Abstract
Accumulating evidence indicates that there is extensive crosstalk between integrins and TGF-β signalling. TGF-β affects integrin-mediated cell adhesion and migration by regulating the expression of integrins, their ligands and integrin-associated proteins. Conversely, several integrins directly control TGF-β activation. In addition, a number of integrins can interfere with both Smad-dependent and Smad-independent TGF-β signalling in different ways, including the regulation of the expression of TGF-β signalling pathway components, the physical association of integrins with TGF-β receptors and the modulation of downstream effectors. Reciprocal TGF-β–integrin signalling is implicated in normal physiology, as well as in a variety of pathological processes including systemic sclerosis, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease and cancer; thus, integrins could provide attractive therapeutic targets to interfere with TGF-β signalling in these processes.
Keywords: integrin, TGF-β, fibrosis, cancer, wound healing
See Glossary for abbreviations used in this article.
Glossary.
- βig-h3
TGF-β-inducible gene-h3
extracellular matrix
epithelial-to-mesenchymal transition
focal adhesion kinase
human epidermal growth factor receptor 2
integrin-linked kinase
latency-associated protein
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- ECM
extracellular matrix
epithelial-to-mesenchymal transition
focal adhesion kinase
human epidermal growth factor receptor 2
integrin-linked kinase
latency-associated protein
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- EMT
epithelial-to-mesenchymal transition
focal adhesion kinase
human epidermal growth factor receptor 2
integrin-linked kinase
latency-associated protein
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- FAK
focal adhesion kinase
human epidermal growth factor receptor 2
integrin-linked kinase
latency-associated protein
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- HER2
human epidermal growth factor receptor 2
integrin-linked kinase
latency-associated protein
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- ILK
integrin-linked kinase
latency-associated protein
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- LAP
latency-associated protein
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- LTBP
latent TGF-β binding protein
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- MAPK
mitogen-activated protein kinase
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- MMP
matrix metalloproteinase
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- PAR1
protease-activated receptor 1
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- PI(3)K
phosphatidylinositol-3-kinase
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- RGD
arginine–glycine–aspartate
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- ROCK
Rho-associated kinase
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- TGF-β
transforming growth factor-β
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- TGF-βRI
transforming growth factor-β type I receptor
transforming growth factor-β type II receptor
- TGF-βRII
transforming growth factor-β type II receptor
Introduction
Integrins—which consist of an α-subunit and a β-subunit—constitute a family of transmembrane receptors that bind extracellularly to the ECM and intracellularly to the cytoskeleton, thereby ‘integrating' the extracellular environment with the cell interior (Hynes, 2002; Legate et al, 2009). Integrins transduce signals from the outside into the cell and vice versa to regulate cell adhesion and cell spreading, as well as migration, proliferation, differentiation and remodelling of the ECM. In addition, integrins can modulate the signalling cascade elicited by several growth factors, including TGF-β. The TGF-β isoforms TGF-β1, TGF-β2 and TGF-β3 are pleiotropic cytokines that mediate a variety of effects on a range of cell types. TGF-βs bind to a heterodimeric serine/threonine kinase receptor complex—which consists of TGF-βRI and TGF-βRII—leading to the recruitment and phosphorylation of the intracellular effector proteins Smad2 and Smad3. Phosphorylated Smad2 and Smad3 subsequently bind to Smad4 and translocate to the nucleus to initiate gene expression. TGF-β signalling is negatively regulated by inhibitory Smads, including Smad6 and Smad7 (Massagué & Chen, 2000). In addition, TGF-β can affect numerous signal transduction pathways in a Smad-independent manner. Although the effect of TGF-β signalling depends on the context and cell type, TGF-β clearly controls a vast number of transcriptional targets, many of which are integrins and their ligands. The connection between integrins and TGF-β is therefore bidirectional, and it is becoming increasingly clear that it is relevant in many physiological and pathological phenomena. Here, we discuss the integrin–TGF-β interplay and highlight its importance in fibrosis, cancer and wound repair.
Integrin regulation by TGF-β
TGF-β controls the transcription of genes that encode numerous integrins (Table 1) in several cell types and tissues, as well as in various human cancers. Although the downregulation of integrin expression—mostly laminin receptors—has also been reported, in most cases TGF-β stimulates integrin expression. Intriguingly, the induction of integrin expression by TGF-β can be driven by cooperative signalling between the integrin and TGF-β, thereby creating a feedforward loop (Pechkovsky et al, 2008). TGF-β not only regulates the expression of integrin ligands—including βig-h3, tenascin, vitronectin, fibronectin, and several members of the laminin and collagen families—but also stimulates the expression of integrin-associated proteins—including disabled 2, ILK, kindlin 1, paxillin and PINCH—which could increase integrin activation. Therefore, the transcriptional control exerted by TGF-β can strongly affect integrin-mediated processes. Finally, TGF-β could also directly regulate integrin activation, by a still unidentified ‘inside–out' mechanism (Fransvea et al, 2009).
Table 1.
