Abstract
The transfer–messenger RNA (tmRNA)-mediated trans-translation mechanism is highly conserved in bacteria and functions primarily as a system for the rescue of stalled ribosomes and the removal of aberrantly produced proteins. Here, we show that in the antibiotic-producing soil bacterium Streptomyces coelicolor, trans-translation has a specialized role in stress management. Analysis of proteins that were carboxy-terminally His8-tagged by a recombinant tmRNA identified only 10 targets, including the stress proteins: DnaK heat-shock protein 70, thiostrepton-induced protein A, universal stress protein A, elongation factor Tu3, and the cell-cycle control proteins DasR, SsgA, SsgF and SsgR. Although tmRNA-tagged proteins are degraded swiftly, the translation of dnaK and dasR messenger RNAs (mRNAs) depends fully on tmRNA, whereas transcription is unaffected. The data unveil a surprisingly dedicated functionality for tmRNA, promoting the translation of the same mRNA it targets, at the expense of sacrificing the first nascent protein. In streptomycetes, tmRNA has evolved into a dedicated task force that ensures the instantaneous response to the exposure to stress.
Keywords: development, ribosome, ssrA, stress, termination
Introduction
Survival under stress conditions is a constant challenge in life, and cells have various defence mechanisms to cope with the consequences of exposure to among others radicals, chemicals, nutrient deprivation, desiccation, and temperature or salt shock.
One consequence is the accumulation of incorrectly folded proteins and damaged messenger RNAs (mRNAs). Ribosomes translating truncated transcripts become stalled because of the lack of a stop codon, causing rapid depletion of the ribosome pool. Bacteria have an elegant quality-control mechanism, trans-translation, to solve this problem (Moore & Sauer, 2007). A chimeric transfer–messenger RNA (tmRNA) encoded by the ssrA gene mimics transfer RNA (tRNA) and mRNA successively (Retallack & Friedman, 1995; Karzai et al, 2000). The tRNA-like structure of tmRNA enters the ribosomal A site and after alanine transfer a peptidyl-tmRNA is formed. Next, the ribosome switches template, translates the coding region of tmRNA, which encodes a degradation tag, and terminates on the tmRNA-specified stop codon (Keiler et al, 1996). Furthermore, more specific functions in gene regulation have been reported for tmRNA, such as controlling the levels of active Lac repressor (Abo et al, 2000), or cell-cycle control in Caulobacter crescentus, mediating the removal of the response regulator CtrA (Keiler & Shapiro, 2003).
Streptomycetes are Gram-positive soil bacteria that produce a mycelium and propagate through sporulation. Their development is intricately controlled (Flärdh & Buttner, 2009). Streptomycetes face a diverse set of stresses in the soil, such as heat, desiccation and competing organisms. Perhaps the ultimate stress response is development; after nutrient depletion, the underlying vegetative mycelium or substrate mycelium is degraded autolytically to yield the necessary building blocks for the aerial mycelium and spores. Several studies have suggested a function of tmRNA in stress control, such as oxidative stress, heat shock and exposure to antibiotics, although the mechanisms and triggers have not been uncovered (Paget et al, 2001; Braud et al, 2006; Paleckova et al, 2006; Yang & Glover, 2009). In this study, we show that in streptomycetes, specific control of gene expression is the main role of tmRNA, with tmRNA-mediated trans-translation tagging almost exclusively the main stress or developmental proteins, which suggests that tmRNA-mediated protein tagging has evolved into a specialized translational control system in these organisms.
Results And Discussion
Mutation and expression of Streptomyces coelicolor ssrA
An ssrA mutant of S. coelicolor was created by removing nucleotide positions 3226924–3227376 of the S. coelicolor M600 genome (Methods and supplementary Fig S1 online). A total of 16 ssrA mutants were obtained with similar phenotypes—small colonies and inhibited development—and enhanced stress sensitivity. Besides the known sensitivity to hygromycin and heat shock (Yang & Glover, 2009), we also observed strongly increased sensitivity of ssrA-null mutants to the antibiotics thiostrepton and rifampicin, as well as to diamine, which causes oxidative stress and was previously shown to induce ssrA transcription (Paget et al, 2001; supplementary Fig S2 online). As predicted from the small colony size, the growth rate of the ssrA mutant was strongly reduced, particularly at challenging temperatures.
