Abstract
The human circadian system is maximally sensitive to short-wavelength (blue) light. In a previous study we found no difference between the magnitude of phase advances produced by bright white versus bright blue-enriched light using light boxes in a practical protocol that could be used in the real world. Since the spectral sensitivity of the circadian system may vary with a circadian rhythm, we tested whether the results of our recent phase-advancing study hold true for phase delays. In a within-subjects counterbalanced design, this study tested whether bright blue-enriched polychromatic light (17000 K, 4000 lux) could produce larger phase delays than bright white light (4100 K, 5000 lux) of equal photon density (4.2 × 1015 photons/cm2/sec). Healthy young subjects (n = 13) received a 2 h phase delaying light pulse before bedtime combined with a gradually delaying sleep/dark schedule on each of 4 consecutive treatment days. On the first treatment day the light pulse began 3 h after the dim light melatonin onset (DLMO). An 8 h sleep episode began at the end of the light pulse. Light treatment and the sleep schedule were delayed 2 h on each subsequent treatment day. A circadian phase assessment was conducted before and after the series of light treatment days to determine the time of the DLMO and DLMOff. Phase delays in the blue-enriched and white conditions were not significantly different (DLMO: −4.45 ± 2.02 versus −4.48 ± 1.97 h; DLMOff: −3.90 ± 1.97 versus −4.35 ± 2.39 h, respectively). These results indicate that at light levels commonly used for circadian phase shifting blue-enriched polychromatic light is no more effective than the white polychromatic lamps of a lower correlated color temperature (CCT) for phase delaying the circadian clock.
Keywords: Blue Light, Human, Circadian, Phase Shifting
Introduction
The circadian clock is most sensitive to short-wavelength (blue) light (Wright & Lack 2001; Lockley et al., 2003; Warman et al., 2003; Wright et al., 2004; Revell et al., 2005a; Revell & Skene, 2007). Although other photoreceptors do contribute to non-image-forming (NIF) responses, this short-wavelength sensitivity is thought to arise primarily from intrinsically photosensitive retinal ganglion cells (ipRGCs) (Berson et al., 2002). Laboratory and field studies are now addressing how this sensitivity could be used to optimize light treatment for circadian rhythm sleep disorders. Several commercially available devices, primarily emitting light in the blue-green portion of the visible light spectrum, have been reported to induce phase delays of the circadian rhythm of melatonin (Paul et al., 2007). At light levels that are commonly used for the treatment of seasonal affective disorder (SAD) and circadian phase shifting in humans, we found no difference in the size of the phase advance in response to bright white or bright blue-enriched polychromatic light (Smith et al., 2009b). Whether blue-enriched polychromatic light could produce larger phase delays than the standard polychromatic white light containing is unknown.
A circadian rhythm in the spectral sensitivity of NIF responses would not be unexpected because the retina contains a circadian clock (Tosini & Menaker, 1998; Witkovsky et al., 2003). Under entrained conditions, levels of melanopsin mRNA and protein in rat models peak near the light/dark transition and reach a nadir near the dark/light transition (Hannibal et al., 2005; Mathes et al., 2007). Whether a similar circadian rhythm of melanopsin expression occurs in humans is unknown. In both rodents and humans, light that occurs in the evening and early nighttime (when melanopsin protein levels in the rat are highest) falls on the delay portion of the light PRC, while light that occurs late in the nighttime and early morning (when melanopsin protein levels in the rat are lowest) falls on the advance portion of the light PRC [see (Revell & Eastman, 2005) for a human light PRC]. It is thus possible that polychromatic light of a higher correlated color temperature (CCT) may produce larger phase delays, but not phase advances, than white polychromatic light of a lower CCT. Lamps with a higher CCT [measured in degrees Kelvin (K)] emit more short-wavelength energy.
This study compared the phase delays produced by bright blue-enriched and bright white polychromatic lights of equal photon density delivered at the same circadian phase. The light levels used were chosen specifically to answer the question of whether bright blue-enriched light can produce larger phase delays than the bright white light treatment that has been used for circadian phase shifting over the last few decades. In addition, light treatment was delivered in a way similar to what most individuals use in the real world. Previous studies demonstrating that circadian phase shifts are sensitive to short-wavelength light have pharmacologically dilated subjects’ pupils (Lockley et al., 2003) and/or have used a specialized light delivery apparatus (e.g., ganzfeld dome or custom made visors) (Lockley et al., 2003; Warman et al., 2003; Revell et al., 2005a). We did not pharmacologically dilate the pupils of subjects and used desktop light boxes because these are the ways most individuals use light treatment in the field.
