This study describes the collection of UBL/UBA domain proteins in Arabidopsis that participate in the ubiquitin/26S proteasome system, with a focus on the RAD23 family. The data point to a specific role for RAD23s in plants and suggest that the four isoforms have both redundant and unique roles in Arabidopsis development by helping shuttle ubiquitin conjugates to the 26S proteasome.
Abstract
The ubiquitin (Ub)/26S proteasome system (UPS) directs the turnover of numerous regulatory proteins, thereby exerting control over many aspects of plant growth, development, and survival. The UPS is directed in part by a group of Ub-like/Ub-associated (UBL/UBA) proteins that help shuttle ubiquitylated proteins to the 26S proteasome for breakdown. Here, we describe the collection of UBL/UBA proteins in Arabidopsis thaliana, including four isoforms that comprise the RADIATION SENSITIVE23 (RAD23) family. The nuclear-enriched RAD23 proteins bind Ub conjugates, especially those linked internally through Lys-48, via their UBA domains, and associate with the 26S proteasome Ub receptor RPN10 via their N-terminal UBL domains. Whereas homozygous mutants individually affecting the four RAD23 genes are without phenotypic consequences (rad23a, rad23c, and rad23d) or induce mild phyllotaxy and sterility defects (rad23b), higher-order mutant combinations generate severely dwarfed plants, with the quadruple mutant displaying reproductive lethality. Both the synergistic effects of a rad23b-1 rpn10-1 combination and the response of rad23b plants to mitomycin C suggest that RAD23b regulates cell division. Taken together, RAD23 proteins appear to play an essential role in the cell cycle, morphology, and fertility of plants through their delivery of UPS substrates to the 26S proteasome.
INTRODUCTION
Protein degradation is a key posttranslational event that controls the levels of numerous cell regulators, thus enabling cells to maintain homeostasis, initiate phase changes, and respond to internal and external stimuli. A major proteolytic pathway in plants and animals is the ubiquitin (Ub)/26S proteasome system (UPS), which removes naturally short-lived proteins as well as aberrant proteins that are improperly assembled or no longer functional (Smalle and Vierstra, 2004; Vierstra, 2009). Prior to breakdown, one or more Ubs becomes attached to appropriate targets via an isopeptide linkage between the C-terminal Gly of Ub and accessible lysl ϵ -amino groups on the target. These Ubs then serve as concatenation sites for the assembly of Ub polymers linked internally through one of the seven Ub lysines (Ravid and Hochstrasser, 2008; Saracco et al., 2009; Xu et al., 2009). Several concatenation topologies (e.g., involving Lys-11 and Lys-48 in Ub) then provide strong signals for recognition by the 26S proteasome. This ATP-dependent protease complex identifies appropriately ubiquitylated targets, releases the Ub moieties for reuse, and cleaves the unfolded target into small peptides by proteolytic activities in its lumen (Finley, 2009). Via the removal of key regulatory proteins, the UPS controls most aspects of a plant's life cycle, including embryogenesis, photomorphogenesis, hormone signaling, circadian rhythms, responses to abiotic and biotic stresses, self-incompatibility, and senescence (Smalle and Vierstra, 2004; Dreher and Callis, 2007; Vierstra, 2009).
Whereas the specificity of ubiquitylation is directed by large families of E3s (or Ub protein ligases; Vierstra, 2009), the detection of these conjugates by the 26S proteasome is achieved by a much smaller set of Ub binding proteins (Finley, 2009). Several are core subunits of the regulatory particle (RP) subcomplex of the 26S proteasome, including RPN1, RPN10 (or S5a), and RPN13, the last two of which have selective affinity for Lys-48–linked Ub polymers (Hartmann-Petersen and Gordon, 2004; Raasi et al., 2005; van Nocker et al., 1996a; Finley, 2009). In addition, a collection of extraproteasomal proteins participates in yeast and animals to stabilize and sequester ubiquitylated proteins and, in some cases, to help deliver them to the 26S proteasome. These shuttle proteins include members of the RADIATION SENSITIVE23 (RAD23) (Lambertson et al., 1999), DOMINANT SUPPRESSOR OF KAR2 (DSK2) (Funakoshi et al., 2002; Kang et al., 2006), DNA DAMAGE-INDUCIBLE1 (DDI1) (Gabriely et al., 2008), NEDD8 ultimate buster 1 (NUB1) (Kito et al., 2001), and possibly the Ub-like 7 (UBL7) families (Liu et al., 2003). They all contain an N-terminal UBL domain that is structurally related to Ub, and one or more Ub-associated (UBA) domains that bind Ub (Finley, 2009). By interacting with the 26S proteasome through contacts between the UBL domain and Ub receptors and with ubiquitylated proteins through contacts between the UBA domain(s) and the appended Ub moiet(ies), these proteins presumably capture UPS targets remotely and then tether them to the protease.
RAD23 proteins, in particular, have emerged as principal shuttles of Ub conjugates. The first member was discovered in yeast (Saccharomyces cerevisiae) based on its role in DNA damage repair, during which it associates with the nucleotide excision repair factor 2 (NEF2) complex (Guzder et al., 1998). Accordingly, mutations in RAD23 family members confer a hypersensitivity to UV light in yeast (Lambertson et al., 1999), cause the light-sensitive skin disease Xeroderma pigmentosum in afflicted humans (Hiyama et al., 1999), and induce severe developmental defects and male sterility in mice (Ng et al., 2003). Subsequent studies connected yeast RAD23 to the UPS, including the demonstration that the UBL domain of yeast RAD23 docks with the Ub-interacting motif(s) (UIM) of RPN10 to promote the release of substrates to the 26S proteasome (Hiyama et al., 1999; Mueller and Feigon, 2003; Heessen et al., 2005). Mutations in RAD23 have a synergistic effect on yeast null for RPN10, including an increased level of Ub conjugates and a reduced turnover rate of specific UPS targets, such as the cyclin-dependent kinase inhibitor Sic1p (Lambertson et al., 1999; Verma et al., 2004). RAD23 has also been demonstrated to dock with RPN1 using its UBL domain to bind the Leu-rich repeats in RPN1 (Elsasser et al., 2002). More recently, RAD23 was connected to the endoplasmic reticulum–associated degradation (ERAD) subpathway within the UPS, which removes incorrectly folded and misassembled secretory proteins via its association with the heterotrimeric CDC48/UFD1/NPL4 complex (Ye et al., 2003; Raasi and Wolf, 2007).
Despite their potential importance to substrate recognition by the UPS, little is known about UBL/UBA proteins in the plant kingdom. A prior study potentially connected a maize (Zea mays) RAD23 isoform to abscisic acid (ABA) signaling by demonstrating that it interacts via yeast two-hybrid with a rice (Oryza sativa) ortholog (VP1) of the Arabidopsis thaliana transcription factor ABI3, which regulates ABA responses in germinating seedlings and is degraded by the UPS (Schultz and Quatrano, 1997; Zhang et al., 2005). Sturm and Lienhard (1998) showed that two carrot (Daucus carota) RAD23 isoforms rescue the UV-sensitive phenotype of rad23 Δ yeast, suggesting that plant RAD23s also participate in DNA damage repair. To extend these analyses, we exhaustively searched various predicted plant proteomes for UBL/UBA proteins and found that Arabidopsis and other plant species express one or more obvious orthologs of yeast RAD23, DSK2, and DDI1, along with a potential ortholog of human NUB1. Subsequent biochemical and genetic analyses of the four Arabidopsis loci encoding RAD23 demonstrated that this set plays an essential role in plant development, presumably by helping deliver ubiquitylated targets to the RPN10 Ub receptor in the 26S proteasome.
RESULTS
The Collection of Arabidopsis UBL/UBA Proteins
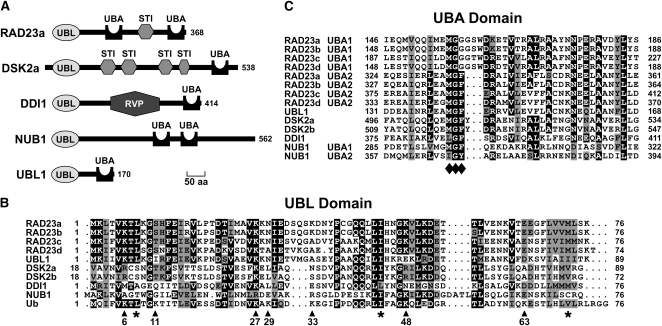
Using the yeast and human RAD23, DSK2, and DDI1 proteins as queries, we identified one or more genes encoding structurally related proteins in the Arabidopsis ecotype Columbia-0 (Col-0) genome database (www.Arabidopsis.org). The collection included one ortholog of DDI1 (At3g13235), two for DSK2 (DSK2a [At2g17190] and DSK2b [At2g17200]), and four for RAD23 (RAD23a [At1g16190], RAD23b [At1g79650], RAD23c [At3g02540], and RAD23d [At5g38470]) that all contain the signature UBL and UBA domains at their N and C termini, respectively (Figure 1A; see Supplemental Figure 1 online). Using human NUB1 and UBL7 as queries (Kito et al., 2001; Liu et al., 2003), we found two additional UBL/UBA loci (Figure 1A). One (At2g12550) is most similar over its entire length to human NUB1 (40%/27% amino acid sequence identity/similarity) and is thus a likely NUB1 ortholog. The other, which we have named UBL1 (At5g16090), is predicted to encode a short protein of 170 amino acids that includes a UBL domain followed by one UBA domain. ESTs could be individually assigned to almost all members of the Arabidopsis UBL/UBA gene collection, indicating that most are transcriptionally active (Figure 2A; www.Arabidopsis.org). The only exception was UBL1, which lacked any EST support (as of August 14, 2009).
Figure 1.
Domain Architectures of the Arabidopsis UBL/UBA Proteins.
(A) Protein domain organization of RAD23a, DSK2a, DDI1, NUB1, and UBL1. Numbers on the right indicate the amino acid length of each protein. STI, stress-inducible-1 domain; RVP, retroviral aspartyl-protease domain.
(B) Amino acid sequence alignment of the UBL domains compared with Ub. Arrowheads indicate the conserved Lys residues in Ub that are used in poly-Ub chain assembly. Asterisks identify residues that form the hydrophobic patch in Ub (Leu-8, Ile-44, and Val-70) needed for RPN10 binding.
(C) Amino acid sequence alignment of the UBA domains. For RAD23a-d and NUB1, both UBA sequences are shown. Diamonds identify residues of the hydrophobic MGF loop (Met-Gly-Phe) that promotes UBA-Ub association. For (B) and (C), black and gray boxes denote conserved and similar residues, respectively. Dots indicate gaps. Numbers on the left and right indicate the amino acid positions of each sequence.
Figure 2.
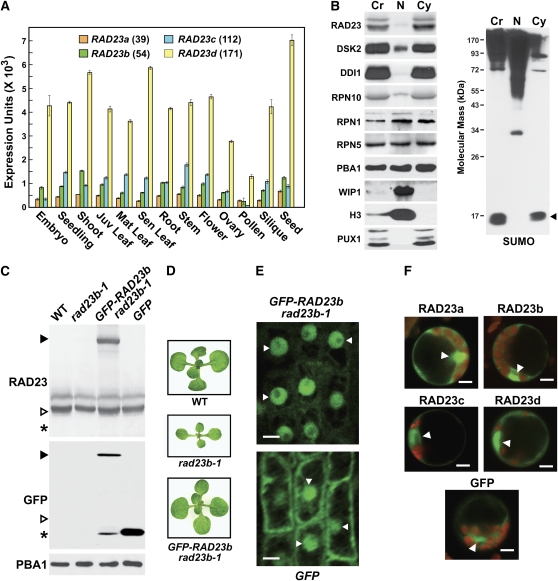
Gene Expression Patterns and Protein Localization for Arabidopsis RAD23 Isoforms.
(A) Relative transcript abundance of RAD23a-d in various tissues determined from the GENEVESTIGATOR DNA microarray data set (https://www.genevestigator.ethz.ch). Number of representatives in the EST database (www.Arabidopsis.org) is listed for each locus. Juv, juvenile; Mat, mature; Sen, senescing. Error bars represent the se from different arrays.
(B) Detection of RAD23, DSK2, and DDI1 proteins in nuclear (N)- and cytosol (Cy)-enriched fractions. The fractions were prepared from 1-week-old wild-type seedlings by Percoll gradient centrifugation and subjected to SDS-PAGE and immunoblot analyses with the indicated antibodies. PBA1, β 1 subunit of the CP; RPN5, lid subunit of the RP; RPN10 and RPN1, base subunits of the RP. Antibodies against WIP-1, histone H3 (H3), PUX1, and SUMO1 were used to verify enrichment of the nuclear and cytosolic fractions, respectively. An equal amount of total protein was analyzed in each lane. Cr, crude extract.
(C) to (F) Subcellular localization of RAD23b fusions to GFP in intact seedlings.