Overview of the regulation of integrin expression by TGF-β
| Integrin | Main ligand | Effect of TGF-β | Cell type | Context |
|---|---|---|---|---|
| α1β1 | Collagens | Upregulation | Fibroblasts | Collagen remodelling and contraction, myofibroblast differentiation during wound healing and fibrosis |
| α2β1 | Collagens | Upregulation, downregulation | Keratinocytes, fibroblasts | Collagen remodelling and contraction, myofibroblast differentiation during wound healing and fibrosis, re-epithelialization during wound healing |
| α3β1 | Laminins | Upregulation, downregulation | Keratinocytes, fibroblasts, carcinoma cells, lung alveolar epithelial cells | Re-epithelialization during wound healing, EMT, cancer cell migration and invasion |
| α5β1 | Fibronectin | Upregulation | Keratinocytes, fibroblasts, carcinoma cells, endothelial cells | Re-epithelialization during wound healing, EMT, cancer cell migration and invasion, endothelial cell migration and tube formation |
| α6β1 | Laminins | Upregulation | Carcinoma cells, lung alveolar epithelial cells, promonocytic leukaemia cells | Macrophage maturation, cancer cell migration and invasion |
| α8β1 | RGD | Upregulation | Fibroblasts, vascular smooth muscle cells | Myofibroblast differentiation, vascular smooth muscle cell contraction |
| α6β4 | Laminins | Upregulation, downregulation | Keratinocytes, carcinoma cells | Re-epithelialization during wound healing, EMT, cancer cell migration and invasion |
| αvβ3 | RGD | Upregulation | Fibroblasts, carcinoma cells, endothelial cells | Myofibroblast differentiation during wound healing and fibrosis, angiogenesis, carcinoma cell migration and invasion |
| αvβ5 | RGD | Upregulation | Keratinocytes, fibroblasts | Myofibroblast differentiation during fibrosis, re-epithelialization during wound healing, EMT, cancer cell migration and invasion |
| αvβ6 | RGD | Upregulation | Keratinocytes, fibroblasts, carcinoma cells, | Myofibroblast differentiation during fibrosis and in tumours, re-epithelialization during wound healing, EMT, cancer cell migration and invasion |
| αLβ2 | ICAM1 | Upregulation | Promonocytic leukaemia cells | Macrophage maturation |
| αΕβ7 | E-cadherin | Upregulation | T lymphocytes | T-lymphocyte infiltration into epithelia |
EMT, epithelial-to-mesenchymal transition; ICAM1, intercellular adhesion molecule 1; RGD, arginine–glycine–aspartate; TGF-β, transforming growth factor-β.
Regulation of TGF-β activation by integrins
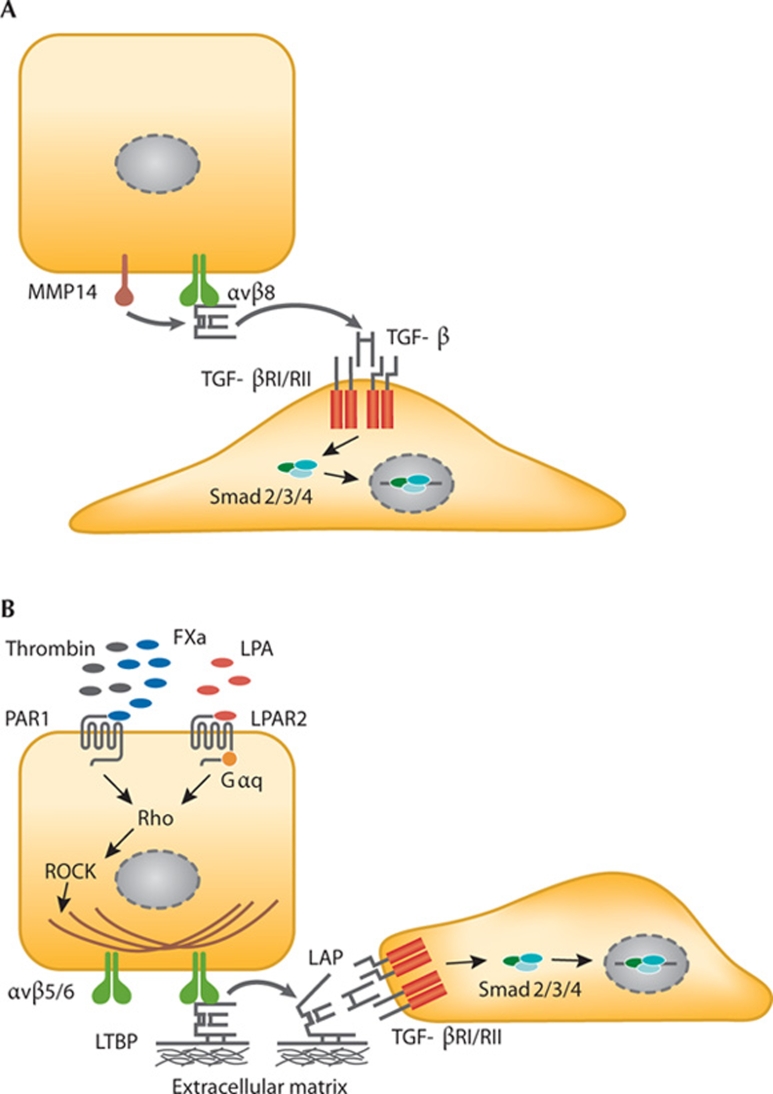
TGF-β is secreted in an inactive (latent) form in a complex with two proteins—LAP and LTBP. Its activation requires the dissociation from the complex, which occurs at low pH or through the action of reactive oxygen species, proteases, thrombospondin 1 or several integrins. The LAPs of TGF-β1 and TGF-β3—but not of TGF-β2—contain an RGD motif that can potentially be bound by the five αv-containing integrins and αIIbβ3, α5β1 and α8β1. Integrin binding to LAP has been demonstrated formally for α8β1 and all αv-integrins, although binding of α8β1 does not seem to lead to activation, and whether αvβ1 can activate TGF-β is also unclear (Table 2; Munger et al, 1999; Lu et al, 2002; Ludbrook et al, 2003). Integrin-mediated TGF-β activation seems to be possible in a protease-dependent or protease-independent manner. Protease-dependent TGF-β activation has only been demonstrated for αvβ8 and depends on the binding of the integrin to the RGD site in LAP and simultaneous recruitment of MMP14, which then releases TGF-β by proteolytic cleavage (Fig 1A; Mu et al, 2002). This mode of activation does not require that the activating cell and target cell be in close proximity. Interestingly, αvβ3 can be a docking site for MMP2 and MMP9 (Brooks et al, 1996; Rolli et al, 2003), although whether this also leads to proteolytic activation of TGF-β remains to be seen. Notably, the genes for these MMPs are TGF-β targets and, therefore, a self-amplifying TGF-β feedforward loop could be envisioned. Non-proteolytic TGF-β activation occurs through cell traction forces exerted by the actin cytoskeleton. These forces are translated by integrins into a conformational change of the TGF-β–LAP–LTBP complex, leading to the presentation of active TGF-β to its receptor (Annes et al, 2004; Fontana et al, 2005; Wipff et al, 2007; Wipff & Hinz, 2008). Hence, non-proteolytic activation requires cytoskeletal integrity, the connection of the β-tail of the integrin to the cytoskeleton, a mechanically resistant matrix, the interaction between LAP and the ECM through LTBP (Fig 1B), and that the target cell be in the direct vicinity of the activating cell. Non-proteolytic activation has been demonstrated in vitro for αvβ3, αvβ5 and αvβ6, as well as for a β1-integrin with a still unidentified α-subunit (Wipff et al, 2007). Whether or not the activation of TGF-β by a β1-integrin is relevant physiologically remains controversial.