Methods
Subjects
This was a within-subjects counterbalanced design. Fourteen healthy young subjects completed the study. Data from one subject was excluded because a number of saliva samples this subject produced also contained blood, precluding an accurate determination of circadian phase. For the remaining 13 subjects (10 male), mean age was 29.5 ± 8.4 (SD) yrs, and mean morningness-eveningness score (Horne & Ostberg 1976) was 54.9 ± 11.2. Subjects were free from medical, psychiatric, and sleep disorders as assessed by a telephone interview, an in-person interview, and several screening questionnaires. Subjects were not color blind according to the Ishihara Color Blindness test. All subjects were non-smokers, typically drank < 300 mg of caffeine and < 2 alcoholic drinks per day, were free from prescription medication, and had body mass indices ≤ 30 kg/m2. A urine toxicology screen at the start of the study verified that subjects were free from recreational drug use. Subjects had not traveled across more than three time zones in the one month prior to or worked a night shift three months prior to starting the study. This study was conducted between January and May, 2008. This study was approved by the Rush University Institutional Review Board and meets the ethical standards of this journal (Portaluppi et al., 2008).
Design
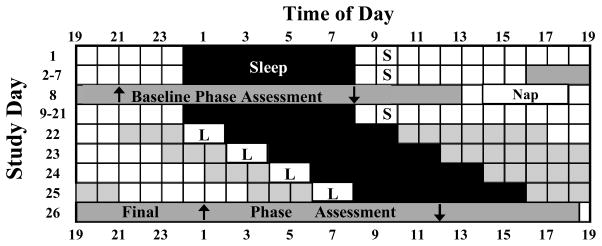
Figure 1 illustrates the study protocol. The study began with seven baseline days in which subjects slept at home on a fixed 8 h schedule similar to their typical sleep schedule. On day 7, subjects came to the lab for a baseline phase assessment. The phase assessment was followed by a compulsory 4 h nap at the subjects’ home centered 12 h from the midpoint of the subjects’ nocturnal sleep episode. Subjects then returned to the baseline sleep schedule for 13 days (days 9–21 in Figure 1). These 13 days were needed to determine the time of the subjects’ dim light melatonin onset (DLMO) from the saliva samples collected during the baseline phase assessment. On days 1–21, subjects were required to get at least 10 min of outdoor light every morning between 1 and 2 h after their wake time. This morning light was expected to stabilize the position of the circadian clock prior to and after the baseline phase assessment.
Figure 1.
Diagram illustrating the study protocol. This schedule is an example for a subject sleeping 00:00–08:00 h during the baseline. On days 22–25, the shaded black area indicates when the subject was in bed in the dark in a laboratory bedroom. “S” during baseline indicates the hour during which the subject was required to go outside for at least 10 min of light exposure. Boxes with “L” indicate the 2 h when the subject sat in front of the light box in the laboratory. Grey areas on days 22–25 indicate times when the subject was required to be in the laboratory (< 60 lux). Upward arrows indicate a typical time of the DLMO. Downward arrows indicate the time of the DLMOff.
On the evening of day 22, subjects came to the lab for the first of four consecutive light treatment days. Subjects arrived at the time of their DLMO, as determined during the baseline phase assessment. A 2 h continuous light pulse began 3 h after the DLMO on day 22. Light at this circadian time delays the circadian clock (Revell & Eastman, 2005). Immediately following the light pulse, subjects spent 8 h in a private dark bedroom in the lab. Previous work in our lab (Revell et al., 2005b) has shown that circadian phase is reproducible under conditions similar to what these subjects experienced on study days 8–22. Because of this, the time of the DLMO during the baseline phase assessment was a good estimate for the time of the DLMO on the first night of light treatment. All subjects remained in the controlled light conditions of the lab (< 60 lux, 4,100 K fluorescent lamps) until 7 h after their waking time on each day of light treatment to avert outside morning light exposure. Subjects were then free to leave the lab, but were required to return to the lab 5 h before their scheduled bedtime the next evening. The sleep episode and the time of the light pulse were delayed by 2 h each successive treatment day to keep up with the expected phase delays of the circadian clock. Subjects were not permitted to leave the lab after awakening on study day 25.
Subjects completed this protocol twice, being exposed to the blue-enriched light in one 26-day session and to white light in the other. The order of light treatment was counterbalanced. Seven subjects received the blue-enriched light first, and 6 received the white light first. To ensure that circadian phase before the second light treatment session was similar to the first session, subjects’ maintained a schedule of sleep and light exposure designed to advance their circadian clocks back to the baseline phase after finishing the first session. Beginning the day after the first final phase assessment, subjects resumed an 8 h sleep episode at home. The time of the sleep episode on this day was the same as on day 24. After awakening from this sleep episode, subjects were required to get at least 30 min of light exposure within the first hour after awakening. Subjects were permitted to get any combination of outside light exposure and/or sitting in front of a light box in their homes for these 30 min. Light at this time was expected to phase advance the circadian clock, facilitating re-entrainment to each subject’s habitual sleep schedule. On each successive day, the sleep episode and the time of the 30 min of morning light exposure were advanced by 1 h. This continued until the sleep episode returned to the subject’s baseline schedule, and then the stable sleep schedule and 10 min of morning light exposure were resumed. There were 16 days between the final phase assessment of the first 26-day session and the baseline phase assessment of the second session. Subjects averaged 9.6 ± 1.3 (mean ± SD) days on their baseline sleep schedule before the second baseline phase assessment.
