Dendritic cells: arbiters of mucosal immunity and tolerance
Dendritic cells consist of a family of potent antigen-capture and antigen-presenting cells that infiltrate the mucosa, skin, and most organs of the body. The dendritic cell subsets that comprise the dendritic cell family and their immune functions have been previously described (28, 29, 104); however, we will briefly describe some aspects of dendritic cell plasticity and the propensity of different dendritic cell subsets to differentiate into osteoclasts. Dendritic cells play an essential role in resistance to infection, by their ability to sense microbes (‘danger signals’), and to respond by undergoing directed migration out of the tissues. The dendritic cells then migrate to the secondary lymphoid organs, where they activate the adaptive immune system. Dendritic cells are also essential for inducing and maintaining mucosal immune homeostasis to harmless commensal bacteria and to self-antigens. They do this by migration in the immature ‘steady-state’ (i.e. in the absence of infection) to lymphoid organs, where they induce antigen-specific unresponsiveness or tolerance (129) by several mechanisms, including induction of regulatory T cells (126). The cytokine microenvironment that dendritic cells encounter in the periphery is critical to the type of adaptive immune response induced by dendritic cells. For example, interleukin-12 and interleukin-23 favor the induction of an inflammatory T helper cell type 1 response (96), while interleukin-10, induced by lipopolysaccharide of the oral pathogen Porphyromonas gingivalis, favors a T helper type 2 effector response (66, 111) and transforming growth factor-β favors an immunosuppressive regulatory T-cell response (97). The consequences of failure to maintain immune homeostasis are exemplified by Crohn's disease (14) and celiac disease (60) of the small intestine, by asthma and allergic rhinitis of the respiratory tract (147) and, as we have proposed, by periodontitis of the supporting soft and hard tissues of the dentition (28–30).
Maintenance of immune homeostasis must be particularly problematic in the oral cavity, which is exposed to thousands of bacteria and other infectious agents as the host respires, eats, or has intimate contact with other humans. In most people, the oral mucosa remains in a relative state of health despite the heavy microbial load (1). Many of these microbes pass into the upper respiratory (95) and gastrointestinal (114) tracts, where they can be eliminated, can colonize, or, in an immunocom-promised host, may cause disease (107). Commensal bacteria may generally protect the host mucosa by preventing colonization by invasive pathogens and by postnatal stimulation of the mucosa-associated lymphoid tissue via some immunoregulatory mechanisms that are not yet totally understood (78, 143).
Commensals are not all beneficial; some provide an attachment substrate for the colonization and biofilm accretion by pathogens (26); others can become pathogenic in response to changes in the host immune status or in the microflora (116). The oral gram-negative anaerobe P. gingivalis, for example, appears to be a commensal in healthy oral mucosa (144), but an opportunistic pathogen in diseased gingival tissues (85, 92). The pathogenetic transition is facilitated by the inflamed tissue microenvironment, which provides the proteins (58) and blood products (71) needed for the bacterial growth and virulence; as well as proinflammatory and anti-inflammatory cytokines that instruct dendritic cells to induce strong cell-mediated and / or humoral type responses (66, 111).
Recent studies make a compelling case for the role of dendritic cell infiltration and response to the oral microbiota in periodontal disease pathogenesis (3, 4, 13, 24, 30, 33, 45, 62, 63, 65, 72, 73, 90). Accordingly, this review will focus on how dendritic cells can mediate periodontal disease pathogenesis, including: (i) expression of mediators of recruitment and trafficking of dendritic cells and other inflammatory cells in and out of the tissues; (ii) pattern recognition by dendritic cells, leading to activation / maturation of dendritic cells and stimulation of an inflammatory T-cell response (or inhibition of dendritic cell maturation, leading to immune suppression); (iii) formation of semi-organized lymphoid aggregates, or oral lymphoid foci in the gingival lamina propria, first described in 2001 (65); and (iv) the potential ability of some dendritic cell subsets to directly develop into osteoclasts, thereby serving as osteoclast precursors.
Trafficking of dendritic cell progenitors, dendritic cells, and T cells into and out of mucosa, towards lymph nodes (Fig. 1)
Fig. 1.
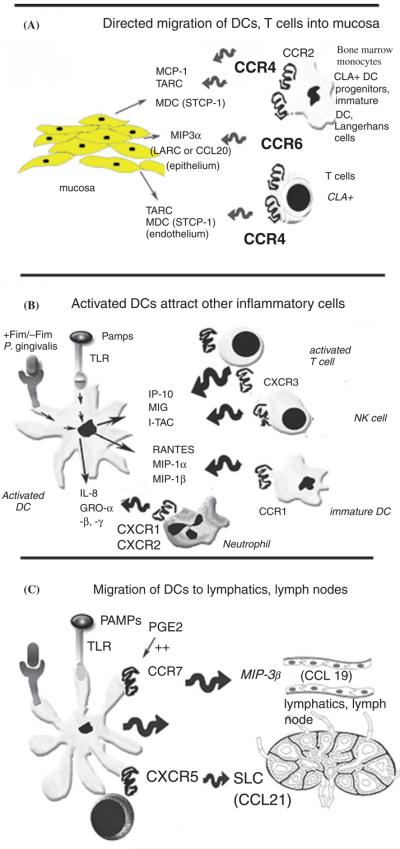
Trafficking of dendritic cells, their precursors tightly regulated by chemokines and chemokine receptors. (A) Chemokines and their receptors mediate the directed migration of dendritic cells, T cells and their progenitors into peripheral tissues. (B) Dendritic cells encounter microbes at infected sites and recognize them by expression of pattern recognition receptors [e.g. Toll-like receptors and nucleotide-binding oligomerization domains (NODs)]. This activates intracellular signaling cascades resulting in transcription of chemokines that, in turn attract more dendritic cells, natural killer cells, T cells, and neutrophils into the site. (C) Dendritic cells are stimulated to migrate through the lymphatics towards the lymph nodes and spleen and to find naive T cells by coordinate regulation of CCR7, CXCR5. CCR, CC chemokine receptor; CLA, cutaneous leukocyte antigen; CXCR, CXC chemokine receptor; CCL, CC chemokine ligand; DC, dendritic cell; Fim, fimbriae; GRO, growth-related oncogene α, β, γ or CXCL1, CXCL2, CXCL3, respectively; IL-8, interleukin-8; IP-10, inter-feron-inducible protein-10; I-TAC, interferon-inducible T-cell A chemoattractant; MCP-1, monocyte chemotactic protein-1; MDC, macrophage-derived chemokine or CCL22; MIP-3α, macrophage-inflammatory protein 3α; NK cell, natural killer cell; PAMPs, pathogen-associated molecular patterns; PGE2, prostaglandin E2; RANTES, regulated on activation normal T cell expressed and secreted (CCL5 or SISd); SLC, secondary lymphoid tissue chemokine or CCL21; TARC, thymus and activation-regulated chemokine or CCL17; TLR, Toll-like receptors.
The proper function of immune surveillance requires well-coordinated mechanisms to guide patrolling immune cells through the peripheral tissues, including oral mucosa, and into secondary organs (39). Control of the immune responses in the intestines, in lymph nodes, and no doubt in the oral mucosa is maintained by differential trafficking patterns of select lymphoid / myeloid subpopulations. Most notable among the molecular signals that regulate cell trafficking are the chemokines and their respective receptors (27). For example, the expression patterns of the chemokines macrophage inflammatory protein-3α (MIP-3α) and macrophage inflammatory protein-3β (MIP-3β), and their respective receptors CCR6 and CCR7, suggest that these molecules mediate dendritic cell influx into tissues (Fig. 1A) and efflux to T-cell-rich regions within human lymph nodes (Fig. 1C; 27, 34, 59). Immature dendritic cells in vitro express high levels of CCR6 and low levels of CCR7, while mature dendritic cells express low levels of CCR6 and high levels of CCR7 (59). Of the respective ligand(s), MIP-3α is predominantly expressed at inflamed tonsillar crypts, while MIP-3β is specifically expressed in T-cell-rich areas, suggesting a role for MIP-3α / CCR6 in the recruitment of immature dendritic cells at sites of injury and for MIP-3β / CCR7 in the accumulation of antigen-loaded mature dendritic cells in T-cell-rich areas (34). The constitutive trafficking to and from the skin of the epidermal dendritic cells, the Langerhans cells, is coupled to the differential expression of MIP-3α / CCR6 and MIP-3β / CCR7 (21). Recent human studies suggest that CCR6 and MIP-3α are expressed in inflamed periodontal tissues and may have an important regulatory role in specific lymphocyte (and dendritic cells) migration (56).