Table 2.
Overview of integrin-mediated TGF-β activation and signalling
| Integrin | Regulation of TGF-β activation or signalling | Context |
|---|---|---|
| αvβ1 | Binding of LAP1 and LAP3, activation of TGF-β is unclear | NA |
| αvβ3 | TGF-β activation in vitro, modulation of TGF-β signalling by physical association with TGF-βRII, control of expression of TGF-βRI and II | Regulation of granulation tissue during wound healing, carcinoma cell migration and invasion, possible role in SS/scleroderma |
| αvβ5 | TGF-β activation in vitro and in vivo, enhancement of TGF-β signalling by physical association with TGF-βRII | Pulmonary fibrosis, possible role in SS/scleroderma |
| αvβ6 | TGF-β activation in vitro and in vivo | Development, IPF, kidney and renal fibrosis, SS, wound healing, EMT, carcinoma migration and invasion |
| αvβ8 | TGF-β activation in vitro and in vivo | Development, suppression of T-cell-mediated immunity, possible role in COPD or wound healing |
| α8β1 | Binding of LAP1 and LAP3, but no activation of TGF-β | NA |
| α5β1 | Control of TGF-βRII expression. NA, binding and activation of LAP | NA |
| α3β1 | Modulation of TGF-β signalling by enabling formation of a β-catenin–Smad2 complex, or by repressing Smad7 expression | EMT during IPF, re-epithelialization during wound healing? |
COPD, chronic obstructive pulmonary disease; EMT, epithelial-to-mesenchymal transition; IPF, idiopathic pulmonary fibrosis; LAP, latency-associated protein; NA, not assessed; SS, systemic sclerosis; TGF-β, transforming growth factor-β; TGF-βR, TGF-β receptor.
Figure 1.
TGF-β activation by integrins. (A) Protease-dependent activation by integrin αvβ8 and MMP14. (B) Protease-independent activation results from a conformational change of LAP–TGF-β induced by cell traction forces. FXa, coagulation factor X; Gαq, G protein αq; LAP, latency-associated protein; LPA, lysophosphatidic acid; LPAR2, lysophosphatidic acid receptor 2; LTBP, latent TGF-β binding protein; MMP14, matrix metalloproteinase 14; PAR1, protease-activated receptor 1; ROCK; Rho-associated kinase; TGF-β, transforming growth factor-β; TGF-βR, transforming growth factor-β receptor.
The activation of TGF-β by integrins can also be initiated by G-protein-coupled receptors. For example, the stimulation of PAR1 with thrombin leads to RhoA-dependent and ROCK-dependent TGF-β activation by integrin αvβ6 in vitro and in vivo (Jenkins et al, 2006). Similarly, PAR1 stimulation with coagulation factor X induces αvβ5-regulated TGF-β activation through ROCK signalling (Scotton et al, 2009). Furthermore, αvβ6-mediated TGF-β activation can be induced by lysophosphatidic acid signalling to RhoA and ROCK, through the lysophosphatidic acid receptor coupled to small G protein Gαq (Fig 1B; Xu et al, 2009). Whether other integrins mediate TGF-β activation through similar signalling pathways remains to be established.
The importance of integrin-mediated activation of TGF-β in vivo is evident, as mutation of the RGD site of LAP leads to defects similar to those observed in TGF-β1-null mice (Yang et al, 2007). In addition, genetic ablation of the β6-subunit, or conditional deletion of αv or β8 from dendritic cells, causes exaggerated inflammation as a result of impaired TGF-β signalling (Lacy-Hulbert et al, 2007; Travis et al, 2007). The phenotype of mice lacking both the αvβ6 and αvβ8 integrins recapitulates the abnormalities observed in TGF-β1 and TGF-β3—but not in TGF-β2—knockout mice, indicating that the integrins αvβ6 and αvβ8 can account for the full activation of TGF-β1 and TGF-β3 in vivo (Aluwihare et al, 2009). Indeed, mice lacking β3, β5, or both do not develop abnormalities similar to those due to deficient TGF-β signalling (Hodivala-Dilke et al, 1999; Huang et al, 2000; Reynolds et al, 2002). Nevertheless, αvβ3-mediated or αvβ5-mediated TGF-β activation could be important in pathological conditions, as increased expression of both of these integrins is observed in the dermis of scleroderma patients, and these integrins elicit autocrine TGF-β signalling in patient fibroblasts in vitro (Asano et al, 2005a, 2006a). In addition, TGF-β activation by αvβ5 is important in pulmonary fibrosis, as discussed below. However, a causal effect of αvβ3-mediated TGF-β activation in human pathology has not yet been established.
Regulation of TGF-β signalling by integrins
In addition to the direct activation of TGF-β, several integrins seem to influence TGF-β-induced signal transduction (Table 2). The effect is almost exclusively an amplification of the signal, that is, increased activation of signalling proteins and/or increased expression of TGF-β target genes. The regulation of TGF-β signalling by integrins occurs at several levels. Integrin-mediated adhesion can potentiate TGF-β-induced signalling and gene expression, in an analogous way to how integrins regulate growth factor signalling through receptor tyrosine kinases. Indeed, TGF-β-induced collagen expression through p42/p44 MAPK requires integrin-mediated FAK activation in mesangial cells (Hayashida et al, 2007). Furthermore, β1-integrins induce TGF-β-dependent p38 MAPK activity during EMT in mammary epithelial cells, and TGF-β-stimulated MMP9 expression in keratinocytes is enhanced by the integrin α3β1 (Bhowmick et al, 2001; Lamar et al, 2008).
