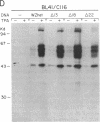
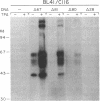
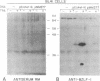
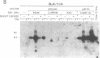
Abstract
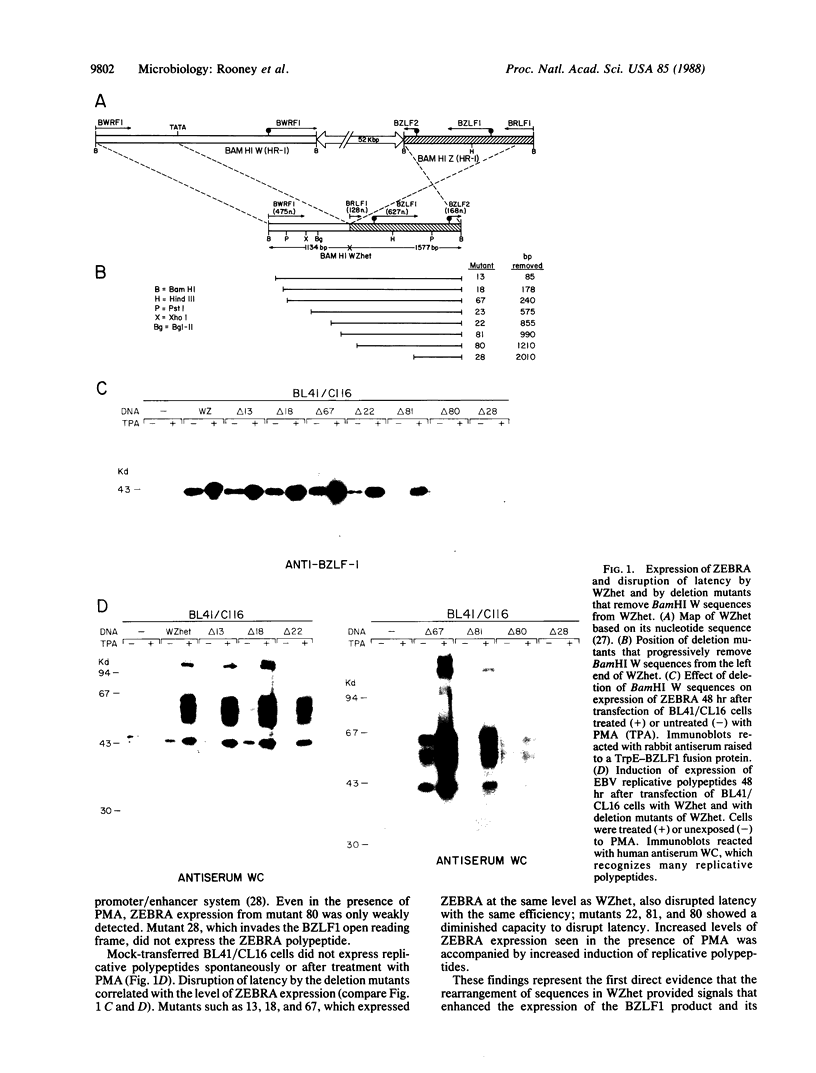
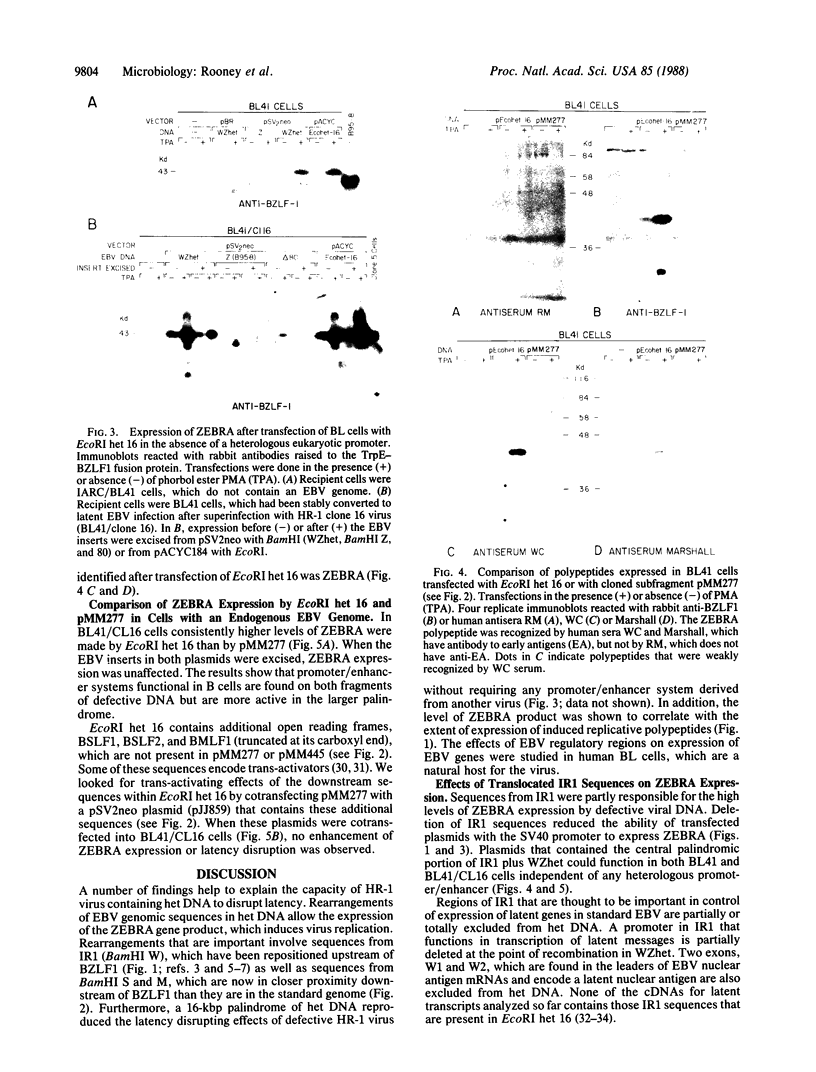
A defective Epstein-Barr virus (EBV) containing a deleted and rearranged genome (het DNA) causes latent EBV to replicate. This activity maps to the 2.7-kilobase-pair WZhet fragment. The BZLF1 open reading frame, present within WZhet as well as in the standard viral BamHI Z fragment, encodes the protein ZEBRA, which induces viral replication. Using gene transfers into Burkitt lymphoma cells, we now demonstrate that rearranged sequences juxtaposed to BZLF1 in het DNA facilitate expression of ZEBRA protein. Two stretches of EBV sequences within a palindromic region of het DNA contain positive regulatory elements. One set, derived from the viral large internal repeat, is newly positioned upstream of BZLF1; the second set is downstream of BZLF1 in het DNA. The capacity of defective HR-1 viruses to disrupt latency of the standard EBV genome is due to abnormal regulation of the BZLF1 gene as a result of genomic rearrangements.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodescot M., Chambraud B., Farrell P., Perricaudet M. Spliced RNA from the IR1-U2 region of Epstein-Barr virus: presence of an open reading frame for a repetitive polypeptide. EMBO J. 1984 Aug;3(8):1913–1917. doi: 10.1002/j.1460-2075.1984.tb02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. S., Bornkamm G. W., zur Hausen H. Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J Virol. 1984 Jul;51(1):199–207. doi: 10.1128/jvi.51.1.199-207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Jenson H., Seibl R., Wolf H., Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987 Dec;61(12):3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Cohen L. K., Henle W., Strominger J. L. An unusually high-titer human anti-Epstein Barr virus (EBV) serum and its use in the study of EBV-specific proteins synthesized in vitro and in vivo. J Immunol. 1983 Feb;130(2):919–924. [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D. K., Robert M. F., Shedd D., Summers W. P., Robinson J. E., Wolak J., Stefano J. E., Miller G. Identification of Epstein-Barr nuclear antigen polypeptide in mouse and monkey cells after gene transfer with a cloned 2.9-kilobase-pair subfragment of the genome. Proc Natl Acad Sci U S A. 1984 Jan;81(1):43–47. doi: 10.1073/pnas.81.