Abstract
Many studies have demonstrated the rapid diversification of reproductive genes that function after mating but before fertilization. This process might lead to the evolution of postmating, prezygotic barriers between species. Here, I investigate the phenotypic and genetic basis of postmating, prezygotic isolation between two closely related species of Drosophila, Drosophila virilis and D. americana. I show that a strong barrier to interspecific fertilization results in a 99% reduction in progeny production. A genetic interaction among maternal and paternal alleles at only a few loci prevents the fertilization of D. virilis females by D. americana males. These loci are autosomal and isolation acts recessively; the fertilization incompatibility is caused by at least two loci in the maternal D. virilis parent in combination with at least three loci in the paternal D. americana parent. These findings, together with results from classical experiments, suggest that male–female coevolution within D. americana may have driven postmating, prezygotic isolation between species.
AN understanding of speciation requires insight into the origins and mechanisms of reproductive isolation. Divergent selection on traits that facilitate mating or fertilization might eventually lead to incompatibilities between males and females of incipient species. In animals, it has long been recognized that sexual selection can promote the evolution of specialized courtship rituals or elaborate phenotypic displays to attract mates (Darwin 1871). Similarly, sexual selection can be a powerful evolutionary force during or after mating by affecting the many biochemical, physiological, and morphological mechanisms involved in fertilization (Eberhard 1996). Postmating reproductive traits might also be subject to sexually antagonistic coevolution, whereby a difference in the reproductive interests of males and females leads to an evolutionary arms race between the sexes (Rice 1996). Just as divergent sexual selection on mate signals and preferences might give rise to premating (sexual) isolation (reviewed in Ritchie 2007), postcopulatory sexual selection and sexual conflict might promote the evolution of postmating barriers to fertilization or hybrid incompatibilities (Howard 1999; Wu and Davis 1993). Indeed, these evolutionary forces have apparently led to competitive gametic isolation (Price 1997; Price et al. 2000; Fishman et al. 2008) and sperm–egg incompatibilities (Galindo et al. 2003). Moreover, because sexual selection and antagonistic coevolution can act rapidly (Fisher 1930; Rice 1996), they might be particularly important in the early stages of speciation.
In diverse animal taxa, sexual selection and/or sexual conflict are thought to drive rapid evolution of a variety of postmating reproductive traits, including male genital morphology (Eberhard 1996), length of sperm and female sperm-storage organs (Pitnick et al. 1997; Miller and Pitnick 2002), ejaculate composition (e.g., Swanson et al. 2001a; Dorus et al. 2004), female reproductive tract proteins (e.g., Lawniczak and Begun 2007; Kelleher et al. 2007), and gamete recognition molecules (e.g., Wyckoff et al. 2000; Swanson et al. 2001b). In recent years, many studies have also documented strong signatures of positive selection in the rapid evolution of reproductive genes (e.g., Haerty et al. 2007; Turner et al. 2008; reviewed in Swanson and Vacquier 2002; Clark et al. 2006). For internally fertilizing species, coevolution between the female reproductive tract and the male ejaculate is particularly dynamic (Pitnick et al. 2007). For example, in Drosophila, hundreds of nonsperm seminal fluid proteins are transferred during mating, including many fast-evolving accessory gland proteins (ACPs) (Swanson et al. 2001a; Wagstaff and Begun 2005). As expected, there is evidence for coordinated evolution of female reproductive tract genes, which also show elevated rates of evolution in Drosophila (Panhuis and Swanson 2006; Prokupek et al. 2008). But what are the consequences of such rapid rates of diversification? How many of these fast-evolving reproductive genes contribute to isolating barriers? Major progress toward addressing these questions would require identifying and characterizing individual loci that cause postmating, prezygotic isolation.
A large body of classical work suggests that the Drosophila virilis species group might represent an ideal model for studying the genetics of reproductive isolation (Patterson and Stone 1952); and importantly, the D. virilis genome sequence is now available. There is also evidence that postmating, prezygotic isolation may be significant among D. virilis and the closely related North American species, D. americana and D. novamexicana. Patterson et al. (1942) describe reproductive isolation due to “gamete mortality” in reciprocal crosses between D. virilis and D. americana. In later studies, these authors discovered that very few eggs from interspecific crosses become fertilized or hatch and speculate that sperm become “immobilized in the reproductive tract of the alien female” (Patterson and Stone 1952). Moreover, a recent study has found a similar problem with fertilization in crosses between D. americana and D. novamexicana (Y. Ahmed and B. McAllister, personal communication). Consistent with the evolution of these interspecific barriers, male and female reproductive tract proteins have been shown to evolve rapidly in the D. virilis species group (Civetta and Singh 1995; Haerty et al. 2007). In addition, females of both D. virilis and D. americana produce a large opaque vaginal mass in response to mating (the “insemination reaction”; Wheeler 1947), which almost certainly reflects an evolutionary history of interaction between the female reproductive tract and male ejaculate (Knowles and Markow 2001).
Despite the potential importance of postmating, prezygotic isolation in D. virilis group divergence, almost nothing is known about its genetic architecture. On the basis of the results from their crosses between D. virilis and D. americana, Patterson et al. (1942) infer that postmating isolation involves recessive autosomal genes. However, their experiments often cannot distinguish between the effects of the apparent fertilization incompatibility and premating isolation, the latter also being strong between D. americana females and D. virilis males (Stalker 1942). Their genetic mapping studies were also crude.
In this study, I have two main objectives. First, I characterize the phenotypic basis of postmating isolation between D. virilis and D. americana. To do so, I perform a series of crosses within and between species. I find that low F1 hybrid production between D. virilis and D. americana is due primarily to a reduction in interspecific fertilization; females presented with heterospecific males almost always become inseminated, but very few eggs are fertilized. Second, I perform a detailed genetic analysis of the fertilization incompatibility between D. virilis females and D. americana males. Using the D. virilis genome assembly, I developed molecular markers targeted to genomic regions of interest for high-resolution genetic mapping of both the maternal and paternal components of isolation. This study is a first step toward understanding the genetic and evolutionary mechanisms of postmating, prezygotic reproductive isolation in Drosophila.