Failure of migrating dendritic cells to mature en route to the lymph nodes (129) or the ability of dendritic cells to depolarize cytokine profiles towards a T helper type 2 profile can lead to immune suppression / tolerance (e.g. towards commensals or self-antigens). P. gingivalis lipopolysaccharide may promote immunosuppression by this route, inducing weak dendritic cell maturation and a T helper type 2 bias both in vitro (66) and in vivo (111); moreover, certain P. gingivalis lipopolysaccharide moieties suppress dendritic cell maturation by lipopolysaccharide from other species (25). This suggests that P. gingivalis may be the proverbial ‘wolf in sheep's clothing’ – appearing to the host as a commensal organism, while invading host tissue and suppressing the immune response towards other species. This characteristic of P. gingivalis is consistent with the presentation of periodontitis: long-standing, low-grade inflammation that is difficult to resolve. Resolution of inflammation is generally favored by migration of fully matured dendritic cells from inflamed sites into lymph nodes, where a Th1 type cell mediated immune response can be induced (19, 98, 130).
After the dendritic cells migrate out of the periphery, monocytic dendritic cell precursors migrate in from the bloodstream (Fig. 1A) and from the bone marrow. Monocytes are recruited from bone marrow by CCR2, and are a primary precursor for Langerhans cells in tissues (122). Dendritic cell precursors are also recruited in elevated numbers to infected sites by chemokines such as thymus and activation-regulated chemokine and macrophage-derived chemokine. Thymus and activation-regulated chemokine and macrophage-derived chemokine are both ligands for CCR4, which is expressed by dendritic cells and T cells. Naive T cells in asthma, for example, produce both thymus and activation-regulated chemokine and macrophage-derived chemokine, which contribute to local migration of T helper type 2 cells into lung and lymphoid tissues (53). These chemokines may have particular relevance to chronic periodontitis, which shows a T helper type 2 bias in many reports (reviewed in ref. 47). In addition, Langerhans cells and T cells express cutaneous leukocyte antigen, which along with CCR6, facilitates their recruitment into mucosa by macrophage inflammatory protein-3α (Fig. 1A). Immature dendritic cells that are activated at the site by pathogen-associated molecular patterns (e.g. lipopolysaccharide), in turn secrete chemokines that attract other inflammatory cells, including activated T cells, natural killer cells, and additional dendritic cells (Fig. 1B). The mediators shown in Fig. 1B have been implicated in rheumatoid arthritis, allergic asthma, atopic dermatitis, and multiple sclerosis [e.g. CCR1, receptor for the CC chemokines CCL5 (regulated on activation normal T cell expressed and secreted; RANTES) and CCL3 (macrophage inflammatory protein-1α)].
The use of receptor antagonists for chemokines is a novel approach to blocking chemokine actions and has the potential to provide novel therapeutics for inflammatory diseases (119). Several pre-clinical studies with CCR1-deficient mice, anti-CCR1 antibodies and CCR1 antagonists, for example, suggest that CCR1 or its ligands may be attractive therapeutic targets for a variety of inflammatory diseases. Publications and patents describing CCR1 antagonists and their pharmacological effects have recently been disclosed (reviewed in refs 17, 50, 70, 116, 117). Recent studies indicate that chemokines and chemokine receptors are differentially expressed in different types of periodontal disease and in health. Most notably, macrophage inflammatory protein-1α, interferon-γ-inducible protein-10, CCR5, and CXCR3 are elevated in aggressive periodontitis (43). Interestingly, activated dendritic cells are significant sources of chemokines such as interleukin-8 and growth-related oncogene, which attract polymorphonuclear leukocytes to the diseased site (121; Fig. 1B). Periodontal disease activity and its resolution have long been ascribed to the function and number of polymorphonuclear leukocytes (reviewed in ref. 68).
Pattern recognition by dendritic cells: both immune-activating and immune-suppressing functions
A valid strategy for dampening immune responses is to interfere with the initial recognition of microbes by innate immune cells, such as dendritic cells and macrophages (110). Different Toll-like receptors on innate immune cells serve as transmembrane receptors for different pathogen-associated molecular patterns (74). Toll-like receptors are transmembrane receptors that translate critical information about the nature of the pathogen / commensal through nuclear factor-κB-dependent signaling pathways (2). For many gram-negative bacteria, Toll-like receptor ligation culminates in increased expression of accessory signals on dendritic cells (e.g. cytokines, costimulatory molecules), which prepares dendritic cells to activate naive T cells, thereby initiating the adaptive immune response. Interestingly, recent studies show that the human gingiva is infiltrated with large numbers of Toll-like receptor 2-positive and Toll-like receptor 4-positive cells, and that their numbers increase significantly in chronic periodontitis, relative to periodontal health; however, Toll-like receptor mRNA expression is down-regulated, probably through a process called endotoxin tolerance (100). This is supported by evidence that the Src homology 2 molecule containing inositol phosphatase (SHIP-1), the mediator of endotoxin tolerance, is up-regulated in situ in chronic periodontitis (99). In vitro, human monocyte-derived dendritic cells express mRNA for Toll-like receptors 1, 2, 3, 4, and 5 (101). Human immature CD11c+ dendritic cells isolated from peripheral blood express high levels of mRNA for Toll-like receptors 1, 2 and 3 and low levels of Toll-like receptors 5, 6, 8, and 10, and undetectable levels of Toll-like receptors 4, 7, and 9 (67). In another study, the expression of Toll-like receptor proteins on human peripheral blood dendritic cells and monocyte-derived dendritic cells were analyzed by flow cytometry – both dendritic cell sources expressed Toll-like receptor 2, but not Toll-like receptor 4; moreover, Toll-like receptor 2 activation resulted in preferential induction of interleukin-12 by dendritic cells (142). Dendritic cells from human gut mucosa have been shown to express Toll-like receptor 2 and Toll-like receptor 4, which may be involved in sensing intestinal flora and in induction of Crohn's disease (84). Expression of specific TLRs by dendritic cells in gingival tissue in situ, however, is presently unclear.
Recently, the NACHT-LRR (domain present in NAIP, CIITA, HET-E and TP1 with leucine-rich repeat) proteins, or nucleotide-binding oligomerization domains (NODs) have been identified on dendritic cells that, like Toll-like receptors, possess the capability to recognize microbes and convert this information to elicit appropriate downstream signaling events. While Toll-like receptor 2 was originally thought to be the receptor for gram-positive bacterial peptidoglycan, nucleotide-binding oligomerization domains 1 and 2 (abbreviated as NOD1 and NOD2) have been reported to recognize specific fragments of peptidoglycan, also known as muropeptides (48). Nucleotide-binding oligomerization domain 1 senses a naturally occurring peptidoglycan breakdown product GlcNAc-MurNAc-l-Ala-zγ-d-Glu-meso-diaminopimelic acid (GMtriDAP), which is found mostly in gram-negative bacteria, while nucleotide-binding oligomerization domain 2 senses muramyl dipeptide MurNAc-l-Ala-d-isoGln (MDP) of the gram-positive peptidoglycan (49). Although human oral epithelial cells have been shown to up-regulate both nucleotide-binding oligomerization domains 1 and 2 in response to peptidoglycans (145), very little is known about expression of the nucleotide-binding oligomerization domains 1 and 2 by oral mucosal dendritic cells and their role in mucosal responsiveness to commensals (reviewed in ref. 133).
Dendritic cells, Langerhans cells and certain tissue macrophages are also equipped to capture micro-organisms and self-antigens by the expression of distinct sets of C-type lectin receptors (reviewed in refs 77, 148). Many of the C-type lectin receptors are expressed in human gingiva, including the Langerhans cell-specific marker Langerin (CD207), macrophage mannose receptor (CD206) and dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN; CD209), with Langerin decreasing in the gingival epithelium in chronic periodontitis, while macrophage mannose receptor and dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin increase in the lamina propria in chronic periodontitis (62, 64; Fig. 2). The C-type lectin receptors consist of type I and II transmembrane receptors, which function primarily to interact with the conserved carbohydrates shared by a large group of microorganisms, and internalize these pathogens for processing and antigen-presentation. Accordingly, C-type lectin receptors contain carbohydrate recognition domains for binding to sugars (e.g. mannose, fucose, galactose and β-glucans) and glycosylated proteins. C-type lectin receptors appear to lack Toll-interleukin-1-receptor activation domains, and thus their ligation does not generally lead to dendritic cell maturation and cytokine secretion, in contrast to the Toll-like receptors (61, 76, 77, 146). Exceptions include the C-type lectin receptors Dectin-1 (15, 42), which appears to collaborate with Toll-like receptor 2 in responding to its natural ligand β-glucan (i.e. yeast). Moreover, mannose receptor (CD206) functions in an antagonistic fashion to the Toll-like receptors, responding to mannosylated lipoarabinomannans (105) by activation of an anti-inflammatory immunosuppressive program (22) and may induce regulatory T cells (76). The Toll-like receptor antagonism of C-type lectin receptors may be mediated by immunoreceptor tyrosine-based activation motifs (ITAMS) or immunoreceptor tyrosine-based inhibitory motifs (ITIMS), respectively (38). Thus although Toll-like receptors and C-type lectin receptors recognize different determinants and have distinct functions, there may be cross-talk between the two; moreover, the balance of Toll-like receptor / C-type lectin receptor triggering can influence the outcome of the immune response (76). The physiological function of C-type lectin receptors may be to recognize glycosylated self-antigens for induction of immune tolerance (44).