Bright-Light Treatment
On day 22, a 2 h continuous light pulse began 3 h after the baseline DLMO. The light was produced by a light box containing fluorescent lamps placed on a desk 40 cm in front of subjects’ eyes. Subjects were instructed to maintain their distance from the light box. The light levels at the angle of gaze and distance to the light box were measured every 10 min during the light treatment to ensure that subjects were at the targeted distance and light intensity. The spectral power distributions of the blue-enriched (17,000 K, Philips Lighting, Eindhoven, The Netherlands) and white (4,100 K, Enviro-Med, Vancouver, WA) lamps have been published (Smith et al., 2009b). The size and shape of the illuminated area for the blue-enriched (57 × 57 cm) and white (54 × 54 cm) light boxes were similar. The white light box was fit with a neutral density filter (#298, Lee Filters, Burbank, California). Light levels were measured (after passing through the diffuser screen and neutral density filter) with an SM240 CCD Spectrometer (Spectral Products, Putnam, CT). The illuminance of the blue-enriched lamps was lower than the white lamps (4000 vs 5000 lux, respectively). The irradiance of the blue-enriched and white light boxes was similar (1640 and 1475 μW/cm2). The blue-enriched and white light boxes emitted the same number of total photons (4.2 × 1015 photons/cm2/sec), but the blue-enriched light box emitted about three times the number of photons in the blue range, between 400–490nm, than the white-light box (19.0 vs. 6.4 × 1014 photons/cm2/sec).
Subjects remained in normal room light (< 60 lux, 4,100K lamps) at all other times while in the lab during the light treatment days. Subjects’ pupils were not dilated during the light treatment. The human pupillary light reflex (PLR) is sensitive to short-wavelength light and is in part driven by melanopsin (Gamlin et al., 2007; Young & Kimura, 2008). In a separate study, pupil diameter of subjects sitting in front of the white and blue-enriched light boxes at the light levels used in the present study was found to be similar (Smith & Eastman, unpublished data). Based on human irradiance response curves for pupillary constriction (Gamlin et al., 2007), both light boxes used in this study delivered light levels that likely elicited maximal pupillary constriction.
Phase Assessments
The time of phase assessments was based on each subject’s sleep schedule. The baseline phase assessment began 8 h before a subject’s baseline bedtime and lasted for 21 h. The final phase assessment began 3 h after each subject’s wake time on day 25 and lasted for 23.5 h. Details of the phase assessment procedures have been described elsewhere (Lee et al., 2006; Smith et al., 2009b)
During phase assessments, subjects remained in dim light [4100K with red filters behind the ceiling diffuser screens; < 5 lux; total irradiance 2.3–3.8 μW/cm2; 0.7 to 1.4 μW/cm2 between 400–490nm]. Saliva samples were collected every 30 min using a salivette (Sarstedt, Newton, NC, USA). Samples were centrifuged immediately upon collection and frozen. These samples were shipped on dry ice to Pharmasan Labs (Osceola, WI) and radioimmunoassayed for melatonin. The sensitivity of the assay was 0.7 pg/ml and the intra- and inter-assay coefficients of variability were 12.1% and 13.2%, respectively.
During phase assessments, subjects were seated in recliner chairs. Restroom trips were not permitted in the 10 min preceding each saliva sample. The restroom and adjoining hallway were dimly lit (< 5 lux). Alcohol was prohibited for 24 h before and during phase assessment sessions as alcohol has been found to alter melatonin level (Rupp et al., 2007). Non-steroidal anti-inflammatory drugs (NSAIDs) were prohibited for 72 h before and during the phase assessments.
Ambient Light Exposure
Subjects wore an actiwatch-L (AWL, Mini-Mitter, Bend, OR) attached to a cord around their neck to measure light exposure. This “light medallion” was worn at all times, except when sleeping and bathing. Using a handheld personal data assistant (PDA), subjects completed daily light logs noting the times they were outside buildings during the daylight hours, and whether sunglasses were worn. Subjects were permitted to wear sunglasses when outside, but were instructed not to use sunglasses during the 10 min of required morning light exposure. Subjects were offered clear UV-blocking glasses to wear when outside for their required morning light. To enforce compliance with the required sleep and outdoor light exposure schedule, subjects came to the lab every 1–3 days to check that the data from the light medallion and a second AWL worn on the wrist to measure activity were consistent with the assigned schedule.
Subjective Impression of Light Boxes
At the end of the study, subjects were asked to rate their impressions of each light box on four 10-point ordinal scales. The endpoints of the four scales were 1) very unpleasant and very pleasant, 2) very painful and very comfortable, 3) sleep-inducing and energizing, and 4) agitating and relaxing. Subjects also answered questions asking which light box they felt was brighter, which light box was more pleasant, and which light box they preferred.
Other Procedures
Subjects were permitted ≤100 mg caffeine within the first 3 h after awakening in the morning. Caffeine was prohibited on the days when phase assessments occurred. Subjects were permitted up to 2 alcoholic drinks per day, but alcohol was prohibited for 24 h before and during each phase assessment, and on days when subjects were in the laboratory for light treatment.