For the expression of tmRNA variants, two low-copy constructs were generated, namely pSsrA expressing wild-type tmRNA and pSsrAHis that expresses a variant of tmRNA designated tmRNA-His. The latter encodes the modified and protease-resistant His8-tag sequence ANTKRDSSHHHHHHHH (instead of the wild-type tag ANTKRDSSQQAFALAA), and thus results in carboxy-terminally His8-tagged proteins that might be purified using Ni2+ affinity chromatography. Compensatory mutations were introduced to ensure that the secondary structure of the recombinant tmRNA-His was maintained (supplementary Fig S3 online). Biochemical analysis revealed that the tmRNA-His variant is fully functional and efficiently aminoacylated by alanyl-tRNA synthetase, and binds to elongation factor (EF)-Tu·GTP and SmpB (supplementary Fig S4 online). Steady-state levels of tmRNA were determined by quantitative reverse transcriptase PCR (RT–PCR), which demonstrated its proper expression from these constructs (supplementary Fig S1 online). As the negative control we used pΔssrA, which contains the flanking regions but not ssrA itself. Introduction of pSsrA and pSsrAHis—but not control plasmids pHJL401 and pΔssrA—restored normal growth and full development of the ssrA mutant.
Low complexity of the tmRNA-tagged proteome
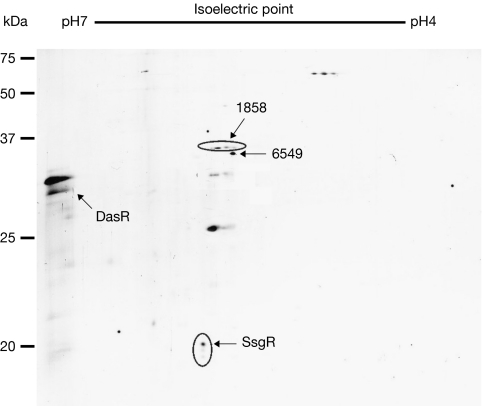
For analysis of tmRNA tagging in S. coelicolor, samples obtained from ssrA mutants harbouring pSsrAHis were separated by two-dimensional gel electrophoresis and analysed by western blotting using His antibodies. This revealed a surprisingly small number of protein spots (Fig 1). A similar experiment in Escherichia coli revealed hundreds of proteins (Roche & Sauer, 2001), whereas the E. coli genome encodes far fewer proteins than that of S. coelicolor. For detailed identification of the tmRNA-tagged proteins, samples were denatured and His-rich proteins were purified by successive rounds of Ni2+ affinity chromatography. The protein samples were digested with trypsin and analysed by capillary liquid chromatography–tandem mass spectrometry (LC-MS/MS), followed by protein identification (Beck et al, 2006). Only 12 His-rich proteins (out of around 7,850 encoded by the genome) were purified, including the naturally occurring His-rich proteins SCO1858 and SCO6549, which were also purified from wild-type cells without tmRNA-His. Thus, only 10 proteins were found to be tagged specifically by tmRNA under the given growth conditions. These were DasR (development of aerial mycelium and spores regulator), DnaK heat-shock protein 70 (Hsp70), SsgA (sporulation of Streptomyces griseus A), SsgF, SsgR, thiostrepton-induced protein A (TipA), universal stress protein A (UspA), EF-Tu3, cystathionine γ-synthase (SCO1294) and a protease (SCO2582) that belongs to the same family as the heat-shock protease HtpX (Pfam PF01435). As a test, several spots were excised from the two-dimensional gel and analysed, and this again identified DasR, SsgR, SCO1858 and SCO6549.
Figure 1.
Specific tagging by tmRNA in Streptomyces coelicolor. Two-dimensional gel electrophoresis. The numbers correspond to the identified proteins and indicate the SCO reference number in the Streptomyces database. The amount of protein loaded for this experiment was equivalent to 100 ml of culture. DasR, development of aerial mycelium and spores regulator; tmRNA, transfer–messenger RNA.
