characterizing the temporal stress-strain behavior of each constituent polymer separately, it becomes possible to predict the behavior of composites comprised of combinations of these polymers under the effects of degradation [127]. With such a methodology, matching the complex mechanical behavior of numerous biologic tissues becomes plausible given a library of polymers with a sufficient range of mechanical behaviors.
Figure 5. Multi-component nanofibrous scaffolds.
Composites of different types of nanofibers can be “mixed and matched” to form composite scaffolds with a range of mechanical and degradation attributes. A) Schematic of an electrospinning system for electrospinning multi-components scaffolds of slow (PCL), medium (PCL/PLGA blend), and fast (PEO) eroding fibers. B) A diagrammatic depiction of the dynamic pore size that results from the gradual loss of fibers through degradation. C) Multi-polymer fibrous scaffolds were formed on a novel setup designed for co-electrospinning with up to three jets. D) Mixing polymer fibers each with unique mechanical properties results in composites that adopt characteristics of the constituents. E) Mechanical modeling of these composites allows production of a wide range of scaffolds with a range of dynamic mechanical properties.
Cellularizing Electrospun Scaffolds
For many tissue engineering applications, especially those where in vitro growth is desired, populating the three-dimensional scaffold is crucial for successful tissue formation. One commonly encountered but oft unmentioned problem is the difficulty of fully colonizing even relatively thin (~1mm) electrospun scaffolds. Inadequate cell infiltration occurs despite the high porosities of these matrices (>80–90% porous), and limits both the rate and distribution of matrix accumulation. Such a slow colonization process would also likely limit integration with native structures when these scaffolds are implanted in vivo. Surface-seeding is the easiest method for populating scaffolds with cells, and thus is the most common. While cells will readily divide and migrate across a biocompatible electrospun surface, their ability to crawl through layers of nanofibers and into the depth of the scaffold is severely limited. This is most likely due to the packing of the sub-micron diameter fibers which results in many small pores, as scaffolds composed of larger, micron-scale fibers are more readily infiltrated [128]. In fact, one strategy to improve cell infiltration centers around the creation of a nanofiber-microfiber layered mesh [129]. In this study, the inclusion of larger fibers interrupts the packing of small nanofibers and increases the pore size of the overall structure, allowing cells to fully colonize the 1 mm thick scaffolds. Fiber alignment may exacerbate this quandary by further reducing pore sizes, as the apparent density in scaffolds is increased compared to non-aligned or random scaffolds [78,130].
As nanofibers better mimic the length-scale of the native ECM and provide control over cell morphology and behavior, inclusion of micro-scale fibers may be undesirable [70]. Several other strategies have thus been developed for augmenting scaffold pore size to facilitate cell infiltration. Nam et al. incorporated salt crystals which were subsequently dissolved away upon hydration of the scaffold. This improved cell infiltration but irregularities introduced throughout the accumulating layers caused scaffold delamination over time [131]. Others have induced the formation of ice crystals from relative humidity with collection on a super-cooled collecting surface to provide solid inclusions around which fibers form [132]. Along similar lines, we uniformly incorporate a sacrificial nanofiber population during the formation of the scaffold which is removed prior to cell seeding. The removal of these space-holding fibers provides the necessary increase in pore size to accelerate cellular ingress [133].
Another method for increasing cell infiltration may be to form ECM proteins directly into nanofiber form. Biologic elements (including collagen) provide a biomimetic environment for cell adhesion (Figure 4) and thus may be more readily colonized. Telemeco and colleagues reported enhanced cell infiltration into pure collagen scaffolds compared to synthetic scaffolds with subcutaneous implantation [134]. In these biologic scaffolds, cells may colonize by one of two routes, either through direct interaction in which they pull themselves through the proteinaceous milieu or they may degrade the ECM by secretion of matrix metalloproteinases. One drawback of this strategy, however, is that the mechanical properties of scaffold formed from biologic polymers are considerably lower than that of common synthetic nanofibrous scaffolds in the hydrated state, and that pre-treatment with crosslinking agents (such as gluteraldehyde) is required for their stabilization [90,100].
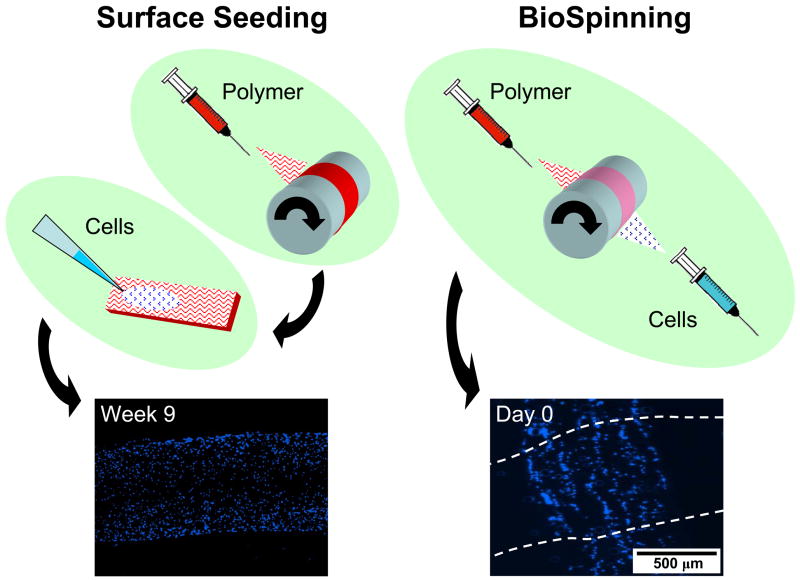
Even when scaffolds are engineered to promote infiltration, constructs that are seeded from the surface will generally contain a gradient of cells, with the highest density at the seeded surface and the lowest density in the scaffold center [133]. The most direct method to overcome this limitation is to place cells directly into the scaffold during formation. Stankus and co-workers accomplished this by simultaneously electrospraying cells and electrospinning fibers onto a common mandrel [135]. We have recently accomplished this in our lab as well, using MSCs electrosprayed in gelatin with PCL electrospun fibers (Figure 6). Jayasinghe and coauthors recently developed a novel method for biospinning, in which encapsulated living cells are formed inside of electrospun fibers using a coaxial needle approach [136,137]. These are exciting new techniques, though issues of solvent lethality, scale-up, and sterility may limit their wide-spread application.
Figure 6. Enhancing cellularity in nanofibrous scaffolds.
The most common method for cellularizing electrospun scaffolds is seeding cells on the outer surfaces of the construct. Cells will migrate inwards with time, a process whose rate can be increased with a number of methods, including the introduction of pores, the addition of biologic fiber constituents, or the use of custom perfusion bioreactor systems. Alternatively, BioSpinning, the direct inclusion of cells within the scaffold substance during fabrication, can produce fully colonized constructs at the outset of cultures.
