Summary
The EMBO Conference on Meiosis held last September highlighted the dynamic aspects of this process, including the variability of hotspots for break formation, switches between partners during repair and the dynamics of sister chromatid cohesion.
The life cycle of eukaryotes oscillates between a diploid and a haploid state; meiosis and fertilization alternate to generate the transitions between these states. Meiosis consists of two special rounds of cell division, preceded by a single phase of DNA replication. The most recent advances in this field were presented at the EMBO Conference on Meiosis, organized by Bernard de Massy and held at Isle sur la Sorgue, France, between 19 and 23 September 2009. The different steps of meiosis were discussed, including the generation of meiotic DNA double-strand breaks (DSBs)—which results in meiotic recombination and is associated with homologous chromosome pairing—and aspects of chromosome segregation and progression through the cell cycle. At the end of the conference, a session on genome evolution brought us back to the beginning of meiotic prophase, providing novel insight into the initiating events of meiotic recombination.
Two key themes emerged from this meeting, in addition to other exciting talks that we do not have space to cover. The first novel viewpoint identified the dynamics of chromosome regulation between individuals or within single cells as important at every level of meiotic regulation—including the initial choice of where to form the meiotic DSBs—in addition to the obvious importance of dynamics associated with chromosome movements during pairing and meiotic divisions. The second theme involved the interactions that take place between sister chromatids in meiotic recombination and chromosome segregation.
A hot topic at the beginning
Meiotic recombination requires the formation of DSBs catalysed by the Spo11 enzyme. These breaks do not occur randomly throughout the genome, but mainly at so-called 'hotspots'. In the opening lecture, Scott Keeney (Memorial Sloan–Kettering Cancer Center) presented results from deep sequencing of the oligonucleotides that remain attached to Spo11 in Saccharomyces cerevisiae after its removal from the break. This approach can locate the DSBs with the highest possible resolution—single nucleotides—thereby providing a wealth of new information about the precise localization of meiotic DSBs. For example, it is now possible to establish a genome-wide overview of the relationship between DSB sites and local chromatin structure in the nucleosome-depleted regions of promoters, which had previously only been documented at a handful of specific locations.
Groundbreaking information on DSB formation was obtained independently by Simon Myers (U. Oxford) and Bernard de Massy (Institut de Génétique Humaine, Montpellier). Starting either from a previously defined consensus DNA sequence that is associated with recombination hotspots in humans (Myers et al, 2008), or from a genetic locus that controls the distribution of DSBs and crossovers in mice (Grey et al, 2009), the two groups converged on the same gene—which has truly remarkable features. It encodes a protein with a histone 3 lysine 4 methylase domain, which is known to be associated with DSB formation, and a stretch of zinc fingers (ZFs) that is predicted to recognize the hotspot consensus DNA sequence in humans. The ZFs are positioned in a tandem array with almost perfect homology between repeats—which provides great potential for variability—and show a high degree of sequence divergence among species and among individuals. Strikingly, there is a marked correlation between allelic variants of the ZF stretch and variation in hotspot usage in humans, strongly suggesting that the modification of this single gene is sufficient to change the DSB map genome-wide.
...the dynamics of chromosome regulation between individuals or within single cells [are] important at every level of meiotic regulation...
These data also provide an explanation for the hotspot paradox, which concerns the fact that, during recombination, the broken strand—that is, the one containing the hotspot—receives its genetic information from the homologous template on which it is repaired. As this homologous strand might not contain such a 'hot' sequence, the repair process could render the hotspot cold and lead to the loss of hotspots. Myers and de Massy now postulate that the high sequence divergence observed for the ZF variable tandem repeat provides a dynamic escape route for the loss of hotspots—new ones are created all the time!