Interestingly, many of the tmRNA targets link directly to observed defects for the tmRNA mutant. The main chaperone and stress control protein DnaK Hsp70 links to reduced growth rates and heat-shock sensitivity (Wickner et al, 1991; Bucca et al, 2003; Guisbert et al, 2004), the drug-resistance regulators, TipA and UspA, to antibiotic sensitivity (Nystrom & Neidhardt, 1992; Kahmann et al, 2003), the cell-division activator SsgA and its specific transcriptional activator SsgR to developmental defects (van Wezel et al, 2000; Traag et al, 2004; Noens et al, 2007), the alternative translation factor EF-Tu3 to nutrient stress (van Wezel et al, 1995) and the global transcriptional regulator and nutrient sensory DasR to oxidative stress, programmed cell death and antibiotic production (Rigali et al, 2006, 2008). Transcription of tmRNA is possibly induced by oxidative stress (Paget et al, 2001) in S. coelicolor, as are dnaK, tipA and dasR (Paget et al, 2001; Paleckova et al, 2006; S.B., B.A.T. and G.P.v W., unpublished data). For most of the identified proteins—with the exception of DnaK—we found good peptide coverage for full-length proteins, strongly suggesting that tagging took place at or near the C-terminal end (Table 1). With the exception of DnaK, none of the identified proteins are products of genes that are highly expressed. Another exception is that dnaK is expressed in an operon. Transcription of the operon is the same in wild-type and ssrA mutant cells, but considering the dependence of dnaK translation on tmRNA, the tmRNA-mediated control might also affect the translation of the downstream-located gene encoding nucleotide exchange factor GrpE (SCO3670). However, GrpE was not identified as a direct target of tmRNA tagging. Finally, DnaK has a clear two-domain structure (Harrison et al, 1997), and we only identified peptides matching the amino-terminal nucleotide-binding (ATPase) domain (coverage up to amino acid 285). The peculiarities relating to the tmRNA-mediated control of dnaK—and perhaps also grpE—should be investigated in more detail.
Table 1.
Main targets for tmRNA-mediated tagging in Streptomyces coelicolor
| ScoDB annotation | Protein name | Protein function and comments | Hits* in sample | ∑‡ | Sequence coverage (%) | Last residue identified§ | Reference (for function) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||
| Stress-related functions | |||||||||
| Development and antibiotic production | |||||||||
| SCO3925 | SsgR | Transcriptional activator of SsgA | 11 | 29 | 28 | 23 | 56.8 | 223/241 (231) | Traag et al (2004) |
| SCO3926 | SsgA | Activator of sporulation-specific cell division | 5 | 6 | 5 | 5 | 43.4 | 89/136 (127) | van Wezel et al (2000) |
| SCO7175 | SsgF | Regulator of sporulation | 2 | 2 | — | 3 | 21.8 | 156/156 (156) | Noens et al (2005) |
| SCO5231 | DasR | Pleiotropic repressor of antibiotic production and N-acetylglucosamine metabolism; nutrient-sensing protein | 5 | 10 | 4 | 11 | 43.7 | 235/254 (250) | Rigali et al (2006, 2008) |
| SCO3413 | TipA | Thiostrepton antibiotic-induced regulator | 6 | 4 | 7 | 10 | 44.7 | 250/253 (250) | Kahmann et al (2003) |
| Stress proteins | |||||||||
| SCO3671 | DnaK | Heat-shock protein 70 (chaperone) | 2 | 3 | 2 | 5 | 10.7 | 285/618 (614) | Bucca et al (2003); Wickner et al (1991) |
| SCO0200 | UspA | Starvation-related | — | 3 | — | 3 | 16.3 | 247/301 (273) | Nystrom & Neidhardt (1992) |
| SCO1321 | EF-Tu3 | Induced by stress conditions and relating to antibiotic resistance | — | 2 | 1 | 3 | 10.7 | 334/392 (381) | van Wezel et al (1994) |
| Other | |||||||||
| SCO1294 | Cystathionine γ-synthase | Cys/Met metabolism; selenate tolerance | — | 8 | 1 | 7 | 25.3 | 352/407 (405) | |
| SCO2582 | Peptidase | Peptidase M48, Ste24 family; highly conserved in actinomycetes | — | 5 | 4 | 5 | 9.7 | 335/402 (399) | |
| Non-specific binding (naturally His-rich proteins, which were also found in the control strain) | |||||||||
| SCO1858 | Hypothetical protein | Unknown function; His-rich carboxy-terminus | 7 | 29 | 10 | 21 | 66.6 | 272/305 (272) | |