(C) Immunoblot analyses of transgenic rad23b-1 plants stably expressing 35S-GFP-RAD23b. Crude seedling extracts were subjected to SDS-PAGE and immunoblot analyses with anti-RAD23b and anti-GFP antibodies. Filled arrowheads indicate GFP-RAD23b, open arrowheads indicate RAD23b, and asterisks indicate free GFP. GFP represents wild-type plants expressing GFP alone. Equal protein loads were verified by immunoblot analysis with antibodies against PBA1.
(D) Rescue of the rad23b-1 phyllotaxy phenotype by the 35S-GFP-RAD23b transgene. Fourteen-day-old plants of the indicated genotypes are shown.
(E) Confocal fluorescence microscopy of root tip cells from 2-week-old 35S-GFP-RAD23b rad23b-1 and GFP plants. Arrowheads identify nuclei. Bars = 5 μ m.
(F) Subcellular localization of GFP-RAD23a-d transiently expressed by the 35S promoter in protoplasts. Protoplasts were prepared from 2-week-old leaves and imaged by confocal fluorescence microscopy 24 h after transfection with plasmids encoding GFP alone or fused to each RAD23 isoform. Green, GFP; red, chloroplasts. Arrowheads identify nuclei. Bars = 5 μ m.
The Arabidopsis UBL and UBA domains are conserved relative to their nonplant counterparts. For example, the UBL domains from the four Arabidopsis RAD23 proteins are nearly collinear and >46 and >41% similar in amino acid sequence compared with Ub and the UBL domain of yeast RAD23, respectively (Figure 1B), strongly suggesting that this domain retains the three-dimensional structure characteristic of Ub-folds (Vijay-Kumar et al., 1987). Amino acid sequence alignments of the UBL domains with Ub also revealed that several of the Lys residues that participate in poly-Ub chain formation (Saracco et al., 2009; Xu et al., 2009) are positionally conserved in Arabidopsis RAD23, DSK2, DDI1, NUB1, and UBL1, implying that these UBL domains could be substrates for ubiquitylation. These Lys residues include Lys-6, Lys-27, and Lys-48 in all four RAD23 isoforms; Lys-27 in DSK2a, DSK2b, DDI1, and UBL1; and Lys-48 in NUB1 (Figure 1B). Additionally, each of the respective UBL domains retained most features of the hydrophobic patch (Leu-8, Ile-44, and Val-70) necessary for the binding of Ub to various Ub receptors, including RPN10 (Beal et al., 1996). Only DSK2a, DSK2b, DDI1, and NUB1 have substantial variations in this patch at residue 8, which may affect their interaction with these receptors (Figure 1B).
Whereas Arabidopsis DSK2a, DSK2b, DDI1, and UBL1 each contain a single UBA domain, two are present in RAD23a-d and NUB1 (Figures 1A and 1C). This ∼ 40–amino acid domain consists of a three α -helix bundle that houses a hydrophobic patch lined with the signature Met-Gly-Phe residues (MGF loop), which is known from yeast RAD23 studies to interact with Ub (Ohno et al., 2005). A comparable MGF loop can be found in the Arabidopsis UBA domains, implying that these proteins also bind Ub (Figure 1C). In addition, the Arabidopsis RAD23 and DSK2 proteins contain one or more of the ∼ 44–amino acid stress-inducible 1 domain, which has been implicated in the binding of yeast RAD23 to the RAD4 subunit in the NEF2 DNA repair complex (Ortolan et al., 2004). Like other DDI1 proteins, the Arabidopsis counterpart has an internal retroviral aspartyl protease (RVP) domain that could impart a novel protease activity to the protein (Gabriely et al., 2008).
The four Arabidopsis RAD23 isoforms clustered phylogenetically into two closely related pairs: RAD23a and b (81.2%/86.1% amino acid identity/similarity) and RAD23c and d (62.3%/70.4%), suggesting that they arose from two separate duplication events (see Supplemental Figure 2 online). In support of this, the RAD23a and b loci are located within duplication blocks on chromosome 1, but are on opposite sides of the centromere (http://bioinfo.genopole-toulouse.prd.fr/PGCViewer). Subsequent searches identified RAD23 orthologs in a variety of other plant genomes, including those for rice (four loci), maize (five loci), grape (Vitis vinifera; two loci), poplar (Populus trichocarpa; six loci), and carrot (two loci; also found in Sturm and Lienhard, 1998). Phylogenetic analysis of the collection revealed two separate clades in higher plants related to Arabidopsis RAD23a/b and RAD23c/d (see Supplemental Figure 2 online). The separation of these clades from the two isoforms in both Physcomitrella patens and Selaginella moellendorffii, suggests that they arose after the evolution of seed plants but before the monocot/eudicot split. Similarly, we identified likely relatives of DSK2, DDI1, and NUB1 in a variety of other higher plants, strongly suggesting that these UBL/UBA proteins are also universal within the plant kingdom. Our failure to find obvious orthologs in other plant species combined with its lack of transcriptional support implies that the Arabidopsis UBL1 locus is a pseudogene.
Expression and Localization of Arabidopsis RAD23a-d
To better understand the functions of the UBL/UBA proteins within the plant UPS, we focused our studies on the Arabidopsis RAD23a-d family. EST numbers (www.Arabidopsis.org) suggested that RAD23c and RAD23d are expressed approximately threefold higher than RAD23a and RAD23b (Figure 2A). Transcript abundance among different tissues as determined by the Genevestigator DNA microarray data set (https://www.genevestigator.ethz.ch) (Hruz et al., 2008) supported this conclusion and demonstrated that RAD23a-d are widely expressed in most, if not all, tissues spanning the Arabidopsis life cycle (Figure 2A).
We generated antibodies against recombinant RAD23b and RAD23c to confirm the accumulation of the proteins and to examine their subcellular localization. Both antibodies recognize recombinant RAD23b and RAD23c proteins equally well (see Supplemental Figure 3 online) but do not recognize DSK2a/b or DDI1 based on immunoblot analyses of corresponding mutants. The antibodies also detected a quartet of proteins in crude seedling extracts subjected to SDS-PAGE (Figure 2B). Their apparent molecular masses (61 to 55 kD) were substantially larger than those predicted from the RAD23a-d coding regions (44 to 38 kD). Fortunately, with the aid of various rad23 mutant combinations (see below), we could assign each band to a specific RAD23 isoform, thus confirming that both antibodies can detect all members of the family (see below).
As a first attempt to define the subcellular location of RAD23a-d, we probed nuclear and cytoplasmic fractions prepared from young seedlings by Percoll gradient centrifugation (Figure 2B). Separation of the compartments was supported by immunoblot analyses with antibodies against the nuclear envelope protein WPP domain-interacting protein 1 (WIP1) (Xu et al., 2007), histone H3, and the soluble, cytoplasmic plant UBX domain-containing protein 1 (PUX1) (Saracco et al., 2007). Whereas several subunits of the 26S proteasome were found in both fractions (RPN1, RPN5, and PBA1; Yang et al., 2004) in agreement with the presence of the 26S proteasome in nuclear and cytoplasmic compartments, we detected little RAD23a-d and its possible binding partner RPN10 in the nuclear fraction (Figure 2B). Similarly low nuclear levels of DSK2a/b and DDI1 were observed using antibodies prepared against the Arabidopsis proteins (Figure 2B). (Lower exposures of the blot detected two DSK2 species, which likely represent the DSK2a and DSK2b isoforms [see Figure 4A].) One possible conclusion was that RAD23a-d, DSK2a, DDI1, and RPN10 are not abundant in the nucleus despite their proposed nuclear functions (Funakoshi et al., 1999; Lambertson et al., 1999; Bertolaet et al., 2001). On the other hand, given the transient nature with which RPN10 and these UBL/UBA proteins associate with the 26S proteasome (Finley, 2009), it was also possible that the nuclei became leaky during isolation, thus allowing these factors to leach out. To test for the latter possibility, we examined the fractionation of SUMO and its conjugates, both of which are present at high levels in the nucleus (Kurepa et al., 2003; Saracco et al., 2007). Whereas most SUMO conjugates were found in the nuclear fraction, almost all of the free SUMO was in the cytoplasmic fraction (Figure 2B), a result consistent with the nuclei becoming more porous during their isolation.
Figure 4.
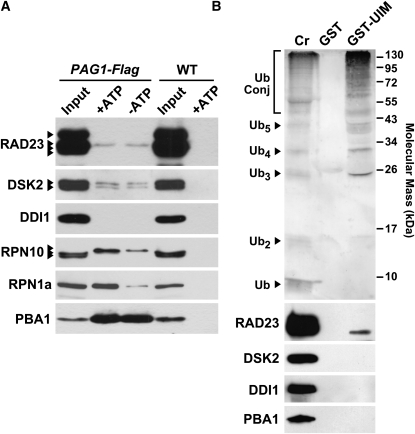
Arabidopsis RAD23 Proteins Interact with the 26S Proteasome
(A) Copurification of RAD23 with the 26S proteasome. The PAG1 subunit of the Arabidopsis CP was replaced by a Flag epitope-tagged version and used to enrich for the 26S proteasome from crude seedling extracts with anti-Flag antibodies. Where indicated, ATP was included to preserve the association of the CP and RP subcomplexes. The immunoprecipitates from PAG1-Flag pag1-1 and wild-type plants were subjected to SDS-PAGE and immunoblot analyses with antibodies against RAD23b, DSK2a, and DDI1 and the RPN10, RPN1a, and PBA1 subunits of the 26S proteasome.
(B) Co-IP of Ub conjugates and RAD23 from wild-type seedlings using the UIM-containing region from Arabidopsis RPN10. Crude extracts (Cr) were incubated with glutathione beads decorated with GST or GST fused to the UIMs. The precipitated fractions were analyzed by SDS-PAGE and immunoblot analyses with antibodies against Ub, RAD23b, DSK2a, DDI1, and PBA1. The migration positions of Ub, free poly-Ub chains, and Ub conjugates are indicated.
As an alternative, we used confocal fluorescence microscopy to determine the subcellular localization of RAD23a-d fused to the C terminus of green fluorescent protein (GFP). For our first approach, we stably expressed the transgene under the control of the cauliflower mosaic virus 35S promoter in homozygous rad23b-1 plants (see below). A GFP-RAD23b fusion protein of the correct size was detected along with a minor amount of free GFP, which likely represented a breakdown product generated either in vivo or in vitro (Figure 2C). Importantly, phenotypic analyses of multiple transgenic lines revealed that the 35S-GFP-RAD23b transgene could rescue most, if not all, of the developmental defects caused by the rad23b-1 mutation (Figure 2D; see below), strongly suggesting that the RAD23b moiety remained functional with the appended GFP. Microscopy analysis of 35S-GFP-RAD23b rad23b-1 root tip cells detected strong GFP fluorescence in the nucleus with weaker fluorescence from the cytoplasm (Figure 2E), which was subsequently confirmed by colocalization of the GFP signal with signal from the DNA stain Vybrant DyeCycle Orange (see Supplemental Figure 4B online). Compared with free GFP, which can distribute between both compartments, the signals from GFP-RAD23b implied that RAD23b is nuclear enriched. Using the GFP-RAD23b marker, we then tested whether nuclei do indeed lyse during enrichment by Percoll gradient centrifugation. Even though confocal microscopy showed substantial amounts of GFP-RAD23b protein in nuclei in planta, we detected very little in the isolated nuclei (see Supplemental Figure 4C online). Consequently, we recommend caution when using cell fractionation to determine nuclear localization.
To test if the other three RAD23 isoforms were similarly nuclear enriched, we transiently expressed each of the RAD23a-d proteins as a C-terminal fusion to GFP in protoplasts and examined their localization by confocal fluorescence microscopy. All four isoforms displayed a similar strong nuclear signal when the 35S promoter was used to drive expression (Figure 2F), a distribution similar to that reported for a RPN10-red fluorescent protein fusion (Yang et al., 2007). We noticed that compared with the stably transformed lines, the cytoplasmic fluorescence from the 35S-GFP-RAD23 transgenes in protoplasts was more obvious. Given the possibility that this cytoplasmic signal was artifactually generated by 35S-driven overexpression, we also examined the distribution of GFP-RAD23 expressed by the native RAD23a-d promoters. For each reporter, the 2-kb region upstream of the translation initiation codon was fused to the GFP-RAD23 cDNA and then introduced into protoplasts. While the GFP signals from these native-promoter fusions were substantially lower than the 35S-promoter fusions, we again observed a strong nuclear fluorescence combined with a weaker cytoplasmic fluorescence for each of the GFP-RAD23 proteins (see Supplemental Figure 4A online).