MATERIALS AND METHODS
Fly lines and genetic crosses:
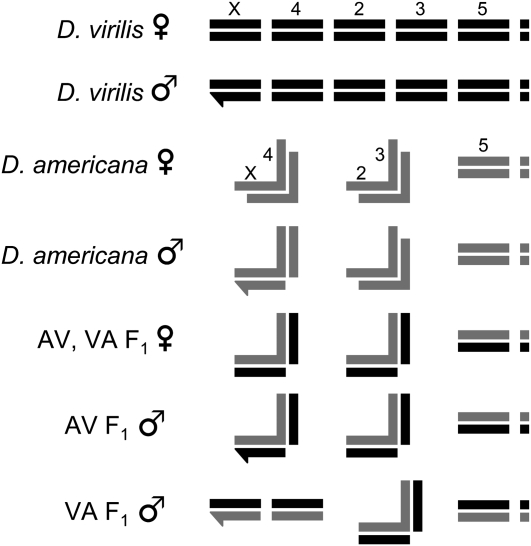
I performed crosses between two closely related species of Drosophila, D. virilis and D. americana. These species are currently allopatric: D. virilis is a worldwide human commensal with natural populations in Asia, and D. americana is associated with riparian habitats throughout much of North America (Throckmorton 1982; McAllister 2002). There is strong pre- and postmating reproductive isolation between these species, but no barrier is complete. Moreover, F1 hybrids are viable and fertile, so later-generation hybrids are easily produced. Both D. virilis and D. americana have six chromosome arms (including a dot chromosome; Figure 1). The D. virilis parental line used here is the genome sequence strain, 15010–1051.87, an inbred line with a visible marker on each of the (nondot) autosomes (b; tb, gp-L2; cd; pe). The D. americana parental line used here (SB02.06) originated as an isofemale line collected by Bryant McAllister in 2002 near the Cedar River, Muscatine County, IA. In D. americana, chromosomes 2 and 3 are fused and therefore do not segregate independently in crosses. In addition, D. americana is characterized by a polymorphic centromeric fusion between the X and fourth chromosomes that is positively correlated with latitude (McAllister 2002). The DaSB02.06 strain carries the X–4 fusion (McAllister and Evans 2006), which affects segregation in certain crosses (see Figure 1). Several chromosomal regions are inverted between D. virilis and D. americana (Hughes 1939). A large inversion on chromosome 2 and a small inversion on chromosome 5 differentiate the Dv1051.87 and DaSB02.06 strains (the former is fixed and the later polymorphic in D. americana). For all crosses, males and females were collected as virgins and maintained separately for 7–10 days to allow them to reach sexual maturity. Following this period, crosses were performed on fresh vials containing standard cornmeal medium at 20° ±1°.
Figure 1.—
Schematic of D. virilis, D. americana, and F1 hybrid chromosomes. For D. virilis and D. americana females, X chromosomes and autosomes are labeled (order is the same for males and hybrids shown below). The Y and dot chromosomes are represented by hooked bars and small squares, respectively. The X–4 and 2–3 chromosomal fusions of D. americana are represented by connected bars (each fusion forms a backward “L”). AV refers to an F1 hybrid with D. americana as the maternal parent, whereas VA refers to an F1 hybrid with D. virilis as the maternal parent. Note that D. americana males carry one unfused chromosome 4. Only in one direction of the interspecific cross (VA) does the F1 male inherit two unfused copies of chromosome 4, which allows the independent assortment of this chromosome in backcrosses.
Assessment of progeny number and male fertility:
To assay progeny number, I placed virgins aged 10–15 days together in vials with new food (made <24 hr before experiment). To increase the probability of mating, individual females were routinely presented with two males. After 10 days together in a vial, parental flies were removed and progeny were allowed to develop. For each vial, the number of progeny were scored as the number of eclosed adult flies.
In crosses between D. virilis females and experimental hybrid males, each male was presented with two females. As before, the number of progeny were scored as the number of eclosed adult flies. To assay male fertility, I measured sperm motility. Testes were dissected in PBS and examined under a compound microscope with dark-field optics. Following Coyne (1984), a male was scored as fertile if at least one motile sperm was observed, and sterile if no motile sperm were detected.
Assessment of rates of mating, egg hatch, and fertilization:
To determine the phenotypic basis of low hybrid production between D. virilis and D. americana, I estimated rates of mating, egg hatch, and fertilization success. Males and females were collected as virgins, aged 14 days, and mass mated in vials for 4 days. Using 10 females and 20 males, I performed reciprocal crosses between D. virilis and D. americana, as well as within-line control crosses. After 4 days of mating, flies were transferred to egg-laying cages with grape juice agar plates and yeast paste. Parental flies were removed after 24 hr, and 100 eggs from each treatment were transferred to a fresh grape juice plate. To assay mating success, I immediately dissected female reproductive tracts in PBS and examined them under a compound microscope. A female was identified as inseminated if sperm was detected in her seminal receptacles and/or spermathecae. After an additional 48 hr, I assayed egg hatch rate by determining the proportion of hatched eggs from each sample of 100 for 10 replicate crosses.
To estimate fertilization success, females were transferred from laying cages after only 3 hr, and eggs were examined 2–3 hr later. Following Patterson and Stone (1952), a single egg was placed on a microscope slide, a cover slip positioned on top, and a drop of water added to the side of the cover slip. The capillary action of the water drop causes the contents of the egg to spill out, including sperm if the egg has been fertilized. All tests of fertilization were conducted blind (i.e., an assistant labeled slides so that I did not know whether eggs derived from interspecific or conspecific crosses). To estimate fertilization success, I determined the proportion of eggs with sperm from a sample of 10 randomly selected eggs for 8 replicate crosses.
Molecular analyses:
The 32 molecular markers used in this study were microsatellites (Table 1). I identified candidate markers from the D. virilis genome sequence using the program Tandem Repeats Finder (Benson 1999) and designed primers using the program Primer3 (Rozen and Skaletsky 2000). Genomic DNA was extracted from whole flies using the protocol of Gloor and Engels (1992). All markers were amplified using standard touchdown PCR conditions (annealing temperatures incremented from 62° to 52° for the first 10 cycles and then an additional 30 cycles at 52°). Marker genotyping was performed by sizing PCR-amplified DNA fragments with an incorporated 5′ fluorescent-labeled primer on an ABI 3700 automated capillary sequencer (Applied Biosystems, Foster City, CA). Marker genotypes were assigned automatically using the program GeneMapper (Applied Biosystems) and then verified by eye.
TABLE 1.