Data Analysis
Circadian Phase Shifts
To determine the time of the DLMO and DLMOff, a threshold was calculated for each melatonin profile. The threshold was the average of the 5 lowest continuous points plus 15% of the average of the five highest continuous points (Revell et al., 2005b; Smith et al., 2009b). Melatonin profiles were smoothed with a locally weighted least squares curve using the fine setting (GraphPad Prism, San Diego, CA). The DLMO was defined as the time that this smoothed curve exceeded and remained above the threshold. The DLMOff was the time that the curve dropped and remained below the threshold. Phase shifts were calculated by taking the difference in the time of the DLMO and DLMOff from the baseline to the final phase assessment. Paired t-tests were used to compare the phase shift of the DLMO and DLMOff in the blue-enriched and white light conditions. To test for order effects, paired t-tests were also used to compare phase shifts of the DLMO and DLMOff during the first and second light treatment sessions. To test whether circadian phase was similar during the baseline phase assessment for the first and second 26-day sessions, paired t-tests were used to test for differences between the DLMOs.
Ambient Light Exposure
Light exposure during the study, as measured with the AWL worn around the neck, was analyzed for two different time periods. First, because photic history can influence the size of NIF responses (Hebert et al., 2002), history of light exposure in the 3 weeks preceding each series of light treatment days was analyzed. Second, light exposure when subjects were permitted to be outside of the laboratory during light-treatment days was analyzed. For each analysis, the average lux/min during waking hours was calculated. All light levels were corrected for the transmission of sunglasses, when worn, using a measurement of subjects’ sunglasses transmission obtained upon enrolling in the study.
For light history, paired t-tests were used to compare the mean light exposure preceding the blue-enriched and white light conditions (days 2–22). Pearson correlations were used to test for an association between light exposure history and phase shift of the DLMO for each light condition.
Light exposure data when subjects were permitted to be outside of the laboratory after the first 3 light treatment days (white squares on days 22–25 in Figure 1) were not normally distributed due to a positive skew. Therefore, light exposure in the blue-enriched and white-light conditions was compared with Wilcoxon Signed Rank tests. In the white-light condition, Spearman rank order correlations were used to assess whether light exposure outside the lab on these days was associated with phase shift of the DLMO. Because light exposure history during the 3 weeks before light treatment was significantly associated with phase shift of the DLMO in the blue-enriched condition (see results), we computed the partial correlation between light exposure when outside of the lab between light treatment days and phase shift of the DLMO, while controlling for history of light exposure (SPSS version 11.0).
Subjective Impression of Light Boxes
The four scales measuring subjects’ impressions of the blue-enriched and white light boxes were compared with paired t-tests. Ratings of which light box a subject thought was brighter, more pleasant, and preferred were compared with a Chi Square test.
Results
Circadian Phase Shifts
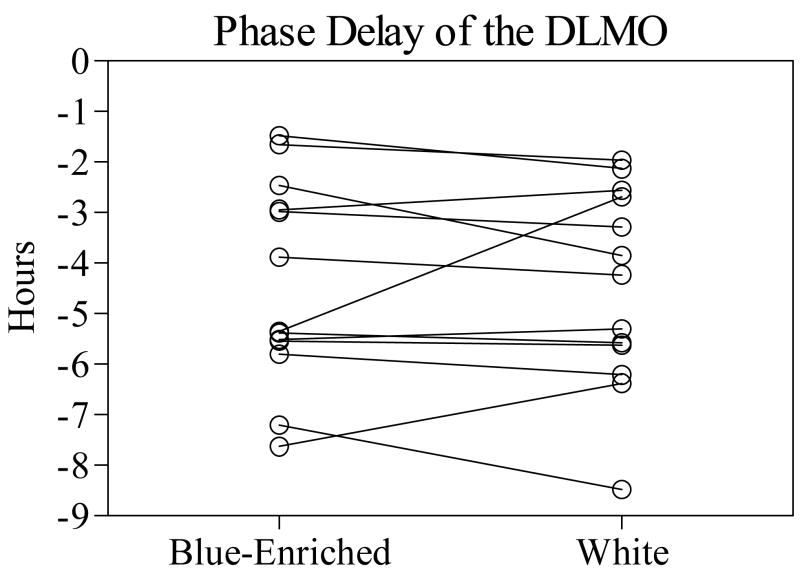
Phase shifts in the blue-enriched and white conditions were of similar magnitude (Figure 2). Phase shifts of the DLMO after treatment with the blue-enriched lamps (−4.45 ± 2.02 h) were not significantly different from the white lamps (−4.48 ± 1.97 h) [t(12) = 0.12, p = 0.99]. Phase shifts of the DLMOff after treatment with the blue-enriched lamps (−3.90 ± 1.97 h) were also not significantly different from white lamps (−4.35 ± 2.39 h) [t(12) = 1.49, p = 0.16]. In each light condition, the phase shift of the DLMO and DLMOff was strongly correlated (blue-enriched, r = .86, p < 0.001; white, r = .96, p < 0.001).
Figure 2.
Phase delay of the DLMO in the blue-enriched and white light conditions. Lines connect the phase shift for the same subject in each condition.