Arabidopsis RAD23 Proteins Interact with Multiple Components of the UPS
Given their proposed shuttle function, Arabidopsis RAD23 proteins should interact with a number of factors either directly or indirectly through ubiquitylation. As a first approach, we screened an Arabidopsis cDNA expression library by yeast two-hybrid analysis using full-length RAD23b as the bait. Of the 45 prey cDNAs identified in this screen, all but one expressed Ub, thus affirming the avidity of RAD23 proteins for Ub (see Supplemental Table 1 online). The list included protein products from the poly-UBQ genes UBQ10 and UBQ11, the Ub extension protein genes UBQ1, UBQ2, and UBQ5, and the UBQ7 gene that expresses a fusion of Ub with Related-to-Ub-1 (Callis et al., 1995). The only non-Ub sequence in this collection was the UPS target indole-3-acetic acid–induced protein 16 (Dreher and Callis, 2007).
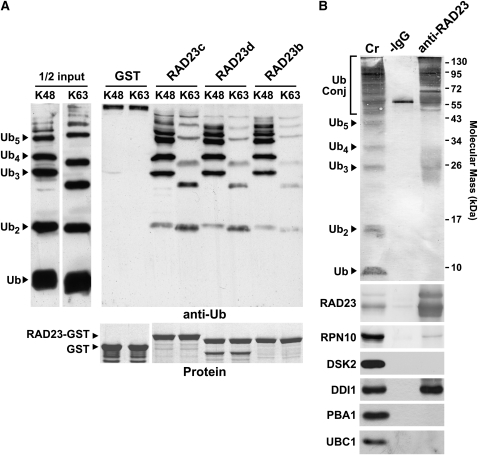
We defined the specificity of the Ub interaction by binding studies with mixtures containing the Ub monomer and poly-Ub chains assembled in vitro via Lys-48 or Lys-63 linkages. Here, purified glutathione S-transferase (GST)-RAD23 fusions were incubated with the Ub mixtures; the binding complexes were then isolated via the GST moiety by in vitro pull down with glutathione beads and analyzed by SDS-PAGE and immunoblot analysis with anti-Ub antibodies. Compared with the input mixtures, RAD23b, c, and d had poor affinity for free Ub and Ub dimers and trimers but bound longer poly-Ub chains well (Figure 3A). Consistent with the preference of the 26S proteasome for Lys-48–linked Ub chains containing at least four Ub monomers (van Nocker et al., 1996a; Hartmann-Petersen and Gordon, 2004; Ravid and Hochstrasser, 2008), all three RAD23 proteins bound long Lys-48–linked Ub chains best.
Figure 3.
Arabidopsis RAD23s Preferentially Bind Lys-48–Linked Poly-Ub Chains and Ub-Conjugates.
(A) Poly-Ub chain binding in vitro. A mixture of Ub and poly-Ub chains linked via Lys-48 (K48) or Lys-63 (K63) was incubated with recombinant GST or GST fused to RAD23b, RAD23c, and RAD23d and precipitated with glutathione beads. The Ub moieties were resolved by SDS-PAGE and detected by immunoblot analysis with anti-Ub antibodies. Left panel, Ub mixtures added to the reactions. Right top panel, poly-Ub chains recovered with the RAD23 proteins. Right bottom panel, amount of GST or GST-RAD23 used in each pull-down assay as shown by staining the gel with Coomassie blue. Arrowheads show the migration of Ub and Ub polymers of various lengths and the RAD23-GST and GST proteins.
(B) Co-IP of RAD23 binding proteins from wild-type Arabidopsis seedlings using anti-RAD23b antibodies. Crude extracts (Cr) from 1-week-old seedlings were incubated with Protein A beads alone or decorated with anti-RAD23b immunoglobulins. The precipitated fractions were analyzed by SDS-PAGE and immunoblot analyses with antibodies against Ub, RAD23b, RPN10, DSK2a, DDI1, and PBA1. Anti-UBC1 antibodies were used as a control for nonspecific binding. The positions of the Ub monomer, free poly-Ub chains, and Ub-protein conjugates are indicated.
To identify interactors in vivo, we tested several likely candidates by coimmunoprecipitation (co-IP) with RAD23a-d from crude seedling extracts. Anti-RAD23b antibodies substantially enriched for high molecular mass Ub-protein conjugates assembled in planta but not free Ub or the Ub dimer (Figure 3B). In addition to recovering the four RAD23 proteins, the antibodies isolated RPN10 and DDI1, but not DSK2a/b (Figure 3B). A strong enrichment for DDI1 raised the possibility that it heterodimerizes with RAD23s. Similar to observations using yeast (Lambertson et al., 1999), only a small fraction of RPN10 was isolated with RAD23, suggesting that the interaction of RAD23a-d with this Ub receptor is weak and/or transient. Moreover, the absence of the 26S proteasome core protease (CP) subunit PBA1 in the co-IPs implied that most of the RPN10 that associated with RAD23a-d was the free form and not that assembled into the 26S proteasome (Figure 3B).
Working under the assumption that Arabidopsis RAD23s and other UBL/UBA proteins associate with the 26S proteasome (Finley, 2009), we attempted to more carefully detect this interaction. Here, we used a recently developed affinity method to rapidly purify Arabidopsis 26S proteasomes engineered with an epitope-tag (A.J. Book and R.D. Vierstra, unpublished data). The tagged proteasomes were created by complementing the lethal pag1-1 mutant with a transgene expressing the corresponding PAG1 CP subunit bearing a C-terminal Flag epitope (PAG1-Flag). The 26S proteasomes were then quickly purified from crude seedling extracts by binding to agarose beads coated with anti-Flag antibodies. ATP was included during the purification to maintain association of the 26S proteasome RP with the CP (Yang et al., 2004). As shown in Figure 4A, we could easily isolate intact 26S proteasomes from PAG1-Flag pag1-1 but not wild-type plants using anti-Flag beads, as judged by the enrichment for the CP subunit PBA1 and the RP subunits RPN1a and RPN10. In agreement with the need for ATP to maintain RP/CP interactions, lower levels of RPN10 and RPN1a were detected when ATP was omitted during purification. When we tested for the presence of RAD23, DSK2, and DDI1, low amounts of DSK2a/b and at least one isoform of RAD23, but not DDI1, was found associated with these purified 26S proteasome preparations (Figure 4A). Unlike RPN1 and RPN10, the interactions of RAD23 and DDI1 with the proteasome were less dependent on ATP, suggesting that some also binds to the CP.
As RAD23 can interact with free RPN10 and with 26S proteasomes containing RPN10, we reasoned that RAD23 binds to the proteasome complex via this receptor, likely using the UIMs of RPN10 for UBL recognition. To directly test this possibility, we fused the region containing the three UIMs of RPN10 (105 amino acids) to GST and examined its ability to bind RAD23, DSK2, and DDI1 from Arabidopsis crude seedling extracts by pull-down assays with glutathione beads. As shown in Figure 4B, GST-UIM interacted well with Ub-protein conjugates present in the seedling extracts and not with the Ub monomer, in agreement with the binding preference of Arabidopsis RPN10 for Ub polymers (van Nocker et al., 1996a; Fu et al., 1998). A small amount of RAD23, but not DSK2 nor DDI1, was also detected by immunoblot analyses of the precipitates, suggesting that RPN10 only binds RAD23 at appreciable levels. Coupled with the analysis of affinity-purified proteasomes, we conclude that Arabidopsis RAD23s associate with the 26S proteasome, but at substoichiometric levels.
Arabidopsis Mutants Affecting the Expression of RAD23a-d
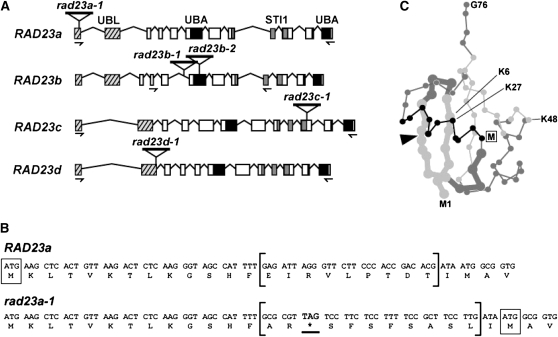
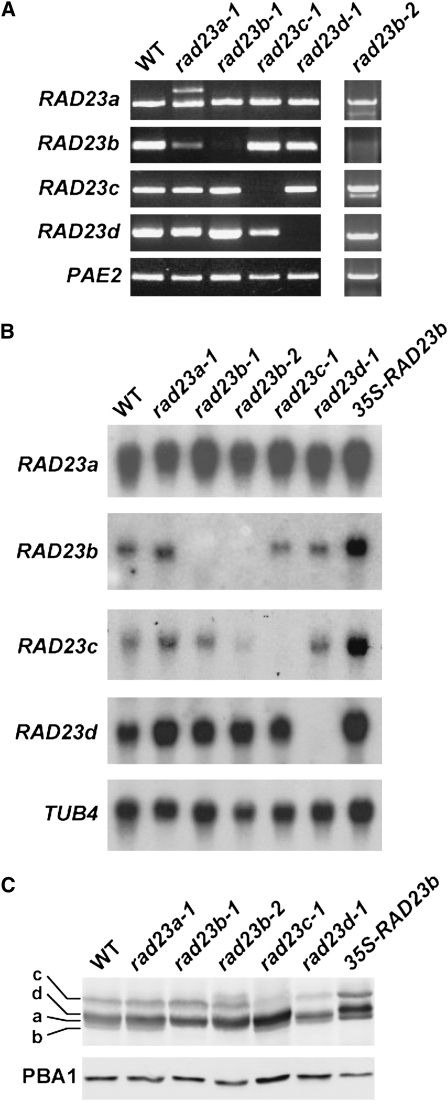
A genetic analysis of the Arabidopsis RAD23 family was performed by assembling a collection of homozygous T-DNA insertion mutants in the Col-0 background that disrupted the transcribed regions of each gene (Figure 5A). The rad23b-1, rad23b-2, rad23c-1, and rad23d-1 alleles blocked accumulation of the full-length transcripts for RAD23b, c, and d (1.1, 1.25, and 1.1 kb, respectively), as determined by RT-PCR with gene-specific primers and by RNA gel blot analyses (Figures 6A and 6B), indicating that these mutations are likely null alleles. Interestingly, none of the mutations altered the mRNA levels for the other RAD23 genes, suggesting that the RAD23 proteins do not autoregulate their expression.
Figure 5.
Organization of the Arabidopsis RAD23 Genes and Descriptions of the rad23 Mutations.
(A) Diagrams of the RAD23a, RAD23b, RAD23c, and RAD23d genes. Boxes and lines denote protein coding regions and introns, respectively. Black boxes, UBA domains; cross-hatched boxes, UBL domains; gray boxes, stress-inducible 1 (STI1) domains. The locations of the various T-DNA insertions are shown. The arrows locate the positions of the primers used for the RT-PCR analyses in Figure 6A.
(B) Effect of the rad23a-1 T-DNA insertion on the RAD23a open reading frame. The nucleotide sequence of rad23a-1 downstream of the ATG initiator codon is aligned with the wild-type RAD23a sequence. The boxes locate the predicted N-terminal Met of each protein. The brackets demarcate the aberrant mRNA sequence generated by the rad23a-1 insertion. The introduced nonsense codon (asterisk) is underlined.
(C) Ball-and-stick three-dimensional structure of Ub highlighting the region predicted to be missing from the rad23a-1 protein. β -Strands are in light gray, random coils are in dark gray, and the α helix is in black. The arrowhead indicates the position of the T-DNA insertion within the coding sequence for rad23a-1 gene. The thicker lines denote the deleted sequence predicted for the rad23a-1 UBL with the box identifying the expected N-terminal Met (M), Met-24. The positions of the Lys residues (K) conserved between Ub and RAD23a-d and the N-terminal Met (M1) and the C-terminal Gly (G76) in Ub are indicated.
Figure 6.
Molecular and Biochemical Descriptions of the Arabidopsis rad23 Mutants.
(A) RT-PCR analyses of the rad23a-1, rad23b-1, rad23b-2, rad23c-1, and rad23d-1 mutants. Total RNA isolated from wild-type and mutant seedlings was subjected to RT-PCR using the primers shown in Figure 5A. A primer pair specific to PAE2 was used as an internal control.
(B) RNA gel blot analyses of total RNA from 1-week-old wild-type, rad23 mutant, and 35S-RAD23b seedlings using probes for RAD23a, RAD23b, RAD23c, and RAD23d. Equal loading of the blot was confirmed by probing with β -tubulin4 (TUB4).
(C) Immunoblot analysis of crude extracts from wild-type, rad23 mutant, and 35S-RAD23b seedlings with anti-RAD23b antibodies. The SDS-PAGE migration positions of the four RAD23 isoforms are indicated. Equal protein loads were confirmed by probing with anti-PBA1 antibodies.