Drug Delivery from Nanofibrous Scaffolds
The above sections demonstrate several key attributes and modifications to nanofibrous scaffolds that endow them with structural and nanotopographical features as well as biologic functionality similar to that of the native tissue ECM. However, another benefit of nanofibers is their potential to mediate the biochemical environment. Native ECM acts to sequester and bind growth factors and other molecules, and so creates local microenvironments that can be enriched with certain factors. As noted above, the surface of nanofibers can be modified with biologic epitopes to serve this function as well. For example, Casper and colleagues functionalized poly(ethylene glycol) (PEG) with low molecular weight heparin and demonstrated improved binding of basic FGF [118]. This same group also developed methods to modify natural protein nanofibers (collagen/gelatin) by functionalization with perlecan domain I, and showed that these fibers were 10 times more effective in binding basic FGF than controls [138]. However, the very high surface area-to-volume ratio of nanofibers provides another method for functionalization. That is, just as nanofibers tend to bind larger amounts of serum proteins compared to microfibers, they might also be used to directly deliver select agents and biofactors from the surface area of the scaffold itself, and do so in a controlled fashion (Figure 7). Below we detail recent progress towards this end for several specific classes of agents, including antibiotics, analgesics, tumor suppressing molecules, and biologic growth factors. Further, we highlight new fabrication methods by which these drug delivery methods may be optimized.
Figure 7. Drug delivering nanofibrous scaffolds.
A) PCL nanofiber scaffold in which an aqueous cell tracker dye was homogenized. Note the minute corpuscular inclusions throughout the nanofiber. B) PCL nanofiber scaffold in which fluorescently labeled microspheres are co-electrospun. These larger (2 micron) inclusions are distributed along the length of the nanofiber, allowing for both dose control and controlled release based on microsphere properties.
Antibiotics
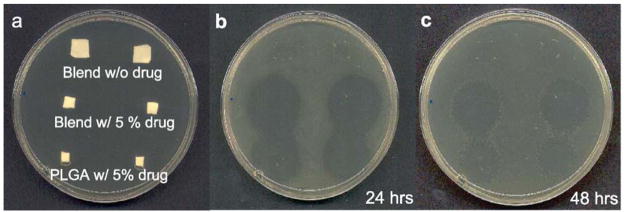
One of the first classes of molecules to be delivered from electrospun fibers was antibiotics. Antibiotic loaded scaffolds can be easily applied as wound dressings or formed into sutures and so prevent infection at an injury or surgical site. Kenawy and colleagues were one of the first to demonstrate this principle, releasing tetracycline hydrochloride (tet), a broad-spectrum antibiotic, from PLA, poly(ethylene-co-vinyl acetate) or a 50:50 blend of the two. They did so by adding tet to the electrospinning solution and were able to show antibiotic release over 5 days, with a significant burst release occurring on day 1 [139]. Similarly, Zong and coworkers released Mefoxin from PLLA fibers and showed that the concentration and ionic salt in the spinning solution influenced fiber morphology [140]. Likewise, Kim and coworkers showed Mefoxin release from PLGA fibers, though they too observed an early burst release. Interestingly, this effect could be minimized by the addition of the amphiphilic block copolymer PEG-b-PLA. These authors also showed that the antibiotic was bioactive on release, with inhibition of growth of Staphylococcus aureus cultures at early time points [141], Figure 8. In addition to these findings, other antibiotics with variable properties have also been blended into electrospun scaffolds. Zeng and co-workers showed that the addition of surfactants or proteinase K decreased the burst release of the antibiotic rifampin from PLLA fibers [142] and Katti and coworkers showed how fabrication parameters, such as needle gauge, concentration, density and voltage influence loading of the antibiotic cefazolin [143].
Figure 8. Antibiotic release from nanofibrous scaffolds.
Release of antibiotic from PLGA and composite nanofibrous scaffolds into agar plates reduces subsequent bacterial colonization for 4 and 24 hours. Reproduced from [141] with permission from Elsevier.
One notable recent study combined multiple fiber populations in which one of the fibers was structural, while the other fiber was designed to deliver the antibiotic. This work was carried out in response to the observation that addition of molecules (and their solvents) can change the mechanical properties of produced fibers. In this work, Hong and co-workers created nanofibrous sheets composed of two fiber populations; biodegradable PEUU fibers to provide mechanical functionality and PLGA fibers loaded with tet to deliver antibiotic. Mechanical properties, including tensile strength and suture retention capacity, were greatly improved by the dual-component scaffold compared to the PLGA-tet fiber system alone. Most illustratively, in vivo application of these scaffolds demonstrated that implantation of the tet-releasing scaffold could prevent abscess formation in a contaminated rat abdominal wall [144].
While the above study obviates the adverse mechanical effects of antibiotic inclusion, and adding the antibiotic directly to the electrospinning solution is a simple process, it may be desirable in some cases to decrease the burst release observed in most of these systems. To address this, some have proposed electrospinning in a co-axial fiber format, with an inner core containing the antibiotic and a protective outer shell modulating the release characteristics. This method can decrease exposure of drugs to harsh fabrication conditions, as well as create a coating to decrease burst release and extend release times. He and coworkers created nanofibers with a PLLA outer shell encapsulating a solution of tet in the interior of the fiber. The resulting fibers showed a sustained tet release profile, with almost no burst release [145]. Huang and coworkers compared the release of reservatrol (an antioxidant) and gentamycin sulfate (an antibiotic) from the inner core of coaxial fibers with a PCL outer shell. The degradation rate was found to be closely related to the hydrophilicity of the drug in the core, and the miscibility of the solvents used influenced mechanical properties of the fibers [146]. While some contest that a sustained release is most ideal, He and coworkers suggest that different release profiles might find use in different applications. For example, an initial burst, as seen with blended fibers, could be applicable to antibacterial release wherein the drug is required from the outset, while a more sustained release, as is achieved with co-axial methods, would be appropriate for the delivery of long-term therapeutic agents [147].
Analgesics
Analgesics have also been incorporated into electrospun fibers for the control of pain. Jiang an coworkers demonstrated that by covalently conjugating ibuprofen with PEG-g-chitosan and electrospinning with PLGA, sustained release of the drug could be attained over 16 days [148]. Also, Qi and colleagues created acid-labile electrospun fibers that released an analgesic (paracetanol) more completely and at a faster rate when placed in acidic environments. Natural decreases in local pH often accompany inflammation, tumor growth, and myocardial ischemia, suggesting that such a system may provide a sophisticated drug delivery capacity that is tuned to the local wound environment [149]. These same authors were also able to demonstrate that paracetanol was released with longer zero-order release profiles with thicker nanofibers [150].