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan E., Jenson H., Countryman J., Heston L., Gradoville L., Miller G. Transfection of a rearranged viral DNA fragment, WZhet, stably converts latent Epstein-Barr viral infection to productive infection in lymphoid cells. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1332–1336. doi: 10.1073/pnas.84.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Wang F., Bushman E. W., Kieff E. Definitive identification of a member of the Epstein-Barr virus nuclear protein 3 family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5693–5697. doi: 10.1073/pnas.83.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Kieff E. Epstein-Barr virus RNA. VIII. Viral RNA in permissively infected B95-8 cells. J Virol. 1982 Jul;43(1):262–272. doi: 10.1128/jvi.43.1.262-272.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbra R. J., Karin M. Phorbol ester induces the transcriptional stimulatory activity of the SV40 enhancer. Nature. 1986 Oct 9;323(6088):555–558. doi: 10.1038/323555a0. [DOI] [PubMed] [Google Scholar]
- Jenson H. B., Farrell P. J., Miller G. Sequences of the Epstein-Barr Virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J Virol. 1987 May;61(5):1495–1506. doi: 10.1128/jvi.61.5.1495-1506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson H. B., Miller G. Polymorphisms of the region of the Epstein-Barr virus genome which disrupts latency. Virology. 1988 Aug;165(2):549–564. doi: 10.1016/0042-6822(88)90599-5. [DOI] [PubMed] [Google Scholar]
- Jenson H. B., Rabson M. S., Miller G. Palindromic structure and polypeptide expression of 36 kilobase pairs of heterogeneous Epstein-Barr virus (P3HR-1) DNA. J Virol. 1986 May;58(2):475–486. doi: 10.1128/jvi.58.2.475-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Dillner J., Ernberg I., Ehlin-Henriksson B., Rosén A., Henle W., Henle G., Klein G. Four virally determined nuclear antigens are expressed in Epstein-Barr virus-transformed cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1499–1503. doi: 10.1073/pnas.83.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux G., Perricaudet M., Farrell P. J. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J. 1988 Mar;7(3):769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., O'Hare P., Hayward G. S., Hayward S. D. Promiscuous trans activation of gene expression by an Epstein-Barr virus-encoded early nuclear protein. J Virol. 