The loss of individual RAD23b-d proteins was confirmed by immunoblot analysis of the crude extracts resolved by SDS-PAGE. The absence of a specific protein species in the single mutants (and later in the double mutants; see below) then allowed us to assign each species to one of the four isoforms with a rank order (high to lower apparent molecular mass) of RAD23c (61 kD), RAD23d (57 kD), RAD23a (56 kD), and RAD23b (55 kD) (Figure 6C). The relative abundances of each as determined with anti-RAD23b antibodies supported the higher expression of RAD23c and RAD23d relative to RAD23a and RAD23b as estimated by EST count (Figure 2A).
The rad23a-1 allele was an unusual T-DNA insertion mutant. Although a large piece of T-DNA interrupts the RAD23a coding region, as determined by genomic sequencing together with cosegregation of the allele with kanamycin resistance conferred by the neomycin phosphotransferase gene encoded within the T-DNA (Figure 5A), a near normal level of the apparently full-length mRNA could be detected by RT-PCR and RNA gel blot analysis of homozygous rad23a-1 plants (Figures 6A and 6B). However, subsequent sequencing of several rad23a-1 transcripts generated by RT-PCR revealed that they all contained the same short sequence near the expected T-DNA insertion site, which was likely left behind after mis-splicing of the T-DNA from the initial transcript. Instead of the 27 nucleotides encoding amino acids 14 to 22 in the wild-type RAD23a mRNA, the rad23a-1 mRNA had a unique sequence of 33 nucleotides predicted to contain an in-frame stop codon (Figure 5B).
Translation of the rad23a-1 mRNA from the normal start codon would generate a truncated RAD23a protein that includes only the first 13 amino acids of the UBL domain followed by Ala-Arg. If translation commenced at the next ATG codon downstream of the insertion (codon 24), a truncated protein missing the first 23 residues of the UBL domain would be generated. Based on the expectation that the UBL domain assembles into a Ub fold, this N-terminal deletion would remove the first two β strands and the region connecting them to helix- α 1, thus profoundly disrupting the three-dimensional structure of the UBL domain (Figure 5C). Accumulation of this predicted truncation could not be unequivocally confirmed in homozygous rad23a-1 plants, likely due to SDS-PAGE migration of this truncated product near the other three RAD23 isoforms (Figure 6C). However, immunoblot analysis of the triple mutant combination rad23a-1 rad23b-1 rad23d-1 detected a low amount of the likely rad23a-1 product, strongly suggesting that the mutant protein accumulates with its translation beginning at Met-24 (see below).
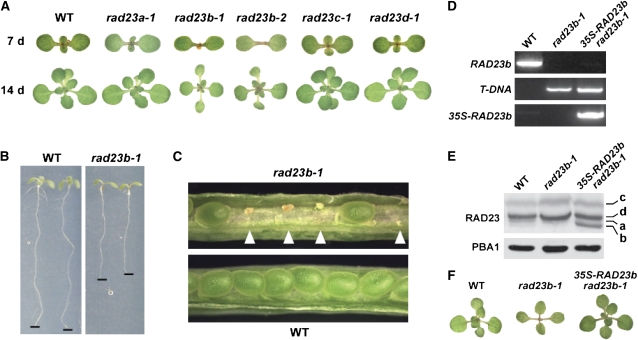
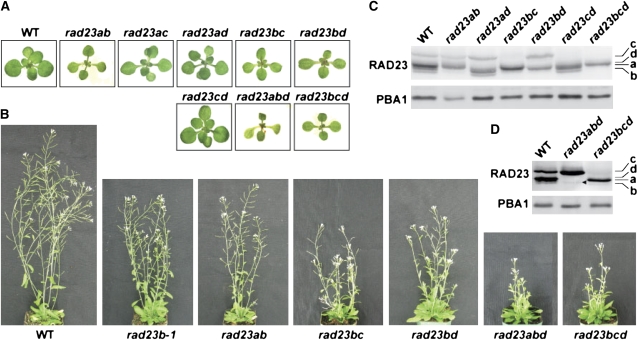
rad23b Mutants Have Pleiotropic Developmental Defects
We could easily generate homozygous lines for each of the rad23 mutants, indicating that none of these loci are essential in Arabidopsis. The rad23a-1, rad23c-1, and rad23d-1 plants were indistinguishable from the wild type, with normal fecundity when grown under a variety of conditions, likely indicating that the encoded proteins have redundant functions (Figure 7A; see Supplemental Figure 5 online). Only the two rad23b alleles caused abnormal development. When grown under a long-day photoperiod (LD; 16 h light/8 h dark), homozygous rad23b-1 and rad23b-2 seedlings grew more slowly than the wild type and displayed abnormal phyllotaxy as young seedlings (Figure 7A). Instead of the true leaves individually emerging at 120 ° angles relative to the prior leaf, the first two emerge simultaneously as a pair 90 ° relative to the cotyledons, thus generating a cruciform arrangement. The third/fourth and fifth/sixth leaves then emerged almost simultaneously as pairs at ∼ 45 ° and 135 ° offsets. Thereafter, each leaf emerged more sequentially. The edges of the older leaves frequently developed a mild serration common among leaf development mutants (e.g., Nikovics et al., 2006). The primary root of rad23b seedlings was shorter with fewer lateral roots (Figure 7B). As the rad23b-1 and rad23b-2 plants matured, they developed shorter inflorescences and smaller siliques and had reduced seed set, with unfertilized ovules interspersed among seeds of normal appearance (Figure 7C). Several mutants affecting individual 26S proteasome subunits, including RPN10, produce enlarged organs (e.g., cotyledons, leaves, flowers, and seeds) due to increases in cell size (Kurepa et al., 2009; Sonoda et al., 2009); a similar effect was not seen for rad23b plants. Furthermore, unlike the rpn10-1 mutant, which stabilizes Ub-protein conjugates (Smalle et al., 2003), none of the rad23 mutations, including the two rad23b alleles, appeared to affect Ub metabolism. The levels of free Ub, Ub polymers, and Ub-protein conjugates for all the single homozygous lines were indistinguishable from the wild type (see Supplemental Figure 6 online).
Figure 7.
Phenotypic Analysis of Arabidopsis rad23b Mutants.
(A) rad23b mutants have altered leaf phyllotaxy. Wild-type and single homozygous rad23 mutant seedlings were grown in LD for 7 (top) or 14 d (bottom).
(B) Homozygous rad23b-1 roots grow slower and have fewer lateral roots. Seedlings were grown for 1 week on vertical hard-agar plates under LD conditions.
(C) rad23b plants are semisterile. Pictured are siliques from self-fertilized wild-type and homozygous rad23b-1 plants. Aborted ovules are indicated by white arrowheads.
(D) to (F) Rescue of the rad23b phenotype with a 35S-RAD23b transgene.
(D) Genotyping of a rad23b-1 complementation line. DNA was isolated from wild-type, rad23b-1, and 35S-RAD23b rad23b-1 seedlings and PCR amplified with primers specific for RAD23b, the T-DNA in rad23b-1, or the 35S-RAD23b transgene.
(E) Immunoblot analysis with anti-RAD23b antibodies showing the reintroduction of the RAD23b protein in 35S-RAD23b rad23b-1 seedlings. Equal protein loads were confirmed by probing with anti-PBA1 antibodies.
(F) Phenotypic rescue of the rad23b-1 mutant. Pictured are 14-d-old plants showing the restoration of normal seedling growth and leaf phyllotaxy for a 35S-RAD23b rad23b-1 line.
As proof that the rad23b-associated phenotypes were directly caused by inactivation of the RAD23b gene, we successfully rescued rad23b-1 plants with a transgene expressing the RAD23b cDNA under control of the 35S promoter (Figure 7D). Several homozygous 35S-RAD23b rad23b-1 lines that expressed high levels of the RAD23b protein restored the wild-type phenotype to rad23b-1 plants, including normal phyllotaxy, root growth, inflorescence development, and seed viability (Figures 7E and 7F; data not shown). We also introduced the 35S-RAD23b transgene into wild-type plants and obtained lines that expressed high levels of the RAD23b mRNA and its corresponding protein (Figures 6B and 6C). Under normal growth conditions, the 35S-RAD23b plants were phenotypically indistinguishable from the wild type, indicating that excess RAD23b protein is not detrimental to Arabidopsis development. The only noticeable change was an increase in the RAD23c transcript and protein in the 35S-RAD23b plants (Figures 6B and 6C).
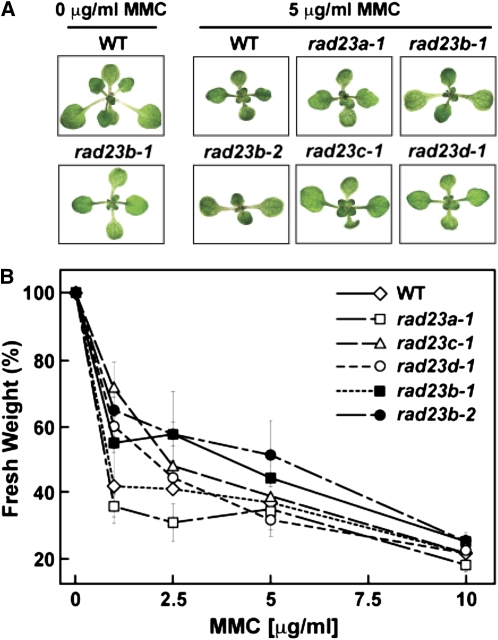
To explore the rad23b phenotypes in depth, we examined the response of homozygous rad23b-1 seedlings to a variety of suboptimal growth conditions known to perturb the UPS in general or yeast rad23 and Arabidopsis rpn10-1 mutants in particular. These included exposure to various hormones, such as ABA, that require RPN10 and UPS for signaling (Smalle et al., 2003; Vierstra, 2009), the amino acid analogs canavanine and p-fluorophenylalanine and the glycosylation inhibitor tunicamycin, which induce the synthesis of abnormal proteins requiring the UPS and possibly RAD23 for removal (Yan et al., 2000; Martinez and Chrispeels, 2003), the proteasome inhibitor MG132 (Yang et al., 2004), and several DNA-damaging agents, such as UV light, methyl methanesulfonate, bleomycin sulfate, and mitomycin C (MMC), that accentuate defects in the UPS, RPN10, and DNA repair (Smalle et al., 2003; Inagaki et al., 2006; Ramirez-Parra and Gutierrez, 2007). rad23b-1 seedlings failed to display a hyper- or hyposensitivity to most of these treatments compared with the wild type. The only notable exception was MMC, a DNA cross-linking agent that mitotically arrests mammalian cells (Cui et al., 1999) and preferentially inhibits the growth of Arabidopsis rpn10-1 seedlings (Smalle et al., 2003). Growth of rad23b-1 and rad23b-2 seedlings (as determined by fresh weight accumulation) was significantly more resistant to intermediate concentrations of MMC compared with the wild type and the rad23a-1, rad23c-1, and rad23d-1 seedlings (Figure 8B). Interestingly, continuous exposure of wild-type, rad23a-1, rad23c-1, and rad23d-1 seedlings to 5.0 μ g/mL MMC generated the same cruciform leaf organization as the rad23b mutants (Figure 8A). The effect of MMC implies that loss of RAD23b generates the phyllotaxy defect by impairing cell division.
Figure 8.
Exposure to MMC Induces the rad23b Phyllotaxy Defect in Wild-Type and rad23a, c, and d Seedlings.
(A) Two-week-old seedlings grown under LD in the absence or presence of 5 μ g/mL MMC.
(B) Fresh weight ( ±sd) of seedlings grown on increasing concentrations of MMC and normalized to their respective growth without MMC. Number of seedlings analyzed per line was 25 (wild type), 21 (rad23a-1), 17 (rad23b-1), 20 (rad23b-2), 20 (rad23c-1), and 20 (rad23d-1).
In addition to the biochemical interaction between RAD23 and RPN10, the corresponding yeast genes also show a synergistic genetic interaction, with the double mutants displaying pleiotropic growth defects, such as G2/M phase delay, increased sensitivity to cold and the amino acid analog canavanine, and a stabilization of Ub-protein conjugates (Lambertson et al., 1999). To test for a similar genetic relationship in Arabidopsis, we combined the rad23b-1 with rpn10-1 mutations. The rpn10-1 allele was created by exon-trap mutagenesis and expresses a fusion of neomycin phosphotransferase with an RPN10 truncation containing just the N-terminal von Willebrand factor A domain that promotes its association with the RP of the 26S proteasome, but is missing the C-terminal UIMs that interact with Ub and UBL/UBA proteins (Figure 4B; Fu et al., 1998; Smalle et al., 2003). rpn10-1 plants have pleiotropic defects, including reduced seed germination, slower growth rate, semisterility, and a hypersensitivity to various stresses caused, in part, by a stabilization of the ABA signaling protein ABA-INSENSITIVE5 (ABI5) (Smalle et al., 2003).