Cancer Therapeutics
Another important class of molecules that has been incorporated and released from electrospun fibers is agents used in the treatment of cancer. Systemic administration of anti-cancer medications often leads to debilitating side-effects and so local delivery through a biodegradable patch might be less noxious to the patient. In a series of experiments, Zeng and colleagues explored the incorporation of anticancer drugs into PLLA fibers. They showed that Paclitaxel incorporated uniformly into fibers, whereas doxorubicin hydrochloride, a hydrophilic drug, appeared to phase-separate onto the surface of the fibers [151,152]. In similar studies, Xie and colleagues incorporated Paclitaxel into electrospun PLGA nanofibers and demonstrated cytotoxicity against C6 glioma cell lines for local applications in brain tumor destruction [153]. Also, Xu and colleagues released BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea) from PEG–PLLA ultrafine fibers and showed sustained release and decreased cell viability of Glioma C6 cells over time [154]. Xu and colleagues also demonstrated that doxorubicin hydrochloride could be loaded into amphiphilic PEG-PLLA diblock copolymer fibers at 1–5 wt%, with release controlled by a combined diffusion and degradation mechanism [155].
Biologics
While delivery of biologically active chemical therapeutics is possible from electrospun fibers produced with a variety of solvents and spinning conditions, biologic molecules such as growth factors pose a slightly more challenging scenario. Proteins and other biomolecules are susceptible to denaturation with harsh solvents and strong electrostatic forces. While challenging, the benefit of release of growth factors could be substantial in a tissue engineering context, in that one could continue to influence cell behavior, matrix deposition, and tissue remodeling long after construct implantation.
Though perhaps difficult, some studies have shown delivery of bioactive molecules directly from blended fibers. Chew and colleagues encapsulated human nerve growth factor (NGF) stabilized by bovine serum albumin (BSA) in a copolymer of PCL and ethyl ethylene phosphate. A bioassay using PC12 neurite outgrowth confirmed that the bioactivity of electrospun NGF was retained, at least partially [156]. Zeng and colleagues electrospun poly(vinyl alcohol) nanofibers loaded with BSA and demonstrated that coating the fibers with poly(p-xylylene) by chemical vapor deposition decreased the burst release and retarded overall release rates, depending on the coating thickness [152]. Also, Sanders and coworkers encapsulated aqueous BSA in poly(ethylene-co-vinyl acetate) and found that based on fabrication parameters, bubbles of liquid could be trapped inside the fibers [157]. Finally, Maretschek and colleagues incorporated cytochrome C into PLLA fibers and modulated the hydrophobicity and resulting release rates by electrospinning emulsions of PLLA and other hydrophilic polymers [158]. One more recent approach involves the replacement of synthetic polymers with natural polymers. This allows for spinning to take place in solvents that are less damaging to protein structure (i.e., water), and has shown some success in terms of growth factor release. For example, Li and coworkers electrospun silk fibroin fiber scaffolds containing bone morphogenetic protein 2 (BMP-2) and/or nanoparticles of hydroxyapatite (nHAP) [159]. Human mesenchymal stem cells were grown on the scaffolds and differentiated towards the osteogenic phenotype for 31 days. Results from this study show that groups containing BMP-2 increased osteogenic marker gene expression compared to controls, indicating the sustained bioactivity of the growth factor in this system.
When harsh solvents are required, co-axial electrospinning, rather than blend electrospinning, allows for improved maintenance of protein activity after processing into fibers. For example, Yang demonstrated that the emulsion co-axial spinning technique protected entrapped proteins from denaturation during fabrication and protected the structural integrity of the protein during incubation [160]. Jiang and colleagues encapsulated BSA and lysozyme inside a PCL shell via co-axial electrospinning and found that the relative thicknesses of the core and PCL shell (and subsequently, the release rates) could be adjusted by modifying flow rates of each stream within the coaxial setup [161]. The addition of water-soluble PEG to the protein containing inner core [162,163] resulted in a more sustained release. Liao and coworkers co-axially electrospun BSA-stabilized platelet-derived growth factor (PDGF) stabilized within a PCL shell. By adding PEG to the shell, these authors were able to fine-tune the release characteristics of the fibers, and showed that released PDGF stimulated proliferation in NIH 3T3 cells over 20 days [164].
An alternative strategy for protecting the biologic material during nanofiber processing can be achieved by the use of micro and nanoparticles. These particles, formed using standard techniques that have been optimized to preserve biofactor activity, can be suspended in the electrospinning solution. An example of this is shown in Figure 7B, where fluorescently labeled 2 micron diameter microspheres are distributed along a PCL fiber [165]. Ding and co-workers recently showed similar findings, and further demonstrated that multiple families of microspheres and/or nanoparticles could be encapsulated along a single fiber, allowing for the potential delivery of multiple factors from a single mesh. In a similar approach that may better retain bioactivity, Qi and colleagues developed a method for electrospinning Ca-alginate microspheres containing BSA into PLLA fibers. These authors found that release from the microsphere/fiber system resulted in less initial burst than the microspheres alone [166]. This brings up an interesting and important point – when the material to deliver the drug is positioned within the nanofiber, then both the delivery polymer degradation and the fiber polymer degradation will control the release rate. On this point, it is not yet clear exactly how molecules diffuse from nanofibers. Recent work by Srikar and colleagues showed that release of rhodamine occurs via the desorption of the embedded compound from nanopores in the fibers or from the outer surface of the fibers in contact with the water bath [167]. An additional consideration for load-bearing applications is that inclusion of any material within a nanofiber strand can change its mechanical properties. For example, retinoic acid added at low levels increased mechanical properties of single fibers, but decreased properties at higher levels [168]. If a fibrous scaffold is to serve multiple roles, for example, load bearing and drug delivery, then this issue should be considered. We recently tailored our system to include drug delivering microspheres within the sacrificial fibers we use for enhancing cell infiltration (described above). This effectively captures the microsphere within the nanofiber network, and decouples drug delivery from the mechanics or degradation rates of the load-bearing fibers [165].
Gene Delivery
Moving beyond antibiotics, anticancer drugs and proteins, other unique molecules have been incorporated into electrospun fibers. Luu and colleagues released plasmid DNA from a mixture of predominantly PLGA random copolymer and a PLA–PEG block copolymer. Release of plasmid DNA from the scaffolds was sustained over a 20-day study period [169]. Similarly, Nie and coworkers encapsulated DNA into chitosan nanoparticles that were electrospun into PLGA/hydroxyapetite fibers and optimized the system for cell attachment, viability and transfection efficiency [170]. Liang and colleagues also created a variation on this them where the nanoparticles possessed core-shell structure in order to better protect the contained DNA from the harsh electrospinning process [171].
Expert Commentary and 5-year View
Engineering replacement tissues requires a deep understanding of native tissue structure and function, as well as the development of enabling technologies that can replicate key features of the normal cellular microenvironment. Nanofibers are a promising vehicle towards this goal, as they replicate many key length scales of the normal cell environment. As the field of tissue engineering with nanofibers progresses, the trend for the next five years will be directed towards the addition of key new functionalities to these already unique scaffolds. For example, novel studies involving ‘writing’ with nanofibers, either through near field electrospinning [172] or melt electrospinning, as demonstrated by Sun et al. and Dalton et al. [173,174], respectively, offers the option of directly forming tissue templates with a desired structural hierarchy, geometry, and organization. Delivery of new biologic agents, in addition to those detailed above, including, but not limited to, micro and small interfering RNAs (miRNA and siRNA) [175,176], may further the ability of nanofibrous scaffolds to impart control on cellular function. These additional functionalities, along with tuning scaffold mechanics in multi-polymer composites comprised of novel polymer inputs, will further our ability to precisely control the complex sequence of tissue formation and maturation after implantation. As this process continues, we must preserve the key nanotopographic features that make such scaffolds so attractive, while at the same time ensuring that the methodologies developed are simple and practical for in vivo application. Towards this end, these new technologies must be tested in rigorous in vivo models of tissue restoration. Based on the already promising literature to date, and the intense interest in these unique materials, the repair and/or replacement of damaged or diseased tissues with nanofiber based scaffolds will quickly become a reality, and will improve the lives of millions suffering from numerous medical conditions and tissue pathologies.