There was no effect of order on the phase shift of the DLMO and DLMOff. Phase shifts of the DLMO for the first light condition (−4.64 ± 1.84 h) were not significantly different from the second light condition (−4.30 ± 2.12 h). Phase shifts of the DLMOff for the first light condition (−4.13 ± 2.04 h) were also not significantly different from the second light condition (−4.11 ± 2.35 h).
After the first 26-day session, the circadian clocks of the subjects returned to their original position before the baseline phase assessment in the second session. The average absolute difference in the DLMO during the first and second baseline phase assessments was 29 ± 38 min, while the average absolute difference in the DLMOff was 23 ± 25 min. There were no significant differences for either phase marker between the two baseline phase assessments.
Ambient Light Exposure
Light exposure during the 3 weeks preceding light treatment in the laboratory was similar for the two lighting conditions. During wakefulness, subjects averaged 459 ± 251 and 451 ± 306 lux/min preceding the blue-enriched and white light treatment days, respectively.
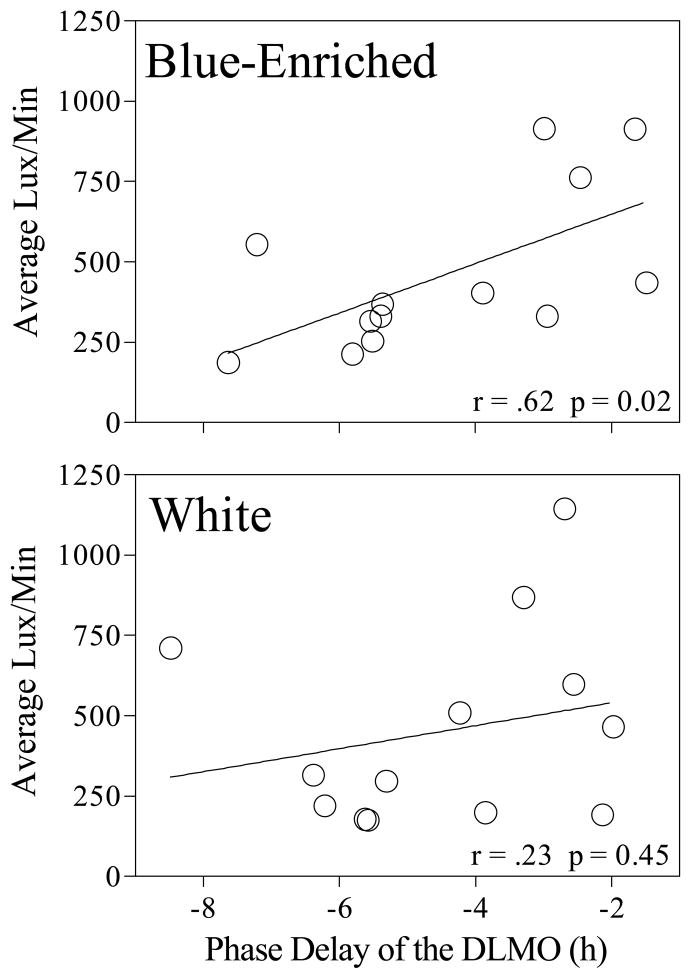
The size of the subsequent phase shift was related to light exposure history. In the blue-enriched condition, increased light exposure (lux/min) was associated with smaller phase delays of the DLMO (Figure 3; r = .62, p = 0.02). In the white light condition, the correlation between light history and phase shift of the DLMO (r = .23, p = 0.45) was not statistically significant. These light history results were similar when analyzed using the number of minutes > 500 lux rather than the average lux/min.
Figure 3.
Light exposure history for the three weeks preceding treatment versus phase delay of the DLMO.
Most subjects were exposed to dim light when outside of the laboratory on light treatment days (white squares on days 22–25 in Figure 1), because the time that they were permitted to be outside the laboratory occurred in the evening and nighttime hours. There were no significant differences in light exposure when outside of the laboratory between the light conditions. There were no significant correlations between light exposure when outside of the laboratory during light treatment days and phase shifts of the DLMO. Light exposure averaged 38 ± 48 lux/min on days 22–23, 40 ± 75 lux/min on day 24, and 8 ± 6 lux/min on day 25 for the two light conditions combined. The amount of light exposure on day 25 was lower because the clock hour that subjects were permitted to leave the laboratory on that day occurred after sunset.
Subjective Impression of Light Boxes
At the end of the study, one subject felt that he could not answer the questions regarding his impression of the two light boxes because he thought they were the same. For the remaining 12 subjects, ratings of comfort level and overall pleasantness of the blue-enriched and white light boxes were similar and rather neutral. On the unpleasant (1) to pleasant (10) scale, average ratings were 4.92 ± 1.94 (blue-enriched) and 5.23 ± 2.01 (white) [t(12) = 0.39, p = 0.70]. Average ratings on the painful (1) to comfortable (10) scale were 6.31 ± 2.06 (blue-enriched) and 6.38 ± 1.81 (white) [t(12) = 0.12, p = 0.90]. Average ratings on the sleep-inducing (1) to energizing (10) scale were 5.08 ± 2.29 (blue-enriched) and 5.31 ± 1.97 (white) [t(12)=0.29, p = 0.80]. Average ratings on the agitating (1) to relaxing (10) scale were 5.00 ± 2.20 (blue-enriched) and 5.31 ± 1.38 (white) [t(12) = 0.43, p = 0.68]. Responses to which light box subjects preferred, thought was brighter, or felt was more comfortable were evenly divided, with 6 subjects choosing each light box for each question [χ(1) = 0.00, p = 1.00].