| SCO6549 | Hypothetical protein | Unknown function; His-rich amino-terminus | 2 | 2 | 3 | 4 | 20.7 | 264/314 (296) | |
| Proteins were identified by capillary LC-MS/MS. Most proteins that are His-tagged by the tmRNA-mediated trans-translation mechanism in a strain expressing the tmRNA-His variant are important cell-cycle control or stress-related proteins. All targets are highly conserved in all streptomycetes. | |||||||||
| *Total number of tandem mass spectra matched to the protein as rank 1 hits by Sequest (sample 1) or Mascot (samples 2 and 3). | |||||||||
| ‡Total sum of unique peptides (that is, not sharing the same sequence) found in the three LC-MS/MS runs; only proteins identified with ⩾3 unique peptides in these three runs are reported. | |||||||||
| §Position of the last identified residue compared with the full length of the protein; the position before the last trypsin cleavage site in the protein is given in brackets. | |||||||||
| EF-Tu3, elongation factor Tu3; LC-MS/MS, liquid chromatography–tandem mass spectrometry; ScoDB, Streptomyces coelicolor database; Ssg, sporulation of Streptomyces griseus; TipA, thiostrepton antibiotic-induced protein; tmRNA, transfer–messenger RNA; Usp, universal stress protein | |||||||||
Translation of dnaK and dasR transcripts depends on tmRNA
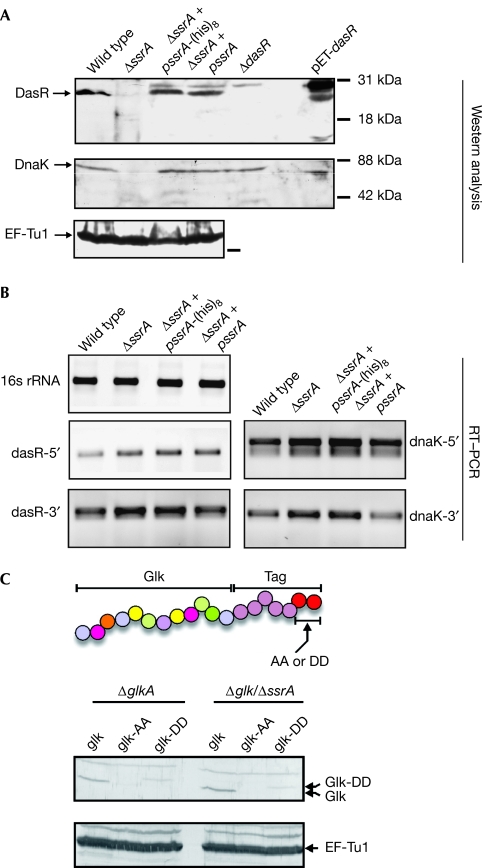
The unexpected outcome that only a few stress and cell-cycle control proteins are tagged and that most proteins are tagged at or near the end of the full-length amino-acid sequence, prompted an investigation into the fate of the tagged proteins in vivo. For this, steady-state levels of the main tmRNA targets DasR and DnaK were analysed by western blotting, using samples isolated from exponentially growing liquid cultures. Much to our surprise, both proteins were hardly detectable in a tmRNA-deficient strain, whereas wild-type protein levels were observed in extracts obtained from ssrA mutants complemented by the expression of wild-type tmRNA or tmRNA-His (Fig 2A). Streptomycetes can survive depletion of DnaK, but the protein is essential for germination and under heat-shock conditions (Bucca et al, 2003). In line with this, ssrA mutants cannot survive elevated temperatures (39°C or higher) and tmRNA tagging strongly increases during development. Quantitative RT–PCR was performed on RNA samples prepared from the same cultures as those used for western analysis, with two different primer pairs for both dnaK and dasR, recognizing sections near the 5′ and 3′ ends of the transcripts, respectively. Thus, steady-state transcript levels were not affected, suggesting that the translation of dasR and dnaK transcripts is defective in ssrA mutants (Fig 2B).
Figure 2.
Translation, but not transcription, of dasR and dnaK depends on tmRNA tagging. (A) Proteins were analysed by western blotting using DasR, DnaK or EF-Tu1 antibodies. Escherichia coli overexpressing DasR (pET-dasR) was used as a control (E. coli DnaK is smaller than that of Streptomyces coelicolor). (B) RT–PCR of total RNA from the different strains, demonstrating that transcription of the dasR and dnaK genes is not affected in ssrA mutants. (C) Engineered carboxy-terminally tagged glucose kinase is degraded in a canonical manner. Protein extracts from transformants expressing wild-type Glk, GlkAA or GlkDD were separated by 12% SDS–PAGE and immunodetected with Glk antibodies. As a control, EF-Tu1 antibodies were used. The experiment demonstrates that tmRNA-tagged proteins are efficiently degraded in Streptomyces. A schematic representation of the translational fusion of wild-type tmRNA tag (AA; giving GlkAA) or a mutated tmRNA tag (DD; giving GlkDD) to glucose kinase is shown. DasR, development of aerial mycelium and spores regulator; EF, elongation factor; Glk, glucose kinase; RT–PCR, reverse transcriptase PCR; SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel electrophoresis; tmRNA, transfer–messenger RNA.