Names and primers for mapped D. virilis microsatellite markers
| Marker name | Forward primer (5′–3′) | Reverse primer (5′–3′) | Chromosome |
|---|---|---|---|
| SSR6 | cggaaattgtcagcttttgg | ctccctacagtacggctcca | 3 |
| SSR7 | acgtccctgacaaactgagc | aaagcggttgccaaattcta | 3 |
| SSR11 | ttggcagagctttctcacct | ctaaacgggcctccacatt | 5 |
| SSR23 | aaactggcagatgggcatag | ccacgatttcagaagcacaa | 5 |
| SSR32 | ctctcacaacgcgtgaacat | ggacctcaaaacggagcata | 2 |
| SSR33 | catttcctgctggctagctt | gtcagacacagcgacgacat | 2 |
| SSR37 | ctctagatagcgccacagca | tgagatccaacagcaggatg | 2 |
| SSR42 | tgcctcataatggccaaaac | cattgcgtcctcgatctgta | 3 |
| SSR44 | cacacgcaaagctcacttgt | gcagtgcttagcaggtagcc | 3 |
| SSR45 | acccaaactgtaagcgtcaa | gtgtgtcatttccgtgcaac | 3 |
| SSR46 | aagagctacttgccgctgac | gtgccattctctggcagttt | 3 |
| SSR58 | tgcctagcatttggcactta | aaaagagcgtggcaaagaaa | 5 |
| SSR60 | caaaagtgttgccttgatgg | gggttctagcccccaaataa | 5 |
| SSR62 | tgttagttggcagcgcaat | gattatgcgtgttgcagtcg | 5 |
| SSR66 | ctcgctgtcgcaatgtttac | gccgcaaataaaatggtgat | 5 |
| SSR72 | tgcagtcaaaactgggtcaa | gcaagaccaaaagtgcgagt | 2 |
| SSR74 | ccttggcatgttttagagcaa | aagcgacgcgcaaaatatac | 2 |
| SSR76 | tgtgtggctaccacccttaat | cggcagttgggagtcatatc | 2 |
| SSR81 | tgcacgtaagtgtgaatctgc | gtgccaatcacatcgcagt | 2 |
| SSR84 | cagcatggagcatctgtgtc | tggaagggatgtcatggact | 5 |
| SSR85 | ctctcgccacgaactctttg | aacgtagacgcgttttcagc | 5 |
| SSR87 | cagcgcgtgctgattagtta | tacagctggctgcgtttatg | 5 |
| SSR88 | ccaaaaggcaggaccataaa | ttgcgtagacaccacaaggt | 5 |
| SSR89 | caacactttttccgccttct | accaactgcgagcttgacat | 5 |
| SSR90 | actttgccaagctgtgaagg | gcgtctcgtatgctctgcta | 5 |
| SSR94 | agttattgcccccagaacag | tgaaaagtgaatggctctcg | 3 |
| SSR95 | tgtgcctgctgacaaaacat | acactgcctgcttgcattta | 5 |
| SSR98 | caacaacagccgacagacaa | ctgccgcttgagagaaaatc | 3 |
| SSR108 | caaatacaagcagctgcaaca | tccgtctcagtgcagttcag | 5 |
| SSR111 | tttgattgtttccctcactcg | tgtcattgtccttggcaaaa | 5 |
| SSR116 | ccccattgaaagttcatcca | gtcaggaggccacattgttt | 5 |
| SSR118 | gcccaaaattcttagccaaa | tggcttgggtactggtttct | 5 |
Genetic mapping and QTL analyses:
Linkage groups that correspond to D. virilis chromosomes 2, 3, and 5 were constructed using JoinMap 4.0 (Van Ooijen 2006) by assessing the genotypes of two different backcross mapping populations (see results for details of these crosses). The group function of JoinMap was used with a LOD score threshold of 10.0 to assign markers to linkage groups. The genetic map created for each linkage group used the Kosambi mapping function, a LOD threshold of 0.5–1.0, a recombination threshold of 0.400–0.500, a jump threshold of 5.00, and a “ripple” after the addition of each locus.
I mapped QTL for low progeny production between D. virilis and D. americana using composite interval mapping (CIM) (Zeng 1993, 1994) using Windows QTL Cartographer V. 2.5 (Wang et al. 2007). Cofactors included in each CIM model were determined with forward-backward stepwise regression, with the critical P-values set at 0.05. Tests were performed at 2-cM intervals with a flanking window size of 10 cM. Significance thresholds were set by permutation (experimentwise type I error rate of α = 0.05, n = 1000).
To allow higher resolution mapping, I performed QTL analyses iteratively: after each round of analysis, I designed additional markers in regions with significant phenotypic effects.
RESULTS
Progeny numbers from conspecific and interspecific crosses:
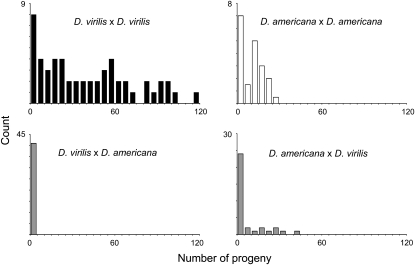
To begin to characterize reproductive isolation between D. virilis and D. americana, I compared the number of progeny that result from crosses within and between species (Figure 2). In 90% of intraspecific crosses between two D. virilis females and one D. virilis male, progeny are produced (N = 54). Of these successful D. virilis crosses, most produce many offspring (mean ± SE = 45.3 ± 8.6). Despite lower fecundity, a similar result is seen when two D. americana females are mated to a D. americana male: of the 84% of crosses that produce any offspring (N = 38), most result in at least several progeny (12.4 ± 1.4). In contrast, interspecific crosses produce fewer progeny. In crosses between two D. americana females and one D. virilis male, only 47% produce any offspring (N = 34). However, among those successful D. americana–D. virilis crosses, progeny numbers (13.8 ± 3.0) are comparable to crosses within D. americana. A more pronounced reduction in progeny number occurs in the reciprocal cross between two D. virilis females and one D. americana male: only 24% of crosses produce any offspring (N = 41). Among these successful D. virilis–D. americana crosses, the number of progeny is very low (1.2 ± 0.2).
Figure 2.—
Histograms of progeny number from vials with two females and one male for crosses within and between D. virilis and D. americana. D. virilis females produce significantly more progeny when crossed to D. virilis males than when crossed to D. americana males (Wilcoxon: Z = −7.53, P = 0.0001). Similarly, D. americana females produce significantly more progeny when crossed to D. americana males than when crossed to D. virilis males (Wilcoxon: Z = −2.34, P = 0.019).