Key Points
The cellular microenvironment is defined by a specialized extracellular matrix that includes structural elements, biologic inputs, and biochemical signals, all of which define and regulate cell function.
Nanotopographical features within this microenvironment regulate cell behavior including division, matrix synthesis, and apoptosis, both in vitro and in vivo.
Nanofibrous scaffolds replicate key length scales and structural features of native fibrous tissues and can control cell shape and differentiation, as well as direct the ordered deposition of new ECM.
New materials are continually being added to the available palette of electrospun fibers. These provide additional biologic and chemical functionality within the scaffold and can enhance tissue formation in vitro and in vivo.
New scaffold fabrication methods can improve cell infiltration and generate composite scaffolds with tunable mechanical and degradation properties.
Nanofibrous scaffolds can serve as implantable vehicles for the controlled release of a number of pharmaceutical and biologic agents.
Progress in the fabrication of multi-functional nanofibrous scaffolds, combining the above elements, holds great promise for soft tissue engineering for the repair and/or replacement of damaged or diseased tissues.
Acknowledgments
This work was supported with funding from the Aircast Foundation, the National Institutes of Health (NIH R01 AR056624 and T32 AR007132), a National Science Foundation Graduate Research Fellowship, and the Penn Center for Musculoskeletal Disorders (NIH P30 AR050950). The authors would also like to acknowledge Dr. Albert Gee for images of stem cell interactions with electrospun collagen and Mr. Ross Marklein for images of nanofibers containing emulsified Cell Tracker aqueous capsules.
References
- 1.Schindler M, Nur EKA, Ahmed I, et al. Living in three dimensions: 3D nanostructured environments for cell culture and regenerative medicine. Cell Biochem Biophys. 2006;45(2):215–227. doi: 10.1385/CBB:45:2:215. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339 (Pt 3):481–488. [PMC free article] [PubMed] [Google Scholar]
- 3.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 4.Katz BZ, Zamir E, Bershadsky A, Kam Z, Yamada KM, Geiger B. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol Biol Cell. 2000;11(3):1047–1060. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulmes DJS. Building collagen molecules, fibrils, and suprafibrillar structures. Journal of Structural Biology. 2002;137(1–2):2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 7.Orgel JP, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U S A. 2006;103(24):9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baselt DR, Revel JP, Baldeschwieler JD. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophys J. 1993;65(6):2644–2655. doi: 10.1016/S0006-3495(93)81329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7(2):157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 11.Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299(1):39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 12.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 13.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour. I. Simple step cues. Development. 1987;99(3):439–448. doi: 10.1242/dev.99.3.439. [DOI] [PubMed] [Google Scholar]
- 14 *.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Cell guidance by ultrafine topography in vitro. J Cell Sci. 1991;99(Pt 1):73–77. doi: 10.1242/jcs.99.1.73. This seminal paper showed how topography can influence cell morphology and guidance in 2D patterned cultures. [DOI] [PubMed] [Google Scholar]
- 15.Gray C, Boyde A, Jones SJ. Topographically induced bone formation in vitro: implications for bone implants and bone grafts. Bone. 1996;18(2):115–123. doi: 10.1016/8756-3282(95)00456-4. [DOI] [PubMed] [Google Scholar]
- 16.Yim EK, Leong KW. Significance of synthetic nanostructures in dictating cellular response. Nanomedicine. 2005;1(1):10–21. doi: 10.1016/j.nano.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Kriparamanan R, Aswath P, Zhou A, Tang LP, Nguyen KT. Nanotopography: Cellular responses to nanostructured materials. Journal of Nanoscience and Nanotechnology. 2006;6(7):1905–1919. doi: 10.1166/jnn.2006.330. [DOI] [PubMed] [Google Scholar]
- 18.Martinez E, Engel E, Planell JA, Samitier J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat. 2009;191(1):126–135. doi: 10.1016/j.aanat.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S. Designer self-assembling Peptide nanofiber scaffolds for study of 3-d cell biology and beyond. Adv Cancer Res. 2008;99:335–362. doi: 10.1016/S0065-230X(07)99005-3. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116(Pt 10):1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson AS, Backhed F, von Euler A, Richter-Dahlfors A, Sutherland D, Kasemo B. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials. 2003;24(20):3427–3436. doi: 10.1016/s0142-9612(03)00208-4. [DOI] [PubMed] [Google Scholar]
- 22.Dalby MJ, Childs S, Riehle MO, Johnstone HJ, Affrossman S, Curtis AS. Fibroblast reaction to island topography: changes in cytoskeleton and morphology with time. Biomaterials. 2003;24(6):927–935. doi: 10.1016/s0142-9612(02)00427-1. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen KT, Shukla KP, Moctezuma M, Tang LP. Cellular and molecular responses of smooth muscle cells to surface nanotopography. Journal of Nanoscience and Nanotechnology. 2007;7(8):2823–2832. doi: 10.1166/jnn.2007.610. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher JOKFM, Wilkinson CDW, Riehle MO. Interaction of animal cells with ordered nanotopography. IEEE Transactions on Nanobioscience. 2002;1(1):24–28. doi: 10.1109/tnb.2002.806918. [DOI] [PubMed] [Google Scholar]
- 25.Baac HW, Lee JH, Seo JM, et al. Submicron-scale topographical control of cell growth using holographic surface relief grating. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2004;24(1–2):209–212. [Google Scholar]
- 26.Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, Spatz JP. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophysical Journal. 2007;92(8):2964–2974. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51(3):475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Wan Y, Wang Y, Liu Z, et al. Adhesion and proliferation of OCT-1 osteoblast-like cells on micro- and nano-scale topography structured poly(L-lactide) Biomaterials. 2005;26(21):4453–4459. doi: 10.1016/j.biomaterials.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 29 *.Thapa A, Miller DC, Webster TJ, Haberstroh KM. Nano-structured polymers enhance bladder smooth muscle cell function. Biomaterials. 2003;24(17):2915–2926. doi: 10.1016/s0142-9612(03)00123-6. This seminal paper shows how nano-scaled features can improve cell attachment compared to micron-scaled features. [DOI] [PubMed] [Google Scholar]
- 30.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials. 2004;25(1):53–61. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 31.Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis AS. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials. 2002;23(14):2945–2954. doi: 10.1016/s0142-9612(01)00424-0. [DOI] [PubMed] [Google Scholar]