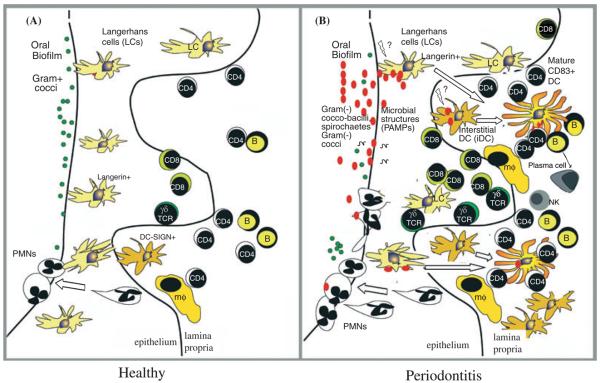
Fig. 2.
Oral lymphoid foci: semi-organized dendritic cell–T-cell infiltrates in gingiva exposed to oral biofilm. The results of recent case–control immunohistochemical studies of healthy (A) and diseased (B) gingival tissues from humans suggest distinct changes in lymphoid and dendritic cell infiltrates. The healthy gingival epithelium is populated by large numbers of resident Langerin+ Langerhans cells; moreover, intraepithelial Langerhans cells decrease in number in periodontitis, undergo efflux towards the lamina propria, and begin to express CD83 at the basement lamina, presumably in response to changes in the oral biofilm, and the release of microbial pathogen-associated molecular patterns (PAMPs). Dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin-positive (DC-SIGN+) interstitial dendritic cells, almost absent in the healthy lamina propria, increase by about four-fold in diseased lamina propria and also begin to express CD83 (62). The increase in CD83+ cells in diseased lamina propria is approximately sixfold (65); moreover CD83+ mature dendritic cells form immune conjugates with CD4+ T cells (62). Note that CD83+ B cells have also been identified in diseased gingiva (45), as have nonconventional T-cell populations that are not involved in recognition of protein antigens (20). These include intraepithelial γδ T cells (46, 86) and natural killer cells (6). Polymorphonuclear leukocytes migrate out through the gingival pocket epithelium towards the oral biofilm. Abbreviations: DC, dendritic cell; Mo, monocyte; Mϕ, macrophages.
Some pathogens appear to subvert the immune response by specifically targeting C-type lectin receptors, most notably dendritic cell-specific inter-cellular adhesion molecule-3 grabbing nonintegrin and macrophage mannose receptor. This is ostensibly beneficial to the pathogen as intracellular signaling in dendritic cells is down-regulated, inhibiting maturation and cytokine secretion; activities that are necessary for effective adaptive immunity. Such pathogens include human immunodeficiency virus type 1, Mycobacterium tuberculosis, Helicobacter pylori, Klebsiella pneumoniae, Candida albicans, and Schistosoma mansoni (reviewed in ref. 148). It has been stated that the central feature of pathogens that interact with dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin is that they cause chronic infections that last a lifetime and that they manipulate the T helper type 1 / type 2 balance to persist at the disease site (77). Notably, high expression of dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin, as we have observed in the gingival lamina propria in chronic periodontitis (62, 64), is dependent on T helper type 2 cytokines (113). Ligation of dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin, in the presence of lipopolysaccharide or tumor necrosis factor, triggers the release of the T helper type 2-biasing cytokine interleukin-10 (18). The lipopolysaccharide of P. gingivalis induces dendritic cells to release interleukin-10, but not interleukin-12 and induces a T helper type 2 effector response in vivo (111) and in vitro (66). The role of C-type lectin receptors in antigen-capture of oral microbes or in the responses of oral mucosal dendritic cells to microbes; however, are presently unclear.
Oral lymphoid foci within interdental papilla: immune effector (inductive?) sites in the oral mucosa
After dendritic cells arrive at immune inductive sites (e.g. lymph nodes, spleen), they locate naive T and B cells to activate them and initiate the adaptive immune response. Dendritic cells also re-stimulate previously activated T and B cells in peripheral tissues, i.e. at immune effector sites. We understand very little about the predominant immune inductive and effector sites in the oral mucosa proper; however, the prototypical oral inductive sites, contained within Waldeyer's ring, are oropharyngeal tissues and nasopharyngeal-associated lymphoid tissue (150). The latter include palatine tonsils, nasopharyngeal tonsils (adenoids) and lingual tonsils (40). In humans, nasopharyngeal-associated lymphoid tissue is ultra-structurally and functionally similar to gut-associated lymphoid tissue, containing Peyer's patches and lymphoid follicles (41). The lymphoid follicles within the villus lamina propria are a dominant gut-associated lymphoid tissue effector site and are covered by a single layer of epithelium containing intraepithelial lymphocytes and lamina propria lymphocytes and dendritic cells (127). Published data (62, 65) suggest that oral lymphoid foci in interdental papillae are evocative of these evolutionarily ancient lymphoid aggregates, which predate lymph nodes and Peyer's patches and play important roles in mucosal and systemic immune responses (10). This comparison is based on double-immunofluorescence analysis of oral mucosa showing organizational structure of lymphoid and myeloid elements, with lamina propria CD83+ dendritic cells engaged with large numbers of CD4+ T cells, which include CD45RA+ and CD45RO+ subsets (62, 65). Langerin+ Langerhans cells infiltrate the oral / gingival epithelium, while large numbers of dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin-positive interstitial dendritic cells infiltrate the gingival lamina propria; particularly in chronic periodontitis. Both Langerhans cells and interstitial dendritic cells appear to contribute to the pool of maturing CD83+ dendritic cells in the lamina propria (62). The presence of germinal centers, however, as observed in lymphoid follicles (103), has not yet been identified in oral lymphoid foci. Murine studies suggest that buccal mucosal dendritic cells capture antigen and migrate to the cervico-mandibular lymph nodes, where antigen is presented (36). However, the identity of the dendritic cell subsets that participate in oral mucosa immune responses or migrate in the steady-state to cervico-mandibular lymph nodes is not clear.
Linking dendritic cells to osteoimmunology in periodontal pathogenesis
While dendritic cells are imbued with a powerful ability to traffic throughout the periphery, under normal conditions dendritic cells are rarely localized in the alveolar bone proper or the adjacent stroma, nor do they contribute to normal bone remodeling, as dendritic cell-deficient animals have no apparent skeletal defects (91). However, the active lesions of inflammatory bone diseases (e.g. periodontal disease and rheumatoid arthritis) harbor both mature and immature dendritic cells located in different compartments of the affected synovial and periodontal tissues surrounding bone (28, 52, 65, 108, 136, 141). Moreover, dendritic cells in the actively inflamed tissues of rheumatoid arthritis and chronic periodontitis form aggregates with T cells, where the dendritic cell / T-cell interactions through receptor activator of nuclear factor-κB–ligand (RANK-RANKL) signaling take place in vivo (109). Leung et al. (83) showed that type-II collagen-pulsed dendritic cells can induce arthritis in DBA / 1 inbred mice after adoptive transfer, suggesting that dendritic cells play a critical role in driving the immunopathology of rheumatoid arthritis leading to chronic synovitis. However, their exact pathophysiological functions and mechanisms involved in rheumatoid arthritis and periodontal disease remain unclear.