Discussion
The finding that the human circadian system is sensitive to short-wavelength light was seminal for the field of circadian rhythms. Two main questions concerning the practical applications of this finding arose, each leading to a divergent line of research. The first question was whether this sensitivity could be used to increase the efficiency of light treatment (i.e., requiring lower light levels to achieve the same magnitude response). The second question was whether this sensitivity could be harnessed to increase the effectiveness of light treatment (i.e., increasing the magnitude of the phase shift).
A number of studies have shown that blue light is more efficient than other wavelengths for phase shifting the circadian clock (Wright & Lack, 2001; Lockley et al., 2003; Warman et al., 2003; Wright et al., 2004; Revell et al., 2005a). An excellent example is the study by Warman et al. (2003) in which dim blue light (28 μW/cm2 or 8 lux, with spectral peaks at 436 and 456 nm) produced similar size phase advances as bright white polychromatic light (4,300 μW/cm2 or 12,000 lux). This efficiency has been used to make dim light-producing devices that, though dim, evoke the desired phase-shifting response. For example, one week of light treatment (2 h/day, upon awakening) with spectacle frames containing blue LEDs (λ max 470 nm), combined with a 30 min advance of wake time/day, advanced the DLMO of patients with delayed sleep phase disorder by 2.5 h (Lack et al., 2007). The light levels in this study were only 65 μW/cm2, which is a small fraction of the irradiance produced by most bright-light boxes.
The present study addresses the question of whether the short-wavelength sensitivity could be used to increase the effectiveness of light treatment; that is, to produce a larger phase delay of the circadian clock. The fluorescent lamps used for circadian phase shifting over the last several decades, where reported in research papers, have been of a lower CCT (typically 4,000 to 6,000K) than the blue-enriched light source used in the present study. Following the discovery of the blue light sensitivity for human NIF responses, lamps of a higher CCT (blue-enriched) were quickly developed by multiple manufacturers. At light levels within the range commonly used in circadian phase shifting studies [2,500–10,000 lux e.g., Eastman & Miescke, 1990; Dawson & Campbell 1991; Campbell, 1995; Duffy et al., 1996; Terman et al., 2001; Boivin & James, 2002; Burgess & Eastman, 2005; Lack et al., 2005; Santhi et al., 2008; Smith et al., 2009a)], we found that bright blue-enriched polychromatic light did not produce larger phase delays than bright white polychromatic light of equal photon density.
Several previous reports support these findings. We found no difference in the size of the phase delays in response to intermittent bright blue-enriched or bright white-light pulses during simulated night shifts (Smith et al., 2006). Another study has found that remission from seasonal depression was similar following treatment with blue-enriched (17,000 K, 10,000 lux) and polychromatic white lamps (5,000 K, 10,000 lux) (Gordijn et al., 2006). In addition, a preliminary assessment of phase shifting in elderly subjects reported findings similar to the present study (Scheuermaier et al., 2008). In this latter study, 10 elderly subjects received a 2 h phase delaying blue-enriched or white light pulse on each of 4 consecutive days, using the same lamps as in the present study, but using much lower light levels (1.0 × 1015 photons/cm2/sec in each condition: 1200 lux and 370 μW/cm2 in the white condition and 500 lux and 320 μW/cm2 in the blue-enriched condition). Even at these lower light levels, the blue-enriched and white light boxes produced similar phase delays. However, the lack of a difference between the blue-enriched and white light conditions at these lower light levels may not be due to similar efficacy of the two light boxes, but rather due to age-related changes in retinal function or uncontrolled morning light to which the elderly subjects were exposed.
It cannot be ruled out that at very low light levels, blue-enriched light could produce larger circadian phase shifts. Polychromatic lamps with a higher CCT (8,000 K) have been shown to produce greater light-induced melatonin suppression than lamps with a lower CCT (4,100 K) at low (100 lux), but not high (1000 lux), light levels (Figueiro et al., 2006). Indeed, near the low threshold level for eliciting a phase shift, the blue-enriched lamps would be expected to produce larger shifts because they emit much more energy in the most potent part of the visible light spectrum for eliciting NIF responses.
It is possible that blue-enriched light may be beneficial for applications other than circadian phase-shifting. Relative to office lighting of a lower CCT, blue-enriched polychromatic light has been reported to increase subjective alertness, concentration, and work performance and to decrease subjective fatigue (Mills et al., 2007; Viola et al., 2008). As these studies had notable shortcomings regarding the subjects’ expectations of the light treatment, clarification of these possible benefits of blue-enriched light in the workplace requires replication with a rigorous experimental design.