- 32.Dalby MJ, Riehle MO, Sutherland DS, Agheli H, Curtis AS. Changes in fibroblast morphology in response to nano-columns produced by colloidal lithography. Biomaterials. 2004;25(23):5415–5422. doi: 10.1016/j.biomaterials.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313(9):1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao X, Yao H, Li Y, Chen G. Preparation of honeycomb scaffold with hierarchical porous structures by core-crosslinked core-corona nanoparticles. J Colloid Interface Sci. 2009;332(1):165–172. doi: 10.1016/j.jcis.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal SK, Sanabria-Delong N, Tew GN, Bhatia SR. Nanoparticle-reinforced associative network hydrogels. Langmuir. 2008;24(22):13148–13154. doi: 10.1021/la8015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiyama H, Ito A, Kawabe Y, Kamihira M. Cell-patterning using poly (ethylene glycol)-modified magnetite nanoparticles. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32313. [DOI] [PubMed] [Google Scholar]
- 37.Frasca G, Gazeau F, Wilhelm C. Formation of a three-dimensional multicellular assembly using magnetic patterning. Langmuir. 2009;25(4):2348–2354. doi: 10.1021/la8030792. [DOI] [PubMed] [Google Scholar]
- 38.Sharma RI, Shreiber DI, Moghe PV. Nanoscale variation of bioadhesive substrates as a tool for engineering of cell matrix assembly. Tissue Eng Part A. 2008;14(7):1237–1250. doi: 10.1089/ten.tea.2007.0279. [DOI] [PubMed] [Google Scholar]
- 39.Lu J, Ma S, Sun J, et al. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials. 2009;30(15):2919–2928. doi: 10.1016/j.biomaterials.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Altinoglu EI, Russin TJ, Kaiser JM, et al. Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano. 2008;2(10):2075–2084. doi: 10.1021/nn800448r. [DOI] [PubMed] [Google Scholar]
- 41.Wu P, He X, Wang K, et al. Imaging breast cancer cells and tissues using peptide-labeled fluorescent silica nanoparticles. J Nanosci Nanotechnol. 2008;8(5):2483–2487. doi: 10.1166/jnn.2008.362. [DOI] [PubMed] [Google Scholar]
- 42.Jung R, Kim Y, Kim HS, Jin HJ. Antimicrobial properties of hydrated cellulose membranes with silver nanoparticles. J Biomater Sci Polym Ed. 2009;20(3):311–324. doi: 10.1163/156856209X412182. [DOI] [PubMed] [Google Scholar]
- 43.Debbage P. Targeted drugs and nanomedicine: present and future. Curr Pharm Des. 2009;15(2):153–172. doi: 10.2174/138161209787002870. [DOI] [PubMed] [Google Scholar]
- 44.Vyas SP, Goyal AK, Khatri K, et al. Development of self-assembled nanoceramic carrier construct(s) for vaccine delivery. J Biomater Appl. 2009 doi: 10.1177/0885328209104018. [DOI] [PubMed] [Google Scholar]
- 45.Grenha A, Gomes ME, Rodrigues M, et al. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32466. [DOI] [PubMed] [Google Scholar]
- 46.Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60(11):1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32441. [DOI] [PubMed] [Google Scholar]
- 48.Veiseh O, Kievit FM, Gunn JW, Ratner BD, Zhang M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials. 2009;30(4):649–657. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen FM, Ma ZW, Dong GY, Wu ZF. Composite glycidyl methacrylated dextran (Dex-GMA)/gelatin nanoparticles for localized protein delivery. Acta Pharmacol Sin. 2009;30(4):485–493. doi: 10.1038/aps.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harush-Frenkel O, Altschuler Y, Benita S. Nanoparticle-cell interactions: drug delivery implications. Crit Rev Ther Drug Carrier Syst. 2008;25(6):485–544. doi: 10.1615/critrevtherdrugcarriersyst.v25.i6.10. [DOI] [PubMed] [Google Scholar]
- 51.Cellot G, Cilia E, Cipollone S, et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009;4(2):126–133. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- 52.Kam NW, Jan E, Kotov NA. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009;9(1):273–278. doi: 10.1021/nl802859a. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald RA, Voge CM, Kariolis M, Stegemann JP. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008;4(6):1583–1592. doi: 10.1016/j.actbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Shokuhfar T, Makradi A, Titus E, et al. Prediction of the mechanical properties of hydroxyapatite/polymethyl methacrylate/carbon nanotubes nanocomposite. J Nanosci Nanotechnol. 2008;8(8):4279–4284. doi: 10.1166/jnn.2008.an26. [DOI] [PubMed] [Google Scholar]
- 55.Lu YL, Cheng CM, LeDuc PR, Ho MS. Controlling the mechanics and nanotopography of biocompatible scaffolds through dielectrophoresis with carbon nanotubes. Electrophoresis. 2008;29(15):3123–3127. doi: 10.1002/elps.200700824. [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya S, Guillot S, Dabboue H, Tranchant JF, Salvetat JP. Carbon nanotubes as structural nanofibers for hyaluronic acid hydrogel scaffolds. Biomacromolecules. 2008;9(2):505–509. doi: 10.1021/bm7009976. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Gao H, Uo M, et al. Effect of carbon nanotubes on cellular functions in vitro. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32203. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Ramsaywack S, Fenniri H, Webster TJ. Enhanced osteoblast adhesion on self-assembled nanostructured hydrogel scaffolds. Tissue Eng Part A. 2008;14(8):1353–1364. doi: 10.1089/ten.tea.2006.0436. [DOI] [PubMed] [Google Scholar]
- 59.Jin H, Heller DA, Kim JH, Strano MS. Stochastic analysis of stepwise fluorescence quenching reactions on single-walled carbon nanotubes: single molecule sensors. Nano Lett. 2008;8(12):4299–4304. doi: 10.1021/nl802010z. [DOI] [PubMed] [Google Scholar]
- 60.Sung J, Barone PW, Kong H, Strano MS. Sequential delivery of dexamethasone and VEGF to control local tissue response for carbon nanotube fluorescence based micro-capillary implantable sensors. Biomaterials. 2009;30(4):622–631. doi: 10.1016/j.biomaterials.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 61 **.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. This seminal manuscript introduced nanofibrous scaffolds for the long-term in vitro tissue engineering of musculoskeletal tissues. [DOI] [PubMed] [Google Scholar]
- 62.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 63.Berndt P, Fields GB, Tirrell M. Synthetic Lipidation of Peptides and Amino-Acids -Monolayer Structure and Properties. Journal of the American Chemical Society. 1995;117(37):9515–9522. [Google Scholar]
- 64.Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2004;39(3):125–131. doi: 10.1016/j.colsurfb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Li WJ. Electrospinning Technology for Nanofibrous Scaffolds in Tissue Engineering. In: Kumar CSSR, editor. Tissue, Cell and Organ Engineering. WILEY-VCH Verlag GmbH & Co; 2006. pp. 135–187. [Google Scholar]
- 66.Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- 67.Tian F, Hosseinkhani H, Hosseinkhani M, et al. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J Biomed Mater Res A. 2008;84(2):291–299. doi: 10.1002/jbm.a.31304. [DOI] [PubMed] [Google Scholar]
- 68.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed I, Ponery AS, Nur EKA, et al. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol Cell Biochem. 2007;301(1–2):241–249. doi: 10.1007/s11010-007-9417-6. [DOI] [PubMed] [Google Scholar]