Phenotypic basis of postmating reproductive isolation:
I next studied the phenotypic basis of reduced offspring production between D. virilis and D. americana. Low hybrid production might be caused by several potential reproductive barriers, including premating isolation, gametic isolation, or hybrid lethality. To distinguish among these possibilities, I mass mated 10 females and 20 males (within and between species) for 5 days, and then determined the rates of female insemination, egg hatch, and fertilization. At the end of the 5-day period, all 10 D. virilis females presented with conspecific males were always inseminated (N = 10 replicate crosses). Similarly, D. americana females presented with conspecific males were usually inseminated (8.30 ± 0.04, N = 10). Rates of insemination were also high in interspecific crosses: most D. virilis females presented with D. americana males were inseminated (8.41 ± 0.03, N = 10), as were D. americana females presented with D. virilis males (7.31 ± 0.08, N = 10). These results indicate that any potential for premating isolation between species is largely overcome during the 5-day mating period. Premating isolation is thus not the primary cause of reduced offspring production seen in crosses between D. virilis and D. americana.
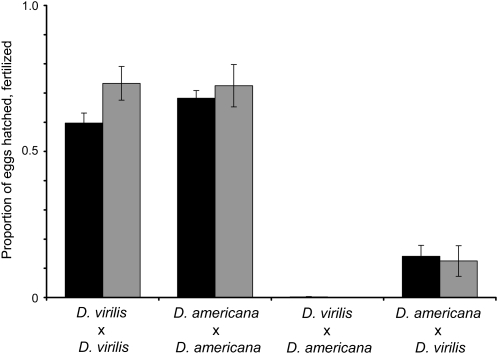
In contrast, egg hatch rates differed dramatically between conspecific and heterospecific treatments (Figure 3). For D. virilis females, egg hatch rate was much higher when mated to D. virilis males (0.597 ± 0.034) vs. D. americana males (0.002 ± 0.001; Wilcoxon: Z = 3.87, P = 0.0001). Similarly, for D. americana females, egg hatch rate was higher when mated to D. americana males (0.698 ± 0.026) vs. D. virilis males (0.141 ± 0.038; Wilcoxon: Z = −3.75, P = 0.0002). Because rates of female insemination were uniformly high and had no effect on egg hatch (data not shown), I investigated the possibility that a postmating, prezygotic barrier might prevent fertilization. For all crosses, the rate of egg fertilization mirrored the rate of egg hatch (Figure 3). Conspecific matings resulted in a high proportion of fertilized eggs for both D. virilis (0.733 ± 0.058) and D. americana (0.725 ± 0.073), whereas heterospecific matings resulted in few or no fertilized eggs (D. americana females × D. virilis males: 0.125 ± 0.053; D. virilis females × D. americana males: 0). Thus, the reduced offspring production between D. virilis and D. americana results from failed egg fertilization. Hybrid inviability appears to play little or no role in isolation: I never observed dead embryos, larvae, or pupae in interspecific crosses (embryonic lethality was assessed by searching for brown embryos). These findings imply that postmating, prezygotic isolation—but not postzygotic isolation—causes reduced offspring production between D. virilis and D. americana.
Figure 3.—
Egg hatch and fertilization rates from conspecific and interspecific crosses with D. virilis and D. americana. Solid bars show the mean proportion of hatched eggs from samples of 100 (N = 10 replicate crosses). Shaded bars show the mean proportion of fertilized eggs from samples of 10 (N = 8 replicate crosses). Bars denote standard errors.
In subsequent experiments, I focused on the direction of the cross showing the stronger postmating, prezygotic isolation: D. virilis females × D. americana males.
Genetic mapping of postmating, prezygotic isolation—D. virilis component:
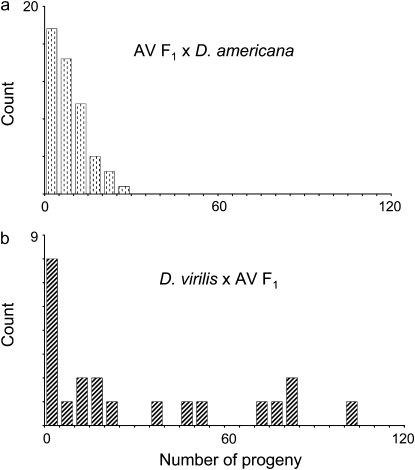
To determine the number, location, and dominance of genetic factors that contribute to the fertilization incompatibility between D. virilis females and D. americana males, I genetically mapped loci affecting offspring production. As a first step, I focused on the maternal component of the interaction. I examined the number of progeny that result from crosses between two D. americana–D. virilis F1 hybrid females and one D. americana male (Figure 4a). Progeny number from these and intraspecific D. americana crosses did not significantly differ (Wilcoxon: Z = 0.68, P = 0.49, compare Figure 4a to Figure 2). Indeed, 95% of the crosses between F1 females and D. americana males produced one or more progeny (N = 61). Of these successful crosses, some produced many offspring (9.2 ± 0.9). This result shows that a severe reduction in progeny number requires that the maternal parent be homozygous for D. virilis alleles at one or more loci.
Figure 4.—
Histograms of progeny number from pairs of males and females for crosses between (a) D. americana–D. virilis (AV) F1 females and D. americana males and (b) D. virilis females and D. americana–D.virilis (AV) F1 males.
To determine whether these isolation loci reside on one or more of the D. virilis chromosomes, I generated a population of D. virilis–backcross females using D. virilis–D. americana F1 hybrids as the paternal parents. Note that the lack of crossing over in Drosophila males means that single markers identify species origin of whole chromosomes. Backcross females heterozygous for chromosomes 2 and 3 (which do not segregate independently in this cross, see Figure 1) had significantly more progeny (7.8 ± 1.5) when paired with D. americana males than did females homozygous for D. virilis chromosomes 2 and 3 (0.5 ± 0.8; Wilcoxon: Z = 4.48, P < 0.0001, N = 40). Neither chromosome 4 nor 5 had a significant effect on progeny number (Wilcoxon: Z = −1.86, P = 0.06 and Z = 0.40, P = 0.69 for chromosomes 4 and 5, respectively). Note that the mean number of progeny from backcross females heterozygous for chromosomes 2 and 3 is similar to that from F1 females (7.8 ± 1.5 vs. 8.7 ± 0.8). The phenotypic effect of the X chromosome is therefore likely modest or negligible; each backcross female is homozygous for the D. virilis X chromosome. Thus, it is clear that low hybrid production in crosses between D. virilis females and D. americana males is largely due to recessive factors on chromosomes 2 and/or 3 in the maternal parent.