To assess whether, similarly to other bacteria, tmRNA-tagged proteins are rapidly degraded in streptomycetes, we used glucose kinase (Glk, encoded by glkA) as a reporter system. The glkA gene was engineered by gene synthesis so as to fuse the S. coelicolor ssrA tag sequence (including the stop codon) directly downstream from the last codon of the glkA mRNA. In this way, a fusion product of Glk, designated GlkAA, is expressed with the wild-type tmRNA tag sequence at its C-terminal end. As a control, we synthesized a similar glkA–ssrA tag fusion gene but encoding a tag ending with two aspartates (GlkDD), which should be resistant to degradation (Keiler et al, 1996). Western analysis of cellular extracts prepared from S. coelicolor M600 ΔglkA and S. coelicolor M600 ΔglkA/ΔssrA (B.A.T and G.P.vW., unpublished data), expressing Glk, GlkAA or GlkDD, showed that GlkAA was readily degraded, whereas steady-state levels of GlkDD were much higher (Fig 2C). This indicates that tmRNA-tagged proteins are degraded in a canonical manner in streptomycetes.
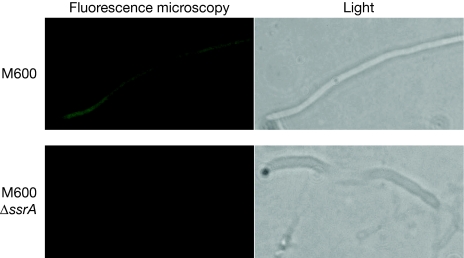
The experiment above suggested that the signal for tmRNA rescue somehow lies in the C-terminal (termination) region of the target genes. To test this hypothesis, we created the reporter construct pGWS537 through gene synthesis, by fusing the termination region of dasR (nucleotide positions +180/+180 relative to the stop codon) behind the gene for enhanced green fluorescent protein (EGFP), with the fusion gene expressed from the ftsZ promoter (the DNA sequence of the insert of pGWS537 is shown in supplementary Fig S5 online). Excitingly, EGFP now became tmRNA dependent, as there was abundant EGFP expression in the wild-type strain S. coelicolor M600, but low levels in the ssrA mutant (Fig 3). With relative fluorescence of 32.6±4.0 for expression in M600 and 6.3±0.4 for M600 ΔssrA (P<0.005), expression was 5–6 times lower in the absence of tmRNA. In a control experiment, EGFP was expressed without the dasR 3′ untranslated region. Expectedly, in the absence of the dasR termination region, EGFP was expressed at comparable levels in wild-type (36.8±2.3) and in tmRNA mutant cells (27.8±1.2), or a 1.3-fold decrease in the mutant (in total, 100 individual spots were measured in 10 hyphae (P<0.005).
Figure 3.
The dasR 3′ UTR dictates tmRNA dependence of egfp translation. Differential expression of a reporter (for DNA sequence see supplementary Fig S5 online), consisting of egfp fused to the 3′ UTR of dasR expressed from the ftsZ promoter in M600 (top) and its ssrA null mutant (bottom). In all hyphae analysed, the expression was much higher in the parent S. coelicolor M600 than in its ssrA mutant derivative (fivefold to sixfold; P<0.005), whereas in the absence of the dasR tag the EGFP expression was only 1.3-fold higher. This strongly suggests that the region around the dasR stop codon contains an intrinsic element that makes gene expression dependent on tmRNA (see Fig 5). EGFP, enhanced green fluorescent protein; tmRNA, transfer–messenger RNA; UTR, untranslated region.
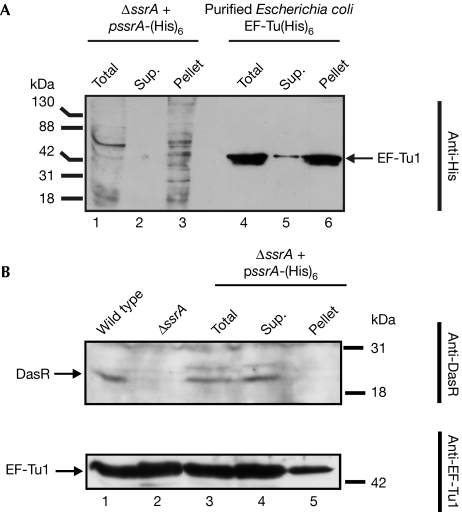
To analyse what proportion of the target proteins is tagged by tmRNA, a pulldown assay was performed using Ni2+–nitrilotriacetic acid affinity chromatography on cell extracts of S. coelicolor ΔssrA complemented with tmRNA-His (Fig 4). Probing with His antibodies indicated complete binding of all tagged proteins (Fig 4A, lanes 2 and 3), and in a second control experiment, in which a large amount of purified EF-Tu1–(His)6 was used, demonstrating that even for such large amounts of His-tagged protein the column capacity was not limiting. Nonetheless, the amount of DasR protein in the bound fraction was below the detection limit in a pulldown assay with DasR antibodies, which shows that only a minute fraction of the total DasR protein pool is tagged by tmRNA in vivo (Fig 4B). Thus, only a small proportion of DasR is tagged by tmRNA during translation and subsequently degraded.