There was a significant correlation between light exposure history and phase shift, such that those subjects that had relatively lower levels of light exposure in the weeks preceding the in-lab light treatment sessions had larger subsequent phase delays of the DLMO in response to the light treatment. Previous reports have shown that reduced prior light exposure history augments subsequent light-induced melatonin suppression (Hebert et al., 2002; Smith et al., 2004; Higuchi et al., 2007). The present study is the first to extend these findings, showing that light history also influences subsequent light-induced circadian phase shifting. Why this relationship would be specific to the blue-enriched lamps in our study is not clear. One possibility is that the lack of a significant correlation between light exposure history and phase shift in the white light condition is due simply to the relatively small sample size. Alternatively, it is possible that light history may have a greater effect on subsequent exposure to short-wavelength light, relative to longer wavelengths. The levels of melanopsin protein and mRNA decrease when animals are housed in constant light (Hannibal et al., 2005; Mathes et al., 2007). If these observations extend to humans living under entrained conditions, they could suggest that a mechanism by which light history influences subsequent NIF responses to light. The photoreceptors mediating the effects of photic history on human NIF responses are not known, but a photopigment with peak spectral sensitivity of 483 nm (the blue portion of the visible light spectrum) has been shown to temporally integrate photic input over a period of at least several hours in humans, and has feedback onto other retinal photoreceptors (Hankins & Lucas, 2002). The spectral sensitivity of longer term (days to weeks) history of light exposure on NIF responses is unknown. Several photosensitive devices measuring illuminance as well as short-wavelength radiation, more akin to the spectral sensitivity of the NIF system, have been developed (Bierman et al., 2005; Hubalek et al., 2006), but have yet to be used to determine whether the influence of long-term light history on subsequent NIF responses is also short-wavelength-sensitive. It is possible that the history of exposure to short-wavelength light is a stronger mediator of subsequent NIF responses to light than the light history of the photopic or scotopic systems.
The sleep episode in this study was delayed by 2 h/day because previous research has demonstrated that under optimal conditions (e.g., potent phase delaying bright light stimulus, dark bedroom with a shift of the sleep schedule, and avoidance of bright morning light) the circadian clock can delay as fast as 2 h/day (Eastman & Miescke, 1990; Eastman, 1992). Contrary to our expectations, the average phase delay across the 4 study days was only 4.5 h. We do not know if the small phase delays observed in some subjects would have been larger if higher light levels were used, although we presume that both lamps delivered a saturating level of energy (discussed below). There were very large individual differences, with some subjects delaying as much as 8 h, and others delaying as little as 2 h over the 4 treatment days. Other laboratory studies that included more rigorous experimental conditions (e.g., subjects’ heads in a Ganzfeld dome for 6.7 h of light exposure, pupils pharmacologically dilated) have also reported very large individual differences in NIF responses (Lockley et al., 2003). In the present study all subjects received the bright light at the same circadian time on the first treatment day, during the high-amplitude delay portion of the light PRC. It is thus not likely that the timing of the light pulse can account for the individual differences in the size of the phase shift. Some of the individual differences, at least in the blue-enriched condition, were due to prior light history. Other factors that could have contributed include each subject’s individual circadian period (tau), or individual differences in light sensitivity or nonphotic zeitgebers. It is also possible that there are individual differences in the maximum speed that the circadian clock can be delayed. Thus, although some individuals clearly have the capacity to delay as much as 2 h/day in the conditions of this study, many do not, and the factor(s) mediating these differences have yet to be fully elucidated.
A dose-response curve for “cool white” fluorescent lamps suggest that circadian phase shifts saturate at less than 1000 lux (Zeitzer et al., 2000). However, in that study subjects remained in the laboratory in < 150 lux for 5 days before, and in less than 10 lux for the 60 h immediately preceding experimental light exposure. These sustained dim light levels are very different from the light levels that individuals in the real world encounter. As decreased prior light history increases sensitivity to subsequent light exposure in humans (Hebert et al., 2002), we speculate that the saturation level under real-world lighting conditions that include outdoor light exposure is probably much higher. The saturation level for circadian phase shifts in response to lamps with a high correlated color temperature and in subjects who are exposed to real-world ambient lighting conditions is unknown. Nonetheless, a parsimonious interpretation of our data is that at the light levels used both the blue-enriched and “white” lamps contain a saturating level of energy.
Acknowledgments
Supported by NIH Grant R01 NR007677. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. Phillips Lighting donated the blue-enriched light boxes. Enviro-Med donated the white light boxes. We thank Erin Cullnan, Tom Molina, Jillian Canton, Meredith Rathert, and Heather Holly for assistance with data collection. We thank Helen Burgess, Ph. D. for comments on the manuscript. We thank Apollo Health for assistance with light measurements.
Footnotes
Declaration of Interest: MRS has received consulting fees from Respironics, Inc. CIE has no conflicts of interest.