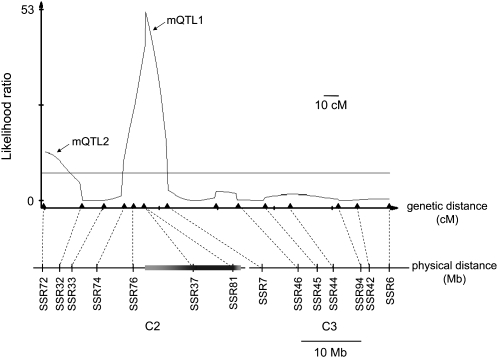
To genetically dissect the effect of chromosomes 2 and 3, I generated a mapping population (N = 368) of D. virilis–backcross females, this time using D. americana–D. virilis F1 hybrids as maternal parents to allow recombination. Note that because chromosomes 2 and 3 are fused in D. americana, they form a single linkage group (with recombination rates between the fused and unfused homologous chromosomes that are apparently normal). I used a quantitative trait locus (QTL) mapping approach to identify genomic regions that contribute to offspring production in backcross females presented with two D. americana males (Figure 5). Incompatibility loci mapped to two regions on chromosome 2. A highly significant QTL (maternal QTL1, hereafter mQTL1) mapped to two genetically inseparable microsatellite markers that reside in an inverted region; this inversion accounts for roughly half the physical length of chromosome 2 (Hughes 1939). A second maternal QTL (mQTL2) mapped to the distal end of chromosome 2.
Figure 5.—
Genetic dissection of the effect of chromosomes 2 and 3 on progeny number from D. virilis–D. americana crosses. Likelihood ratio (LR) test statistic profile from composite interval mapping (CIM) of progeny number in the D. virilis–backcross female mapping population. A horizontal line marks the LR significance threshold of 7.8. The genetic positions of molecular markers are indicated by triangles, and the corresponding physical locations along chromosomes 2 and 3 (based on the D. virilis genome assembly) are indicated below by vertical bars. The shaded horizontal bar on chromosome 2 denotes an inverted region, with lighter shading representing uncertainty in its precise physical location.
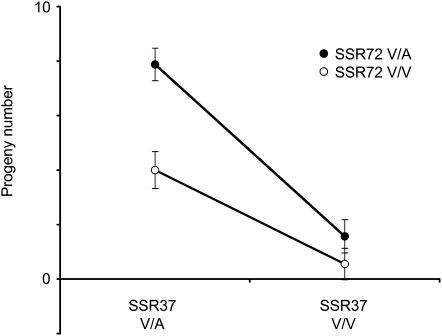
To examine the QTL effects, I measured the contribution of genotypic variation at the marker most tightly linked to each QTL (SSR37 and SSR72 for mQTL1 and 2, respectively) to offspring production (Figure 6). The number of progeny produced by D. virilis–backcross females was significantly affected by mQTL1 (ANOVA: F = 61.87, P < 0.0001), mQTL2 (F = 15.55, P < 0.0001), and the interaction between the two QTL (F = 5.35, P = 0.02). On average, females heterozygous for markers linked to both QTL produced roughly the same number of progeny as F1 hybrid females (7.9 ± 0.6 vs. 8.7 ± 0.8), whereas females homozygous for D. virilis alleles at both QTL-linked markers produced almost no progeny (0.55 ± 0.58). Note that backcross females homozygous for D. virilis alleles at mQTL1 had very few progeny, regardless of their genotypes at mQTL2 (Figure 6). Moreover, this major-effect mQTL1 is not associated with hybrid female sterility: females homozygous for the D. virilis chromosomal arrangement containing mQTL1 (identified by the visible marker b) produce many offspring when mated to D. virilis males (25.0 ± 3.4, N = 41). Instead, mQTL1 likely contributes to the fertilization incompatibility between D. virilis females and D. americana males. (However, note that these experiments cannot rule out the possibility that mQTL2 affects hybrid female fertility.)
Figure 6.—
Least square means of progeny number vary among SSR72–SSR37 genotypes in D. virilis–backcross females (N = 368). Heterozygous genotypes are indicated by “V/A” and homozygous D. virilis genotypes are indicated by “V/V”. Bars indicate standard errors.
The fact that mQTL1 maps to an inversion precludes further genetic mapping in this region, but future genetic dissection of mQTL2 might reveal one or more of the maternal genes responsible for the D. virilis–D. americana fertilization incompatibility. In addition, further progress may be possible in identifying partner loci from D. americana.
Genetic mapping of postmating, prezygotic isolation—D. americana component:
I next characterized the genetic basis of the paternal contribution to the D. virilis–D. americana fertilization incompatibility. First, I tested the potential for D. americana–D. virilis F1 hybrid males to sire offspring when presented with two D. virilis females (Figure 4b). The majority of these crosses produce offspring (71%, N = 21). Among these successful crosses, average progeny number is high (43.5 ± 8.6). Indeed, progeny production from D. virilis females mated to F1 males does not significantly differ from that of conspecific D. virilis crosses (Wilcoxon: Z = −1.48, P = 0.138, compare Figure 4b to Figure 2). This result suggests that the paternal contribution to the fertilization incompatibility acts recessively: a reduction in D. virilis–D. americana hybrid progeny number requires that the male parent be homozygous for D. americana alleles at one or more loci.
To localize these D. americana factors, I generated D. americana–backcross males using D. virilis–D. americana F1 hybrids as the paternal parents. D. virilis females had significantly more progeny when presented with backcross males heterozygous for chromosome 5 (12.3 ± 3.1) than with males homozygous for D. americana (0.8 ± 0.2; Wilcoxon: Z = −3.73, P = 0.0002, N = 46). A more modest effect was seen for chromosomes 2 and 3: D. virilis females had significantly more progeny when crossed to males heterozygous for these chromosomes (11.1 ± 3.2) than to males homozygous for D. americana (2.4 ± 0.9; Wilcoxon: Z = −2.62, P = 0.009). In contrast, chromosome 4 had no significant phenotypic effect (Wilcoxon: Z = −0.33, P = 0.74). Similarly, neither the X nor Y chromosome appears to contribute to the D. americana component of the fertilization incompatibility: despite the fact that D. americana–backcross males carry the D. americana X and Y chromosomes, several vials produced >10 progeny. In addition, D. virilis females can produce many progeny when paired with either D. americana–D. virilis F1 or D. virilis–D. americana F1 males (Figure 4 and data not shown), which carry a D. americana X and Y chromosome, respectively. Thus, it is clear that the paternal contribution to D. virilis–D. americana isolation is caused by two or more recessive factors on chromosomes 2–3 and 5.