Bone remodeling and homeostasis are tightly regulated and controlled by a number of osteogenic cytokines, growth factors, and hormones that exert their effects via osteoblasts and osteoclasts. Osteo-blasts and osteoclasts work together to maintain bone homeostasis in health. In contrast, in rheumatoid arthritis, osteomyelitis and periodontitis, and in metabolic bone disorders like osteoporosis, bone remodeling becomes unbalanced and is accompanied by increased osteoclast numbers and activity, leading to irreversible bone loss and resulting in morbidity and / or perturbation of life quality (140). Active osteoclasts are defined as multinucleated giant cells expressing tartrate-resistant acid phosphatase (TRAP), calcitonin receptor, cathepsin-K and inte-grins αvβ3, and capable of dissolving bone matrix (133, 140). Importantly, the recently identified tumor necrosis factor family molecule, RANKL, its receptor RANK, and the natural antagonist osteoprotegerin (79), have been shown to be the essential regulators of bone remodeling and are directly involved in the differentiation, activation, and survival of osteoclasts and osteoclast precursors (79, 125, 149, 151). Mice deficient in RANKL, RANK, and macrophage colony-stimulating factor all carry a similar phenotype of severe osteopetrosis, indicating these molecules, play a critical role in osteoclastogenesis (35, 131, 152). RANKL is expressed by osteoblasts, stromal cells, chondrocytes, and mesenchymal cells (140). In addition, RANKL–RANK signaling is involved in dendritic cell survival, lymph node formation and organogenesis, and critical dendritic cell / T-cell interactions (7, 75). By using experimental mouse models, CD4+ T cells have been shown to be critically involved in regulating alveolar bone loss in vivo (11, 12, 69, 132, 148), and activated CD4+ T cells to express RANKL, which can directly trigger osteoclastogenesis and alveolar bone loss associated with periodontitis in vivo (134, 135, 137–139). Interestingly, blocking RANKL activity via osteoprotegerin injections has been shown to result in significantly reduced bone loss in arthritis (75), periodontitis (139, 140), osteoporosis (54, 94), cancer-related bone metastasis (16, 55), and the enhanced alveolar bone loss associated with type-1 diabetes in vivo (89). In particular, osteoprotegerin injections into both human peripheral blood lymphocytes-nonobese diabetic / severe combined immunodeficient (NOD / SCID) and diabetic nonobese diabetic (NOD) mice, orally infected by live microorganisms to induce experimental periodontitis, consistently prevented and inhibited alveolar bone loss up to 80% and 90%, respectively, suggesting that the RANKL–RANK / osteoprotegerin axis is the central pathway of controlling osteoclastogenesis in the periodontium (89, 137, 139). More recent studies have shown that periodontally resident cells (i.e. periodontal ligament fibroblasts or mesenchymal tissues) can also be induced to express RANKL / osteoprotegerin by microbes or microbial product-induced inflammatory conditions in vivo or in vitro (51, 102, 106, 112), suggesting the potentially broad range and contributions of the RANKL–RANK / osteoprotegerin signaling network in periodontal disease and tissues. Together, these studies have supported the new paradigm of linking adaptive immunity to bone–skeletal biology (i.e. osteoimmunology) associated with inflammatory periodontal disease.
Based on recent findings, the fine balance between T helper types 1 and 2 cytokines and RANKL–RANK under various inflammatory conditions (i.e. presence of tumor necrosis factor-α or interleukin-1) may contribute directly (or indirectly) to osteoclastogenesis and subsequent bone remodeling in vivo (134, 135, 137, 138, 153). Thus, there are more complex cytokine networks involved in the regulation of RANKL–RANK / osteoprotegerin signaling pathways leading to osteoclastogenesis in vivo than those suggested to date (for the ‘RANKL and Cytokine Interactions Network’: called ‘RACIN’; see refs 135, 137, 152). Further research is required to decipher the molecular interactions and mechanisms to develop future therapeutics to modulate the host immune responses in human periodontal infection.
Although dendritic cells have long been thought to contribute indirectly to inflammation-induced bone loss (i.e. as antigen-presenting cells) (118), Rivollier et al. (115) recently showed that human peripheral blood monocyte-derived dendritic cells can transdifferentiate into osteoclasts in the presence of macrophage colony-stimulating factor and RANKL in vitro, suggesting a direct involvement of dendritic cells in osteoclastogenesis. However, whether dendritic cell / T-cell interactions can support dendritic cell-derived osteoclast development remains unclear. Studies have been interested in investigating the osteoclastogenic potential of dendritic cells to establish whether those cells can give rise to osteoclasts during dendritic cell / T-cell interactions under inflammatory conditions, because it has been suggested that dendritic cells and osteoclasts may share common progenitors (93). This hypothesis was pursued by establishing a unique co-culture system, which consists of highly purified murine bone marrow- or spleen-derived granulocyte–macrophage colony-stimulating factor and interleukin-4-derived CD11c+ dendritic cells. These purified cultures lack classic osteoclast precursors (120, 133) and are cocultured with CD4+ T cells plus microbial or protein antigens [i.e. human pathogens, Aggregatibacter (Actinobacillus) actinomycetemcomitans, P. gingivalis, or Escherichia coli] on bone-matrix-coated plates (5, 139). The results suggest that immature CD11c+ dendritic cells can act like osteoclast precursors during immune interactions with CD4+ T cells and develop into functional osteoclasts capable of resorbing bone in vitro and, after adoptive transfer, in vivo, in a RANKL / RANK signaling-dependent fashion (5). Hence, these cells are referred to as dendritic cell-derived osteoclasts. These findings indicate a potentially critical contribution of CD11c+ dendritic cell subset(s) to elevated osteoclastogenesis associated with inflammatory bone disorders where they can not only act as potent antigen-presenting cells for immune activation and regulation, but also directly contribute to bone destruction (5). An alternative pathway of osteoclastogenesis involving dendritic cell-derived osteoclasts and inflammation-induced bone loss is proposed here (Fig. 3) and further discussed below.
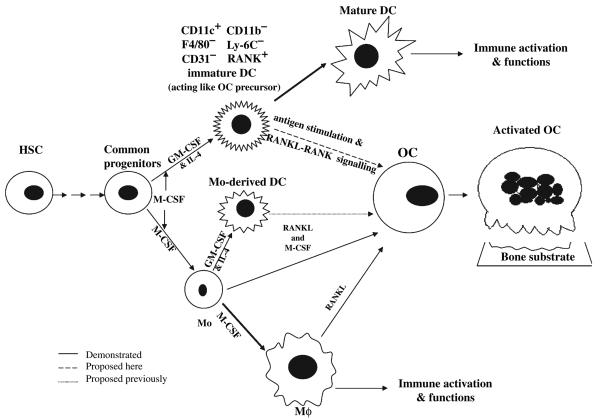
Fig. 3.
Dendritic cell-derived osteoclasts (DCOC): alternative players in inflammation-induced osteoclastogenesis. Immature CD11c+ dendritic cells may act as osteoclast precursors and thus develop into functional osteoclasts in the bone environment in response to RANKL / RANK signaling during the immune interactions with CD4+ T cells in the presence of microbial products (or protein) antigens (5) or via macrophage colony-stimulating factor signaling (115). DC, dendritic cell; GM-CSF, granulocyte-macrophage colony stimulating factor; HSC, human stem cell; Mϕ, macrophage; M-CSF, monocytercolony stimulating factor; OC, osteoclast; RANK, receptor activator of NF-kappaB; RANKL, RANK-ligand.
Dendritic cell plasticity and dendritic cell-derived osteoclasts: an alternative player in osteoclastogenesis and the new link between innate immunity and osteoimmunology
Dendritic cells are a heterogeneous population of bone marrow-derived leukocytes classified into different subsets based on their phenotypic and functional properties, as well as their anatomical locations. Although the details of dendritic cell origin and developmental pathways remain inconclusive, their development from myeloid, lymphoid, and plasmacytoid progenitors have been proposed. To date, it remains unclear whether these lineages represent distinct subsets or different maturational stages (8); however, several lines of evidence demonstrate that dendritic cells can arise from monocytes in vitro and in vivo (82), suggesting that they may share common progenitors with osteoclast precursors (93).
It was recently suggested that precursors of monocyte / macrophage lineages may differentiate into macrophages, dendritic cells, or osteoclasts in response to environmental cues, whereby mouse myeloid dendritic cells are shown to share the Flt3+ marker with osteoclast precursors (123). This is consistent with reports that, in response to macrophage colony-stimulating factor, myeloid precursors develop into macrophages, while in response to granulocyte–macrophage colony-stimulating factor they develop into dendritic cells, a process regulated in part by the transcription factor c-fos (93). Injection of Flt3-ligand into mice induces a significant increase of dendritic cell numbers in spleen and lymph nodes as well as in peripheral blood, lungs, liver, Peyer's patches, and thymus (124); while injection of Flt3-ligand into colony-stimulating factor-deficient op / op mice rescues the osteopetrosis (81). These findings suggest that Flt3-ligand can compensate for macrophage colony-stimulating factor signaling during the development of osteoclasts and dendritic cell progenitors. However, granulocyte–macrophage colony-stimulating factor-deficient mice are not defective in dendritic cell development or function (128), but macrophage colony-stimulating factor-deficient mice are osteopetrotic and have a 50–70% reduction in their dendritic cell numbers (88), thus challenging the paradigm that macrophage colony-stimulating factor and granulocyte–macrophage colony-stimulating factor are critical for macrophage and dendritic cell development, respectively. This notion is further complicated by recent reports showing that inflammation in the environment can polarize the development of monocytes to either dendritic cells or macrophages. For example, interferon-γ can switch monocyte differentiation to macrophages rather than dendritic cells at least through autocrine feedback of macrophage colony-stimulating factor and interleukin-6 expression (32). Tumor necrosis factor-a has been proposed to skew monocyte differentiation from macrophages to dendritic cells by downregulating c-fms expression (23). In contrast, another study shows that tumor necrosis factor-α up-regulates c-fms expression in osteoclast precursors and thus increases the number of circulating osteoclast precursors (80), suggesting that the effects of tumor necrosis factor-α signaling may be dependent on the developmental stage of the target cells. Together, these studies do suggest that dendritic cells, macrophages, and osteoclasts may share common precursor pool(s); however, definitive differentiation pathways and the exact lineage relationships among monocytes, macrophages, dendritic cells, and osteoclasts will require further investigation.