Integrins can also indirectly control the expression of components of the TGF-β pathway. For example, the ectopic expression of the integrin α5-subunit induces TGF-βRII expression, which is potentiated further by α5β1 ligation to fibronectin, rendering cells responsive to TGF-β (Wang et al, 1999). In fibroblasts deficient in the integrin β3-subunit, TGF-β signalling is enhanced owing to an increased expression of both TGF-βRI and TGF-βRII, suggesting that the expression of these receptors is repressed by αvβ3 (Reynolds et al, 2005). In addition, TGF-β signalling is repressed in α3-deficient keratinocytes due to an elevated expression of the inhibitory Smad7, which could mean that α3β1 can downregulate Smad7 to enhance TGF-β signalling (Reynolds et al, 2008).
Integrins might also regulate TGF-β signalling synergistically, through their physical interaction with TGF-βRs. For example, TGF-β stimulation induces the association of integrin αvβ3 with TGF-βRII in both breast cancer cells and lung fibroblasts, initiating cooperative signalling to c-Src and MAPKs (Scaffidi et al, 2004; Galliher & Schiemann, 2006). Similarly, TGF-βRII associates with αvβ5 in sclerodermal fibroblasts, and integrin signalling through FAK is necessary for TGF-β-induced myofibroblastic differentiation (Asano et al, 2006b). Furthermore, α3β1 association with E-cadherin and TGF-βRs mediates the TGF-β-stimulated phosphorylation of β-catenin and its association with phosphorylated Smad2, as well as the subsequent nuclear translocation of the Smad2–β-catenin complex. Interestingly, both phenomena are independent of ligand binding by α3β1 (Kim et al, 2009a,b). Finally, in mammary epithelial cells overexpressing HER2, TGF-β stimulates integrin clustering with HER2 and their association with the cytoskeleton, leading to PI(3)K signalling through c-Src and FAK (Wang et al, 2009).
In conclusion, integrins can control TGF-β signalling directly by TGF-β activation, or indirectly by affecting Smad-dependent and Smad-independent signalling pathways at various levels (Table 2). Although the physiological relevance of some of the proposed mechanisms needs to be clarified, others are clearly important in the context of EMT, cancer, fibrosis and wound healing, as will be described below.
Integrin–TGF-β crosstalk in fibrosis
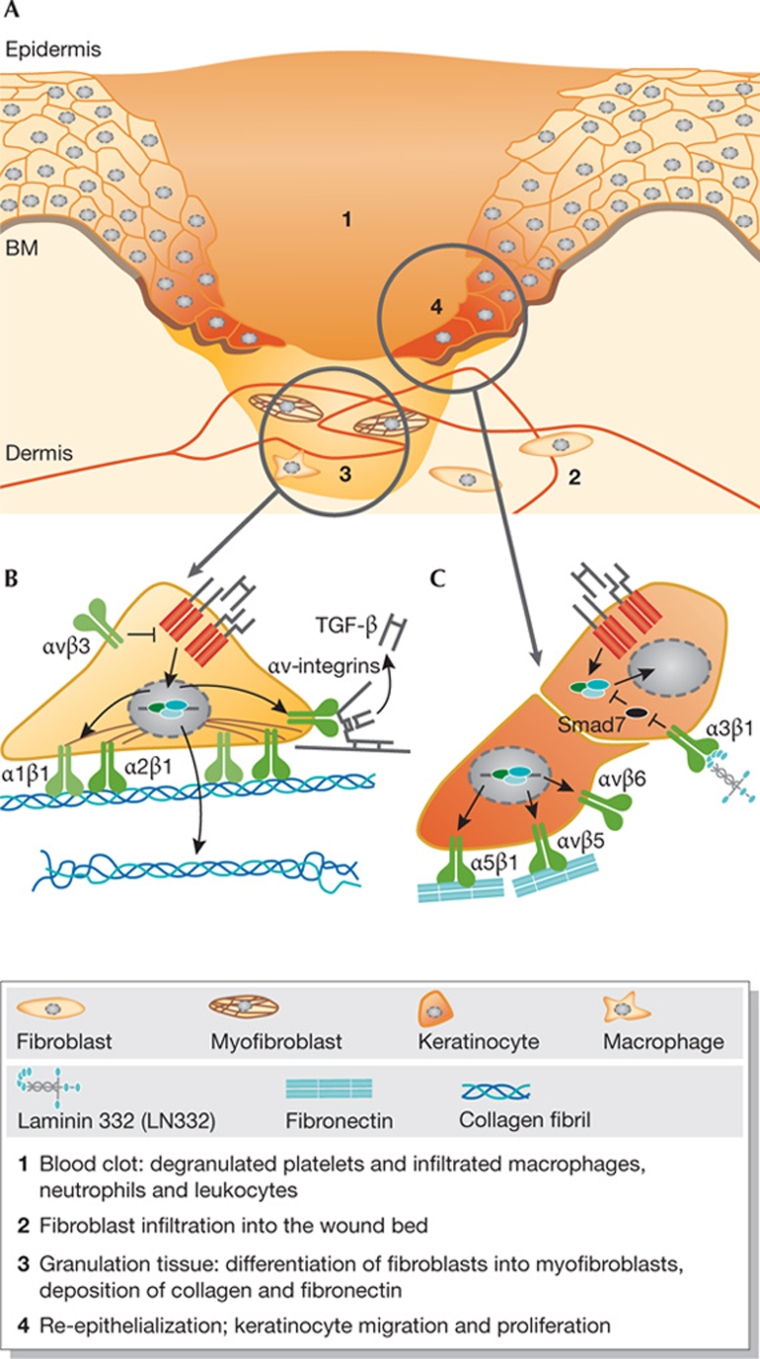
Fibrosis results from an aberrant response to organ injury and is characterized by the proliferation of fibroblasts, their differentiation into myofibroblasts, and excessive ECM production and deposition; these processes are all mediated by TGF-β. Fibrosis can ultimately lead to major organ failure and even death. Increasing evidence points to the integrin–TGF-β crosstalk as crucial for the development and pathogenesis of fibrosis. TGF-β induces the expression of the integrins α1β1 and α2β1, which mediate collagen remodelling and myofibroblast contraction (Fig 2A). Furthermore, the integrins α3β1, αvβ5 and—most notably—αvβ6 control TGF-β activity or signalling in fibrosis.