Figure 4.
Efficiency of tagging of tmRNA targets. ΔssrA expressing tmRNA-His was used to estimate the fraction of DasR protein, which becomes tagged by the tmRNA-mediated trans-translation mechanism. (A) Western analysis using His antibodies of tmRNA-tagged proteins and of purified EF-Tu1(His)6 (excess of 10 pmol; control for column capacity). (B) Analysis of tmRNA-tagged fractions using DasR and EF-Tu1 antibodies and compared with Streptomyces coelicolor M600 and its ssrA mutant. The amount of protein loaded for this experiment was equivalent to 100 μl of culture. DasR, development of aerial mycelium and spores regulator; EF, elongation factor; pellet, bound fraction; sup., unbound fraction; tmRNA, transfer–messenger RNA; total, extract before separation.
In summary, our data strongly suggest a new tmRNA-based suicide rescue mechanism that allows an instantaneous response to stress conditions. A transcript pre-loaded with ribosomes provides a jump start for a rapid response to changing conditions, circumventing the need for the rate-limiting processes of translation initiation (in the order of minutes) and translation elongation (at least 40 s for a dnaK transcript at the rate of 15 codons/s). In addition, polysomes will protect the transcripts from degradation. This mechanism might also explain specific translational control mechanisms that are active in other bacteria, such as the situation in E. coli in which translation of the stress RNA polymerase-σ factor RpoS depends on the presence of tmRNA (Ranquet & Gottesman, 2007).
A speculative model
The main question that remains unanswered is what causes the unprecedented specificity of the tmRNA system in streptomycetes. We propose a structural element that affects the stalling of ribosomes near the 3′ end of the mRNAs, mediated by binding of a protein or small non-coding RNA. Such a structural element in the mRNA would be removed during trans-translation through ‘edge cleavage', which probably cleaves the mRNA 10–20 bases 3′ to the A site (Sunohara et al, 2004; Li et al, 2006). During stress tmRNA is induced, and after trans-translation the first ribosome is removed. This and the removal of the proposed structural element allow a rapid burst of translation by already loaded and translation-committed polysomes (Fig 5). The exact mechanism that forces the stalling of ribosomes specifically on mRNAs of important cell-cycle and stress-control proteins is under investigation.
Figure 5.
Model for tmRNA-mediated translational control in Streptomyces coelicolor. Ribosomes translating mRNAs, such as that of dasR or dnaK, are stalled at the 3′ end, and the first nascent peptide chain becomes tagged for proteolytic degradation. During this tagging process the mRNA is cleaved to remove a yet unknown structural element in the mRNA. As a result, the ribosomes are able to finish translation without further tmRNA tagging. This mechanism allows the instantaneous production of crucial stress proteins after stress. See Discussion section for details. mRNA, messenger RNA; tmRNA, transfer–messenger RNA.
Methods
Strains and culturing conditions. E. coli JM109 (Sambrook et al, 1989) and ET12567 (Kieser et al, 2000) were used for routine cloning procedures. S. coelicolor M600 was obtained from the John Innes Centre, Norwich, UK. All media and routine Streptomyces techniques are described in the Streptomyces manual (Kieser et al, 2000). SFM (soy flower mannitol) agar plates were used for making spore suspensions, R2YE (regeneration media with yeast extract) agar plates for selecting recombinants and YEME (yeast extract/malt extract), tryptone soy broth and minimal media for Streptomyces cultivation and plasmid isolation (Kieser et al, 2000).
Plasmids and gene replacement. The tmRNA disruption construct, pΔssrA, contains the −1333/−13 and +441/+1605 regions relative to the start of ssrA in pSET151 (Kieser et al, 2000; supplementary Fig S1 and Table S1 online). pssrA-(His)8 was constructed by fusion PCRs using strep1 and strep5-3, and strep2 and strep4-3, producing a 2,950 bp fragment encompassing a mutated ssrA gene and approximately 1350 bp upstream and approximately 1200 bp downstream region in pSET151 (for gene disruption) or into the low-copy vector pHJL401 (for complementation; Kieser et al, 2000). pΔssrA was conjugated to S. coelicolor M600 and double recombination events were screened by loss of thiostrepton resistance. The mutants were checked by PCR using strep6 and strep9 and by Southern hybridization with a 32P-labelled PCR fragment (oligonucleotides strep14 and strep15) as probe (supplementary Fig S1 online). For details about construction of pGWS537, see supplementary information online.