The RANK–RANKL signaling pathways in osteoclasts and osteoclast precursors have been extensively studied (133, 140). However, the intracellular signaling pathways downstream of RANK in different dendritic cell subsets and whether any of the known signaling adaptors or regulators can lead to the induction of osteoclastogenesis in vivo, remain largely unknown. RANKL-deficient and RANK-deficient mice have normal dendritic cell numbers and functions (7, 140), indicating that RANKL / RANK signaling is not essential for dendritic cell development or function. Nevertheless, it is clear that RANK / RANKL signaling in dendritic cells can: (i) enhance dendritic cell survival via induction of bcl-x (149) and promote antigen-presenting cell function via mechanisms independent of CD80 / 86 co-stimulation (7); (ii) be actively involved during dendritic cell / T-cell interactions in the local tissues of rheumatoid arthritis synovium (109) and of periodontitis (7); and (iii) mediate CD40 ligand-independent T helper cell activation during viral infection (9). Yet, it is also evident that environmental stimuli can affect dendritic cell behavior and function. For example, in the arthritic synovium, local dendritic cells not only manifest a prolonged life span and are resistant to the immunosuppressive effects of interleukin-10 when compared with healthy counterparts (87), but also their development is skewed towards the myeloid subset (118).
The developmental plasticity of the mononuclear phagocytes and myeloid cells has since been demonstrated, most notably that of bone-marrow-derived plasmacytoid dendritic cells (57, 154), which can become myeloid dendritic cells after down-regulation of the expression of Toll-like receptors 3 and 7 (specific for plasmacytoid dendritic cells) upon lymphocytic choriomeningitis viral infection (154). The inflammatory environment may mediate osteoclast development, as findings show that infiltrating immature dendritic cells upon proper activation by microbial product or protein antigen (e.g. bovine insulin and outer membrane protein-1) (5) during immune interactions with CD4+ T cells in vitro, give rise to the development of murine CD11c+ dendritic cells (also being CD11b− F4 / 80− Ly-6C− CD31−) into tartrate-resistant acid phosphatase-positive, calcitonin receptor-positive, cathepsin-k-positive osteoclasts in a RANKL / RANK-dependent manner. Further rescue and blocking experiments using CD11c+ dendritic cells derived from colony-stimulating factor-1-deficient op / op mice clearly show that macrophage colony-stimulating factor is required for the development of osteoclastogenic potential upstream of RANKL / RANK signaling. This suggests that immature CD11c+ dendritic cells can indeed act like osteoclast precursors (5). Interestingly, these CD11c+ dendritic cells lose their osteoclastogenic potential if they are activated first or become fully mature in the absence of RANKL-RANK signaling (unpublished data), indicating a distinctive property of dendritic cell-derived osteoclasts. These findings raise the issue of whether the dendritic cell plasticity is restricted to the context of immune responses to environmental factors (i.e. feedback signaling from T cells expressing RANKL or other modulating signals) or whether it extends beyond their immune functions. Further study of these underlying processes will facilitate our understanding of the plasticity and contribution of dendritic cells to inflammation-induced bone loss under various pathological conditions.
As human CD14+ monocyte-derived dendritic cells can transdifferentiate into osteoclasts in the presence of both macrophage colony-stimulating factor and RANKL in vitro (115), it was recently demonstrated that the interactions of murine CD11c+ dendritic cells with CD4+ T cells in the bone environment promote dendritic cell-derived osteoclast development in response to microbial (or protein) antigens and RANKL-RANK signaling (Fig. 3). Although dendritic cell-derived osteoclasts may develop under different conditions and kinetics, they appear to carry similar phenotypes with distinct differences (i.e. murine CD11c+ dendritic cells are CD86− CD11b− with dendrites). Together, it is conceivable that dendritic cell-derived osteoclasts can develop in the presence of: (i) macrophage colony-stimulating factor and RANKL, possibly from osteoblasts, synovial or stromal cells, as part of the innate immune responses, and (ii) inflammatory stimulation from RANKL+ T cells with activating antigens during adaptive immunity in the bone marrow or bony environment. Meanwhile, the study of Da Costa et al. (31). provides another line of evidence whereby osteoclast-like multinucleated giant cells expressing CD11c and human leukocyte antigen-DR are found in the local Langerhans cell lesions of patients with histiocytosis, echoing the concept that dendritic cell-derived osteoclasts may develop as the result or part of disease-associated osteoclastogenesis in vivo. Moreover, this finding is consistent with the results of our recent immunohistochemical analysis in the arthritis mouse model, revealing the presence of CD11c+ tartrate-resistant acid phosphatase + multi-nucleated cells (possibly dendritic cell-derived osteoclasts) located on the eroding sub-chondral bone surface of the arthritic joints (DBA mice immunized and boosted with chicken type II collagen) but not in the healthy controls (ref. 5) and unpublished data). Since dendritic cells can prime T cells against blood-borne antigens in bone marrow (37), it is possible that activation of the osteoclastogenesis program(s) can occur in some local dendritic cells present in the inflamed tissues adjacent to bone, whereby upon receiving specific stimuli including RANKL-RANK signaling they develop into CD11c+ major histo-compatibility complex-II+ tartrate-resistant acid phosphatase+ calcitonin receptor+ dendritic cell-derived osteoclasts, which consequently contribute to inflammation-induced bone loss in situ. The search for critical genetic factors involved in dendritic cell-derived osteoclast development is in progress and the results will likely shed some light on the regulatory mechanisms involved in dendritic cell-derived osteoclasts and inflammation-induced bone loss.
Based on recent evidence, it is proposed that dendritic cell-derived osteoclasts may represent a previously unrecognized subset of osteoclasts produced during interactions of dendritic cells with T cells, stromal cells or osteoblasts under distinct inflammatory conditions (see Fig. 3; refs 29, 135). However, whether other dendritic cell subsets (e.g. plasmacytoid dendritic cells) may have the potential of developing into osteoclasts under various pathological conditions will require more study in the future.
Summary
As scientists and clinicians continue to explore and dissect the links between mucosal immunology, osteoimmunology, and periodontal pathogenesis, it is becoming clearer that such links are more complex than previously thought. Indeed, while studies to improve our understanding of the mechanisms involved at the mucosal and osteo-immune interfaces and their roles in inflammation-induced alveolar bone loss is in progress, further research is required to investigate the physiological relevance and significance of the development of oral lymphoid foci and of dendritic cell-derived osteoclasts in vivo, so as to reveal the implications of dendritic cell responses in the oral mucosa and dendritic cell-derived osteoclast development for innate / adaptive immunity and periodontal osteoclastogenesis, and to highlight their therapeutic potential in the future.
Acknowledgments
This work was supported by U.S. Public Health Service grant from the NIH / NIDCR DE14328 (CWC) and by grants from the Ministry of Health of Ontario and the Canadian Institute of Health Research, MOP-37960, Canada; University of Rochester, Rochester, New York; and by the U.S. Public Health Service Grant NIH / NIDCR DE14473 and DE15786 (Y-T.A.T.).
