Heat shock proteins (HSPs) of the Hsp70 family play a number of roles as molecular chaperones in eukaryotic cells, participating in the folding of newly synthesized proteins, transport of proteins across membranes, and disassembly of stress-induced protein aggregates. Hsp70s function with cochaperones, including J domain proteins and nucleotide exchange factors, in chaperone cycles consisting of repetitive cycles of peptide binding, ATP hydrolysis, and peptide release (reviewed in Wang et al., 2004). The Arabidopsis genome encodes at least 14 Hsp70s, which fall into four classes, each of which localizes to a separate subcellular compartment, viz. cytosol, endoplasmic reticulum, plastids, or mitochondria. Hsp70 function in transport across the endoplasmic reticulum and mitochondrial membranes is fairly well characterized, and there is some evidence that they perform a similar function in chloroplasts. In new work, Shi and Theg (pages 205–220) show that a stromal Hsp70 plays a key role in the import of proteins across the chloroplast envelope membranes.
Chloroplast protein import depends on the action of translocon complexes termed Toc (translocon at the chloroplast outer envelope membrane) and Tic (translocon at the chloroplast inner envelope membrane). Tic components are known to interact with the chaperones Hsp93 and Cpn60. It is thought that stromal Hsp93 provides a major driving force for chloroplast protein import, analogous to the role of mitochondrial Hsp70 in mitochondrial import (Kessler and Schnell, 2006). Both stromal and chloroplast membrane Hsp70s have been implicated in import across the chloroplast envelope membranes, but their precise role and possible association with the Toc/Tic translocon is not well understood. Shi and Theg focused on the function of stromal Hsp70 using the model system Physcomitrellla patens, which has chloroplast protein transport machinery that is functionally equivalent to that of flowering plants and presents a tractable system for the study of chloroplast import (Hofmann and Theg, 2003).
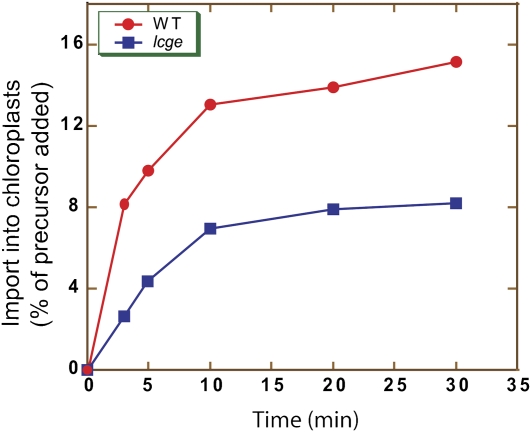
The authors identified three Hsp70s in Physcomitrella that are imported into chloroplasts and localized within the stroma. They used homologous recombination to create targeted knockouts of these genes and found that Hsp70-2 is an essential gene: the endogenous Hsp70-2 could be knocked out only with cotransformation with a functionally expressed cDNA copy of the gene. They created a temperature-sensitive mutant form of the gene using one or two amino acid changes, and experiments with isolated chloroplasts containing the temperature-sensitive mutant form provided strong evidence that Hsp70-2 is involved in protein import into chloroplasts. In addition, they isolated two mutants of putative chloroplast Hsp-70 cochaperones, disrupted in genes homologous to GrpE nucleotide exchange cochaperones, named Chloroplast GrpE 1 (CGE1) and CGE2. A double knockout of both of these genes was lethal, whereas a partial double knockout that retained a low level of CGE2 showed severe defects in chloroplast protein import (see figure). Immunoprecipation experiments showed that Hsp70-2 is present in the actively translocating Toc/Tic complex and that this association is stabilized under conditions in which CGE is limiting. Taken together, the data suggest that stromal Hsp70-2 and the cochaperones CGE1 and CGE2 play a crucial role in chloroplast protein import in concert with the Toc/Tic translocon. This work thus demonstrates the power of reverse genetics in Physcomitrella to provide important insight into our understanding of how proteins are transported across the chloroplast envelope.
Chloroplast protein import is compromised in cge mutant chloroplasts. Protein import assays conducted with isolated chloroplasts from the wild type and a cge1 cge2 double mutant with a low level CGE2 (lcge). (Adapted from Figure 7D of Shi and Theg [2009].)
References
- Hofmann N.R., Theg S.M. (2003). Physcomitrella patens as a model for the study of chloroplast protein transport: Conserved machineries between vascular and non-vascular plants. Plant Mol. Biol. 53: 643–654 [DOI] [PubMed] [Google Scholar]
- Kessler F., Schnell D.J. (2006). The function and diversity of plastid protein import pathways: A multilane GTPase highway into plastids. Traffic 7: 248–257 [DOI] [PubMed] [Google Scholar]
- Shi L.-X., Theg S.M. (2009). A stromal heat shock protein 70 system functions in protein import into chloroplasts in the moss Physcomitrella patens. Plant Cell 21: 205–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Vinocur B., Shoseyov O., Altman A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9: 244–252 [DOI] [PubMed] [Google Scholar]