Surprisingly, introgression of the rad23b-1 mutation accentuated the developmental abnormalities of the rpn10-1 background and elicited some new defects, even in the heterozygous state (rpn10-1/rpn10-1 rad23b-1/+). These new phenotypes included unusual pin-like structures emerging from the axillary meristems in the rosette, which partially resembled those seen for the pinhead mutants (Lynn et al., 1999; Figure 9B). Similar pinhead-type organs occasionally appeared at the presumptive branch points of the primary inflorescence, at the tips of the inflorescence stems, and on the abaxial face of the leaf, which could be seen as a trichome-covered projection separated from the midvein and pointing out toward the leaf tip (Figures 9C to 9E). The rpn10-1/rpn10-1 rad23b-1/+ plants also developed aerial rosettes and abnormal flowers often containing extra stamens and had drastically reduced fertility (Figure 9A; data not shown). Double homozygous rad23b-1 rpn10-1 plants were even more compromised. Early on, they produced small and highly lanceolate cotyledons and leaves (Figures 9F to 9H) and then after several months in continuous, long-day, or short-day photoperiods, they generated only a small dense clump of poorly organized, lanceolate leaves with no floral structures. Given that some of the rpn10-1 phenotypes in young seedlings are caused by a dramatic stabilization of ABI5 (Smalle et al., 2003), it was possible that loss of RAD23b or the other isoforms might similarly stabilize this ABA regulator. However, none of the single rad23a-d mutants had a significant effect on ABI5 protein levels detected immunologically either before or after treating young seedlings with high concentrations of ABA (50 μ M).
Figure 9.
The rad23b-1 Mutant Acts Synergistically with the rpn10-1 Mutant.
(A) to (E) Phenotypes of Arabidopsis plants homozygous for rpn10-1 and heterozygous for rad23b-1 when grown under a short-day photoperiod (8 h light/16 h dark). Bars = 2 mm.
(A) Ten-week-old plant showing a disorganized rosette, reduced inflorescence branches, and secondary rosettes (arrowheads) on the inflorescence stem.
(B) Six-week-old rosette with unusual pin-like organs emerging at or near the axillary meristems. Inset: 2-week old seedling displaying the rpn10-1 phenotype (Smalle et al., 2003).
(C) An inflorescence terminating in a pin-like structure.
(D) Pin-like structures emerging at or near the axillary meristems on the inflorescence stem.
(E) A pin-like structure (arrowhead) covered in trichomes emerging from the midvein on the abaxial surface.
(F) and (G) Two-week-old seedling phenotype of double homozygous rpn10-1 rad23b-1 plants.
(F) Homozygous rpn10-1 seedling.
(G) Homozygous rad23b-1 seedling.
(H) Double homozygous rpn10-1 rad23b-1 seedling.
DDI1 Is Not Essential in Arabidopsis
The interaction of RAD23 proteins with DDI1 implied that some of the rad23b phenotypes could be induced indirectly by impaired DDI1-RAD23 heterodimer formation. To test this possibility, we examined a T-DNA mutant allele that abrogates expression of the single DDI1 gene. The insertion is within the 12th intron and blocks accumulation of the DDI1 transcript (as determined by RT-PCR) and the corresponding protein (as determined by immunoblot analysis with anti-DDI1 antibodies) (see Supplemental ). Homozygous ddi1-1 plants did not have elevated levels of Ub, poly-Ub chains, or Ub-protein conjugates, indicating that Ub metabolism was unaffected (see Supplemental Figure 7D online). We also failed to observe compensatory effects of the ddi1-1 and rad23a-d single mutants on RAD23a-d and DDI1 protein levels, suggesting that the corresponding genes are not coregulated (see Supplemental ). And finally, when grown under normal conditions, homozygous ddi1-1 plants resembled wild-type and not rad23b-1 seedlings, with no problems in phyllotaxy or fertility (see Supplemental Figure 7E online). Taken together, we conclude that DDI1 is not essential in Arabidopsis and that the rad23b phenotypes are not generated by the absence of the DDI1-RAD23b heterodimer.
Attempts at Higher-Order Mutant Combinations Indicate That the RAD23 Family Is Essential in Arabidopsis
To examine the global significance of the RAD23 family on Arabidopsis development, we attempted to generate all higher-order mutant combinations affecting the four RAD23 loci by appropriate crosses followed by selfing, with the combinations then verified by PCR genotyping and by immunoblot analysis (Figure 10C). The double mutants generated with the rad23a-1, rad23c-1, and rad23d-1 alleles were phenotypically normal as both young and mature plants, whereas all the double mutant combinations containing the rad23b-1 allele displayed slightly enhanced phyllotaxy, root growth, stature, and semisterility defects compared with the single rad23b mutants (Figures 7B and 10A; see Supplemental Figure 5 online; data not shown). The viability of the rad23ab and rad23cd lines indicates that the a/b and c/d subfamilies of plant RAD23s by themselves are not essential despite their predicted ancient origins. Like the single rad23 mutants, none of the double mutant combinations had altered pools of free Ub, Ub polymers, and Ub-protein conjugates compared with the wild type (see Supplemental Figure 6 online).
Figure 10.
Phenotypic and Biochemical Descriptions of the Combinatorial rad23 Mutants.
(A) Two-week-old seedlings grown under LD.
(B) Flowering plants grown for 8 weeks under LD.
(C) and (D) Immunoblot analyses of crude extracts prepared from 2-week-old seedlings with anti-RAD23b antibodies. The migration positions of the RAD23a-d isoforms are shown on the right. The arrowhead in (D) marks the position of the presumed rad23a-1 truncation. Immunoblot analysis with anti-PBA1 antibodies was included to verify equal loading.
We attempted to generate the ensemble of triple mutants and the quadruple rad23abcd mutant by first creating a quadruple heterozygous individual via a rad23ac and rad23bd double mutant cross and then allowing the AaBbCcDd plants to self-fertilize to create an F2 population potentially containing all of the rad23 mutant combinations (if viable). We then identified individuals with appropriate heterozygous/homozygous combinations that might simplify isolation of the triple and quadruple mutant combinations upon selfing.
Despite testing numerous progeny from several self-fertilized homozygous/heterozygous mutant combinations (e.g., AabbCcDD, AaBBCcdd, and AabbCcdd parents), we failed to find the rad23abc and rad23acd triple mutants or the quadruple mutant, implying that the complete absence of RAD23 proteins is lethal in Arabidopsis and that some triple mutants are nonviable as well. By contrast, we easily identified rad23bcd triple mutants from a selfed AAbbccDd parent as confirmed by immunoblot analysis, which showed that these plants accumulate only the RAD23a protein (Figures 10C and 10D). rad23bcd individuals appeared at the typical 1/4 frequency from the AAbbccDd parent (n = 30), indicating that both Abcd gametes are viable. Young rad23bcd plants displayed the same cruciform phyllotaxy defect as rad23b-1 seedlings but were even shorter and more disorganized as mature plants compared with the single and double mutants carrying these mutations (Figures 10A and 10B). They flowered and produced viable seed but generated numerous unfertilized/aborted ovules upon selfing, indicating that fertilization/embryogenesis was substantially compromised. Despite this growth defect, the levels of Ub and Ub conjugates in rad23bcd plants were indistinguishable from the wild type (see Supplemental Figure 6 online).
We also found the homozygous rad23abd triple mutant, but it appeared at a more rare frequency than expected, indicative of defects in gametogenesis and/or embryogenesis. Using an AabbCcdd parent in a self-cross, we found one homozygous rad23abd individual after screening 24 progeny; while using an aabbCCDd parent in a self-cross, we failed to find any homozygous rad23abd individuals after screening 143 progeny (instead of the expected 1/16 and 1/4 segregation ratios, respectively). The sole rad23abd plant produced numerous flowers with normal-looking pollen, but the resulting siliques contained mostly aborted seeds, implying that the mutant is nearly sterile. The few mature seeds that were produced ( ∼ 10 per plant) successfully germinated to produce seedlings of the expected rad23abd genotype. Like the rad23bcd plants, the rad23abd plants had a cruciform phyllotaxy as young seedlings and generated short and disorganized inflorescences as mature plants (Figures 10A and 10B). The rad23abd plants accumulated two RAD23 proteins: one was RAD23c at 61 kD, whereas the other faint species at ∼ 55 kD was slightly smaller in apparent molecular mass compared with the wild-type RAD23a protein and likely represented the rad23a-1 truncation (Figure 10D). Taken together, our genetic analyses indicate that Arabidopsis requires at least one functional copy of the RAD23.
DISCUSSION
UBL/UBA proteins are emerging as important regulators of Ub-protein conjugate functions/dynamics by helping stabilize and tether these conjugates to appropriate receptors that bind Ub and/or UBL domains. Within the UPS, the main receptors are the Ub/UBL binding subunits of the 26S proteasome (RPN1, RPN10, and RPN13) with additional receptors present within the ERAD subpathway that engage substrates before the 26S proteasome (Finley, 2009). UBL/UBA proteins also likely participate in extraproteasomal events, such as vesicular trafficking, DNA repair, and transcriptional regulation by docking ubiquitylated proteins with appropriate components (Mueller and Smerdon, 1996; Ortolan et al., 2004; Gabriely et al., 2008).
Plants express a collection of UBL/UBA proteins that includes orthologs of yeast RAD23, DSK2, and DDI1 and a possible ortholog of human NUB1. Their N-terminal UBL domains have strong sequence homology to Ub, including one or more positionally conserved Lys residues that could participate in poly-Ub chain formation and a hydrophobic patch that likely facilitates binding of the UBL domain to various Ub receptors, such as RPN10. Their more C-terminal UBA domain(s) presumably dock with Ub covalently attached to protein substrates and possibly with UBL domains either present in other UBL/UBA proteins or connected in the same polypeptide. We found that each UBL/UBA gene is actively transcribed in Arabidopsis with immunological evidence that all four RAD23 isoforms, both isoforms of DSK2, and DDI1 accumulate in planta. The expression of Arabidopsis NUB1 is supported by EST evidence, but the lack of ESTs for UBL1 combined with the absence of this gene in other plant species suggest that this locus is nonfunctional.
For the RAD23 family in particular, we demonstrated by several assays that this group binds Ub-protein conjugates with a strong preference for those bearing poly-Ub chains linked internally through Lys-48 compared with Lys-63. Since both Lys-48 and Lys-63 are common sites for Ub concatenation in planta (Maor et al., 2007; Saracco et al., 2009), such specificity implies that the RAD23 family is not involved in all aspects of poly-Ub dynamics in Arabidopsis. Given the importance of Lys-48 chains as a determinant for protein breakdown by the 26S proteasome (Hartmann-Petersen and Gordon, 2004; Ravid and Hochstrasser, 2008), a role of RAD23 proteins in proteolysis is expected. In support of this, our binding assays showed that one or more Arabidopsis RAD23 proteins associate with the 26S proteasome and with the UIMs of RPN10. At least part of the RPN10 bound to RAD23 appeared to be derived from the free pool of RPN10 and not that integrated within the 26S proteasome. An intriguing possibility is that this pool represents a free, trimeric Ub-protein conjugate/RPN10/RAD23 intermediate delivering ubiquitylated cargo to the protease.
In animals and yeast, various members of the UBL/UBA protein group have been shown to heterodimerize (Bertolaet et al., 2001; Rao and Sastry, 2002). While our co-IP experiments with RAD23 failed to find evidence that Arabidopsis RAD23 and DSK2 proteins associate, a strong contact between one or more RAD23s and DDI1 was detected. This association could occur via not yet identified heterodimerization domains, by binding of the UBL domain of one protein with the UBA domain(s) of the other, or indirectly via binding to a common poly-Ub chain either free or attached to a third protein. Conversely, we detected an association of DSK2 and RAD23s, but not DDI1, from the analysis of affinity-purified 26S proteasomes. Given that DSK2 does not appear to bind the UIMs of RPN10, its binding to the proteasome suggests that alternate receptor(s), such as RPN1 and RPN13, are involved. The binding data collectively imply that a complex web of interactions among the UBL/UBA proteins is possible, some of which could be substrate and/or route specific. Clearly, a deeper understanding of the binding interactions among the UBL/UBA proteins, their specificities for the various types of poly-Ub linkages, and more in-depth combinatorial genetic analyses of RAD23, DSK2, DDI1, and NUB1 loci are needed to sort out these roles.
Genetic analyses revealed that the RAD23 family, but not DDI1, is essential in Arabidopsis. Whereas the quadruple rad23abcd mutant and some of the triple mutants (rad23abc and rad23acd) appeared by segregation analyses to be nonviable, the single and double mutants were relatively healthy and capable of producing viable progeny. Coupled with their overlapping expression patterns, their colocalization in Arabidopsis cells, and the failure of the single and double mutants to noticeably affect Ub pool dynamics, it appears that the four isoforms have largely redundant functions. However, the analysis of two independent alleles combined with complementation indicate that RAD23b also has a nonoverlapping role. The rad23b phenotypes included a defect in leaf phyllotaxy, slower root growth, reduced apical dominance, and semisterility. A possible nonoverlapping function for RAD23c during fertilization is also indicated by segregation analyses. Whereas the rad23bcd triple mutant (which lacks RAD23c expression) produced numerous unfertilized ovules, the rad23abd triple mutant mostly generated aborted seeds. A special requirement for RAD23a during embryogenesis could be inferred from (1) our failure to find the rad23abc and rad23acd triple mutants, (2) our difficulty in finding the rad23abd triple mutant, and (3) the ease of finding a rad23bcd triple mutant. However, it remains possible that this segregation distortion is caused by the unique nature of the rad23a-1 allele.