Figure 2.
Integrin–TGF-β crosstalk mechanisms. (A) In fibrosis and sclerosis, TGF-β signalling induces fibroblast differentiation into contractile myofibroblasts. The myofibroblasts express and deposit collagen (1), express α1β1- and α2β1-integrins that mediate collagen remodelling and contraction (2), and express αv-integrins that activate latent TGF-β from the matrix (3). (B) During TGF-β-mediated EMT of alveolar epithelial cells, integrin α3β1 forms a complex with TGF-βRs and E-cadherin, facilitating β-catenin–Smad2 complex formation and nuclear translocation. (C) During malignant progression, TGF-β frequently represses the expression of laminin and/or laminin-binding integrins α3β1 and α6β4, and induces the expression of fibronectin and integrins α5β1 and αvβ6. αvβ6 mediates migration and invasion and generates new active TGF-β, stimulating other tumour cells as well as myofibroblast differentiation in the tumour stroma. β-cat, β-catenin; Col, collagen; E-cadh, E-cadherin; EMT, epithelial-to-mesenchymal transition; FN, fibronectin; LN332, laminin 332; TGF-β, transforming growth factor-β; TGF-βR, transforming growth factor-β receptor.
The first clue that the integrin–TGF-β interplay was important in fibrosis came from the observation that mice lacking the β6-subunit are protected from bleomycin-induced pulmonary fibrosis (Munger et al, 1999). The importance of αvβ6 for fibrogenesis has been demonstrated subsequently in several models; αvβ6 is not normally expressed in healthy epithelia but its expression is induced in many human fibrotic disorders in the kidney (such as diabetes mellitus, progressive fibrosing glomerulonephritis and Alport syndrome), the liver (acute biliary fibrosis) and the lung (sclerosis and idiopathic pulmonary fibrosis (IPF)). In mice, the constitutive expression of αvβ6 in the basal layer of the epidermis leads to elevated TGF-β1 activation and the development of spontaneous chronic ulcers with severe fibrosis (Häkkinen et al, 2004). Conversely, β6 knockout mice are partly or completely protected from pulmonary fibrosis induced by radiation, tubulointerstitial fibrosis as a response to kidney obstruction, or acute biliary fibrosis caused by bile duct ligation. In wild-type mice, fibrosis can be equally inhibited by treatment with antagonists of TGF-β signalling or by using a blocking antibody against αvβ6 (Ma et al, 2003; Hahm et al, 2007; Wang et al, 2007; Patsenker et al, 2008). In fact, given that blocking the TGF-β pathway has serious adverse effects—such as the development of autoimmunity—the specific inhibition of αvβ6-induced TGF-β activation at sites of injury is a promising therapeutic tool to combat TGF-β-mediated fibrosis. Indeed, low doses of antibodies against αvβ6 prevent radiation-induced or bleomycin-induced pulmonary fibrosis in mice, without causing inflammation (Puthawala et al, 2008; Horan et al, 2007).
Observations suggest that the integrins αvβ3, αvβ5 and αvβ8 provide additional therapeutic targets for this pathology. As mentioned above, αvβ3 and αvβ5 are thought to contribute to the pathogenesis of systemic sclerosis and scleroderma through TGF-β activation (Asano et al, 2005b, 2006a). In human fibrotic lungs, epithelial cells expressing αvβ5 and PAR1 co-localize with myofibroblasts, and TGF-β-mediated pulmonary fibrosis is reduced by the blockade of αvβ5 in a mouse model (Scotton et al, 2009). Furthermore, TGF-β activation by αvβ8 can induce the differentiation of airway fibroblasts into myofibroblasts, and the expression of αvβ8 is increased in the airways of chronic obstructive pulmonary disease patients, correlating with the severity of the obstruction (Araya et al, 2006, 2007). However, the importance of αvβ8 in this process has not been corroborated by knockout or targeting studies. Finally, α3β1 also contributes to the development of IPF through a β-catenin–Smad2-dependent mechanism, as described above (Fig 2B). In IPF, a subset of differentiating fibroblasts is derived initially from alveolar epithelial cells by EMT (Kim et al, 2006). The lung-specific deletion of the α3-subunit in a mouse model of IPF reduces myofibroblast accumulation, collagen deposition, expression of EMT-associated genes and progression to fibrosis, suggesting that blocking α3β1 could also be effective against fibrosis (Kim et al, 2009a,b).
Collectively, these results show that several integrins aggravate TGF-β-mediated fibrotic disorders, either by direct activation of TGF-β, or by affecting downstream signalling. Thus, targeting these integrins could prove to be a valuable anti-fibrotic therapy in humans. Alternatively, integrin-associated proteins might represent targets for therapeutic intervention. For example, ILK is essential for TGF-β-induced kidney and liver fibrosis, although whether this depends on the modulation of integrin activity, or is an integrin-independent effect of ILK, is unknown (Li et al, 2003).
Integrin–TGF-β crosstalk in carcinoma progression
TGF-β has a dual role in the development and progression of epithelial tumours: initially, it acts as a tumour suppressor for epithelial cells, but at a later stage can also promote growth, invasion and metastasis. The ability of TGF-β to promote or suppress carcinoma progression is at least partly dependent on the tumour microenvironment (Bierie & Moses, 2006; Massagué, 2008). The interactions between TGF-β and integrins can affect tumorigenesis and malignant progression in several ways. For example, an inappropriate suprabasal expression of α6β4 in stratified squamous epithelia inhibits TGF-β signalling, thereby enhancing tumorigenesis by relieving the inhibitory effects of TGF-β on epithelial proliferation (Owens et al, 2003). In addition, squamous cell carcinomas develop in stratified epithelia after the abrogation of TGF-β signalling, which could be associated with enhanced integrin activity and would suggest that, under normal circumstances, TGF-β has a suppressive effect on integrins (Guasch et al, 2007). However, it should be noted that most studies support a role for TGF-β in inducing the de novo expression of several integrins that are not normally expressed in epithelial cells—such as α5β1, αvβ3, αvβ5 and αvβ6—thereby enhancing the migratory and invasive behaviour of carcinoma cells, particularly in conjunction with newly expressed MMPs and ECM components such as fibronectin (Fig 2C). Indeed, antagonizing the TGF-β pathway blocks the induction of the expression of these integrins, as well as TGF-β-mediated invasion and metastasis, without affecting the growth of the primary tumour, suggesting that inhibiting integrin upregulation by TGF-β is sufficient to block metastasis (Bandyopadhyay et al, 2006; Kawajiri et al, 2008).