1986 Oct;60(1):140–148. doi: 10.1128/jvi.60.1.140-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Raab-Traub N. Two strains of Epstein-Barr virus (B95-8 and a P3HR-1 subclone) that lack defective genomes induce early antigen and cause abortive infection of Raji cells. J Virol. 1987 Jun;61(6):1985–1991. doi: 10.1128/jvi.61.6.1985-1991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Rabson M., Heston L. Epstein-Barr virus with heterogeneous DNA disrupts latency. J Virol. 1984 Apr;50(1):174–182. doi: 10.1128/jvi.50.1.174-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Heston L., Miller G. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2762–2766. doi: 10.1073/pnas.80.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Farrell P. J., Miller G. Novel nuclear antigens recognized by human sera in lymphocytes latently infected by Epstein-Barr virus. Virology. 1987 Jan;156(1):153–162. doi: 10.1016/0042-6822(87)90446-6. [DOI] [PubMed] [Google Scholar]
- Rowe D., Heston L., Metlay J., Miller G. Identification and expression of a nuclear antigen from the genomic region of the Jijoye strain of Epstein-Barr virus that is missing in its nonimmortalizing deletion mutant, P3HR-1. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7429–7433. doi: 10.1073/pnas.82.21.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Speck S. H., Pfitzner A., Strominger J. L. An Epstein-Barr virus transcript from a latently infected, growth-transformed B-cell line encodes a highly repetitive polypeptide. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9298–9302. doi: 10.1073/pnas.83.24.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986 Mar;57(3):1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel R., Miller G. Latent and viral replicative transcription in vivo from the BamHI K fragment of Epstein-Barr virus DNA. J Virol. 1985 May;54(2):501–508. doi: 10.1128/jvi.54.2.501-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. M., Levine A. J. Identification and mapping of Epstein-Barr virus early antigens and demonstration of a viral gene activator that functions in trans. J Virol. 1986 Oct;60(1):149–156. doi: 10.1128/jvi.60.1.149-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Bornkamm G. W., Schmidt R., Hecker E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):782–785. doi: 10.1073/pnas.76.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]