It is unclear why elimination of RAD23b by itself induced the phyllotaxy, growth, and fertility defects. Neither its sequence, expression patterns, nor subcellular location point to a unique function. RAD23b is also not the most abundant isoform, implying that the defects are not caused by an insufficient amount of total RAD23. In light of the roles of yeast and mammalian RAD23 proteins in the cell cycle and the hyposensitivity of Arabidopsis rad23b plants to MMC, an obvious possibility is that RAD23b has a special role in the breakdown of one or more plant cell cycle checkpoint proteins. Stabilization of these checkpoint proteins could slow cell division, thereby compromising meristem function and gametogenesis in rad23b plants. One possible set of targets is the KRP family of cyclin-dependent kinase inhibitors. Their counterparts in yeast and mammalian cells (Sic1p and p27, respectively; Verma et al., 2004; Hara et al., 2005) are removed by a UBL/UBA-dependent process during the G1-to-S phase transition to allow cell division to proceed. In support of this, overexpression of KRP1 generates some of the same phenotypes seen for rad23b and higher-order mutant plants (dwarfed plants, serrated leaves, and infertility; De Veylder et al., 2001), while the KRP1 and KRP6/7 isoforms have been shown recently to be targets of the UPS, with impaired KRP6/7 breakdown leading to an arrest of cell division for the male gametes (Kim et al., 2008; Ren et al., 2008).
Like their yeast counterparts (Lambertson et al., 1999), the Arabidopsis rad23b-1 rpn10-1 double mutants display a synergistic developmental phenotype (Smalle et al., 2003). This synergy was even evident in rpn10-1/rpn10-1 rad23b-1/+ plants, indicating that in the complete absence of UIMs of RPN10, levels of RAD23b become limiting in the heterozygous rad23b-1 state. One striking aspect of this haplo-insufficiency was the terminally developed pinhead-like structures that routinely emerged from meristems and from the midvein of leaves. Because these pin structures arose from positions normally reserved for more fully developed organs, such as leaves, axillary branches, and flowers, their appearance supports the proposal that meristem function is substantially compromised under limiting RAD23b levels. The biochemical origin(s) of these defects is intriguing. It could reflect a reinforced problem in a common pathway, a strong compromise of separate RPN10- and RAD23-dependent pathways leading targets to the 26S proteasome, and/or a dual function of RAD23b (one facilitating interaction with RPN10 and other receptors in the 26S proteasome, and another representing RAD23b working in an extraproteasomal context such as DNA repair and/or ERAD). Certainly, a continued phenotypic analysis of double mutants combining rpn10-1 with mutants affecting all four RAD23 loci is needed to resolve this issue.
METHODS
Analysis of UBL/UBA Genes
UBL/UBA loci were identified in the Arabidopsis thaliana ecotype Col-0 genomic database (www.Arabidopsis.org) by BLAST (Swarbreck et al., 2008) using yeast Rad23p, Dsk2p, and DDI1p and human NUB1 amino acid sequences as queries. Intron/exon positions of RAD23b-d were determined by alignment of the genomic sequences with full-length cDNAs (GenBank accession numbers AY063103, AY113034, and AY081835, respectively) acquired from the ABRC. The RAD23a genomic region was annotated by comparison with that for RAD23b and confirmed by alignment with a full-length RAD23a cDNA sequence generated by RT-PCR.
Oryza sativa (cv Japonica) RAD23a-d genes were identified by a BLAST search of the rice EST database (http://tigrblast.tigr.org/tgi) with the AtRAD23b coding sequence. Full-length rice ESTs were used to search the rice genomic database to verify the exact number of RAD23 genes. RAD23 loci in other species were identified by iterative tBLASTn searches of The Institute for Genomic Research EST database, the National Center for Biotechnology Information, and the Joint Genome Institute (JGI) Selaginella moellendorffii, Physcomitrella patens, and Populus trichocarpa databases and supported by the analysis of ESTs. Predicted protein domains were confirmed by SMART (http://smart.embl-heidelberg.de) (Letunic et al., 2006).
Sequence Alignment and Phylogenetic Analysis
Amino acid sequences were aligned using the ClustalX Multiple Sequence Alignment Program v1.63b and MACBOXSHADE v2.15 (Institute of Animal Health). A phylogenetic tree of full-length protein sequences (aligned with ClustalX; see Supplemental Data Set 1 online) was generated in MEGA 4.0.1 (Tamura et al., 2007) by the neighbor-joining method, using the Poisson distance calculation, pairwise deletion of gaps, and the default assumptions that the substitution patterns among lineages and substitution rates among sites were homogeneous.
Antibody Production and Immunoblot Analyses
Anti-RAD23b antibodies were produced using recombinant full-length protein bearing a C-terminal 6His tag. The cDNA was amplified with bracketing NdeI and HindIII sites using the P1 and P2 primer pairs (see Supplemental Table 2 online for all primer sequences used in this study), inserted into pET28a(+) plasmid (Novagen), and expressed at 37 ° C in the Escherichia coli strain BL21(DE3) pLysS cells using a 3-h induction with 1 mM isopropyl 1-thio- β -d-galactopyranoside. The 6His-RAD23b protein was released with the Bug Buster reagent (Novagen), purified via nickel nitrilotriacetic acid agarose (Qiagen Sciences) at 4 ° C under native conditions, and injected directly into rabbits (University of Wisconsin Polyclonal Antibody Service). Antibodies against Arabidopsis DSK2a and DDI1 were generated against 6His-tagged recombinant proteins purified in the same manner (IgMedica Biotechnology). Antibodies against RPN1a, RPN5a, RPN10, PBA1, UBC1, and Ub were as described (Sullivan et al., 1994; van Nocker et al., 1996b; Smalle et al., 2002, 2003; Yang et al., 2004). Anti-histone H3 antibodies were purchased from Abcam (ab1791), and anti-WIP1 and anti-PUX1 antibodies were gifts from Iris Meier (Ohio State University) and Sebastian Bednarek (University of Wisconsin, Madison), respectively.
Immunoblot analyses were performed according to Smalle et al. (2002). Primary antibodies were visualized with alkaline phosphatase–conjugated goat anti-rabbit immunoglobulins (for RAD23 and RPN10) or with horseradish peroxidase–conjugated goat anti-rabbit antibodies (rest of primary antibodies) (Kirkegaard and Perry Laboratories). Signal was detected using Classic Autoradiography Film (MidSci). Exposure times were adjusted to remain in the linear range of the film.
Nuclei Isolation
Nuclei were enriched using the Percoll gradient method as described (Saracco et al., 2007) from 10 g of wild-type Col-0 seedlings grown for 7 d on liquid Gamborg's B-5 growth medium (GM). ATP (10 mM) was included throughout the protocol. Equal amounts of total protein (as determined by the Bradford assay; Bio-Rad) were subjected to SDS-PAGE and immunoblot analysis. Nuclei were stored at –80 ° C in 20% glycerol, 50 mM Tris-HCl, pH 7.8, and 5 mM MgCl2 prior to SDS-PAGE.
Protoplast Isolation and Confocal Microscopy
Protoplasts were prepared from 2-week-old, plate-grown Col-0 seedlings according to Lee et al. (2001). For transient expression, protoplasts were transfected by the polyethylene glycol method with pMDC43 plasmids (Curtis and Grossniklaus, 2003) expressing GFP, GFP-RAD23a, GFP-RAD23b, GFP-RAD23c, and GFP-RAD23d under the control of the 35S promoter or their native promoters. The 35S promoter constructions were generated by amplifying the full-length coding sequences of RAD23a-d with the primer pairs P3 and P4, P5 and P6, P7 and P8, P9 and P10, respectively, and the fragments were ligated into the Gateway vector pENTR-D/TOPO (Invitrogen). The plasmids were subsequently linearized with NruI, and the coding sequences were recombined in-frame using the Gateway LR Clonase Enzyme Mix (Invitrogen) to the 3 ′ end of GFP in the pMDC43 vector. The native promoter constructions were generated using a PCR fragment amplified from the chromosomal region of each RAD23 gene beginning at the ATG initiation codon and extending ∼ 2 kb upstream. The primer pairs used for the promoter segments were P11 and P12 (RAD23a), P13 and P14 (RAD23b), P15 and P16 (RAD23c), and P17 and P18 (RAD23d). After transfection, protoplasts were incubated at least 12 h in the dark at 25 ° C. GFP was visualized within 24 h after transformation by fluorescence microscopy using a Zeiss 510-Meta confocal laser scanning microscope and the BP505-530 (excitation 488 nm; emission 505 to 530 nm) and Chs 650-670 (excitation 633 nm; emission 650 to 670 nm) filter sets to detect GFP and chlorophyll autofluorescence, respectively (Thompson et al., 2005). Nuclei were visualized in roots by fluorescence following a 1-h incubation with 10 μ M Vybrant DyeCycle Orange (Invitrogen; Wu and Spalding, 2007).
Ubiquitin Chain Binding Assays
Full-length coding regions for RAD23b, RAD23c, and RAD23d proteins were PCR amplified from size-selected cDNA libraries (CD4-14; ABRC) using primer pairs P19 and P20, P21 and P22, P23 and P24, respectively, which added SacI/HindIII, SacI/XhoI, and BamHI/XhoI sites to the 5 ′ and 3 ′ ends, respectively, and then inserted into similarly digested pET42a (Novagen) to generate in-frame fusions to the C terminus of GST. RAD23-GST proteins were expressed in BL21 (DE3) E. coli cells using 1 mM isopropyl 1-thio- β -d-galactopyranoside, released by sonication of the cells, and affinity purified from the crude extracts using immobilized glutathione beads (Pierce). The beads were washed with Buffer A (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM Na2EDTA, and 0.1% Nonidet P-40), incubated at 4 ° C for 2 h with 5 μ g of purified Lys-48– or Lys-63–linked chains (Ub2-7) (BIOMOL) in Buffer A, and then extensively washed in Buffer A. Bound proteins were eluted by heating the beads in SDS-PAGE sample buffer and subjected to SDS-PAGE and immunoblot analysis with anti-Ub antibodies (Santa Cruz Biotechnology).
Co-IP and GST-UIM Pull-Down Assays
Anti-RAD23b agarose was created by cross-linking anti-RAD23b IgGs from whole serum to Protein A agarose (GE Healthcare) using disuccinimidyl suberate (Thermo Scientific). Liquid-grown seedlings were frozen, pulverized at liquid nitrogen temperatures, and homogenized in Extraction Buffer (25 mM Tris-HCl, pH 7.2, 200 mM NaCl, 0.25% Triton X-100, 10 mM iodoacetamide, and 2 mM phenylmethylsulfonyl fluoride). Extracts were filtered through Miracloth (Calbiochem), clarified at 15,000g, and then incubated with anti-RAD23b agarose by rotating for 2 h at 4 ° C. Bound proteins were eluted by heating the beads in SDS-PAGE sample buffer and then were subjected to SDS-PAGE and immunoblot analyses as above.
The UIM coding region from Arabidopsis RPN10 was PCR amplified from cDNA (Smalle et al., 2003) using the primers P25 and P26, ligated into the Gateway pENTR-D/TOPO vector (Invitrogen), and then recombined in-frame to the 3 ′ end of the GST coding sequence into the pDEST15 vector using the Gateway LR Clonase Enzyme Mix (Invitrogen). Following expression in BL21(DE3) cells, the GST-UIM polypeptide was adsorbed onto GST • Bind Resin (Novagen), washed with and eluted with 10 mM glutathione in wash buffer. GST-UIM [dialyzed into 50 mM 3-(N-morpholino)propanesulfonic acid, pH 7.5] was bound to Affigel 15 agarose (Bio-Rad). GST protein was prepared in the same manner following expression from the vector pGEX-4T (GE Healthcare). The GST-UIM and GST beads were incubated with clarified seedling extracts as above and washed with extraction buffer containing 2 M NaCl. Bound proteins were eluted in Extraction Buffer containing 8 M urea.