As in fibrosis, αvβ6 seems to have a crucial role in the TGF-β–integrin crosstalk in carcinomas. TGF-β induces the expression of αvβ6 during EMT in vitro and in vivo, and αvβ6 is upregulated at the tumour–stromal interface of several aggressive squamous cell carcinomas—including cervical, colorectal, esophageal, head and neck, and skin carcinomas—and its upregulation is a prognostic factor for decreased survival (Bates et al, 2005; Hazelbag et al, 2007; van Aarsen et al, 2008; Marsh et al, 2008). αvβ6 can mediate migration and invasion, but can also establish a self-amplifying loop by activating TGF-β; the interruption of this feedforward mechanism could be an important step to arrest malignant progression. Although the blockade of αvβ6 had no effect on TGF-β-mediated proliferation of tumour cells in vitro, it did successfully inhibit the growth of xenograft tumours in vivo, suggesting that the tumour microenvironment has an important regulatory role (van Aarsen et al, 2008). Indeed, αvβ6-mediated TGF-β activation in an organotypic culture system for basal cell carcinoma induced differentiation of fibroblasts into myofibroblasts, which subsequently induced tumour cell invasion by the secretion of hepatocyte growth factor. Interestingly, the stroma of high-risk basal cell carcinomas is rich in myofibroblasts that express hepatocyte growth factor, and its receptor—c-Met—is expressed on the tumour cells, suggesting that a similar tumour–stromal interaction can occur in patients (Marsh et al, 2008). Therefore, although the blockade of several TGF-β-induced integrins might inhibit the migratory and invasive behaviour of tumour cells, antagonizing αvβ6 could also be important for interfering with self-amplifying, TGF-β-mediated tumour–stromal interactions. This approach could ultimately become an effective treatment for various carcinomas.
Integrin–TGF-β crosstalk during wound healing
The repair of cutaneous wounds is achieved through the concerted efforts of many cell types (Fig 3A; Singer & Clark, 1999). TGF-β is involved in every phase of wound repair and is released by platelets, neutrophils, macrophages, fibroblasts and migrating keratinocytes. TGF-β suppresses the inflammatory response and promotes the formation of granulation tissue by inducing fibroblast proliferation and differentiation, the expression of integrins and deposition of ECM proteins by fibroblasts, and endothelial cell migration and angiogenesis (Fig 3B; Werner & Grose, 2003). However, there are conflicting results as to the role of TGF-β during re-epithelialization. On the one hand, TGF-β stimulates the expression of fibronectin and the integrins α5β1, αvβ5 and αvβ6 in keratinocytes, thereby inducing a migratory phenotype (Fig 3C). On the other hand, TGF-β inhibits keratinocyte proliferation, and there is evidence to indicate that the net result of TGF-β signalling on re-epithelialization is inhibitory. For example, re-epithelialization is delayed in mice that overexpress TGF-β1 in the basal layer of the epidermis (Yang et al, 2001; Chan et al, 2002; Tredget et al, 2005), whereas it is accelerated and keratinocyte proliferation is increased in mice that express a dominant negative TGF-βRII in basal keratinocytes or those that lack TGF-βRII (Amendt et al, 2002; Guasch et al, 2007). In addition, re-epithelialization is accelerated in Smad3 knockout mice (Ashcroft et al, 1999; Falanga et al, 2004).
Figure 3.
Overview of proposed integrin–TGF-β interactions during wound healing. (A) Schematic representation of the main phases in wound healing, which are explained in the figure key. (B) In the granulation tissue, TGF-β induces expression of integrins α1β1 and α2β1, which mediate fibroblast contraction, and of αv-integrins, which activate latent TGF-β. Furthermore, αvβ3 might repress TGF-β signalling by inhibiting TGF-βR expression. (C) During re-epithelialization, TGF-β stimulates the expression of fibronectin and integrins, which mediate keratinocyte migration or activate latent TGF-β. Integrin α3β1 could enhance TGF-β signalling by controlling the expression of Smad7. BM, basement membrane; Col, collagen; FN, fibronectin; LN332, laminin 332.
Integrins mediate adhesion and migration during re-epithelialization (Grose et al, 2002), and emerging evidence suggests that several can modulate TGF-β signalling during wound healing, although the precise mechanisms are controversial and poorly understood. Re-epithelialization is accelerated in β3-null mice, which is accompanied by enhanced fibroblast infiltration, fibronectin deposition and neo-angiogenesis, and elevated TGF-β levels in the granulation tissue, suggesting that αvβ3 suppresses TGF-β signalling (Reynolds et al, 2005). However, this is inconsistent with both the activation of TGF-β by αvβ3 and the inhibitory effects of TGF-β on re-epithelialization. In addition, although the targeted deletion of the β6-subunit does not affect wound healing, abnormal wound healing is observed in β6-null mice when TGF-β signalling is disturbed—for example, in the presence of glucocorticoids (Huang et al, 1996, 2000; AlDahlawi et al, 2006; Xie et al, 2009)—suggesting that rather than maintaining adhesion and mediating migration, αvβ6 functions as a safeguard in wounds, ensuring sufficient supply of TGF-β when required. The activation of TGF-β by αvβ8 has also been seen to delay the closure of scratch wounds in vitro, although whether it has a physiological role during re-epithelialization in vivo is unknown (Fjellbirkeland et al, 2003; Neurohr et al, 2006). Finally, delayed wound re-epithelialization has been observed in full-thickness skin explants from α3-null mice, supposedly owing to repressed TGF-β signalling caused by an upregulation of Smad7 in the absence of integrin α3β1 (Fig 3C; Reynolds et al, 2008). However, these data are controversial in the light of the evidence that TGF-β signalling inhibits re-epithelialization, and because the targeted deletion of α3 from the basal layer of the epidermis has been recently shown not to inhibit re-epithelialization (Margadant et al, 2009; Mitchell et al, 2009). Therefore, although regulation of TGF-β signalling by integrins is potentially important in many aspects of the wound healing process, it is not fully understood. Future studies should shed more light on the exact mechanisms involved.