- 70.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12(7):1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 71.Abbott J, Holtzer H. The loss of phenotypic traits by differentiated cells. 3. The reversible behavior of chondrocytes in primary cultures. J Cell Biol. 1966;28(3):473–487. doi: 10.1083/jcb.28.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 73 **.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. This striking work shows how regulation of cell shape can influence the lineage commitment of mesenchymal progenitor cells. [DOI] [PubMed] [Google Scholar]
- 74 *.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three dimensional nanofibrillar surfaces induce activation of Rac. Biochem Biophys Res Commun. 2005;331(2):428–434. doi: 10.1016/j.bbrc.2005.03.195. This manuscript demonstrates that nanofibrous scaffolds modulate internal signaling pathways compared to traditional cell culture surfaces. [DOI] [PubMed] [Google Scholar]
- 75.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 76.Tsuji T, Ishizaki T, Okamoto M, et al. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J Cell Biol. 2002;157(5):819–830. doi: 10.1083/jcb.200112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie J, Willerth SM, Li X, et al. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30(3):354–362. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40(8):1686–1693. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 80.Nerurkar NL, Mauck RL, Elliott DM. ISSLS prize winner: Integrating theoretical and experimental methods for functional tissue engineering of the annulus fibrosus. Spine. 2008;33(25):2691–2701. doi: 10.1097/BRS.0b013e31818e61f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81 *.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28(11):1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. Refs #80 and 81 demonstrate that aligned nanofibrous scaffolds can direct ordered matrix deposition, a key feature of soft tissues of the musculoskeletal system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baker BM, Nathan AS, Huffman GR, Mauck RL. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthritis Cartilage. 2009;17(3):336–345. doi: 10.1016/j.joca.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83 *.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotech. 1996;7:216–223. One of the seminal papers on controlling nanofiber morphology via control of fabrication variables. [Google Scholar]
- 84.Li D, Xia YN. Electrospinning of nanofibers: Reinventing the wheel? Advanced Materials. 2004;16(14):1151–1170. [Google Scholar]
- 85.Li WJ, Mauck RL, Tuan RS. Electrospun nanofibrous scaffolds: production, characterization, and applications for tissue engineering and drug delivery. J Biomed Nanotech. 2005;1 (3):259–275. [Google Scholar]
- 86.Burger C, Hsiao BS, Chu B. Nanofibrous materials and their applications. Annual Review of Materials Research. 2006;36:333–368. [Google Scholar]
- 87.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17(14):R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 88.Mauck RL, Baker BM, Nerurkar NL, et al. Engineering on the Straight and Narrow: The Mechanics of Nanofibrous Assemblies for Fiber-Reinforced Tissue Regeneration. Tissue Eng Part B Rev. 2009 doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261–272. [Google Scholar]
- 90.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59(14):1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 91.Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res B Appl Biomater. 2003;67(2):675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- 92.Xu C, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10(7–8):1160–1168. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 93.Venugopal J, Ma LL, Yong T, Ramakrishna S. In vitro study of smooth muscle cells on polycaprolactone and collagen nanofibrous matrices. Cell Biol Int. 2005;29(10):861–867. doi: 10.1016/j.cellbi.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 94.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 95.Boland ED, Telemeco TA, Simpson DG, Wnek GE, Bowlin GL. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly(glycolic acid) for tissue engineering. J Biomed Mater Res B Appl Biomater. 2004;71(1):144–152. doi: 10.1002/jbm.b.30105. [DOI] [PubMed] [Google Scholar]
- 96.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 97.Yang F, Xu CY, Kotaki M, Wang S, Ramakrishna S. Characterization of neural stem cells on electrospun poly(L-lactic acid) nanofibrous scaffold. J Biomater Sci Polym Ed. 2004;15(12):1483–1497. doi: 10.1163/1568562042459733. [DOI] [PubMed] [Google Scholar]
- 98.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2(4):377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 99.Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005;1(1):115–123. doi: 10.1016/j.actbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 100 *.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–238. doi: 10.1021/bm015533u. One of the early papers describing the electrospinning of biologic ECM components. [DOI] [PubMed] [Google Scholar]
- 101.Rho KS, Jeong L, Lee G, et al. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27(8):1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27(5):724–734. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 103.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26(30):5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 104.Min BM, Jeong L, Nam YS, Kim JM, Kim JY, Park WH. Formation of silk fibroin matrices with different texture and its cellular response to normal human keratinocytes. Int J Biol Macromol. 2004;34(5):281–288. doi: 10.1016/j.ijbiomac.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25(7–8):1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 106.Zhang X, Baughman CB, Kaplan DL. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials. 2008;29(14):2217–2227. doi: 10.1016/j.biomaterials.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soffer L, Wang X, Zhang X, et al. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J Biomater Sci Polym Ed. 2008;19(5):653–664. doi: 10.1163/156856208784089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geng X, Kwon OH, Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26(27):5427–5432. doi: 10.1016/j.biomaterials.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 109.Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 2005;26(31):6176–6184. doi: 10.1016/j.biomaterials.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 110.Jiang H, Fang D, Hsiao BS, Chu B, Chen W. Optimization and characterization of dextran membranes prepared by electrospinning. Biomacromolecules. 2004;5(2):326–333. doi: 10.1021/bm034345w. [DOI] [PubMed] [Google Scholar]
- 111.Woerdeman DL, Ye P, Shenoy S, Parnas RS, Wnek GE, Trofimova O. Electrospun fibers from wheat protein: investigation of the interplay between molecular structure and the fluid dynamics of the electrospinning process. Biomacromolecules. 2005;6(2):707–712. doi: 10.1021/bm0494545. [DOI] [PubMed] [Google Scholar]