Affinity Purification of Proteasomes
The pag1-1 (SALK_114864) T-DNA insertional mutant in the Arabidopsis ecotype Col-0 background was identified in the SIGNAL T-DNA collection (http://signal.salk.edu/) and obtained from the ABRC (Alonso et al., 2003). The T-DNA insertion was identified via PCR using the SALK T-DNA–specific left border primer P38 and the gene-specific primer P27 and found to be in the 5th intron of PAG1. Heterozygous pag1-1 plants were transformed by floral dip with the pEarleyGate 202 plasmid (Earley et al., 2006) containing 2 kb of the native promoter region and the coding sequence of PAG1 in-frame with the Flag epitope sequence (Smalle et al., 2003). Basta resistance and PCR genotyping were used to select for T0 transformants containing the pag1-1 T-DNA. Double heterozygous T1 plants were allowed to self-pollinate, and PAG1-Flag pag1-1 homozygous mutants were identified in the T2 progeny. Immunoblot analysis verified that these plants express PAG1-Flag and not the wild-type PAG1 protein.
PAG1-Flag pag1-1 and Col-0 seedlings were grown in liquid GM under continuous light for 10 d, harvested, and frozen in liquid nitrogen. Ten grams of tissue was ground in liquid N2 and homogenized in 12.5 mL of Buffer A (50 mM Tris-HCl, pH 7.5, 25 mM NaCl, 2 mM MgCl2, 1 mM EDTA, 10 mM ATP, and 5% glycerol) plus 2 mM phenylmethylsulfonyl fluoride and 50 μ M MG132 added before use to prevent proteolysis during preparation. Extracts were filtered through Miracloth and clarified at 30,000g for 20 min. Soluble extract was incubated with 50 μ L of anti-Flag M2 Affinity Gel (Sigma-Aldrich) and passed over the column three times to ensure optimal binding. The column was washed three times with 2 mL of Buffer A, and bound protein was competitively eluted with purified Flag peptide (DYKDDDDK) prepared by the University of Wisconsin-Madison Peptide Synthesis Facility) in Buffer A (500 ng/ μ L) at 4 ° C for 30 min while rotating. Purified proteasomes were precipitated with trichloroacetic acid, acetone washed, resuspended in protein sample buffer, and subjected to SDS-PAGE and immunoblot analysis.
RAD23a-d and DDI1 Mutants
The rad23a-1, rad23b-1, rad23b-2, rad23c-1, rad23d-1, and ddi1-1 alleles in the Col-0 background were created by T-DNA insertional mutagenesis (Alonso et al., 2003) and were obtained from the ABRC (SALK lines 064980, 075940, 076360, 068091, 014137, and 152214, respectively). Gene-specific primer pairs used for PCR genotyping of the T-DNA insertions were P28 and P29 (rad23a-1), P30 and P31 (rad23b-1 and rad23b-2), P32 and P33 (rad23c-1), P34 and P35 (rad23d-1), and P36 and P37 (ddi1-1). Left border primer used to amplify the T-DNA insertions was P38 for all mutants. The rad23b-1 mutant was backcrossed three times to the wild-type Col-0 parent and then made homozygous by selfing. The mutant alleles were tracked in the progeny by phenotype, PCR genotyping, and by kanamycin resistance for the rad23a-1 and rad23b-1 alleles. Double rad23 mutant combinations were generated by fertilizing single homozygous plants. Quadruple heterozygous mutant plants (AaBbCcDd) were generated using pollen from homozygous rad23ac plants to fertilize rad23bd flowers. Selfed progeny were then screened by genomic PCR from the various heterozygous and homozygous triple mutant combinations. Where possible, the genotypes were confirmed by immunoblot analysis of crude extracts from individual plants with anti-RAD23b antibodies.
For complementation, the full-length RAD23b coding region was amplified using primers P39 and P40, which bracket the segment with XbaI sites. The product was digested with XbaI and then inserted into the similarly digested binary vector pGSVE9 (Smalle et al., 2003) for expression under control of the 35S promoter. The 35S:RAD23b transgene was introduced into wild-type Col-0 plants by the floral dip method using the Agrobacterium tumefaciens strain GV3101 (Smalle et al., 2003) and then introgressed into the rad23b-1 mutant background. The transgene was followed by hygromycin resistance conferred by pGSVE9 and by PCR using the pGSVE9-specific primers P41 and P42.
Unless otherwise noted, plants were grown at 22 ° C under sterile conditions on solid GM (Sigma-Aldrich) containing 2% sucrose and 0.7% agar. After stratification, the seeds were placed under continuous irradiation or under an LD photoperiod (16 h light/8 h dark). Root elongation was measured on seedlings grown vertically for 7 d on 1.2% agar. Roots were scanned on a Gel Doc System (Bio-Rad) and measured by Image J 3.0 (http://rsbweb.nih.gov/ij/).
RT-PCR and RNA Gel Blot Analyses
For RT-PCR, total RNA was extracted from 2-week-old seedlings using the Trizol reagent (Invitrogen) and treated with RQ1 DNase (Promega) prior to RT-PCR. First-strand cDNA synthesis was performed with 1 μ g of RNA using the Superscript II reverse transcriptase system (Invitrogen) in conjunction with the gene-specific primers P42 (RAD23b), P43 (RAD23c), P44 (RAD23d), P45 (PAE2), and P46 (DDI1). RT-PCRs were performed with this template using the first-strand primers in combination with the 5 ′ gene-specific primers P47, P48, P49, P50, and P51, respectively. The RAD23a cDNA was amplified using an Oligo(dT)18 primer (Fermentas) and the 5 ′ gene-specific primer P52. RNA gel blot analyses were conducted according to Smalle et al. (2002) using 10 μ g of total RNA extracted from 1-week-old seedlings by the Trizol reagent. 32P-labeled riboprobes were synthesized with T7 or SP6 RNA polymerase using the appropriate linearized plasmids and the Riboprobe SP6 system (Promega). Full-length RAD23b, RAD23c, and RAD23d templates were provided by the ABRC clones U09913, U12782, and U12657, respectively, and the RAD23a template was created from a 5 ′ EST fragment (M26H7; ABRC). The PAE2 and β -TUBULIN4 templates were used as controls. Radiographic signals were detected using Classic autoradiography film (MidSci) with the exposure times kept within the linear range of the film.
Yeast Two-Hybrid Analyses
The coding region of RAD23b was amplified by PCR from a full-length cDNA clone (ABRC; U09913) using the primers P53 and P54. A second round of PCR with the primers P55 and P56 was used to appended flanking sequences, which facilitated in vivo homologous recombination into the pGBKT7 vector that generated a fusion of RAD23b to the C terminus of the GAL4 activation domain (Clontech). The construction was transformed into yeast and used as bait to screen an Arabidopsis green seedling cDNA library inserted into the pGAD10 vector (Clontech). Prey plasmids were purified from stationary phase yeast cultures lysed in equal volumes of 2% Triton X-100, 1% SDS, 100 mM NaCl, 10 mM Tris-HCl, pH 8.0, and 1 mM EDTA, extracted with 0.2 mL of phenol/chloroform/isoamyl alcohol (25:24:1), and precipitated with 100% isopropanol. DNA was sequenced by the dideoxy method using the primers P57 and P58; these sequences were used as queries in BLAST searches of the Arabidopsis genome database (www.Arabidopsis.org).
Accession Numbers
RAD23 protein sequences from this article can be found in the EMBL/GenBank data libraries under accession numbers NP_173070 (At RAD23a), NP_850982 (At RAD23b), NP_186903 (AtRAD23c), NP_198663 (At RAD23d), BAD28007 (Os RAD23a; Os02g08300), BAD54370 (Os RAD23c; Os06g15360), BAD01169 (Os RAD23d.1; Os08g33340), BAD36295 (Os RAD23d;2; Os09g24200), XP_001780766 (Pp RAD23a), XP_001763027 (Pp RAD23b), AAB28441 (ScRAD23), CAA72741 (Dc RAD23d; formerly isoform I), CAA72742 (Dc RAD23a; formerly isoform II), ACF87572 (Zm RAD23c;1), ACF84553 (Zm RAD23d;1), ACF84200 (Zm RAD23d;2), and ABK95041 (Pt RAD23b;2). The JGI (Department of Energy Joint Genome Institute) accession numbers are E.GW1.1.1607.1 (Sm RAD23a) and E.GW1.5.1119.1 (Sm RAD23b) for Selaginella moellendorffii and C_LG_I000167 (Pt RAD23b;1), gw.123.228.1 (Pt RAD23c;1), Ext_fgenesh4_pm.C_LG-IV0203 (Pt RAD23c;2), gw1.X.249.1 (Pt RAD23d;1), and gw1.VIII.1139.1 (Pt RAD23d;2) for Populus trichocarpa. Maize sequences were verified using the Harvard Computational Biology and Functional Genomics Gene Indices database (http://compbio.dfci.harvard.edu), whereas the rice sequences were verified using the Michigan State University Rice Genome Annotation database (http://rice.plantbiology.msu.edu/). Sequences for other Arabidopsis UBL/UBA proteins can be found in the EMBL/GenBank data libraries under accession numbers NP_565407 (DSK2a), NP_179311 (DSK2b), NP_566451 (DDI1), NP_178939 (NUB1), and NP_197113 (UBL1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Amino Acid Sequence Comparison of RAD23 Proteins in Arabidopsis and Rice.
Supplemental Figure 2. Phylogenetic Comparison of Plant RAD23 Proteins.
Supplemental Figure 3. Specificity of Anti-RAD23 Antibodies.
Supplemental Figure 4. Nuclear Enrichment of RAD23a-d Proteins in Arabidopsis.
Supplemental Figure 5. Mature rad23 Mutants Bearing the rad23a-1, rad23c-1, and rad23d-1 Alleles Resemble Wild-Type Plants.
Supplemental Figure 6. Ub-Conjugate Profiles of the Single and Combinatorial rad23 Mutants.
Supplemental Figure 7. Molecular and Biochemical Characterizations of the Arabidopsis ddi1-1 Mutant.
Supplemental Table 1. RAD23b Interactors Identified by Yeast Two-Hybrid Analysis.
Supplemental Table 2. Oligonucleotide Primers Used in This Study
Supplemental Data Set 1. Text File of the RAD23 Protein Sequence Alignment Used to Create the Phylogenetic Tree in Supplemental Figure 2.
Supplementary Material
Acknowledgments
We thank Sebastian Bednarek and Iris Meier for providing the anti-PUX1 and anti-WIP1 antibodies, respectively, Joachim Uhrig for helping with the yeast two-hybrid studies, Tyler Vits for contributing to the characterization of DDI1, and the ABRC for providing the EST clones and SALK T-DNA lines. This work was supported by grants from the U.S. Department of Energy Basic Energy Sciences program (DE-FG02-88ER13968) and the National Science Foundation Arabidopsis 2010 Program (MCB-0115870) to R.D.V., the Taiwan National Science Council (NSC 88-2311-B001-127, NSC 89-2311-B001-125, and NSC 89-2311-B001-044) and Academia Sinica (AS-94-TP-B08 and AS-97-TP-B03) to H.F., a National Institutes of Health Ruth L. Kirschstein predoctoral fellowship to A.J.B., and a National Institutes of Health predoctoral fellowship to the University of Wisconsin Genetics Training Program (to L.M.F.). Y.-L.L. was supported by the Taiwan International Graduate Program, Academia Sinica, and National Chung-Hsing University, Taiwan.