Conclusion
Extensive interactions undoubtedly exist between integrins and the TGF-β pathway. Although our knowledge of the wide implications of this crosstalk and the underlying mechanisms has increased greatly in recent years, there are still several outstanding questions to address (Sidebar A). Clarification of these issues is important as it will not only increase our understanding of integrin signalling, TGF-β signalling and integrin–TGF-β crosstalk, but—importantly—could also lead to new treatment strategies for several human pathologies.
Sidebar A | In need of answers.
(i) Do integrins αIIbβ3 and α5β1 interact with LAP TGF-β and therefore have a role in TGF-β activation?
(ii) Do αvβ1 and α8β1 activate TGF-β?
(iii) What is the exact nature and function of αvβ3–TGF-β crosstalk, and is it important in vivo—for example, in wound healing?
(iv) Can αvβ6 be a therapeutic target in cancer and fibrosis?
(v) Will antagonism of αvβ8 be effective against chronic obstructive pulmonary disease?
(vi) Are the effects of ILK on pulmonary fibrosis dependent on integrins?

Coert Margadant

Arnoud Sonnenberg
Acknowledgments
We thank Rabab Charafeddine for stimulating discussions. We apologize to colleagues whose work might have been omitted owing to space constraints. This study was supported by grants from the Dystrophic Epidermolysis Bullosa Research Association (Crowthorne, UK) and the Dutch Cancer Society.
References
- AlDahlawi S, Eslami A, Häkkinen L, Larjava HS (2006) The αvβ6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen 14: 289–297 [DOI] [PubMed] [Google Scholar]
- Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS (2009) Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122: 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendt C, Mann A, Schirmacher P, Blessing M (2002) Resistance of keratinocytes to TGF-β-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci 115: 2189–2198 [DOI] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB (2004) Integrin αvβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol 165: 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J, Cambier S, Morris A, Finkbeiner W, Nishimura SL (2006) Integrin-mediated transforming growth factor-β activation regulates homeostasis of the pulmonary epithelial–mesenchymal trophic unit. Am J Pathol 169: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J et al. (2007) Squamous metaplasia amplifies pathologic epithelial–mesenchymal interactions in COPD patients. J Clin Invest 117: 3551–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K (2005a) Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol 175: 7708–7718 [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K (2005b) Involvement of αvβ5 integrin-mediated activation of latent transforming growth factor β1 in autocrine transforming growth factor-β signaling in systemic sclerosis fibroblasts. Arthritis Rheum 52: 2897–2905 [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K (2006a) Involvement of αvβ5 integrin in the establishment of autocrine TGF-β signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol 126: 1761–1769 [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K (2006b) Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 168: 499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS et al. (1999) Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1: 260–266 [DOI] [PubMed] [Google Scholar]
- Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM (2005) Transcriptional activation of integrin β6 during the epithelial–mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest 115: 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A, Agyin JK, Wang L, Tang Y, Lei X, Story BM, Cornell JE, Pollock BH, Mundy GR, Sun LZ (2006) Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-β type I receptor kinase inhibitor. Cancer Res 66: 6714–6721 [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL (2001) Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J Biol Chem 276: 46707–46713 [DOI] [PubMed] [Google Scholar]
- Bierie B, Moses HL (2006) Tumour microenvironment: TGF-β: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6: 506–520 [DOI] [PubMed] [Google Scholar]
- Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 85: 683–693 [DOI] [PubMed] [Google Scholar]
- Chan T, Ghahary A, Demare J, Yang L, Iwashina T, Scott PG, Tredget EE (2002) Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-β1 transgenic mouse. Wound Repair Regen 10: 177–187 [DOI] [PubMed] [Google Scholar]
- Falanga V, Schrayer D, Cha J, Butmarc J, Carson P, Roberts AB, Kim SJ (2004) Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad2 knockout mice. Wound Repair Regen 12: 320–326 [DOI] [PubMed] [Google Scholar]
- Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL (2003) Integrin αvβ8-mediated activation of transforming growth factor-β inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol 163: 533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Chen Y, Prijatelj P, Sakai T, Fässler R, Sakai LY, Rifkin DB (2005) Fibronectin is required for integrin αvβ6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J 19: 1798–1808 [DOI] [PubMed] [Google Scholar]
- Fransvea E, Mazzocca A, Antonaci S, Giannelli G (2009) Targeting transforming growth factor (TGF)-βRI inhibits activation of β1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology 49: 839–850 [DOI] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP (2006) β3 integrin and Src facilitate transforming growth factor-β mediated induction of epithelial–mesenchymal transition in mammary epithelial cells. Breast Cancer Res 8: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fässler R, Brakebusch C, Werner S (2002) A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315 [DOI] [PubMed] [Google Scholar]
- Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E (2007) Loss of TGF-β signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12: 313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm K et al. (2007) αvβ6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170: 110–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häkkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, Laato M, Heino J, Larjava H (2004) Increased expression of β6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol 164: 229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida T, Wu MH, Pierce A, Poncelet AC, Varga J, Schnaper HW (2007) MAP-kinase activity necessary for TGF-β1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci 120: 4230–4240 [DOI] [PubMed] [Google Scholar]
- Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, Violette SM, Weinreb PH, Fleuren GJ (2007) Overexpression of the αvβ6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol 212: 316–324 [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Culleré M, Ross FP, Coller BS, Teitelbaum S, Hynes RO (1999) β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 103: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan GS et al. (2008) Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 177: 56–65 [DOI] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Sheppard D (1996) Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 133: 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Sheppard D (2000) Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol Cell Biol 20: 755–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687 [DOI] [PubMed] [Google Scholar]
- Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D (2006) Ligation of protease-activated receptor 1 enhances αvβ6 integrin-dependent TGF-β activation and promotes acute lung injury. J Clin Invest 116: 1606–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri H et al. (2008) A novel transforming growth factor-β receptor kinase inhibitor, A-77, prevents the peritoneal dissemination of scirrhous gastric carcinoma. Clin Cancer Res 14: 2850–2860 [DOI] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK et al. (2009a) Epithelial cell α3β1 integrin links β-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119: 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA (2009b) Integrin α3β1-dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol 184: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson R, Roes JT, Savill JS, Hynes RO (2007) Ulcerative colitis and autoimmunity induced by the loss of myeloid αv integrins. Proc Natl Acad Sci USA 104: 15823–15828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar JM, Iyer V, DiPersio CM (2008) Integrin α3β1 potentiates TGF-β-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol 128: 575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Wickström SA, Fässler R (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23: 397–418 [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Dai C, Wu C, Liu Y (2003) Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest 112: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM (2002) Integrin α8β1 mediates adhesion to LAP–TGF-β1. J Cell Sci 115: 4641–4648 [DOI] [PubMed] [Google Scholar]
- Ludbrook SB, Barry ST, Delves CJ, Horgan CM (2003) The integrin αvβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem J 369: 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB (2003) Transforming growth factor-β-dependent and -independent pathways of induction of tubulointerstitial fibrosis in β6(-/-) mice. Am J Pathol 163: 1261–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A (2009) Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 122: 278–288 [DOI] [PubMed] [Google Scholar]
- Marsh D, Dickinson S, Neill GW, Marshall JF, Hart IR, Thomas GJ (2008) αvβ6 integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res 68: 3295–3303 [DOI] [PubMed] [Google Scholar]
- Massagué J (2008) TGF-β in cancer. Cell 134: 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Chen YG (2000) Controlling TGF-β signaling. Genes Dev 14: 627–644 [PubMed] [Google Scholar]
- Mitchell K, Szekeres C, Milano V, Nilsen-Hamilton M, Kreidberg JA, DiPersio CM (2009) Integrin α3β1 in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial cell crosstalk through induction of MRP3/Prl2c4. J Cell Sci 122: 1778–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL (2002) The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 157: 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS et al. (1999) The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328 [DOI] [PubMed] [Google Scholar]
- Neurohr C, Nishimura SL, Sheppard D (2006) Activation of transforming growth factor-β by the integrin αvβ8 delays epithelial wound closure. Am J Respir Cell Mol Biol 35: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DM, Romero MR, Gardner C, Watt FM (2003) Suprabasal α6β4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGF-β signalling. J Cell Sci 116: 3783–3791 [DOI] [PubMed] [Google Scholar]
- Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D (2008) Inhibition of integrin αvβ6 on cholangiocytes blocks transforming growth factor-β activation and retards biliary fibrosis progression. Gastroenterology 135: 660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, Thompson PJ, Thannickal VJ, Knight DA (2008) TGF-β1 induces αvβ3 integrin expression in human lung fibroblasts via a β3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J Biol Chem 283: 12898–12908 [DOI] [PubMed] [Google Scholar]
- Puthawala K et al. (2008) Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177: 82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM (2002) Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med 8: 27–34 [DOI] [PubMed] [Google Scholar]
- Reynolds LE et al. (2005) Accelerated re-epithelialization in β3 integrin-deficient mice is associated with enhanced TGF-β1 signaling. Nat Med 11: 167–174 [DOI] [PubMed] [Google Scholar]
- Reynolds LE et al. (2008) α3β1 integrin-controlled Smad7 regulates re-epithelialization during wound healing in mice. J Clin Invest 118: 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B (2003) Activated integrin αvβ3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA 100: 9482–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA (2004) αvβ3 integrin interacts with the transforming growth factor-β (TGF-β) type II receptor to potentiate the proliferative effects of TGF-β1 in living human lung fibroblasts. J Biol Chem 279: 37726–37733 [DOI] [PubMed] [Google Scholar]
- Scotton CJ et al. (2009) Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest 119: 2550–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341: 738–746 [DOI] [PubMed] [Google Scholar]
- Travis MA et al. (2007) Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tredget EB, Demare J, Chandran G, Tredget EE, Yang L, Ghahary A (2005) Transforming growth factor-β and its effect on re-epithelialization of partial-thickness ear wounds in transgenic mice. Wound Repair Regen 13: 61–67 [DOI] [PubMed] [Google Scholar]
- van Aarsen LA et al. (2008) Antibody-mediated blockade of integrin αvβ6 inhibits tumor progression in vivo by a transforming growth factor-β-regulated mechanism. Cancer Res 68: 561–570 [DOI] [PubMed] [Google Scholar]
- Wang D, Sun L, Zborowska E, Willson JK, Gong J, Verraraghavan J, Brattain MG (1999) Control of type II transforming growth factor-β receptor expression by integrin ligation. J Biol Chem 274: 12840–12847 [DOI] [PubMed] [Google Scholar]
- Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM (2007) Role of αvβ6 integrin in acute biliary fibrosis. Hepatology 46: 1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Xiang B, Zent R, Quaranta V, Pozzi A, Arteaga CL (2009) Transforming growth factor-β induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res 69: 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83: 835–870 [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B (2008) Integrins and activation of latent transforming growth factor-β1: an intimate relationship. Eur J Cell Biol 87: 601–615 [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B (2007) Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol 179: 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Gao K, Häkkinen L, Larjava HS (2009) Mice lacking β6 integrin in skin show accelerated wound repair in dexamethasone impaired wound healing model. Wound Repair Regen 17: 326–339 [DOI] [PubMed] [Google Scholar]
- Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G (2009) Lysophosphatidic acid induces αvβ6 integrin-mediated TGF-β activation via the LPA2 receptor and the small G protein Gα(q). Am J Pathol 174: 1264–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chan T, Demare J, Iwashina T, Ghahary A, Scott PG, Tredget EE (2001) Healing of burn wounds in transgenic mice overexpressing transforming growth factor-β1 in the epidermis. Am J Pathol 159: 2147–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS (2007) Absence of integrin-mediated TGF-β1 activation in vivo recapitulates the phenotype of TGF-β1-null mice. J Cell Biol 176: 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]