References
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Al-Darmaki S, Knightshead K, Ishihara Y, Best A, Schenkein HA, Tew JG, Barbour SE. Delineation of the role of platelet-activating factor in the immunoglobulin G2 antibody response. Clin Diagn Lab Immunol. 2004;11:720–728. doi: 10.1128/CDLI.11.4.720-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Darmaki S, Schenkein HA, Tew JG, Barbour SE. Differential expression of platelet-activating factor acetylhydrolase in macrophages and monocyte-derived dendritic cells. J Immunol. 2003;170:167–173. doi: 10.4049/jimmunol.170.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Alnaeeli M, Penninger JM, Teng Y-TA. Immune interactions with CD4+ T-cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006;177:3314–3326. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]
- 6.Amanuma R, Nakajima T, Yoshie H, Yamazaki K. Increased infiltration of CD1d+ and natural killer T cells in periodontal disease tissues. J Periodontal Res. 2006;41:73–79. doi: 10.1111/j.1600-0765.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DM, Maraskovsky E, Billingsley WL, Dougall W, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 8.Ardavin C. Origin, precursors and differentiation of mouse dendritic cells. Nat Rev Immunol. 2003;3:582–590. doi: 10.1038/nri1127. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey M, Haverson K. The postnatal development of the mucosal immune system and mucosal tolerance in domestic animals. Vet Res. 2006;37:443–453. doi: 10.1051/vetres:2006013. [DOI] [PubMed] [Google Scholar]
- 11.Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181–1192. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- 12.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbour SE, Ishihara Y, Fakher M, Al-Darmaki S, Caven TH, Shelburne CP, Best AM, Schenkein HA, Tew JG. Monocyte differentiation in localized juvenile periodontitis is skewed toward the dendritic cell phenotype. Infect Immun. 2002;7:2780–2786. doi: 10.1128/IAI.70.6.2780-2786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgartner DC, Metzke D, Schmitz J, Scheffold A, Sturm A, Wiedenmann B, Dignass AU. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–236. doi: 10.1136/gut.2004.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JM, Zhang J, Keller ET. OPG, RANKL and RANK in cancer metastasis: expression and regulation. Cancer Treat Res. 2004;118:149–172. doi: 10.1007/978-1-4419-9129-4_7. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 18.Caparros E, Munoz P, Sierra-Filardi E, Serrano-Gomez D, Puig-Kroger A, Rodriguez-Fernandez JL, Mellado M, Sancho J, Zubiaur M, Corbi AL. DC-SIGN ligation on dendritic cells results in ERK and PI3 K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. Epub 24 January 2006. [DOI] [PubMed] [Google Scholar]
- 19.Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448–462. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 20.Champagne E, Martinez LO, Vantourout P, Collet X, Barbaras R. Role of apolipoproteins in gammadelta and NKT cell-mediated innate immunity. Immunol Res. 2005;33:241–255. doi: 10.1385/ir:33:3:241. [DOI] [PubMed] [Google Scholar]
- 21.Charbonnier AS, Kohrgruber N, Kriehuber E, Stingl G, Rot A, Maurer D. Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal langerhans cells. J Exp Med. 1999;190:1755–1768. doi: 10.1084/jem.190.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 23.Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171:2262–2269. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- 24.Cirrincione C, Pimpinelli N, Orlando L, Romagnoli P. Lamina propria dendritic cells express activation markers and contact lymphocytes in chronic periodontitis. J Periodontol. 2002;73:45–52. doi: 10.1902/jop.2002.73.1.45. [DOI] [PubMed] [Google Scholar]
- 25.Cohen N, Morisset J, Emilie D. Induction of tolerance by Porphyromonas gingivalis on APCS: a mechanism implicated in periodontal infection. J Dent Res. 2004;83:429–433. doi: 10.1177/154405910408300515. [DOI] [PubMed] [Google Scholar]
- 26.Cook GS, Costerton JW, Lamont RJ. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J Periodontal Res. 1998;33:323–327. doi: 10.1111/j.1600-0765.1998.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 27.Cook DN, Prosser DM, Foster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis and immune responses in mucosal tissue. Immunity. 2000;12:495. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 28.Cutler CW, Jotwani R. Antigen-presentation and dendritic cells in periodontitis. Periodontol 2000. 2004;35:135–157. doi: 10.1111/j.0906-6713.2004.003560.x. [DOI] [PubMed] [Google Scholar]
- 29.Cutler CW, Jotwani R. Dendritic cells at the oral mucosal interface. Crit Rev Oral Biol Med J Dent Res. 2006;85:678–689. doi: 10.1177/154405910608500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutler CW, Jotwani R, Palucka KA, Davoust J, Bell D, Banchereau J. Evidence and a novel hypothesis for the role of dendritic cells and Porphyromonas gingivalis in adult periodontitis. J Periodontal Res. 1999;34:406–412. doi: 10.1111/j.1600-0765.1999.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 31.Da Costa C, Annels NE, Faaji CMJM, Forsyth RG, Hogendoorn PCW, Egeler RM. Presence of osteoclast-like multinucleated giant cells in the bone and nonostotic lesions of Langerhan's cell histiocytosis. J Exp Med. 2005;201:687–693. doi: 10.1084/jem.20041785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delneste Y, Charbonnier P, Herbult N, Magistrell G, Caron G, Bonnefoy J-Y, Jeamin P. Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–150. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 33.Dereka XE, Tosios KI, Chrysomali E, Angelopoulou E. Factor XIIIa + dendritic cells and S-100 protein + Langerhans' cells in adult periodontitis. J Periodontal Res. 2004;39:447–452. doi: 10.1111/j.1600-0765.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- 34.Dieu M-C, Vanbervliet B, Vicari A, Bridon J-M, Oldham E, Ait-Yahia S, Briere F, Zlotnik S, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cell by distinct chemokines in distinct anatomic sites. J Exp Med. 1998;188:373. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;18:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eriksson K, Ahlfors E, George-Chandy A, Kaiserlian D, Czerkinsky C. Antigen presentation in the murine oral epithelium. Immunology. 1996;88:147–152. doi: 10.1046/j.1365-2567.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hammerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Immunol. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 38.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 39.Föster R, Schubel A, Brietfeld D, Kremmer E, RennerMüller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 40.Fujimura Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. 2000;436:560–566. doi: 10.1007/s004289900177. [DOI] [PubMed] [Google Scholar]
- 41.Fujimura Y, Takeda M, Ikai H, Haruma K, Akisada T, Harada T, Sakai T, Ohuchi M. The role of M cells of human nasopharyngeal lymphoid tissue in influenza virus sampling. Virchows Arch. 2004;444:36–42. doi: 10.1007/s00428-003-0898-8. [DOI] [PubMed] [Google Scholar]
- 42.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by Dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garlet GP, Martins W, Jr, Ferreira BR, Milanezi CM, Silva JS. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003;38:210–217. doi: 10.1034/j.1600-0765.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- 44.Geijtenbeek TB, van Vliet SJ, Engering A, 't Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 45.Gemmell E, Carter CL, Hart DN, Drysdale KE, Seymour GJ. Antigen-presenting cells in human periodontal disease tissues. Oral Microbiol Immunol. 2002;17:388–393. doi: 10.1034/j.1399-302x.2002.170609.x. [DOI] [PubMed] [Google Scholar]
- 46.Gemmell E, Seymour GJ. Gamma delta T lymphocytes in human periodontal disease tissue. J Periodontol. 1995;66:780–785. doi: 10.1902/jop.1995.66.9.780. [DOI] [PubMed] [Google Scholar]
- 47.Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response.Crit. Rev Oral Biol Med. 2002;13:17–34. doi: 10.1177/154411130201300104. [DOI] [PubMed] [Google Scholar]
- 48.Girardin SE, Philpott DJ. Mini-review: the role of peptidoglycan recognition in innate immunity. Eur J Immunol. 2004;34:1777–1782. doi: 10.1002/eji.200425095. [DOI] [PubMed] [Google Scholar]
- 49.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 50.Grainger DJ, Reckless J. Broad-spectrum chemokine inhibitors (BSCIs) and their anti-inflammatory effects in vivo. Biochem Pharmacol. 2003;65:1027–1034. doi: 10.1016/s0006-2952(02)01626-x. [DOI] [PubMed] [Google Scholar]
- 51.Hasegawa T, Yoshimura Y, Kikuiri T, Yawaka Y, Takeyama S, Matsumoto A, Oguchi H, Shirakawa T. Expression of receptor activator of NF-kappa B ligand and osteoprotegerin in culture of human periodontal ligament cells. J Periodontal Res. 2002;37:405–411. doi: 10.1034/j.1600-0765.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 52.Highton J, Kean A, Hessian PA, Thomson J, Rietveld J, Hart DN. Cells expressing dendritic cell markers are present in the rheumatoid nodule. J Rheumatol. 2000;27:339–346. [PubMed] [Google Scholar]