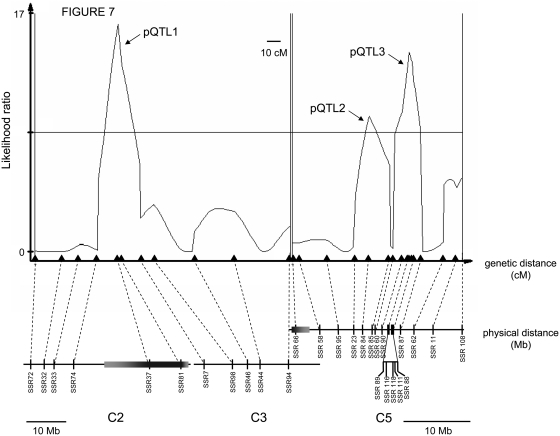
To map isolation loci on chromosomes 2, 3, and 5, I generated a mapping population (N = 368) of D. americana–backcross males, using D. americana–D. virilis F1 hybrids as the maternal parents. Each recombinant male was then presented with two D. virilis females. I excluded males that were sterile (7.8% of backcross males) or found dead after the mating period (modified mapping population: N = 326). Incompatibility loci mapped to three regions (Figure 7). One highly significant paternal QTL mapped to the inverted region of chromosome 2 (pQTL1). Two additional QTL mapped to a region corresponding to roughly 10 Mb on chromosome 5 (pQTL2 and pQTL3).
Figure 7.—
Genetic dissection of the effect of chromosomes 2, 3, and 5 on progeny number from D. virilis–D. americana crosses. Likelihood ratio (LR) test statistic profile from composite interval mapping of progeny number in the D. americana–backcross male mapping population. A horizontal line marks the LR significance threshold of 8.5. The genetic positions of molecular markers are indicated by triangles, and the corresponding physical locations along chromosomes 2, 3, and 5 (based on the D. virilis genome assembly) are indicated below by vertical bars. Shaded horizontal bars on chromosomes 2 and 5 denote inverted regions, with lighter shading representing uncertainty in precise physical position. Note that physical distances for chromosomes 2–3 and 5 are shown at different scales.
The number of progeny produced by D. americana–backcross males was significantly affected by an interaction among the three molecular markers most tightly linked to each QTL (SSR81 for pQTL1, SSR84 for pQTL2, and SSR116 for pQTL3; ANOVA: F = 4.01, P = 0.046). Backcross males heterozygous at markers tightly linked to all three QTL sired more than 30 times the number of progeny as males homozygous for D. americana alleles (13.6 ± 1.1 vs. 0.4 ± 1.5 for heterozygotes and homozygotes, respectively). However, because F1 males presented with D. virilis females sire more offspring than these triple heterozygotes (31.1 ± 7.5 vs. 13.6 ± 1.1), it is possible that additional small-effect modifier loci are involved. Note that these QTL are not associated with hybrid male sterility: with only one exception, backcross male genotype at QTL-linked markers SSR81, SSR84, and SSR116 had no significant effect (individually or in combination) on progeny number when males were mated to D. americana females (ANOVA: FSSR81 = 0.004, P = 0.947; FSSR84 = 0.03, P = 0.868; FSSR116 = 0.40, P = 0.529, N = 61; interaction statistics not shown). The one exception involved a significant interaction between markers linked to QTL2 and 3 (ANOVA: F = 5.42, P = 0.024, N = 61); however, individuals homozygous for D. americana alleles at these QTL actually produced more progeny than heterozygotes.
Because pQTL1 maps to an inversion on chromosome 2, further genetic dissection of this region is not possible. For chromosome 5, however, the current genetic analyses have already localized two or more of the genes causing D. virilis–D. americana isolation to a region of only 10 Mb; fine-mapping pQTL2 and pQTL3 should be possible in this highly recombining region.
DISCUSSION
Here I have characterized the phenotypic and genetic basis of strong postmating, prezygotic reproductive isolation between two closely related species of Drosophila, D. virilis and D. americana. With respect to the phenotypic basis of isolation, I have shown that normal fertilization is disrupted in crosses between these species. Whereas D. virilis and D. americana females mated to conspecific males begin to lay fertilized eggs within a few hours of insemination, females mated to heterospecific males lay almost no fertilized eggs. This difference is not due to premating isolation; I found high rates of heterospecific mating, and females stored heterospecific sperm for at least 24 hr. Instead, D. americana sperm is apparently incompatible with the D. virilis female reproductive tract and/or egg (as is D. virilis sperm with a D. americana female, though to a far lesser degree). A similar, though less severe, fertilization incompatibility has been observed between races of D. melanogaster (Alipaz et al. 2001). Although further studies will be needed to identify the precise timing and mechanism of D. virilis–D. americana reproductive isolation, it is clear that this fertilization incompatibility represents a strong barrier to interspecific hybridization: D. virilis females mated to D. americana males produced <1% of the offspring of those mated to D. virilis males. These findings agree with results from the classic experiments of Patterson et al. (1942), which showed that no matter which of several wild-collected strains were used, egg hatch rate was invariably low (usually much less than 10%) from crosses between D. virilis females and D. americana males.
To characterize the genetic basis of this fertilization incompatibility, I have performed several mapping experiments. I have found that isolation between D. virilis females and D. americana males involves a genetic incompatibility among recessive, heterospecific alleles at loci that map to only four autosomal regions. It is thus possible that postmating, prezygotic isolation between D. virilis and D. americana may result from a relatively simple genetic interaction.
The maternal component of the fertilization incompatibility maps to only two QTL on chromosome 2; however, each QTL corresponds to a large genomic region that might contain more than one isolation locus. The paternal component of D. virilis–D. americana isolation maps to three QTL: pQTL1 localizes to the same chromosome 2 inversion as mQTL1, whereas both pQTL2 and pQTL3 map to a large, collinear region of chromosome 5. Because marker density is high on chromosome 5 (see Figure 7), future experiments to identify the paternal component genes will focus on generating additional recombinants in the relevant region. In any case, given that entire chromosomes have no detectable effect on reproductive isolation, it is unlikely that the D. virilis–D. americana fertilization incompatibility is highly polygenic. In contrast, reproductive isolation due to competitive gametic interactions (i.e., competitive sperm/pollen precedence) has been shown to have a polygenic basis in other Drosophila species, crickets, and monkeyflowers (Civetta et al. 2002; Britch et al. 2007; Fishman et al. 2008).