- 112.Tan AR, Ifkovits JL, Baker BM, Brey DM, Mauck RL, Burdick JA. Electrospinning of photocrosslinked and degradable fibrous scaffolds. J Biomed Mater Res A. 2008;87A(4):1034–1043. doi: 10.1002/jbm.a.31853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ifkovits JL, Padera RF, Burdick JA. Biodegradable and radically polymerized elastomers with enhanced processing capabilities. Biomed Mater. 2008;3(3):034104. doi: 10.1088/1748-6041/3/3/034104. [DOI] [PubMed] [Google Scholar]
- 114.Lee SJ, Yoo JJ, Lim GJ, Atala A, Stitzel J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J Biomed Mater Res A. 2007;83(4):999–1008. doi: 10.1002/jbm.a.31287. [DOI] [PubMed] [Google Scholar]
- 115.Stitzel J, Liu J, Lee SJ, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27(7):1088–1094. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 116 *.Sell SA, McClure MJ, Barnes CP, et al. Electrospun polydioxanone-elastin blends: potential for bioresorbable vascular grafts. Biomed Mater. 2006;1(2):72–80. doi: 10.1088/1748-6041/1/2/004. An important work defining how intra-fiber composites can be created from mixed solutions, with resulting meshes possessing biologic features and controllable mechanical properties. [DOI] [PubMed] [Google Scholar]
- 117.Stankus JJ, Freytes DO, Badylak SF, Wagner WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. Journal of Biomaterials Science-Polymer Edition. 2008;19(5):635–652. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Casper CL, Yamaguchi N, Kiick KL, Rabolt JF. Functionalizing Electrospun Fibers with Biologically Relevant Macromolecules. Biomacromolecules. 2005;6(4):1998–2007. doi: 10.1021/bm050007e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Casper CL, Yang W, Farach-Carson MC, Rabolt JF. Coating electrospun collagen and gelatin fibers with perlecan domain I for increased growth factor binding. Biomacromolecules. 2007;8(4):1116–1123. doi: 10.1021/bm061003s. [DOI] [PubMed] [Google Scholar]
- 120.Ma Z, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell Orientation. Tissue Eng. 2005;11(7–8):1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 121.Pornsopone V, Supaphol P, Rangkupan R, Tantayanon S. Electrospinning of methacrylate-based copolymers: Effects of solution concentration and applied electrical potential on morphological appearance of as-spun fibers. Polymer Engineering and Science. 2005;45(8):1073–1080. [Google Scholar]
- 122.Kim SH, Nair S, Moore E. Reactive electrospinning of cross-linked poly(2-hydroxyethyl methacrylate) nanofibers and elastic properties of individual hydrogel nanofibers in aqueous solutions. Macromolecules. 2005;38(9):3719–3723. [Google Scholar]
- 123 *.Gupta P, Wilkes GL. Some investigations on the fiber formation by utilizing a side-by-side bicomponent electrospinning approach. Polymer. 2003;44(20):6353–6359. One of the first papers demonstrating the fabrication of electrospun scaffolds with multiple fiber populations. [Google Scholar]
- 124.Ding B, Kimura E, Sato T, Fujita S, Shiratori S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer. 2004;45(6):1895–1902. [Google Scholar]
- 125.Baker BM, Nerurkar NL, Burdick J, Elliott DM, Mauck RL. Instilling Time-Dependent Behavior in Electrospun, Multi-Polymer Nanofibrous Composites. Trans of the 55th Annual Meeting of the Orthopaedic Research Society. 2009;34:473. [Google Scholar]
- 126.Baker BM, Nerurkar NL, Burdick JA, Elliott DM, Mauck RL. Fabrication and modeling of an electrospun tri-polymer composite for the engineering of fibrous tissues. Proc BIO2008 Summer Bioengineering Conference; 2008. [Google Scholar]
- 127.Baker BM, Nerurkar NL, Burdick JA, Elliott DM, Mauck RL. Fabrication and modeling of dynamic multi-component nanofibrous scaffolds. Journal of Biomechanical Engineering. 2008 doi: 10.1115/1.3192140. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Balguid A, Mol A, van Marion MH, Bank RA, Bouten CV, Baaijens FP. Tailoring Fiber Diameter in Electrospun Poly(epsilon-Caprolactone) Scaffolds for Optimal Cellular Infiltration in Cardiovascular Tissue Engineering. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 129.Pham QP, Sharma U, Mikos AG. Electrospun Poly(epsilon-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 130.Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel Nanofiber-Based Scaffold for Rotator Cuff Repair and Augmentation. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13(9):2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simonet M, Schneider OD, Neuenschwander P, Stark WJ. Ultraporous 3D polymer meshes by low-temperature electrospinning: Use of ice crystals as a removable void template. Polymer Engineering and Science. 2007;47(12):2020–2026. [Google Scholar]
- 133 *.Baker BM, Gee AO, Metter RB, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29(15):2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. An important work introducing the concept of sacrificial fibers in a mixed nanofibrous composite for the purposes of increasing cell infiltration rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Telemeco TA, Ayres C, Bowlin GL, et al. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005;1(4):377–385. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 135.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jayasinghe SN, Irvine S, McEwan JR. Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomed. 2007;2(4):555–567. doi: 10.2217/17435889.2.4.555. [DOI] [PubMed] [Google Scholar]
- 137.Townsend-Nicholson A, Jayasinghe SN. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006;7(12):3364–3369. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- 138.Casper CL, Yang W, Farach-Carson MC, Rabolt JF. Coating Electrospun Collagen and Gelatin Fibers with Perlecan Domain I for Increased Growth Factor Binding. Biomacromolecules. 2007;8(4):1116–1123. doi: 10.1021/bm061003s. [DOI] [PubMed] [Google Scholar]
- 139 **.Kenawy el-R BG, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release. 2002;17(81):57–64. doi: 10.1016/s0168-3659(02)00041-x. One of the first manuscripts to show the controlled release of an antibiotic from nanofibrous scaffolds. [DOI] [PubMed] [Google Scholar]
- 140.Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43(16):4403–4412. [Google Scholar]
- 141.Kim K, Luu YK, Chang C, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. Journal of Controlled Release. 2004;98(1):47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 142.Zeng J, Xu X, Chen X, et al. Biodegradable electrospun fibers for drug delivery. Journal of Controlled Release. 2003;92(3):227–231. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 143.Katti DS, Robinson Kyle W, Ko Frank K, Laurencin Cato T. Bioresorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parameters. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2004;70B(2):286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 144 **.Hong Y, Fujimoto K, Hashizume R, et al. Generating Elastic, Biodegradable Polyurethane/Poly(lactide-co-glycolide) Fibrous Sheets with Controlled Antibiotic Release via Two-Stream Electrospinning. Biomacromolecules. 2008;9(4):1200–1207. doi: 10.1021/bm701201w. An important work demonstrating that two fiber populations can be used to create scaffolds with appropriate drug release and mechanical properties by segregating these functions in different fiber populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He C, Huang Z-M, Han X-J, Liu L, Zhang H-S, Chen L-S. Journal of Macromolecular Science: Physics. Taylor & Francis Ltd; 2006. Coaxial Electrospun Poly(L-Lactic Acid) Ultrafine Fibers for Sustained Drug Delivery; pp. 515–524. [Google Scholar]