References
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bierman A, Klein T, Rea M. The daysimeter: a device for measuring optical radiation as a stimulus for the human circadian system. Measure Sci Technol. 2005;16:2292–2299. [Google Scholar]
- Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–567. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Short nights attenuate light-induced circadian phase advances in humans. J Clin Endocrinol Metabol. 2005;90:4437–4440. doi: 10.1210/jc.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS. Effects of times bright-light exposure on shift-work adaptation in middle-aged subjects. Sleep. 1995;18:408–416. doi: 10.1093/sleep/18.6.408. [DOI] [PubMed] [Google Scholar]
- Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14:511–516. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: Influence of sleep timing, social contact and light exposure. J Physiol. 1996;495:289–297. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI. High intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol. 1992;263:R428–R436. doi: 10.1152/ajpregu.1992.263.2.R428. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Miescke KJ. Entrainment of circadian rhythms with 26-hr bright light and sleep-wake schedules. Am J Physiol. 1990;259:R1189–R1197. doi: 10.1152/ajpregu.1990.259.6.R1189. [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Rea MS, Bullough JD. Circadian effectiveness of two polychromatic lights in suppressing human nocturnal melatonin. Neurosci Lett. 2006;406(3):293–297. doi: 10.1016/j.neulet.2006.07.069. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis Res. 2007;47(7):946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordijn M, Mannetje D, Meesters Y. The effects of blue enriched light treatment compared to standard light treatment in SAD. Society for Light Treatment and Biological Rhythms Abstracts. 2006;18:6. doi: 10.1016/j.jad.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Cur Biol. 2002;12(3):191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Georg B, Hindersson P, Fahrenkrug J. Light and darkness regulate melanopsin in the retinal ganglion cells of the albino Wistar rat. J Mol Neurosci. 2005;27(2):147–155. doi: 10.1385/JMN:27:2:147. [DOI] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Motohashi Y, Ishibashi K, Maeda T. Less exposure to daily ambient light in winter increases sensitivity of melatonin to light suppression. Chronobiol Int. 2007;24:63–85. doi: 10.1080/07420520601139805. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Hubalek S, Zoschg D, Schierz C. Ambulant recording of light for vision and non-visual biological effects. Lighting Res Technol. 2006;38(4):314–324. [Google Scholar]
- Lack L, Wright H, Kemp K, Gibbon S. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28:616–623. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- Lack L, Bramwell CT, Wright HR, Kemp K. Morning blue light can advance the melatonin rhythm in mild delayed sleep phase syndrome. Sleep Biol Rhythms. 2007;5:78–80. [Google Scholar]
- Lee C, Smith MR, Eastman C. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23(4):859–875. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Met. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Mathes A, Engel L, Holthues H, Wolloscheck T, Spessert R. Daily profile in melanopsin transcripts depends on seasonal lighting conditions in the rat retina. J Neuroendocrinol. 2007;19(12):952–957. doi: 10.1111/j.1365-2826.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- Mills PR, Tomkins SC, Schlangen LJ. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J Circadian Rhythms. 2007;5:2. doi: 10.1186/1740-3391-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M, Miller J, Gray G, Buick F, Blazeski S, Arendt J. Circadian phase delay induced by phototherapeutic devices. Aviat Space Environ l Med. 2007;78(7):645–652. [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol Int. 2008;25(6):999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Arendt J, Terman M, Skene DJ. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms. 2005a;20:270–272. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J Pineal Res. 2005b;39:195–200. doi: 10.1111/j.1600-079X.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- Revell VL, Skene DJ. Light-induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiol Int. 2007;24:1125–1137. doi: 10.1080/07420520701800652. [DOI] [PubMed] [Google Scholar]
- Rupp TL, Acebo C, Carskadon MA. Evening alcohol suppresses salivary melatonin in young adults. Chronobiol Int. 2007;24:463–470. doi: 10.1080/07420520701420675. [DOI] [PubMed] [Google Scholar]
- Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23(4):341–352. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermaier KD, Munch M, Guzik A, Silva EJ, Ronda JM, Duffy JF. Phase delay shifts to blue-enriched vs. standard polychromatic white light in healthy older people in a semi-ambulatory setting. Sleep. 2008;31:A44. [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Met. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Smith MR, Lee C, Revell VL, Eastman CI. Blue-enriched versus white light for circadian phase delays. Abstracts of the Society for Research on Biological Rhythms. 2006;131 [Google Scholar]
- Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: Study 4. J Biol Rhythms. 2009a;24:161–172. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009b;10:287–294. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work, Environ Hlth. 2008;34(4):297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon D, Silver R. Cellular location and circadian rhythm of expression of the biological clock gene period 1 in the mouse retina. J Neurosci. 2003;23:7670–7676. doi: 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18:801–808. doi: 10.1081/cbi-100107515. [DOI] [PubMed] [Google Scholar]
- Wright HR, Lack LC, Kennaway DJ. Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res. 2004;36:140–144. doi: 10.1046/j.1600-079x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- Young RS, Kimura E. Pupillary correlates of light-evoked melanopsin activity in humans. Vis Res. 2008;48(7):862–871. doi: 10.1016/j.visres.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526.3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]