- 53.Hirata H, Arima M, Cheng G, Honda K, Fukushima F, Yoshida N, Eda F, Fukuda T. Production of TARC and MDC by naive T cells in asthmatic patients. J Clin Immunol. 2003;23:34–45. doi: 10.1023/a:1021948214742. [DOI] [PubMed] [Google Scholar]
- 54.Hofbauer LC, Schoppet M. Clinical implication of the osteoprotegerin / RANKL / RANK system for bone and vascular diseases. JAMA. 2004;28:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 55.Honore P, Luger NM, Sabino MAC, Schwei MJ, Rogers SD, Mach DB. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. 2000;6:521–528. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 56.Hosokawa Y, Nakanishi T, Yamaguchi D, Takahashi K, Yumoto H, Ozaki K, Matsuo T. Macrophage inflammatory protein 3alpha-CC chemokine receptor 6 interactions play an important role in CD4+ T-cell accumulation in periodontal diseased tissue. Clin Exp Immunol. 2002;128:548–554. doi: 10.1046/j.1365-2249.2002.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Imamura T, Travis J, Potempa J. The biphasic virulence activities of gingipains: activation and inactivation of host proteins. Curr Protein Pept Sci. 2003;4:443–450. doi: 10.2174/1389203033487027. [DOI] [PubMed] [Google Scholar]
- 59.Iwasaki A. Kelsall BL Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.James SP. Prototypic disorders of gastrointestinal mucosal immune function: celiac disease and Crohn's disease. J Allergy Clin Immunol. 2005;115:25–30. doi: 10.1016/j.jaci.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 62.Jotwani R, Cutler CW. Multiple dendritic cell (DC) sub-populations in human gingiva and association of mature DC with CD4+ T-cells in situ. J Dent Res. 2003;82:736–741. doi: 10.1177/154405910308200915. [DOI] [PubMed] [Google Scholar]
- 63.Jotwani R, Cutler CW. Fimbriated Porphyromonas gingivalis is more efficient than fimbria-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infect Immun. 2004;72:1725–1732. doi: 10.1128/IAI.72.3.1725-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jotwani R, Muthukuru M, Cutler CW. Increase in HIV receptors / co-receptors / α-defensins in inflamed human gingiva. J Dent Res. 2004;83:371–378. doi: 10.1177/154405910408300504. [DOI] [PubMed] [Google Scholar]
- 65.Jotwani R, Palucka AK, Al-Quotub M, Kim J, Bell D, Banchereau J, Cutler CW. Dendritic cells infiltrate T-cell rich oral mucosa in chronic periodontitis: in situ, in vivo and in vitro studies. J Immunol. 2001;167:4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting TH2 effector response in vitro. Eur J Immunol. 2003;33:2980–2986. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 67.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kantarci A, Van Dyke TE. Resolution of inflammation in periodontitis. J Periodontol. 2005;76(Suppl):2168–2174. doi: 10.1902/jop.2005.76.11-S.2168. [DOI] [PubMed] [Google Scholar]
- 69.Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–2109. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 70.Kawasaki S, Matsushima K. Chemokines and chemokine receptors in collagen diseases. Nippon Rinsho. 1999;57:278–282. [PubMed] [Google Scholar]
- 71.Kesavalu L, Holt SC, Ebersole JL. In vitro environmental regulation of Porphyromonas gingivalis growth and virulence. Oral Microbiol Immunol. 2003;18:226–233. doi: 10.1034/j.1399-302x.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 72.Kikuchi T, Hahn CL, Tanaka S, Barbour SE, Schenkein HA, Tew JG. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect Immun. 2004;72:5089–5096. doi: 10.1128/IAI.72.9.5089-5096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kikuchi T, Willis DL, Liu M, Purkall DB, Sukumar S, Barbour SE, Schenkein HA, Tew JG. Dendritic-natural killer cell interactions in P. gingivalis-specific responses. J Dent Res. 2005;84:858–862. doi: 10.1177/154405910508400915. [DOI] [PubMed] [Google Scholar]
- 74.Kinane DF, Demuth DR, Gorr SU, Hajishengallis G, Martin M. Human variability in innate immunity. Periodontol 2000. 2007;45:14–34. doi: 10.1111/j.1600-0757.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 75.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–308. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 76.van Kooyk Y, Engering A, Lekkerkerker AN, Ludwig IS, Geijtenbeek TB. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr Opin Immunol. 2004;16:488–493. doi: 10.1016/j.coi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 77.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 78.Kunisawa J, Fukuyama S, Kiyono H. Mucosa-associated lymphoid tissues in the aerodigestive tract: their shared and divergent traits and their importance to the orchestration of the mucosal immune system. Curr Mol Med. 2005;5:557–572. doi: 10.2174/1566524054863924. [DOI] [PubMed] [Google Scholar]
- 79.Lacey DL, Timms E, Tan HL, Kelly MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 80.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lean JM, Fuller K, Chambers T. FLT3-ligand can substitute for macrophage colony-stimulating factor in support of osteoclast differentiation and function. Blood. 2001;98:2707–2713. doi: 10.1182/blood.v98.9.2707. [DOI] [PubMed] [Google Scholar]
- 82.Leon B, Martinez del Hoyo G, Parrillas V, Vargas HH, Sanchez-Mateos P, Longo N, Lopez-Bravo M, Ardavin C. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8− and CD8+ splenic dendritic cells. Blood. 2004;103:2668–2676. doi: 10.1182/blood-2003-01-0286. [DOI] [PubMed] [Google Scholar]
- 83.Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1 / ST2. J Immunol. 2004;173:145–150. doi: 10.4049/jimmunol.173.1.145. [DOI] [PubMed] [Google Scholar]
- 84.Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N, Kamm MA, Knight SC, Forbes A. Clinical, microbiological, and immunological effects of fructooligosaccharide in patients with Crohn's disease. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loos BG, Dyer DW, Whittam TS, Selander RK. Genetic structure of populations of Porphyromonas gingivalis associated with periodontitis and other oral infections. Infect Immun. 1993;61:204–212. doi: 10.1128/iai.61.1.204-212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lundqvist C, Hammarstrom S, Hammarstrom ML. Intraepithelial lymphocytes expressing TCR gamma delta in human gingival tissues. Adv Exp Med Biol. 1995;371B:1097–1102. [PubMed] [Google Scholar]
- 87.MacDonald KP, Pettit AR, Quinn C, Thomas GJ, Thomas R. Resistance of rheumatoid synovial dendritic cells to the immunosuppressive effects of interleukin-10. J Immunol. 1999;163:5599–5607. [PubMed] [Google Scholar]
- 88.MacDonald KPA, Rowe V, Bofinger HM, Thomas R, Sasmono T, Hume DA, Hill GR. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol. 2005;175:1399–1405. doi: 10.4049/jimmunol.175.3.1399. [DOI] [PubMed] [Google Scholar]
- 89.Mahamed D, Marleau A, Alnaeeli M, Singh B, Zhang X, Penninger JM, Teng Y-TA. G(−) anaerobe-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes. 2005;54:1477–1486. doi: 10.2337/diabetes.54.5.1477. [DOI] [PubMed] [Google Scholar]
- 90.Mahanonda R, Sa-Ard-Iam N, Yongvanitchit K, Wisetchang M, Ishikawa I, Nagasawa T, Walsh DS, Pichyangkul S. Upregulation of co-stimulatory molecule expression and dendritic cell marker (CD83) on B cells in periodontal disease. J Periodontal Res. 2002;37:177–183. doi: 10.1034/j.1600-0765.2002.00664.x. [DOI] [PubMed] [Google Scholar]
- 91.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovesky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 92.Menard C, Mouton C. Genetic structure of populations of Porphyromonas gingivalis associated with periodontitis and other oral infections. Infect Immun. 1993;61:204–212. doi: 10.1128/iai.61.1.204-212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, Anderson DM, Suda T. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001;98:2544–2554. doi: 10.1182/blood.v98.8.2544. [DOI] [PubMed] [Google Scholar]
- 94.Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor / osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 95.Mojon P, Bureau J. Respiratory infection: how important is oral health? Curr Opin Pulm Med. 2003;9:166–170. doi: 10.1097/00063198-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Monteleone G, Fina D, Caruso R, Pallone F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22:361–364. doi: 10.1097/01.mog.0000231808.10773.8e. [DOI] [PubMed] [Google Scholar]
- 97.Mowat AM, Parker LA, Beacock-Sharp H, Millington OR, Chirdo F. Oral tolerance: overview and historical perspectives. Ann N Y Acad Sci. 2004;1029:1–8. doi: 10.1196/annals.1309.001. [DOI] [PubMed] [Google Scholar]
- 98.Muller G, Hopken UE, Stein H, Lipp M. Systemic immunoregulatory and pathogenic functions of homeostatic chemokine receptors. J Leukoc Biol. 2002;72:1–8. [PubMed] [Google Scholar]
- 99.Muthukuru M, Cutler CW. Upregulation of immunoregulatory Src homology 2 molecule containing inositol phosphatase and mononuclear cell hyporesponsiveness in oral mucosa during chronic periodontitis. Infect Immun. 2006;74:1431–1435. doi: 10.1128/IAI.74.2.1431-1435.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muthukuru M, Jotwani R, Cutler CW. Induction of oral mucosal endotoxin tolerance in chronic periodontitis. Infect Immun. 2005;73:687–694. doi: 10.1128/IAI.73.2.687-694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 102.Nagasawa T, Kobayashi H, Kiji M, Aramaki M, Mahanonda R, Kojima T, et al. LPS-stimulated human gingival fibroblasts inhibit the differentiation of monocytes into osteoclasts through the production of osteoprotegerin. Clin Exp Immunol. 2002;130:338–344. doi: 10.1046/j.1365-2249.2002.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Newberry RD, Lorenz RG. Organizing a mucosal defense. Immunol Rev. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 104.Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Curr Opin Gastroenterol. 2006;22:354–360. doi: 10.1097/01.mog.0000231807.03149.54. [DOI] [PubMed] [Google Scholar]