Western analysis. Samples (1 ml) were taken from transition phase liquid cultures, sonicated and proteins detected by western blot analysis using His (TebuBio, Paris, France), EF-Tu1, DasR, DnaK and Glk antibodies (van Wezel et al, 2007).
Protein sample preparation and LC-MS. S. coelicolor ΔssrA expressing wild-type tmRNA or tmRNA-His were grown in TSBS with 5 μg/ml thiostrepton until mid-log phase. Cells were collected, sonicated in buffer (0.1 M NaH2PO4, 10 mM Tris-base, 8 M urea; pH adjusted to 8.0) and cleared supernatant bound to Ni2+–nitrilotriacetic acid. The beads were subsequently washed rigorously four times with 15 ml of buffer (pH 6.3) and eluted with the same buffer at pH 5.5 or pH 4.5. The fractions were pooled, pH set to 8.0 and purification was repeated under identical conditions. Detailed procedures for proteomics experiments and LC-MS analysis are presented in the supplementary information online. The LC-MS/MS analysis of proteins was performed as described previously (Beck et al, 2006).
RT–PCR. The RNA was extracted from cells grown in liquid cultures as described by the supplier (Promega, Madison, WI, USA). Primers for quantitative RT–PCR (Invitrogen, Carlsbad, CA, USA) for the detection of dasR and dnaK mRNAs and for the control 16S ribosomal RNA are listed in supplementary Table S1 online. For the detection of tmRNA, quantitative RT–PCR was performed using primers strep19 and strep20 that hybridized to the tmRNA extremities.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Fig 1–5 and Table 1
Acknowledgments
We thank P. Mazodier (Paris, France) and S. Douthwaite (Odense, Denmark) for discussions; and G. Bucca and C. Smith (Surrey, United Kingdom), and F. Titgemeyer (Erlangen, Germany) for antibodies and discussions. The study was supported by grants from the Netherlands Organization for Scientific Research (a VENI grant to S.B. and an ECHO grant to G.P.vW.), and by grants from the Danish Research Agency and the European Union (MRTN-CT-2005-019566) to M.Z. and O.N.J.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abo T, Inada T, Ogawa K, Aiba H (2000) SsrA-mediated tagging and proteolysis of LacI and its role in the regulation of lac operon. EMBO J 19: 3762–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck HC, Nielsen EC, Matthiesen R, Jensen LH, Sehested M, Finn P, Grauslund M, Hansen AM, Jensen ON (2006) Quantitative proteomic analysis of post-translational modifications of human histones. Mol Cell Proteomics 5: 1314–1325 [DOI] [PubMed] [Google Scholar]
- Braud S, Lavire C, Bellier A, Mazodier P (2006) Effect of SsrA (tmRNA) tagging system on translational regulation in Streptomyces. Arch Microbiol 184: 343–352 [DOI] [PubMed] [Google Scholar]
- Bucca G, Brassington AM, Hotchkiss G, Mersinias V, Smith CP (2003) Negative feedback regulation of dnaK,clpB and lon expression by the DnaK chaperone machine in Streptomyces coelicolor, identified by transcriptome and in vivo DnaK-depletion analysis. Mol Microbiol 50: 153–166 [DOI] [PubMed] [Google Scholar]
- Flärdh K, Buttner MJ (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7: 36–49 [DOI] [PubMed] [Google Scholar]
- Guisbert E, Herman C, Lu CZ, Gross CA (2004) A chaperone network controls the heat shock response in E. coli. Genes Dev 18: 2812–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F, Kuriyan J (1997) Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science 276: 431–435 [DOI] [PubMed] [Google Scholar]
- Kahmann JD, Sass HJ, Allan MG, Seto H, Thompson CJ, Grzesiek S (2003) Structural basis for antibiotic recognition by the TipA class of multidrug-resistance transcriptional regulators. EMBO J 22: 1824–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzai AW, Roche ED, Sauer RT (2000) The SsrA–SmpB system for protein tagging, directed degradation and ribosome rescue. Nat Struct Biol 7: 449–455 [DOI] [PubMed] [Google Scholar]
- Keiler KC, Shapiro L (2003) tmRNA in Caulobacter crescentus is cell cycle regulated by temporally controlled transcription and RNA degradation. J Bacteriol 185: 1825–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiler KC, Waller PR, Sauer RT (1996) Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271: 990–993 [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces Genetics. Norwich, UK: John Innes Foundation [Google Scholar]