A primary goal of speciation research is to identify the evolutionary processes within populations and species that eventually give rise to isolating barriers. Because the D. virilis–D. americana fertilization incompatibility is due to an interaction between male and female genotypes, it is almost certainly a result of coevolution between the sexes, potentially caused by sexual conflict (Rice 1996) and/or cryptic female choice (Eberhard 1996). By examining results from classic and recent crossing experiments with D. novamexicana, it is possible to infer the history of male–female coevolution in the D. virilis species group. With respect to the fertilization incompatibility, D. novamexicana is phenotypically similar to D. virilis despite a much closer evolutionary relationship with D. americana. Crosses between D. virilis females and D. novamexicana males produce many progeny (Patterson and Stone 1949), suggesting that D. americana alleles at paternal loci are derived. Moreover, crosses between D. novamexicana females and D. americana males show a fertilization incompatibility (Y. Ahmed and B. McAllister, personal communication), suggesting that D. americana alleles at the maternal loci are also derived. Interestingly, D. virilis and D. novamexicana are collinear for the region of chromosome 2 that contains mQTL1 and pQTL1, whereas D. americana is fixed for an inversion. However, the evolutionary history of this chromosomal region is more complicated than it first appears; D. americana and D. novamexicana share the same initial chromosome 2 inversion, but D. novamexicana carries an additional, unique inversion that reverses this genomic region to the standard D. virilis arrangement (Hsu 1952). An intriguing possibility is that these chromosome 2 inversions might have promoted a genetic correlation through physical linkage between maternal and paternal fertilization factors, thereby facilitating their coevolution (as suggested for mating signal and preference loci; Kronforst et al. 2006; Shaw and Lesnick 2009).
Taken together, these results suggest that male–female coevolution within D. americana may have given rise to postmating, prezygotic isolation between species. This evolutionary change might have been rapid: D. americana and D. novamexicana likely diverged <400,000 years ago (Morales-Hojas et al. 2008). My crossing experiments also showed a milder fertilization incompatibility between D. americana females and D. virilis males. A key question for future studies is whether the genetic basis for reproductive isolation is the same in reciprocal crosses, or alternatively, whether multiple, independent bouts of coevolution in the D. virilis species group may have given rise to multiple genetic incompatibilities.
Acknowledgments
I am grateful to Bryant McAllister for generously providing the wild-collected strain of D. americana. I thank Y. Ahmed, D. McNabney, C. Meiklejohn, D. Presgraves, and especially H. A. Orr for helpful discussions about this project. I also thank Y. Ahmed, D. Begun, M. V. Cattani, D. McNabney, C. Meiklejohn, H. A. Orr, D. Presgraves, R. Unckless, and two anonymous reviewers for helpful comments on a draft of this article. This research was supported by funds from a National Institutes of Health Ruth L. Kirschstein national research service award postdoctoral fellowship (GM-078974) to the author and by funds from the National Institutes of Health (GM-51932) to H. A. Orr.
References
- Alipaz, J. A., C.-I. Wu and T. L. Karr, 2001. Gametic incompatibilities between races of Drosophila melanogaster. Proc. R. Soc. Lond. Ser. B 268 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, G., 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britch, S. C., E. J. Swartout, D. D. Hampton, M. L. Draney, J. Chu et al., 2007. Genetic architecture of conspecific sperm precedence in Allonemobius fasciatus and A. socius. Genetics 176 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta, A., and R. S. Singh, 1995. High divergence of reproductive tract proteins and their association with postzygotic reproductive isolation in Drosophila melanogaster and Drosophila virilis groups species. J. Mol. Evol. 41 1085–1095. [DOI] [PubMed] [Google Scholar]
- Civetta, A., H. M. Waldrip-Dail and A. G. Clark, 2002. An introgression approach to mapping differences in mating success and sperm competitive ability in Drosophila simulans and D. sechellia. Genet. Res. 79 65–74. [DOI] [PubMed] [Google Scholar]
- Clark, N. L., J. E. Aagaard and W. J. Swanson, 2006. Evolution of reproductive proteins from animals and plants. Reproduction 131 11–22. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1984. Genetic basis of male sterility in hybrids between two closely related species of Drosophila. Proc. Natl. Acad. Sci. USA 81 4444–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, C., 1871. The Descent of Man, and Selection in Relation to Sex. Princeton University Press, Princeton.
- Dorus, S., P. D. Evans, G. J. Wyckoff, S. S. Choi and B. T. Lahn, 2004. Rate of molecular evolution of the seminal protein gene SEMG2 correlates with levels of female promiscuity. Nat. Genet. 36 1326–1329. [DOI] [PubMed] [Google Scholar]
- Eberhard, W. G., 1996. Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press, Princeton.
- Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Clarenden Press, Oxford.