Partner switches in recombination
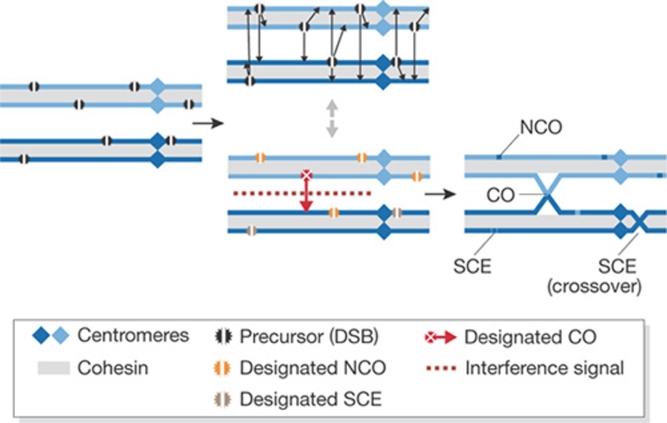
After the removal of Spo11, the DSB ends are processed and coated with the DSB repair protein Rad51 and/or its meiosis-specific paralogue Dmc1. Meiotic DSB repair can occur through several pathways that lead to four different end-products: crossovers or non-crossovers that use either the sister chromatid or a chromatid from the homologous chromosome as a template for repair (Fig 1). Crossovers that result from recombination between non-sister chromatids are required to ensure proper chromosome segregation. Repair using the sister chromatid was thought to be repressed in meiosis—a phenomenon know as inter-homologue bias—but several new results presented at this conference showed that joint molecule formation between sister chromatids occurs more frequently than previously thought. A special mechanism ensures that crossovers with the homologous chromosome do not occur randomly, but at least once per chromosome pair (obligatory chiasma), even if the average crossover number is low. In addition, crossover interference reduces the chance of two crossovers occurring in close proximity (Fig 1).
...strongly suggesting that the modification of [a] single gene is sufficient to change the DSB map genome-wide
Figure 1.
Dynamics of partner choice in meiotic recombination and final formation of crossover and non-crossover products. Two homologous chromosomes are shown, each consisting of two sister chromatids. Meiotic DSBs are induced at multiple sites and each will be repaired by using either the sister chromatid or a chromatid from the homologous chromosome as a template. In addition, each recombination event can lead to either a CO or an NCO. This process seems to be highly dynamic and each event can oscillate between different recombination intermediates (indicated by arrows). Ultimately, each chromosome pair will have at least one CO (obligatory chiasmata) and the occurrence of multiple COs in close proximity will be prevented (interference). CO, crossover; DSB, double-strand break; NCO, non-crossover; SCE, sister chromatid exchange.
The yeast Schizosaccharomyces pombe is a special organism because it has only three chromosome pairs and generates 10–20 crossovers per chromosome in the absence of crossover intereference and inter-homologue bias. Gerry Smith (Fred Hutchinson Cancer Research Center) reported that repair of DSBs at hotspots in S. pombe occurs preferentially using the sister chromatid and without Dmc1 activity, whereas DSBs in colder regions are repaired primarily using the homologous chromatid and require Dmc1. These findings shed light on how crossovers are maintained at a uniform density in spite of widely spaced DSB hotspots, and explain the observed discrepancy between DSB and crossover distributions—not all DSBs are equivalent. Michael Lichten (National Cancer Institute, Bethesda) showed that DSB repair using the sister chromatid as a template is considerably more frequent in S. cerevisiae than was previously thought. He suggested that up to one-third of the breaks could be repaired with the sister chromatid in wild-type budding yeast—as would be expected by chance, as there are two homologous chromatids for one sister! Lichten proposed a model in which a meiosis-specific kinase, which localizes along the chromosomal axes, creates a kinetic constraint that slows but does not abolish inter-sister interaction. He also suggested that switches between inter-homologue and inter-sister interactions might occur frequently, in a highly dynamic process of transient strand invasions.
Nancy Kleckner (Harvard U.) showed that for a DSB to engage in a recombination event with a non-sister chromatid, sister chromatid cohesion must be released around the site of the break. Additional evidence led to the proposition that this effect might release one DSB end, thus allowing the formation of a liberated tentacle that can probe for homologous regions on the non-sister chromatid. Conversely, sister chromatid cohesion at the other end of the break might be required to keep this second end quiescent until it is incorporated into the developing recombination complex. Anne Villeneuve (Stanford U.) presented a system for evaluating the outcome of recombination events initiated by DSBs at a defined site during meiosis in Caenorhabditis elegans. Her group determined that DSBs induced at different times during the meiotic prophase give rise to both crossover and non-crossover products in similar ratios. The induced crossovers impose interference—as do endogenous crossovers—thereby preventing the occurrence of another crossover on the same chromosome. Moreover, DSBs induced at pachytene can still compete to become a crossover, suggesting that a crossover/non-crossover is not specified irreversibly early in prophase.