- Li X, Hirano R, Tagami H, Aiba H (2006) Protein tagging at rare codons is caused by tmRNA action at the 3′ end of nonstop mRNA generated in response to ribosome stalling. RNA 12: 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SD, Sauer RT (2007) The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem 76: 101–124 [DOI] [PubMed] [Google Scholar]
- Noens EE, Mersinias V, Willemse J, Traag BA, Laing E, Chater KF, Smith CP, Koerten HK, van Wezel GP (2007) Loss of the controlled localization of growth stage-specific cell-wall synthesis pleiotropically affects developmental gene expression in an ssgA mutant of Streptomyces coelicolor. Mol Microbiol 64: 1244–1259 [DOI] [PubMed] [Google Scholar]
- Noens EE, Mersinias V, Traag BA, Smith CP, Koerten HK, van Wezel GP (2005) SsgA-like proteins determine the fate of peptidoglycan during sporulation of Streptomyces coelicolor. Mol Microbiol 58: 929–944 [DOI] [PubMed] [Google Scholar]
- Nystrom T, Neidhardt FC (1992) Cloning, mapping and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol Microbiol 6: 3187–3198 [DOI] [PubMed] [Google Scholar]
- Paget MS, Molle V, Cohen G, Aharonowitz Y, Buttner MJ (2001) Defining the disulphide stress response in Streptomyces coelicolor A3(2): identification of the sigmaR regulon. Mol Microbiol 42: 1007–1020 [DOI] [PubMed] [Google Scholar]
- Paleckova P, Bobek J, Felsberg J, Mikulik K (2006) Activity of translation system and abundance of tmRNA during development of Streptomyces aureofaciens producing tetracycline. Folia Microbiol (Praha) 51: 517–524 [DOI] [PubMed] [Google Scholar]
- Ranquet C, Gottesman S (2007) Translational regulation of the Escherichia coli stress factor RpoS: a role for SsrA and Lon. J Bacteriol 189: 4872–4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack DM, Friedman DI (1995) A role for a small stable RNA in modulating the activity of DNA-binding proteins. Cell 83: 227–235 [DOI] [PubMed] [Google Scholar]
- Rigali S et al. (2006) The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol Microbiol 61: 1237–1251 [DOI] [PubMed] [Google Scholar]
- Rigali S, Titgemeyer F, Barends S, Mulder S, Thomae AW, Hopwood DA, van Wezel GP (2008) Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep 9: 670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche ED, Sauer RT (2001) Identification of endogenous SsrA-tagged proteins reveals tagging at positions corresponding to stop codons. J Biol Chem 276: 28509–28515 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Sunohara T, Jojima K, Tagami H, Inada T, Aiba H (2004) Ribosome stalling during translation elongation induces cleavage of mRNA being translated in Escherichia coli. J Biol Chem 279: 15368–15375 [DOI] [PubMed] [Google Scholar]
- Traag BA, Kelemen GH, Van Wezel GP (2004) Transcription of the sporulation gene ssgA is activated by the IclR-type regulator SsgR in a whi-independent manner in Streptomyces coelicolor A3(2). Mol Microbiol 53: 985–1000 [DOI] [PubMed] [Google Scholar]
- van Wezel GP, Woudt LP, Vervenne R, Verdurmen MLA, Vijgenboom E, Bosch L (1994) Cloning and sequencing of the tuf genes of Streptomyces coelicolor A3(2). Biochim Biophys Acta 1219: 543–547 [DOI] [PubMed] [Google Scholar]
- van Wezel GP, Takano E, Vijgenboom E, Bosch L, Bibb MJ (1995) The tuf3 gene of Streptomyces coelicolor A3(2) encodes an inessential elongation factor Tu that is apparently subject to positive stringent control. Microbiology 141: 2519–2528 [DOI] [PubMed] [Google Scholar]
- van Wezel GP, van der Meulen J, Kawamoto S, Luiten RGM, Koerten HK, Kraal B (2000) ssgA is essential for sporulation of Streptomyces coelicolor A3(2) and affects hyphal development by stimulating septum formation. J Bacteriol 182: 5653–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezel GP, Konig M, Mahr K, Nothaft H, Thomae AW, Bibb M, Titgemeyer F (2007) A new piece of an old jigsaw: glucose kinase is activated posttranslationally in a glucose transport-dependent manner in Streptomyces coelicolor A3(2). J Mol Microbiol Biotechnol 12: 67–74 [DOI] [PubMed] [Google Scholar]
- Wickner S, Hoskins J, McKenney K (1991) Function of DnaJ and DnaK as chaperones in origin-specific DNA binding by RepA. Nature 350: 165–167 [DOI] [PubMed] [Google Scholar]
- Yang C, Glover JR (2009) The SmpB-tmRNA tagging system plays important roles in Streptomyces coelicolor growth and development. PLoS ONE 4: e4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1–5 and Table 1