- 105.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 106.Nukaga J, Kobayashi M, Shinki T, Song H, Takada T, Takiguchi T, Kamijo R, Hasegawa K. Regulatory effects of interleukin-1beta and prostaglandin E2 on expression of receptor activator of nuclear factor-kappa B ligand in human periodontal ligament cells. J Periodontol. 2004;75:249–259. doi: 10.1902/jop.2004.75.2.249. [DOI] [PubMed] [Google Scholar]
- 107.Okuda K, Kimizuka R, Abe S, Kato T, Ishihara K. Involvement of periodontopathic anaerobes in aspiration pneumonia. J Periodontol. 2005;76(11 Suppl):2154–2160. doi: 10.1902/jop.2005.76.11-S.2154. [DOI] [PubMed] [Google Scholar]
- 108.Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective expression in rheumatoid synovium. J Immunol. 2002;168:5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- 109.Page G, Miossec P. RANK and RANKL expression as markers of dendritic cell T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis Rheum. 2005;52:2307–2312. doi: 10.1002/art.21211. [DOI] [PubMed] [Google Scholar]
- 110.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol 2002. 2002;10:s32–s37. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 111.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rani CS, MacDougall M. Dental cells express factors that regulate bone resorption. Mol Cell Biol Res Commun. 2000;3:145–152. doi: 10.1006/mcbr.2000.0205. [DOI] [PubMed] [Google Scholar]
- 113.Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, Navarro J, Munoz-Fernandez MA, Sanchez-Mateos P, Corbi AL. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 114.Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- 115.Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, Jurdic P, Servet-Delprat C. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by rheumatoid arthritis microenvironment. Blood. 2004;104:4029–4037. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 116.Rozell B, Ljungdahl PO, Martinez P. Host-pathogen interactions and the pathological consequences of acute systemic Candida albicans infections in mice. Curr Drug Targets. 2006;7:483–494. doi: 10.2174/138945006776359449. [DOI] [PubMed] [Google Scholar]
- 117.Saeki T, Naya A. CCR1 chemokine receptor antagonist. Curr Pharm Des. 2003;9:1201–1208. doi: 10.2174/1381612033454937. [DOI] [PubMed] [Google Scholar]
- 118.Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001;67:1758–1768. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 119.Sasaki M, Hasegawa H, Kohno M, Inoue A, Ito MR, Fujita S. Antagonist of secondary lymphoid-tissue chemokine (CCR ligand 21) prevents the development of chronic graft-versus-host disease in mice. J Immunol. 2003;170:588–596. doi: 10.4049/jimmunol.170.1.588. [DOI] [PubMed] [Google Scholar]
- 120.Schuler G, Lutz MB, Bender A, Thurner B, Röder C, Young J, Romani N. A guide to the isolation and propagation of dendritic cells. In: Lotze MT, Thomson AW, editors. Dendritic Cells: Biology and Clinical Applications Chapter 27. Academic; San Diego, CA: 1999. p. 27. [Google Scholar]
- 121.Scimone ML, Lutzky VP, Zittermann SI, Maffia P, Jancic C, Buzzola F, Issekutz AC, Chuluyan HE. Migration of polymorphonuclear leucocytes is influenced by dendritic cells. Immunology. 2005;114:375–385. doi: 10.1111/j.1365-2567.2005.02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 123.Servet-Delprat C, Arnaud S, Jurdic P, Nataf S, Grasset MF, Soulas C, Domenget C, Destaing O, Rivollier A, Perret M, Dumontel C, Hanau D, Gilmore GL, Belin MF, Rabourdin-Combe C, Mouchiroud G. Flt3+ macrophage precursors commit sequentially to osteoclasts, dendritic cells and microglia. BMC Immunol. 2002;3:15. doi: 10.1186/1471-2172-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shurin MR, Pandharipande PP, Zorina TD, Haluszczak C, Subbotin VM, Hunter O, Brumfield A, Storkus WJ, Maraskovsky E, Lotze MT. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–184. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 125.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 126.Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 127.Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA, Rennert PD, Weiner HL. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol. 2001;31:1278–1287. doi: 10.1002/1521-4141(200104)31:4<1278::aid-immu1278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 128.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JAM, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte / macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 130.Steinman R, Hoffman L, Pope M. Maturation and migration of cutaneous dendritic cells. J Invest Dermatol. 1995;105(11 Suppl):2S–7S. doi: 10.1111/1523-1747.ep12315162. [DOI] [PubMed] [Google Scholar]
- 131.Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, Kurokawa T, Suda T. Macrophage colony stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001;12:125–135. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 133.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;8:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 134.Teng Y-TA. Mixed periodontal Th1 / Th2 cytokines in Actinobacillus actinomycetemcomitans-specific osteoprotegerin ligand (or RANK-L)-mediated alveolar bone destruction in vivo. Infect Immun (Notes) 2002;70:5269–5273. doi: 10.1128/IAI.70.9.5269-5273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teng Y-TA. The role of acquired immunity in periodontal disease progression. Crit Rev Oral Biol Med. 2003;14:237–252. doi: 10.1177/154411130301400402. [DOI] [PubMed] [Google Scholar]
- 136.Teng Y-TA. Protective and destructive immunity in the periodontium: (Part 1) – innate and humoral immunity and the periodontium. J Dent Res. 2006;85:198–208. doi: 10.1177/154405910608500301. [DOI] [PubMed] [Google Scholar]
- 137.Teng Y-TA. Protective and destructive immunity in the periodontium. Part 2: T-cell-mediated immunity in the periodontium. J Dent Res. 2006;85:209–219. doi: 10.1177/154405910608500302. [DOI] [PubMed] [Google Scholar]
- 138.Teng Y-TA, Mahamed D, Singh B. IFN-γ positively modulates Actinobacillus actinomycetemcomitans-specific CD4+ T cells-mediated alveolar bone destruction in vivo. Infect Immun. 2005;73:3453–3461. doi: 10.1128/IAI.73.6.3453-3461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Teng YA, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin-ligand (OPG-L) control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–R67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cell, bone loss and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 141.Thomas R, MacDonald KP, Pettit AR, Cavanagh LL, Padmanabha J, Zehntner S. Dendritic cells and the pathogenesis of rheumatoid arthritis. J Leukoc Biol. 1999;66:286–292. doi: 10.1002/jlb.66.2.286. [DOI] [PubMed] [Google Scholar]
- 142.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, Bouis DA, Norgard MV, Miyake K, Godowski PJ, Roth MD, Modlin RL. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165:3804–3810. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 143.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, Rehakova Z, Sinkora J, Hofman J, Drastich P, Kokesova A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 144.Tuite-McDonnell M, Griffen AL, Moeschberger ML, Dalton RE, Fuerst PA, Leys EJ. Concordance of Porphyromonas gingivalis colonization in families. J Clin Microbiol. 1997;35:455–461. doi: 10.1128/jcm.35.2.455-461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Uehara A, Sugawara Y, Kurata S, Fujimoto Y, Fukase K, Kusumoto S, Satta Y, Sasano T, Sugawara S, Takada H. Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells. Cell Microbiol. 2005;7:675–686. doi: 10.1111/j.1462-5822.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 146.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 147.Upham JW, Stumbles PA. Why are dendritic cells important in allergic diseases of the respiratory tract? Pharmacol Ther. 2003;100:75–87. doi: 10.1016/s0163-7258(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 148.Valverde P, Kawai T, Taubman MA. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Miner Res. 2004;19:155–164. doi: 10.1359/JBMR.0301213. [DOI] [PubMed] [Google Scholar]
- 149.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 151.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin / osteoclastogenesis-inhibitory factor and is identical to TRANCE / RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 153.Zhang X, Teng Y-TA. IL-10 inhibits G(−) microbe-specific RANKL + CD4+ Th1 cells-associated alveolar bone loss in vivo. Infect Immun (Notes) 2006;74:4927–4931. doi: 10.1128/IAI.00491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Old-stone MBA. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]