...joint molecule formation between sister chromatids occurs more frequently than previously thought
An analysis performed by Denise Zickler (Université Paris-Sud) of the interlockings that occur in Sordaria provided hints towards the dynamics of meiotic DSB repair. Interlockings occur when a chromosome or a bivalent is trapped between the partly closed synaptonemal complexes that connect two homologous chromosomes. The resolution of interlocking requires not only a regularization of the topological relationships among entrapped chromosomes, but also the elimination of DNA recombination intermediates, which requires the repair protein Mlh1. Taken together, these new data show that the meiotic recombination process does not follow a fixed sequence of events, but goes back and forth dynamically between different recombination intermediates for quite some time before finally forming a crossover or non-crossover. Furthermore, we now know that the sister chromatid is also allowed to join this dance (Fig 1).
Girlpower
The cohesin complex connects sister chromatids and consists of several subunits that are thought to form a ring around the two sister chromatids. During the meiotic divisions, sister chromatid cohesion is lost in a stepwise manner. Sister chromatid cohesion is removed by the cleavage of one of its subunits, Rec8, which opens the ring. The removal of sister chromatid cohesion along chromosome arms allows a separation of homologues during the first metaphase to anaphase transition, whereas sister chromatid centromeres remain attached until the second division. Meiosis-specific cohesin components—such as Rec8 and a protein that protects Rec8 from cleavage at centromeres during meiosis I, shugoshin (Sgo)—are required for this specialized regulation of sister chromatid cohesion during meiosis. At this conference, Yoshinori Wanatabe (U. Tokyo) and Wolfgang Zachariae (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden), in collaboration with Kim Nasmyth (U. Oxford), demonstrated that Rec8 is primed for cleavage along chromosome arms through phosphorylation by the kinases CK1 and DDK, and protected at centromeres through a specific dephosphorylation mediated by the phosphatase PP2A in complex with Sgo. This process requires strict control of Sgo localization. By using genetic and biochemical screens in fission yeast, Watanabe's group found that the kinase Bub1 is crucial for Sgo recruitment to the centromere through the phosphorylation of H2A. This is apparently a fundamental regulatory mechanism, which is conserved in budding yeast and human cells.
...the cohesion complex in oocytes might be extremely stable, and persist for months, or even years in humans
In mammalian female reproduction, the meiotic prophase is extremely prolonged; all oogonia enter the first meiotic prophase before birth and all oocytes are subsequently arrested late in meiotic prophase. Meiosis is only resumed when ovulation is imminent and again halts at metaphase II until fertilization by a sperm cell releases this block. Thus, sister chromatid cohesion in oocytes needs to be maintained for months in mice—and even for decades in humans—to ensure a proper segregation of homologous chromosomes during the first meiotic division. Kim Nasmyth (U. Oxford) addressed the loading and turnover of cohesins in mouse oocytes. By using a modified version of the meiosis-specific cohesion component REC8 that can be cleaved at will during meiosis progression, he showed that REC8 is able to ensure sister chromatid cohesion if it is expressed before meiotic S phase in the embryo, but not when expressed in growing oocytes in the adult. Thus, the cohesion complex in oocytes might be extremely stable, and persist for months, or even years in humans. These results suggest that the inability of oocytes to regenerate cohesion might contribute to age-related errors in meiosis I.
Conclusion
This meeting illustrated perfectly the diverse approaches—which ranged from detailed analyses of molecular recombination intermediates, to real-life imaging of chromosome movement and genome evolution studies—that are now being used to decipher the fundamental process of heredity, meiosis, in a variety of organisms. In addition, the availability of many sequenced genomes has facilitated the identification of new family members of meiotic genes in different species. Several new homologues of genes involved in meiotic pairing and DSB repair were presented at the meeting, such as mammalian homologues of yeast Mei4 (Rajeev Kumar, from the de Massy group, Montpellier), Hop1 (Attila Toth, Institute of Physiological Chemistry, Dresden), and Zip3 (Neil Hunter, U. California) and the rice homologue of Zip1 (Norio Komeda, from the Ken-ichi Nonomura group, National Institute of Genetics, Japan). We expect that these newcomers will take centre stage and help to decipher the basics of meiosis in the coming years.
References
- Grey C et al. (2009) PLoS Biol 7: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S et al. (2008) Nat Genet 40: 1124–1129 [DOI] [PubMed] [Google Scholar]