- 146.Huang ZM, He Chuang-Long, Yang Aizhao, et al. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. Journal of Biomedical Materials Research Part A. 2006;77A(1):169–179. doi: 10.1002/jbm.a.30564. [DOI] [PubMed] [Google Scholar]
- 147.He C-L, Huang Z-M, Han X-J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. Journal of Biomedical Materials Research Part A. 2009;89A(1):80–95. doi: 10.1002/jbm.a.32004. [DOI] [PubMed] [Google Scholar]
- 148.Jiang H, Fang D, Hsiao B, Chu B, Chen W. Preparation and characterization of ibuprofen-loaded poly(lactide-co-glycolide)/poly(ethylene glycol)-g-chitosan electrospun membranes. Journal of Biomaterials Science, Polymer Edition. 2004;15:279–296. doi: 10.1163/156856204322977184. [DOI] [PubMed] [Google Scholar]
- 149.Cui W, Qi M, Li X, Huang S, Zhou S, Weng J. Electrospun fibers of acid-labile biodegradable polymers with acetal groups as potential drug carriers. International Journal of Pharmaceutics. 2008;361(1–2):47–55. doi: 10.1016/j.ijpharm.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 150.Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. Investigation of Drug Release and Matrix Degradation of Electrospun Poly(dl-lactide) Fibers with Paracetanol Inoculation. Biomacromolecules. 2006;7(5):1623–1629. doi: 10.1021/bm060057z. [DOI] [PubMed] [Google Scholar]
- 151.Zeng J, Yang L, Liang Q, et al. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. Journal of Controlled Release. 2005;105(1–2):43–51. doi: 10.1016/j.jconrel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 152.Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff JH, Greiner A. Poly(vinyl alcohol) Nanofibers by Electrospinning as a Protein Delivery System and the Retardation of Enzyme Release by Additional Polymer Coatings. Biomacromolecules. 2005;6(3):1484–1488. doi: 10.1021/bm0492576. [DOI] [PubMed] [Google Scholar]
- 153.Xie J, Wang C-H. Electrospun Micro- and Nanofibers for Sustained Delivery of Paclitaxel to Treat C6 Glioma in Vitro. Pharmaceutical Research. 2006;23(8):1817–1826. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 154.Xu X, Chen X, Xu X, et al. BCNU-loaded PEG-PLLA ultrafine fibers and their in vitro antitumor activity against Glioma C6 cells. Journal of Controlled Release. 2006;114(3):307–316. doi: 10.1016/j.jconrel.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 155.Xu X, Yang L, Xu X, et al. Ultrafine medicated fibers electrospun from W/O emulsions. Journal of Controlled Release. 2005;108(1):33–42. doi: 10.1016/j.jconrel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 156.Chew SY, Wen J, Yim EKF, Leong KW. Sustained Release of Proteins from Electrospun Biodegradable Fibers. Biomacromolecules. 2005;6(4):2017–2024. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- 157.Sanders EH, Kloefkorn Rene, Bowlin Gary L, Simpson David G, Wnek GE. Two-Phase Electrospinning from a Single Electrified Jet: Microencapsulation of Aqueous Reservoirs in Poly(ethylene-co-vinyl acetate) Fibers. Macromolecules. 2003;36(11):3803–3805. [Google Scholar]
- 158.Maretschek S, Greiner A, Kissel T. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. Journal of Controlled Release. 2008;127(2):180–187. doi: 10.1016/j.jconrel.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 159.Li C, Vepari C, Jin H-J, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 160.Yang Y, Li X, Qi M, Zhou S, Weng J. Release pattern and structural integrity of lysozyme encapsulated in core-sheath structured poly(dl-lactide) ultrafine fibers prepared by emulsion electrospinning. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69(1):106–116. doi: 10.1016/j.ejpb.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 161 *.Jiang H, Hu Y, Li Y, Zhao P, Zhu K, Chen W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. Journal of Controlled Release. 2005;108(2–3):237–243. doi: 10.1016/j.jconrel.2005.08.006. An important work describing the use of co-axial electrospinning for release of active biologic molecules. [DOI] [PubMed] [Google Scholar]
- 162.Jiang H, Hu Y, Zhao P, Li Y, Zhu K. Modulation of protein release from biodegradable core-shell structured fibers prepared by coaxial electrospinning. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;79B(1):50–57. doi: 10.1002/jbm.b.30510. [DOI] [PubMed] [Google Scholar]
- 163.Zhang YZ, Wang X, Feng Y, Li J, Lim CT, Ramakrishna S. Coaxial Electrospinning of (Fluorescein Isothiocyanate-Conjugated Bovine Serum Albumin)-Encapsulated Poly(e-caprolactone) Nanofibers for Sustained Release. Biomacromolecules. 2006;7(4):1049–1057. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- 164.Liao I, Chew S, Leong K. Aligned core-shell nanofibers delivering bioactive proteins. Nanomedicine. 2006;1(4):465–471. doi: 10.2217/17435889.1.4.465. [DOI] [PubMed] [Google Scholar]
- 165.Ionescu L, Baker BM, Burdick JA, Mauck RL. Fabrication of Drug Delivering Biodegradable Microsphere/Nanofiber Networks. 55th Annual Meeting of the Orthopaedic Research Society; 2009. [Google Scholar]
- 166.Qi, Hu P, Xu J, Wang Encapsulation of Drug Reservoirs in Fibers by Emulsion Electrospinning: Morphology Characterization and Preliminary Release Assessment. Biomacromolecules. 2006;7(8):2327–2330. doi: 10.1021/bm060264z. [DOI] [PubMed] [Google Scholar]
- 167.Srikar R, Yarin AL, Megaridis CM, Bazilevsky AV, Kelley E. Desorption-Limited Mechanism of Release from Polymer Nanofibers. Langmuir. 2008;24(3):965–974. doi: 10.1021/la702449k. [DOI] [PubMed] [Google Scholar]
- 168.Chew SY, Hufnagel TC, Lim CT, Leong KW. Mechanical properties of single electrospun drug-encapsulated nanofibres. Nanotechnology. 2006;17(15):3880–3891. doi: 10.1088/0957-4484/17/15/045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. Journal of Controlled Release. 2003;89(2):341–353. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 170.Nie H, Wang C-H. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. Journal of Controlled Release. 2007;120(1–2):111–121. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 171.Liang D, Luu YK, Kim K, Hsiao BS, Hadjiargyrou M, Chu B. In vitro non-viral gene delivery with nanofibrous scaffolds. Nucleic Acids Res. 2005;33(19):e170. doi: 10.1093/nar/gni171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sun D, Chang C, Li S, Lin L. Near-field electrospinning. Nano Lett. 2006;6(4):839–842. doi: 10.1021/nl0602701. [DOI] [PubMed] [Google Scholar]
- 173.Dalton PD, Joergensen NT, Groll J, Moeller M. Patterned melt electrospun substrates for tissue engineering. Biomed Mater. 2008;3(3):034109. doi: 10.1088/1748-6041/3/3/034109. [DOI] [PubMed] [Google Scholar]
- 174.Dalton PD, Lleixa Calvet J, Mourran A, Klee D, Moller M. Melt electrospinning of poly-(ethylene glycol-block-epsilon-caprolactone) Biotechnol J. 2006;1(9):998–1006. doi: 10.1002/biot.200600064. [DOI] [PubMed] [Google Scholar]
- 175.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]