- Fishman, L., J. Aagaard and J. C. Tuthill, 2008. Toward the evolutionary genomics of gametophytic divergence: patterns of transmission ratio distortion in monkeyflower (Mimulus) hybrids reveal a complex genetic basis for conspecific pollen precedence. Evolution 62 2958–2970. [DOI] [PubMed] [Google Scholar]
- Galindo, B. E., V. D. Vacquier and W. J. Swanson, 2003. Positive selection in the egg receptor for abalone sperm lysin. Proc. Natl. Acad. Sci. USA 100 4639–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor, G. B., and W. R. Engels, 1992. Single-fly DNA preps for PCR. Dros. Inf. Serv. 71 148–149. [Google Scholar]
- Haerty, W., S. Jagadeeshan, R. J. Kulathinal, A. Wong, K. Ravi Ram et al., 2007. Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics 177 1321–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, D. J., 1999. Conspecific sperm and pollen precedence and speciation. Annu. Rev. Ecol. Syst. 30 109–132. [Google Scholar]
- Hsu, T. C., 1952. Chromosomal variation and evolution in the virilis group of Drosophila. Univ. Texas Publ 5204 35–72. [Google Scholar]
- Hughes, R. D., 1939. An analysis of the chromosomes of the two sub-species Drosophila virilis virilis and Drosophila virilis americana. Genetics 24 811–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher, E. S., W. J. Swanson and T. A. Markow, 2007. Gene duplication and adaptive evolution of digestive proteases in Drosophila female reproductive tracts. PLoS Genet. 3 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles, L. L., and T. A. Markow, 2001. Sexually antagonistic coevolution of a postmating-prezygotic reproductive character in desert Drosophila. Proc. Natl. Acad. Sci. USA 98 8692–8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst, M. R., L. G. Young, D. D. Kapan, C. McNeely, R. J. O'Neill et al., 2006. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl. Acad. Sci. USA 103 6575–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak, M. K., and D. J. Begun, 2007. Molecular population genetics of female-expressed mating-induced serine proteases in Drosophila melanogaster. Mol. Biol. Evol. 24 1944–1951. [DOI] [PubMed] [Google Scholar]
- McAllister, B. F., 2002. Chromosomal and allelic variation in Drosophila americana: selective maintenance of a chromosomal cline. Genome 45 13–21. [DOI] [PubMed] [Google Scholar]
- McAllister, B. F., and A. L. Evans, 2006. Increased nucleotide diversity with transient Y linkage in Drosophila americana. PLoS ONE 1 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. T., and S. Pitnick, 2002. Sperm-female coevolution in Drosophila. Science 298 1230–1233. [DOI] [PubMed] [Google Scholar]
- Morales-Hojas, R., C. P. Vieira and J. Vieira, 2008. Inferring the evolutionary history of Drosophila americana and Drosophila novamexicana using a multilocus approach and the influence of chromosomal rearrangements in single gene analyses. Mol. Ecol. 17 2910–2926. [DOI] [PubMed] [Google Scholar]
- Panhuis, T. M., and W. J. Swanson, 2006. Molecular evolution and population genetic analysis of candidate female reproductive genes in Drosophila. Genetics 173 2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, J. T., and W. S. Stone, 1949. The relationship of novamexicana to the other members of the virilis group. Univ. Texas Publ. 4920 7–17. [Google Scholar]
- Patterson, J. T., and W. S. Stone, 1952. Evolution in the Genus Drosophila. Macmillan, New York.
- Patterson, J. T., W. S. Stone and R. K. Griffin, 1942. Genetic and cytological analysis of the virilis species group. Univ. Texas Publ. 4228 162–200. [Google Scholar]
- Pitnick, S., T. Markow and G. S. Spicer, 1997. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution 53 1804–1822. [DOI] [PubMed] [Google Scholar]
- Pitnick, S., M. F. Wolfner and S. S. Suarez, 2007. Ejaculate-female and sperm-female interactions, pp. 247–304 in Sperm Evolution, edited by T. R. Birkhead, D. Hosken and S. Pitnick. Academic Press, Oxford.
- Prokupek, A., F. Hoffmann, S. Eyun, E. Moriyama, M. Zhou et al., 2008. An evolutionary expressed sequence tag analysis of Drosophila spermatheca genes. Evolution 62 2936–2947. [DOI] [PubMed] [Google Scholar]
- Price, C. S. C., 1997. Conspecific sperm precedence in Drosophila. Nature 388 663–666. [DOI] [PubMed] [Google Scholar]
- Price, C. S. C., C. H. Kim, J. Posluszny and J. A. Coyne, 2000. Mechanisms of conspecific sperm precedence in Drosophila. Evolution 54 2028–2037. [DOI] [PubMed] [Google Scholar]
- Rice, W. R., 1996. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381 232–234. [DOI] [PubMed] [Google Scholar]
- Ritchie, M. G., 2007. Sexual selection and speciation. Annu. Rev. Ecol. Syst. 38 79–102. [Google Scholar]
- Rozen, S., and H. J. Skaletsky, 2000. Primer3 on the WWW for general users and for biologist programmers, pp. 365–386 in Bioinformatics Methods and Protocols: Methods in Molecular Biology, edited by S. Krawetz and S. Misener. Humana Press, Totowa, NJ. [DOI] [PubMed]
- Shaw, K. L., and S. C. Lesnick, 2009. Genomic linkage of male song and female acoustic preference QTL underlying a rapid species radiation. Proc. Natl. Acad. Sci. USA 106 9737–9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker, H. D., 1942. Sexual isolation studies in the species complex Drosophila virilis. Genetics 27 238–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. J., and V. D. Vacquier, 2002. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 3 137–144. [DOI] [PubMed] [Google Scholar]
- Swanson, W. J., A. G. Clark, H. M. Waldrip-Dail, M. F. Wolfner and C. F. Aquadro, 2001. a Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl. Acad. Sci. USA 98 7375–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. J., Z. Yang, M. F. Wolfner and C. F. Aquadro, 2001. b Positive Darwinian selection drives the evolution of several female reproductive proteins in mammals. Proc. Natl. Acad. Sci. USA 98 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throckmorton, L. H., 1982. The virilis species group, pp. 227–296 in The Genetics and Biology of Drosophila, Vol. 3b, edited by M. Ashburner, H. L. Carson and J. N. Thompson, Jr. Academic Press, London.
- Turner, L. M., E. B. Chuong and H. E. Hoekstra, 2008. Comparative analysis of testis protein evolution in rodents. Genetics 179 2075–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen, J. W., 2006. JoinMap 4, software for the calculation of genetic linkage maps in experimental populations. Kyazma, Wageningen, The Netherlands.
- Wagstaff, B. J., and D. J. Begun, 2005. Molecular population genetics of accessory gland protein genes and testis-expressed genes in Drosophila mojavensis and D. arizonae. Genetics 171 1083–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., C. J. Basten and Z.-B. Zeng, 2007. Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC.
- Wheeler, M. R., 1947. The insemination reaction in intraspecific matings of Drosophila. Univ. Texas Publ. 4720 78–115. [Google Scholar]
- Wu, C.-I., and A. W. Davis, 1993. Evolution of postmating reproductive isolation—the composite nature of Haldane's rule and its genetic bases. Am. Nat. 142 187–212. [DOI] [PubMed] [Google Scholar]
- Wyckoff, G. J., W. Wang and C.-I. Wu, 2000. Rapid evolution of male reproductive genes in the descent of man. Nature 403 304–309. [DOI] [PubMed] [Google Scholar]
- Zeng, Z. B., 1993. Theoretical basis for separation of multiple linked gene effects in mapping quantitative trait loci. Proc. Natl. Acad. Sci. USA 90 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Z. B., 1994. Precision mapping of quantitative trait loci. Genetics 136 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]