References
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Beal R., Deveraux Q., Xia G., Rechsteiner M., Pickart C. (1996). Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc. Natl. Acad. Sci. USA 93: 861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolaet B.L., Clarke D.J., Wolff M., Watson M.H., Henze M., Divita G., Reed S.I. (2001). UBA domains mediate protein-protein interactions between two DNA damage-inducible proteins. J. Mol. Biol. 313: 955–963 [DOI] [PubMed] [Google Scholar]
- Callis J., Carpenter T., Sun C.W., Vierstra R.D. (1995). Structure and evolution of genes encoding polyubiquitin and ubiquitin-like proteins in Arabidopsis thaliana ecotype Columbia. Genetics 139: 921–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Brenneman M., Meyne J., Oshimura M., Goodwin E.H., Chen D.J. (1999). The XRCC2 and XRCC3 repair genes are required for chromosome stability in mammalian cells. Mutat. Res. 434: 75–88 [DOI] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L., Beeckman T., Beemster G.T., Krols L., Terras F., Landrieu I., van der Schueren E., Maes S., Naudts M., Inze D. (2001). Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher K., Callis J. (2007). Ubiquitin, hormones and biotic stress in plants. Ann. Bot. (Lond.) 99: 787–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley K.W., Haag J.R., Pontes O., Opper K., Juehne T., Song K., Pikaard C.S. (2006). Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Elsasser S., Gali R.R., Schwickart M., Larsen C.N., Leggett D.S., Muller B., Feng M.T., Tubing F., Dittmar G.A., Finley D. (2002). Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat. Cell Biol. 4: 725–730 [DOI] [PubMed] [Google Scholar]
- Finley D. (2009). Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78: 477–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Sadis S., Rubin D.M., Glickman M., van Nocker S., Finley D., Vierstra R.D. (1998). Multiubiquitin chain binding and protein degradation are mediated by distinct domains within the 26S proteasome subunit Mcb1. J. Biol. Chem. 273: 1970–1981 [DOI] [PubMed] [Google Scholar]
- Funakoshi M., Geley S., Hunt T., Nishimoto T., Kobayashi H. (1999). Identification of XDRP1; a Xenopus protein related to yeast Dsk2p binds to the N-terminus of cyclin A and inhibits its degradation. EMBO J. 18: 5009–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi M., Sasaki T., Nishimoto T., Kobayashi H. (2002). Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc. Natl. Acad. Sci. USA 99: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely G., Kama R., Gelin-Licht R., Gerst J.E. (2008). Different domains of the UBL-UBA ubiquitin receptor, Ddi1/Vsm1, are involved in its multiple cellular roles. Mol. Biol. Cell 19: 3625–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzder S.N., Sung P., Prakash L., Prakash S. (1998). Affinity of yeast nucleotide excision repair factor 2, consisting of the Rad4 and Rad23 proteins, for ultraviolet damaged DNA. J. Biol. Chem. 273: 31541–31546 [DOI] [PubMed] [Google Scholar]
- Hara T., Kamura T., Kotoshiba S., Takahashi H., Fujiwara K., Onoyama I., Shirakawa M., Mizushima N., Nakayama K.I. (2005). Role of the UBL-UBA protein KPC2 in degradation of p27 at G1 phase of the cell cycle. Mol. Cell. Biol. 25: 9292–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Petersen R., Gordon C. (2004). Protein degradation: Recognition of ubiquitinylated substrates. Curr. Biol. 14: R754–R756 [DOI] [PubMed] [Google Scholar]
- Heessen S., Masucci M.G., Dantuma N.P. (2005). The UBA2 domain functions as an intrinsic stabilization signal that protects Rad23 from proteasomal degradation. Mol. Cell 18: 225–235 [DOI] [PubMed] [Google Scholar]
- Hiyama H., Yokoi M., Masutani C., Sugasawa K., Maekawa T., Tanaka K., Hoeijmakers J.H., Hanaoka F. (1999). Interaction of hHR23 with S5a. The ubiquitin-like domain of hHR23 mediates interaction with S5a subunit of 26S proteasome. J. Biol. Chem. 274: 28019–28025 [DOI] [PubMed] [Google Scholar]
- Hruz T., Laule O., Szabo G., Wessendorp F., Bleuler S., Oertle L., Widmayer P., Gruissem W., Zimmermann P. (2008). Genevestigator V3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki S., Suzuki T., Ohto M.A., Urawa H., Horiuchi T., Nakamura K., Morikami A. (2006). Arabidopsis TEBICHI, with helicase and DNA polymerase domains, is required for regulated cell division and differentiation in meristems. Plant Cell 18: 879–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Vossler R.A., Diaz-Martinez L.A., Winter N.S., Clarke D.J., Walters K.J. (2006). UBL/UBA ubiquitin receptor proteins bind a common tetraubiquitin chain. J. Mol. Biol. 356: 1027–1035 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Oh S.A., Brownfield L., Hong S.H., Ryu H., Hwang I., Twell D., Nam H.G. (2008). Control of plant germline proliferation by SCF(FBL17) degradation of cell cycle inhibitors. Nature 455: 1134–1137 [DOI] [PubMed] [Google Scholar]
- Kito K., Yeh E.T., Kamitani T. (2001). NUB1, a NEDD8-interacting protein, is induced by interferon and down-regulates the NEDD8 expression. J. Biol. Chem. 276: 20603–20609 [DOI] [PubMed] [Google Scholar]
- Kurepa J., Walker J.M., Smalle J., Gosink M.M., Davis S.J., Durham T.L., Sung D.Y., Vierstra R.D. (2003). The small ubiquitin-like modifier (SUMO) protein modification system in Arabidopsis. Accumulation of SUMO1 and -2 conjugates is increased by stress. J. Biol. Chem. 278: 6862–6872 [DOI] [PubMed] [Google Scholar]
- Kurepa J., Wang S., Li Y., Zaitlin D., Pierce A.J., Smalle J.A. (2009). Loss of 26S proteasome function leads to increased cell size and decreased cell number in Arabidopsis shoot organs. Plant Physiol. 150: 178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertson D., Chen L., Madura K. (1999). Pleiotropic defects caused by loss of the proteasome-interacting factors Rad23 and Rpn10 of Saccharomyces cerevisiae. Genetics 153: 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Kim D.H., Kim Y.W., Hwang I. (2001). Identification of a signal that distinguishes between the chloroplast outer envelope membrane and the endomembrane system in vivo. Plant Cell. 13: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Copley R.R., Pils B., Pinkert S., Schultz J., Bork P. (2006). SMART 5: Domains in the context of genomes and networks. Nucleic Acids Res. 34: D257–D260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yu Y., An H., Li N., Lin N., Wang W., Zhang W., Wan T., Cao X. (2003). Cloning and identification of a novel ubiquitin-like protein, BMSC-UbP, from human bone marrow stromal cells. Immunol. Lett. 86: 169–175 [DOI] [PubMed] [Google Scholar]
- Lynn K., Fernandez A., Aida M., Sedbrook J., Tasaka M., Masson P., Barton M.K. (1999). The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development 126: 469–481 [DOI] [PubMed] [Google Scholar]
- Maor R., Jones A., Nuhse T.S., Studholme D.J., Peck S.C., Shirasu K. (2007). Multidimensional protein identification technology (MudPIT) analysis of ubiquitinated proteins in plants. Mol. Cell. Proteomics 6: 601–610 [DOI] [PubMed] [Google Scholar]
- Martinez I.M., Chrispeels M.J. (2003). Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15: 561–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J.P., Smerdon M.J. (1996). Rad23 is required for transcription-coupled repair and efficient overrall repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2361–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T.D., Feigon J. (2003). Structural determinants for the binding of ubiquitin-like domains to the proteasome. EMBO J. 22: 4634–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J.M., Vermeulen W., van der Horst G.T., Bergink S., Sugasawa K., Vrieling H., Hoeijmakers J.H. (2003). A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev. 17: 1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikovics K., Blein T., Peaucelle A., Ishida T., Morin H., Aida M., Laufs P. (2006). The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18: 2929–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno A., Jee J., Fujiwara K., Tenno T., Goda N., Tochio H., Kobayashi H., Hiroaki H., Shirakawa M. (2005). Structure of the UBA domain of Dsk2p in complex with ubiquitin molecular determinants for ubiquitin recognition. Structure 13: 521–532 [DOI] [PubMed] [Google Scholar]
- Ortolan T.G., Chen L., Tongaonkar P., Madura K. (2004). Rad23 stabilizes Rad4 from degradation by the Ub/proteasome pathway. Nucleic Acids Res. 32: 6490–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasi S., Varadan R., Fushman D., Pickart C.M. (2005). Diverse polyubiquitin interaction properties of ubiquitin-associated domains. Nat. Struct. Mol. Biol. 12: 708–714 [DOI] [PubMed] [Google Scholar]
- Raasi S., Wolf D.H. (2007). Ubiquitin receptors and ERAD: a network of pathways to the proteasome. Semin. Cell Dev. Biol. 18: 780–791 [DOI] [PubMed] [Google Scholar]
- Ramirez-Parra E., Gutierrez C. (2007). E2F regulates FASCIATA1, a chromatin assembly gene whose loss switches on the endocycle and activates gene expression by changing the epigenetic status. Plant Physiol. 144: 105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H., Sastry A. (2002). Recognition of specific ubiquitin conjugates is important for the proteolytic functions of the ubiquitin-associated domain proteins Dsk2 and Rad23. J. Biol. Chem. 277: 11691–11695 [DOI] [PubMed] [Google Scholar]
- Ravid T., Hochstrasser M. (2008). Diversity of degradation signals in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell Biol. 9: 679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Santner A., del Pozo J.C., Murray J.A., Estelle M. (2008). Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. Plant J. 53: 705–716 [DOI] [PubMed] [Google Scholar]
- Saracco S.A., Hansson M., Scalf M., Walker J.M., Smith L.M., Vierstra R.D. (2009). Tandem affinity purification and mass spectrometric analysis of ubiquitylated proteins in Arabidopsis. Plant J. 59: 344–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracco S.A., Miller M.J., Kurepa J., Vierstra R.D. (2007). Genetic analysis of SUMOylation in Arabidopsis: conjugation of SUMO1 and SUMO2 to nuclear proteins is essential. Plant Physiol. 145: 119–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz T.F., Quatrano R.S. (1997). Characterization and expression of a rice RAD23 gene. Plant Mol. Biol. 34: 557–562 [DOI] [PubMed] [Google Scholar]
- Smalle J., Kurepa J., Yang P., Babiychuk E., Kushnir S., Durski A., Vierstra R.D. (2002). Cytokinin growth responses in Arabidopsis involve the 26S proteasome subunit RPN12. Plant Cell 14: 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J., Kurepa J., Yang P., Emborg T.J., Babiychuk E., Kushnir S., Vierstra R.D. (2003). The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. Plant Cell 15: 965–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J., Vierstra R.D. (2004). The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 55: 555–590 [DOI] [PubMed] [Google Scholar]
- Sonoda Y., Sako K., Maki Y., Yamazaki N., Yamamoto H., Ikeda A., Yamaguchi J. (2009). Regulation of leaf organ size by the Arabidopsis RPT2a 19S proteasome subunit. Plant J. 60: 68–78 [DOI] [PubMed] [Google Scholar]
- Sturm A., Lienhard S. (1998). Two isoforms of plant RAD23 complement a UV-sensitive rad23 mutant in yeast. Plant J. 13: 815–821 [DOI] [PubMed] [Google Scholar]
- Sullivan M.L., Carpenter T.B., Vierstra R.D. (1994). Homologues of wheat ubiquitin-conjugating enzymes–TaUBC1 and TaUBC4 are encoded by small multigene families in Arabidopsis thaliana. Plant Mol. Biol. 24: 651–661 [DOI] [PubMed] [Google Scholar]
- Swarbreck D., et al. (2008). The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 36: D1009–D1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Thompson A.R., Doelling J.H., Suttangkakul A., Vierstra R.D. (2005). Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 138: 2097–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nocker S., Deveraux Q., Rechsteiner M., Vierstra R.D. (1996a). Arabidopsis MBP1 gene encodes a conserved ubiquitin recognition component of the 26S proteasome. Proc. Natl. Acad. Sci. USA 93: 856–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nocker S., Sadis S., Rubin D.M., Glickman M., Fu H., Coux O., Wefes I., Finley D., Vierstra R.D. (1996b). The multiubiquitin-chain-binding protein Mcb1 is a component of the 26S proteasome in Saccharomyces cerevisiae and plays a nonessential, substrate-specific role in protein turnover. Mol. Cell. Biol. 16: 6020–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Oania R., Graumann J., Deshaies R.J. (2004). Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell 118: 99–110 [DOI] [PubMed] [Google Scholar]
- Vierstra R.D. (2009). The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 10: 385–397 [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar S., Bugg C.E., Wilkinson K.D., Vierstra R.D., Hatfield P.M., Cook W.J. (1987). Comparison of the three-dimensional structures of human, yeast, and oat ubiquitin. J. Biol. Chem. 262: 6396–6399 [PubMed] [Google Scholar]
- Wu G., Spalding E.P. (2007). Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 104: 18813–18818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Duong D.M., Seyfried N.T., Cheng D., Xie Y., Robert J., Rush J., Hochstrasser M., Finley D., Peng J. (2009). Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137: 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.M., Rose A., Muthuswamy S., Jeong S.Y., Venkatakrishnan S., Zhao Q., Meier I. (2007). NUCLEAR PORE ANCHOR, the Arabidopsis homolog of Tpr/Mlp1/Mlp2/megator, is involved in mRNA export and SUMO homeostasis and affects diverse aspects of plant development. Plant Cell 19: 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N., Doelling J.H., Falbel T.G., Durski A.M., Vierstra R.D. (2000). The ubiquitin-specific protease family from Arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol. 124: 1828–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Fu H., Walker J., Papa C.M., Smalle J., Ju Y.M., Vierstra R.D. (2004). Purification of the Arabidopsis 26S proteasome: biochemical and molecular analyses revealed the presence of multiple isoforms. J. Biol. Chem. 279: 6401–6413 [DOI] [PubMed] [Google Scholar]
- Yang P., Smalle J., Lee S., Yan N., Emborg T.J., Vierstra R.D. (2007). Ubiquitin C-terminal hydrolases 1 and 2 affect shoot architecture in Arabidopsis. Plant J. 51: 441–457 [DOI] [PubMed] [Google Scholar]
- Ye Y., Meyer H.H., Rapoport T.A. (2003). Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: Dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J. Cell Biol. 162: 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Garreton V., Chua N.H. (2005). The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev. 19: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.